Abstract
Yellow Himalayan raspberry, a wild edible fruit, was analyzed for phenolic contents, and antioxidant, antibacterial and antiproliferative activities. Phenolics were extracted using 80 % aqueous solvents containing methanol, acidic methanol, acetone and acidic acetone. Our analysis revealed that the acidic acetone extracts recovered the highest level of total phenolics (899 mg GAE/100 g FW) and flavonoids (433.5 mg CE/100 g FW). Free radical scavenging activities (DPPH, ABTS, superoxide and linoleate hydroperoxide radicals) and ferric reducing activity were highest in the acetone and acidic acetone extracts. No metal chelating or antibacterial activity was detected in any of the extracts. Acetone and methanol extracts showed potent antiproliferative activity against human cervical cancer cells (C33A) with an EC50 of inhibition at 5.04 and 4. 9 mg/ml fruit concentration respectively, while showing no cytotoxicity to normal PBMCs cells. Therefore, the present study concluded that the yellow Himalayan raspberry is a potent source of phytochemicals having super antioxidant and potent antiproliferative activities.
Keywords: Polyphenols, Antioxidant activity, Antiproliferative, Phenolics, Flavonoids, Raspberry
Introduction
Cell damage caused by free radicals appears to be a major contributor to aging and degenerative diseases such as cancer, cardiovascular diseases, cataracts, diabetes, immune system decline and brain dysfunction (Sies 1992; Vendemiale et al. 1999). To protect the cell from damage caused by free radicals, cells have evolved a highly sophisticated and complex antioxidant protection system which involves a variety of components, both endogenous and exogenous in origin, that function interactively and synergistically to neutralize free radicals (Jacob 1995). Among the various types of exogenous antioxidants, phenolic compounds constitute the major group of phytonutrients with multiple biological effects including anti-inflammatory, anti-allergic, antiviral, anti-aging and anti-carcinogenic activities (Cody et al. 1986; Kuhnau 1976; Middleton and Kandaswami 1986; Peterson and Dwyer 1998; Gulcin 2012). A wide variety of fruits such as blueberry, blackberry, raspberry, pomegranate, plum, peach, apple, quince, pear, guava, banana and citrus fruits have been shown to possess high phenolic contents and super antioxidant activities (Wang et al. 1996; Gil et al. 2002; Kahkonen et al. 2001; Moyer et al. 2002; Karadeniz et al. 2005; Mokbel and Hashinaga 2005; Lim et al. 2007; Meda et al. 2008). Additionally, fruits phenolics have also been demonstrated to possess strong antiproliferative properties (Johnson et al. 2011; Sun et al. 2002; Liu et al. 2002; Seerama et al. 2005). Due to the cancer protective effects, and super antioxidant properties, fruits are gaining world wide interest for the exploration of their polyphenolic contents and their various health promoting properties (Kahkonen et al. 2001; Leong and Shui 2002; Moyer et al. 2002; Karadeniz et al. 2005; Marinova et al. 2005; Barreira et al. 2008; Lim et al. 2007; Meda et al. 2008).
R. ellipticus, Smith. (Family Rosaceae), commonly known as yellow Himalayan raspberry (Yellow Hissar), is one of the common wild edible fruits of the Uttarakhand, India (Samant and Dhar 1997). Although fruits of R. ellipticus are shown to be highly nutritious, delicious, and rich in vitamins and sugars (Parmar and Kaushal 1982), their antioxidant and antiproliferative potentials remain under explored. Therefore, the present study is aimed to (1) assess the total phenolics and flavonoid contents in the fruits of R. ellipticus using different solvent systems; (2) to evaluate the extracts for antioxidant activities using various biochemical assays; (3) determine the antibacterial and antiproliferative activities of the extracts.
Material and methods
Chemicals and reagents
All the chemicals were of analytical grade and more than 99 % pure. 2, 2-Diphenyl-1-picrylhydrazyl (DPPH), catechin, nicotinamide adenine dinucleotide (NADH), phenyl methosulfate (PMS), nitro blue tetrazolium (NBT), β-carotene, linoleic acid and ferrozine were procured from Sigma–Aldrich (Steinheim, Germany). 2,2′-Azinobis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) was obtained from Calbiochem, Merck Company (Darmstadt, Germany). Other chemicals and reagents were purchased from HiMedia Pvt Ltd (Mumbai, India).
Collection, identification and authentication of the R. ellipticus fruit
Fresh ripe fruit samples (along with the small twig containing leaves) of R. ellipticus (yellow hissar), were harvested from Narender Nagar locations of Tehri Garhwal, Uttarakhand, India. Fruits were cleaned under running tap water and kept at −20°C till use. The herbarium of the R. ellipticus fruit was deposited to Systematic Botany Division, Forest Research Institute (FRI), Deharadun, Uttarakhand, India for botanical identification and authentication.
Preparation of extracts
Phenolic compounds were extracted by four different aqueous solvents namely 80 % each of methanol; acid methanol (pH 2.0); acetone and acid acetone (pH 2.0) (Meda et al. 2008). Briefly, 25 g frozen fruits with seeds were homogenized in a mixer grinder for 5 min to make a homogeneous slurry. The fruit slurry (5 g) was extracted thrice with 25 ml of each solvent for 30 min with constant stirring at room temperature (RT). The extracts were filtered, pooled and centrifuged to obtain the clear extracts. The clarified extracts were stored at −20 °C prior to use within 1 month.
Evaluation of total phenolics and total flavonoid contents
Total phenolic contents were determined by the Folin-Ciocalteau method (Singleton et al. 1999). Each extracts (0.1 ml) was mixed with Folin-Ciocalteau reagent (0.2 N, 2.5 ml) and allowed to stand at RT for 5 min. Thereafter, sodium carbonate solution (75 g/l in water, 2 ml) was added. After 2 h of incubation, the absorbance was measured using UV–vis spectrophotometer (Model No. 119, Systronics, India) at 760 nm against a water control. A standard calibration curve was plotted using gallic acid (0–200 mg/l). The total phenolic contents were expressed as mg of gallic acid equivalents (GAE)/100 g of frozen fruit.
Total flavonoid contents were determined according to Meda et al. (2008). The diluted extract (6.0 ml) was mixed with sodium nitrite solution (5 %, 0.3 ml) and incubated for 5 min at RT. Afterwards, aluminium trichloride solution (10 %, 0.6 ml) was added and incubated further for 5 min at RT. The absorbances of the reaction mixtures were measured at 510 nm against a water blank. A standard calibration curve of catechin (0.5 mg/ml in 80 % methanol) was plotted and the results were expressed as mg catechin equivalents (CE)/100 g FW.
Assessment of free radical activity
DPPH radical scavenging actvity
The DPPH free radical scavenging activity was determined according to Singh et al. (2002). The diluted extract (0.1 ml) was mixed with DPPH solution, (5 ml, 0.1 mM in methanol) and allowed to stand in dark at RT for 20 min. The control was prepared as above without any extract. The reduction in the absorbance of the control and samples was measured at 517 nm against a water blank. The DPPH free radical scavenging activity was expressed as mg CE/100 g FW.
ABTS cation radical scavenging activity
The ABTS cation radical scavenging activity was determined as described (Barreira et al. 2008). The diluted fruit extracts (50 μl) were allowed to react with fresh ABTS solution (3.0 ml) for 6 min, and then absorbance was measured at 734 nm. BHA was used as standard and the capacity of ABTS cation radical scavenging activity was expressed as mg BHA equivalent (BHAE)/100 g FW.
Superoxide anion radicals scavenging activity
Superoxide radical scavenging was determined using PMS-NADH systems (Liu et al. 1997). The reaction mixture contained Tris–HCl buffer (3 ml, 16 mM, pH 8.0), NBT (1 ml, 50 μM), NADH (1 ml, 78 μM) and the fruit extracts of varying dilutions in water. The reactions were initiated by addition of PMS (1 ml, 10 μM) and incubated further in the dark at 25°C for 5 min. The absorbance was measured at 560 nm against a water control. The superoxide anion scavenging activity was determined as mg of ascorbic acid equivalents (AAE)/100 g FW.
Ferric reducing power assay
Ferric reducing power assay was performed according to Barreira et al. (2008). The diluted extract was mixed with sodium phosphate buffer (2.5 ml, 200 mM, pH 6.6) and potassium ferricyanide solution (1 % w/v, 2.5 ml) and incubated at 50 °C for 20 min. Thereafter, TCA (2.5 ml, 10 % w/v) was added and mixture was centrifuged. The upper layer (5 ml) was removed, mixed with distilled water (5 ml) and ferric chloride solution (1 ml, 0.1 % w/v). Subsequently, the absorbance was recorded at 700 nm and the ferric reducing activity was expressed as mg of AAE/100 g FW.
Inhibition of β-carotene bleaching
The inhibition of β-carotene bleaching was evaluated by the β - carotene linoleate model system (Velioglu et al. 1998). β-carotene solution (2 ml, 0.2 mg/ml in chloroform) was pipetted into a round-bottom flask. After the removal of chloroform at 40°C under vacuum, 40 mg of linoleic acid, 400 mg of Tween 80 emulsifier, and 100 ml of distilled water were added to the flask with vigorous shaking. Aliquots (4.9 ml) of this emulsion were incubated with the diluted extracts (0.1 ml) at 50°C in a water bath. Absorbance (470 nm) was recorded at zero time and at 20 min regular interval until the control sample has changed the color. A blank, devoid of β-carotene, was also prepared for background subtraction.
Ferrous metal ion chelating activity
In ferrous metal chelating activity assay (Dinis et al. 1994), diluted fruit extract (1 ml) was mixed with ferric chloride solution (0.05 ml of 2 mM) and ferrozine solution (2 ml, 5 mM). After 10 min of incubation the absorbance (562 nm) was recorded against water blank. The metal chelating activity was expressed as mg EDTA equivalent chelating activity (EE)/100 g FW.
Antibacterial activity
Antibacterial activity was carried out by determining the zone of inhibition through the disc diffusion method (Gulcin et al. 2010b). 100 μl (~107 cells) each of the test bacteria (Escherichia coli MTCC739, Bacillus subtilis MTCC441, and Staphylococcus aureus MTCC 96) were spread plated on nutrient agar plate. Sterilized filter paper discs (Whatmann No # 1, 5 mm in diameter), each impregnated with the undiluted fruit extracts (1 ml) for 48 h were placed on test organism spreaded plates. Simultaneously, streptomycin (10 mcg/disc) and respective solvents used as positive and negative control. The bacterial cultures were incubated at 37 °C for 24 h, and the zones of inhibition were measured in mm.
Inhibition of cancer cell proliferation
Antiproliferative potentials of R. ellipticus fruit extracts were analyzed against two human cervical cancer cell lines, namely, C33A and HeLa cell lines (American Type Culture Collection, ATCC, Pune, India). Peripheral Blood Mononuclear Cells (PBMCs) isolated from the blood of a healthy individual. The primary culture was established in RPMI 1,640 medium supplemented with 10 % FBS. PBMCs were used as a negative control to analyze the extracts induced cytotoxicity. C33A cells were maintained in Minimum Essential Medium (MEM) while, HeLa cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10 % fetal bovine serum (FBS), 1 % antibiotic and antimycotic (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere with 5 % CO2 at 37 °C. Cell concentration of 5 × 103 in the respective phenol free culture media were placed in each well of a 96 well flat bottom plate and allowed to grow for 24 h at 37 °C in 5 % CO2. After incubation, the medium was removed and fresh media (100 μl) containing various concentrations of R. ellipticus extracts were added to the well. Control cultures received the extraction solution minus the fruit extract and blank wells contain 100 μl of growth medium with no cells. After the 48 h of further growth at 37 °C in 5 % CO2, cell proliferation was determined using colorimetric MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazoliumbromide based cell titer 96 nonradioactive cell proliferation assay]. Absorbances were read at 450 nm (Fluostar Omega Spectrofluorometer, BMG Technologies, Offenburg, Germany). Each extract was checked at least in triplicate to determine the percent cell proliferation values.
HPLC analysis of phenolic compounds
Extracts of R. ellipticus were analyzed using a high performance liquid chromatography (HPLC) system equipped with LC-20AT series pump (Shimadzu) and SPD-20A UV–VIS detector (Shimadzu) according to Rawat et al. (2010). The HPLC profiles of the extracts were compared with those of standard phenolics and flavonoids, namely catechin, gallic acid, caffic acid, ellagic acid, tannic acid and transcinnamic acid.
Statistical analysis
To rule out any discrepancy, three independent extractions were performed. The results were expressed as the means of three independent experiments. Microsoft Excel software was used to calculate catechin, gallic acid, ascorbic acid, BHA, and EDTA equivalents, inhibition percentage, linear equations and correlation co-efficient, while State Differences tests were determined using the SPSS software.
Results and discussion
Phenolic and flavonoid contents
The total phenolic contents were evaluated using gallic acid as standard (R2 = 0.9928), that between 550 and 899 mg GAE/100 g FW in the various extracts (Table 1). The phenolic contents were found to be highest in the acid-acetone extract (899 mg GAE/100 g FW) and lowest in 80 % methanol (550 mg GAE/100 g FW), and was intermediate in the acidic-methanol and acetone extracts (690 mg and 782.7 mg GAE/100 g of FW, respectively).
Table 1.
Total phenol and flavonoid contents in the R.ellipticus fruits extracted with different solvents and their antioxidant activities
| Activities in Yellow Himalayan Raspberries | Methanol (ReM) | Acid Methanol pH-2 (ReAM) | Acetone (ReA) | Acid Acetone pH-2 (ReAA) |
|---|---|---|---|---|
| Total Phenolics (mg GAE/100 g FW) | 550.0 ± 12.33a | 690.0 ± 31.12b | 782.7 ± 19.13c | 899.0 ± 4.78d |
| Total Flavonoids (mg CE/100 g FW) | 179.0 ± 8.32a | 276.6 ± 12.13b | 307.4 ± 12.31b | 433.5 ± 13.39c |
| DPPH Scavenging Activity (mg CE/100 g FW) | 359.2 ± 22.34a | 502.2 ± 26.33b | 619.3 ± 32.14c | 521.0 ± 9.48b |
| ABTS Scavenging Activity (mg BHAE/100 g FW) | 619.6 ± 31.13a | 704.9 ± 29.74a | 1072.6 ± 42.11b | 857.8 ± 38.91c |
| Superoxide anion scavenging activity (mg AAE/100 g FW) | 155.0 ± 18.32a | 565.6 ± 28.14b | 581.9 ± 11.32b | 1083.0 ± 2.23c |
| Ferric reducing activity (mg AAE/100 g FW) | 695.7 ± 11.91a | 956.7 ± 17.81b | 1193.2 ± 41.34c | 1389.8 ± 49.22d |
Each value is expressed as mean ± standard error (n = 3). Means with different letters within a row are significantly different (p < 0.05)
ReM Rubus ellipticus Methanol extract; ReAM Rubus ellipticus Acid Methanol extract; ReA Rubus ellipticus Acetonic extract; ReAM Rubus ellipticus Acid Acetonic extract. Total phenolic contents expressed as mg gallic acid equivalents per 100 g of Fruit weight. Total flavonoids contents and DPPH scavenging activity expressed as mg Catechin equivalents per 100 g of Fruit weight. ABTS scavenging activity expressed as mg Butylated hydroxyanisole per 100 g of Fruit weight. Superoxide anion scavenging activity and Ferric reducing activity expressed as mg Ascorbic acid per 100 g of Fruit weight
The total flavonoid content was determined using catechin as standard (R2 = 0.9994). The acidic-acetone fruit extract showed highest flavonoid content (433.5 mg CE/100 g FW) and 80 % methanol showed the lowest flavonoid content (179 mg CE/100 g FW). The total flavonoid content in the acid-methanol and 80 % acetone was found to be 276.56 and 307.38 mg CE/100 g FW respectively. These observations evidently demonstrated higher recovery of total phenolics and flavonoids in the acidified acetone and neutral acetone than that in the acid or neutral methanol extracts suggesting acetone as better solvent system for the optimum extraction of polyphenols from R. ellipticus fruits. Similar observations showing significantly higher extraction of polyphenols in the acetone extracts than in methanol extracts were earlier reported (Meda et al. 2008; Weber et al. 2008).
Antioxidant activity
The antioxidant activities of R. ellipticus fruit extracts were determined using various parameters such as free radical scavenging activities, ferric reducing and β-carotene bleaching inhibitory activities.
The DPPH test determines the antioxidant activity based on the ability of the phenols to neutralize the stable free radical DPPH (Gulcin et al. 2010a). The DPPH free radical scavenging activity ranged from 359.2 to 619.3 mg CE/100 g FW in various extracts (Table 1). It was highest in the acetone extract (619.3 mg CE/100 g FW) and lowest in methanol extract (359.2 mg CE/100 g FW), while intermediate in acidic methanol (502.2 mg CE/100 g FW) and acidic acetone (521 mg CE/100 g FW). The ABTS assay measures the ability of the fruit extracts to scavenge the cationic radical ABTS.+ produced by the oxidation of ABTS. The blue green colored ABTS.+ cation radicals are reduced to its non-radical, colorless ABTS form by the fruit antioxidants. We observed significantly high level of ABTS scavenging activity in all the extracts. It was highest in the acetone extract (1072.6 mg BHAE/100 g FW) and lowest in methanol extract (619.6 mg BHAE/100 g FW), and was intermediate in the acidic methanol and acidic acetone extracts (704.9 and 857.8 mg BHAE/100 g FW) (Table 1).
Superoxide (O−2) is the reactive oxygen radical produced by one-electron reduction of molecular oxygen in metabolic processes and is the major source of other radicals (Liu et al. 1997). Therefore, it was very important to examine the superoxide anion radical scavenging ability of R. ellipticus fruit extracts. This assay determines the ability of fruit extracts to neutralize the superoxide anion radicals generated in the PMS/NADH system which subsequently leads to a decrease in the reduction of nitroblue tetrazolium to a chromogenic product. The highest superoxide scavenging activity was seen in the acidic acetone extract (1,083 mg AAE/100 g FW) and lowest in the methanol extracts (155 mg AAE/100 g FW). The levels were found to be 565.6 and 581.98 mg AAE/100 g FW in acidic methanol and acetone extracts respectively.
The ferric reducing assay measures the ability of phenolics to reduce Fe3+ to Fe2+. The ferric reducing activity ranged from 695.7 to 1389.8 mg AAE/100 g FW in various extracts (Table 1). In this assay, the methanol extract showed a significantly lower value compared to the acetone extract. Among the methanolic extracts, the methanol extracts exhibited lower ferric reducing activity (695.7 mg AAE/100 g FW) than the acidic methanol extracts (956.7 mg AAE/100 g FW). Similarly the acid acetone extract exhibited higher ferric reducing activity (1389.82 mg AAE/100 g FW) than the acetone extract (1193.26 mg AAE/100 g FW) (Table 1).
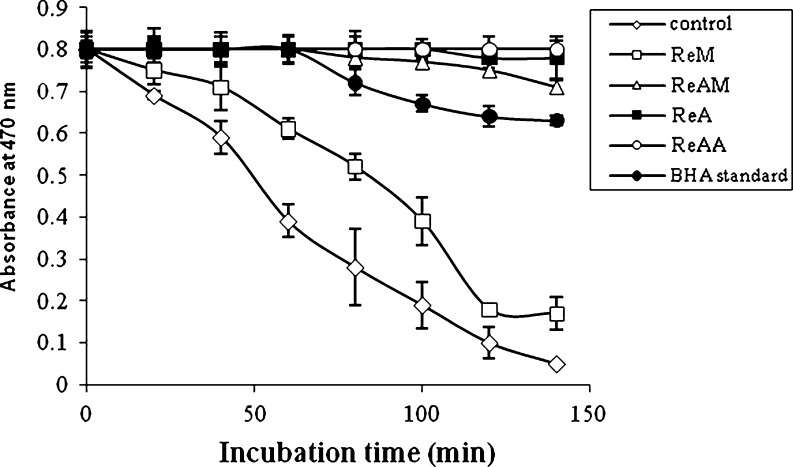
In the β-carotene bleaching test, linoleic acid produces hydroperoxides as free radicals during incubation at 50 °C. These interact with β-carotene, leading to its bleaching. Thus, the β carotene bleaching rate is inversely proportional to the antioxidant activity of the extracts that neutralize the free radical formed in the system. Inhibition of β carotene bleaching activity was much higher and for the prolonged period in acetone, acid acetone and in acid methanol extracts, in comparison to that of control, methanol extracts and standard BHA (Fig. 1). The ferrous ion chelating activity of the extracts was checked for all the extracts. The results showed lack of any activity among the extracts.
Fig. 1.
β-carotene bleaching inhibition activity of R. ellipticus fruits, extracted in different solvent systems. Each value is expressed as mean ± standard error (n = 3)
It was very much clear from the aforementioned observations that the free radical scavenging activities were higher in the acetone extracts as compared to that in the methanol extracts. The neutral and cation free radical scavenging activities (DPPH assay and ABTS assay), were found to be highest in the acetone extract while the ferric reducing activity, superoxide anion radical scavenging activity and inhibition of lipid peroxidation activities were found to be highest in the acidic acetone extract. This indicated that the polyphenol with high neutral and cation free radical scavenging activity, and those with high lipid peroxidation inhibition, ferric reducing activity and superoxide anion radical scavenging activity might be differentially extracted in neutral and acidified acetone. Significant similarities in phytochemical profile would however be expected to be obtained in both extracts.
Antibacterial activity
The antibacterial activitiy of R. ellipticus fruit extracts were studied using E. coli, B. subtilis and S. aureus. However, no zone of inhibition was demonstrated by any of the fruit extracts against any of the tested bacterial species
Inhibition of cancer cell proliferation
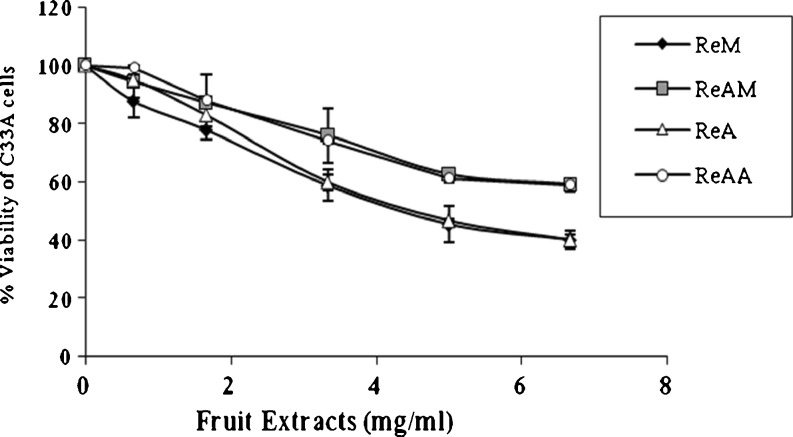
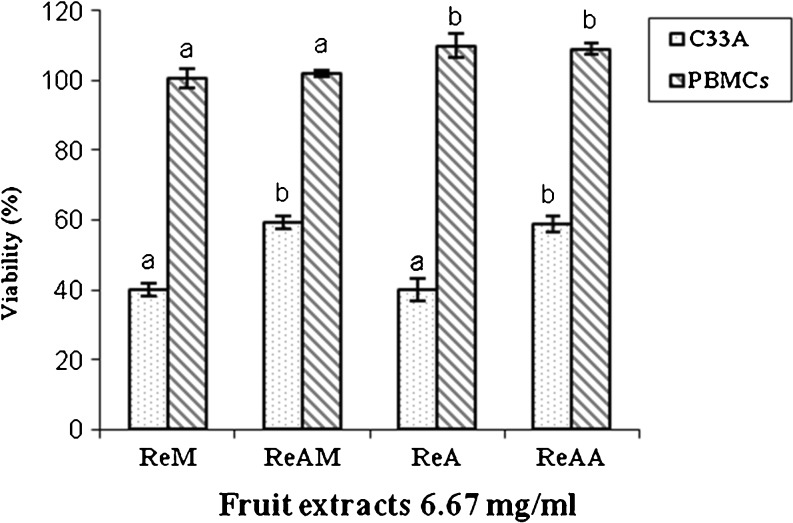
Antiproliferative activity of R. ellipticus fruit extracts were analyzed against two cervical cancer cell lines namely C33A, HeLa and one normal PBM Cells using MTT assays. C33A and HeLa, cells were cultured with an extract concentration equivalent to 0.667, 1.66, 3.33, 5.0 and 6.67 mg/ml of R. ellipticus fruit while primary culture of PBMCs were incubated with 5.0 and 6.67 mg/ml fruit extracts concentration. The extracts demonstrated a potent antiproliferative activity against C33A cells (40–60 %), while no inhibition activity was detected against HeLa cells. Inhibition of C33A cell proliferation was dose dependent, and the degree of inhibition was more or less similar for the different extracts (Fig. 2). Methanol and acetone extracts reduced the viability of C33A cells to 40 % showing ~60 % inhibition, while both the acidic extracts reduced the viability to 60 % demonstrating ~40 % inhibition of C33A proliferation (Fig. 2). The median effective concentration (EC50) was also lower for methanol and acetone extracts (4.9 and 5.04 mg/mL) as compared to that of acid methanol and acid acetone extracts (7.57 and 7.28 mg/ml). None of the extracts showed cytotoxicity to PBMCs rather demonstrated growth promoting effect on their proliferation (1–10 %). Methanol extracts showed negligible, while acetone extracts showed ~10 % enhancement in the proliferation of PBMCs (Fig. 3). This clearly indicated that the R. ellipticus fruit extracts possessed preferential anticancer activity against cervical cancer cells while remaining nontoxic to the normal cells.
Fig. 2.
Antiproliferative activities of R. ellipticus fruit extracts against C33A cervical cancer cells. Each value is expressed as mean ± standard error (n = 3)
Fig. 3.
Comparison of effects of R. ellipticus fruit extracts on C33A cervical cancer cells and PBMCs of a normal individual. Each value is expressed as mean ± standard error (n = 3). Means with different letters are significantly different (p < 0.05)
HPLC analysis of phenolic compounds
Out of six phenolics (namely catechin, caffic acid, ellagic acid, gallic acid, tannic acid and transcinnamic acid) analyzed, only the peaks of ellagic acid and gallic acid peaks were prominently detected in all the extracts. In addition, many other small but intense peaks with different retention times were also detected whose characterization are under study. We did not observe the presence of catechin, caffeic acid, tannic acid and transcinnamic acid in the any of the extracts of R. ellipticus fruit using the current protocol (Rawat et al. 2010).
The present study showed existence of a potent free radical scavenging activity along with strong ferric reducing and lipid peroxidation inhibition activities in the R. ellipticus fruit extracts. In addition, R. ellipticus fruit polyphenols also possessed potent anticancer activity against cervical cancer cells C33A. To the best of our knowledge, the present report is the first study showing the anticancer potential of the R. ellipticus fruits. It is very significant that the R. ellipticus fruit extracts exhibited no cytotoxicity on normal noncancerous cells, but rather showed stimulatory effects on their proliferation at the tested fruit extracts concentrations in which cervical cancerous cells were highly sensitive. The antiproliferative activity of R. ellipticus fruit extracts against cervical cancer cells is supported by the HPLC analysis which showed presence of high gallic acid and ellagic acid contents in the fruit extracts. Both the gallic acid and ellagic acid were earlier shown to possess antiproliferative activity against cervical cancer cells (You et al. 2010; Losso et al. 2004). It is also possible that differential cytotoxicity could be only elicited at the lower fruit extracts concentrations and the higher fruit concentrations might show cytotoxicity to normal cells. Our observations of high antioxidant and antiproliferative activity of R. ellipticus are in consonance with the previous studies on various species of raspberries, including yellow raspberry, which showed high polyphenol contents and their high antioxidant capacities and antiproliferative activities (Wang and Lin 2000; Liu et al. 2002; Ross et al. 2007; Coates et al. 2007; Weber et al. 2008; Gansch et al. 2009; Rao and Snyder 2010; Gulcin et al. 2011). Significantly higher contents of polyphenols and their antioxidant capacities and anticancer activities observed in the yellow Himalayan raspberry R. ellipticus in the present study were much higher than those reported earlier for yellow raspberry (Liu et al. 2002; Gansch et al. 2009). The antiproliferative action of R. ellipticus fruits (~60 % inhibition at 6.6 mg/ml FW) in present study was comparably better than that reported in golden raspberry (Liu et al. 2002).
Conclusion
The abilities of R. ellipticus fruit extracts to scavenge all types of reactive radicals such as DPPH, ABTS, superoxide radicals, linoleate-peroxide free radicals, and to inhibit the proliferation of cervical cells C33A are highly appreciable. The present study ascertained that the fruits of yellow Himalayan raspberry R. ellipticus are an important source of natural antioxidants, therefore, their consumption may play vital role in reducing the oxidative stress and preventing the degenerative diseases including cancer.
Acknowledgments
We acknowledge the contribution of Prof Aditya Shastri, Vice Chancellor, Banasthali University Rajasthan, India for providing facilities in the Dept. of Bioscience and Biotechnology at this university.
References
- Barreira JCM, Ferreira ICFR, Oliveira MBPP, Pereira JA. Antioxidant activity and bioactive compounds of ten Portuguese regional and commercial almond cultivars. Food Chem Toxicol. 2008;46:2230–2235. doi: 10.1016/j.fct.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Coates EM, Popa G, Gill CI, McCann MJ, McDougall GJ, Stewart D, Rowland I. Colon-available raspberry polyphenols exhibit anti-cancer effects on in vitro models of colon cancer. J Carcinog. 2007;6:4–16. doi: 10.1186/1477-3163-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody V, Middleton E, Herborne JB. Plant flavonoids in Biology and Medicine: biochemical, pharmacological and structure activity relationships. New York: Alan R. Liss; 1986. [Google Scholar]
- Dinis TCP, Madeira VMC, Almeida MLM. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radicalscavengers. Arch Biochem Biophys. 1994;315:161–169. doi: 10.1006/abbi.1994.1485. [DOI] [PubMed] [Google Scholar]
- Gansch H, Weber CA, Lee CY. Antioxidant capacity and phenolic phytochemicals in black raspberries. New York Fruit Quat. 2009;17:20–13. [Google Scholar]
- Gil MI, Tamas-Braberan FA, Hess-Pierce B, Kader AA. Antioxidant capacity, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach and plum cultivars from California. J Agric Food Chem. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- Gulcin I. Antioxidant activity of food constituents: an overview. Arch Toxicol. 2012;86:345–391. doi: 10.1007/s00204-011-0774-2. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Bursal E, Sehitoglu MH, Bilsel M, Goren AC. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum. Turk Food and Chem Toxicol. 2010;48:2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- Gulcin I, Kirecci E, Akkemik E, Topal F, Hisar O. Antioxidant, antibacterial, and anticandidal activities of an aquatic plant: duckweed (Lemna minor L. Lemnaceae) Turk J Biol. 2010;34:175–188. [Google Scholar]
- Gulcin I, Topal F, Cakmakc R, Bilsel M, Goren AC, Erdogan U. Pomological features, nutritional quality, polyphenol content analysis, and antioxidant properties of domesticated and 3 wild ecotype forms of raspberries (Rubus idaeus L.) J Food Sci. 2011;76:4. doi: 10.1111/j.1750-3841.2011.02142.x. [DOI] [PubMed] [Google Scholar]
- Jacob RA. The integrated antioxidant system. Nutr Res. 1995;15:755–766. doi: 10.1016/0271-5317(95)00041-G. [DOI] [Google Scholar]
- Johnson JL, Bomser JA, Scheerens JC, Giusti MM. Effect of black raspberry (Rubus occidentalis L.) extract variation conditioned by cultivar, production site, and fruit maturity stage on colon cancer cell proliferation. J Agric Food Chem. 2011;59:1638–1645. doi: 10.1021/jf1023388. [DOI] [PubMed] [Google Scholar]
- Kahkonen MP, Hopia AI, Heinonen M. Berry phenolics and their antioxidant activity. J Agric Food Chem. 2001;49:4076–4082. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- Karadeniz F, Burdurlu HS, Koca N, Soyer Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk J Agric For. 2005;29:297–303. [Google Scholar]
- Kuhnau J. The flavonoids: a class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. doi: 10.1016/S0308-8146(01)00251-5. [DOI] [Google Scholar]
- Lim YY, Lim TT, Tee JJ. Antioxidant properties of several tropical fruits: a comparative study. Food Chem. 2007;103:1003–1008. doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- Liu F, Ooi VEC, Chang ST. Free radical scavenging activity of mushroom polysaccarides extract. Life Sci. 1997;60:763–771. doi: 10.1016/S0024-3205(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Liu M, Li XQ, Weber C, Lee CY, Brown J, Liu RH. Antioxidant and antiproliferative activities of raspberries. J Agric Food Chem. 2002;50:2926–2930. doi: 10.1021/jf0111209. [DOI] [PubMed] [Google Scholar]
- Losso JN, Bansode RR, Trappey A, Bawadi HA, Truax R. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004;15:672–678. doi: 10.1016/j.jnutbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Univ Chem Technol Metall. 2005;40:255–260. [Google Scholar]
- Meda AL, Lamien CE, Compaore MMY, Meda RNT. Polyphenol content and antioxidant activities of fourteen wild edible fruits from Burkina. Molecules. 2008;13:581–594. doi: 10.3390/molecules13030581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EJR, Kandaswami C. The impact of plant flavonoids on mammalian biology: implications for immunity, inflammation and cancer. In: Harborne JB, editor. The flavonoids. London: Chapman and Hall; 1986. pp. 619–651. [Google Scholar]
- Mokbel MS, Hashinaga F. Antibactierail and antioxidant activities of banana fruits peel. Amm J Biochem Biotech. 2005;1:125–131. doi: 10.3844/ajbbsp.2006.125.131. [DOI] [Google Scholar]
- Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE. Anthocyanins, phenolics and antioxidant capacity in diverse small fruits: vaccinium, rubus and ribes. J Agric Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- Parmar C, Kaushal MK (1982) Rubus ellipticus. In: Wild fruits. Kalyani Publishers, New Delhi, India, 84–87
- Peterson J, Dwyer J. Flavonoids: dietary occurrence and biochemical activity. Nutr Res. 1998;18:1995–2018. doi: 10.1016/S0271-5317(98)00169-9. [DOI] [Google Scholar]
- Rao AV, Snyder DM. Raspberries and human health: a review. J Agric Food Chem. 2010;58:3871–3883. doi: 10.1021/jf903484g. [DOI] [PubMed] [Google Scholar]
- Rawat S, Jugran A, Lalit G, Bhatt ID, Rawal RS (2010) Assessment of antioxidant properties in fruits of Myrica esculenta: a popular wild edible species in Indian Himalayan region eCAM published by Oxford University Press doi:10.1093/ecam/neq055 [DOI] [PMC free article] [PubMed]
- Ross HA, McDougall GJ, Stewart D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry. 2007;68:218–228. doi: 10.1016/j.phytochem.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Samant SS, Dhar U. Diversity endemism and economic potential of wild edibles plants of Indian Himalaya. Int J Sust Dev World Ecol. 1997;4:179–191. doi: 10.1080/13504509709469953. [DOI] [Google Scholar]
- Seerama NP, Adamsa LS, Henninga SM, Niua Y, Zhangb Y, Nairb MG, Heber D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Sies H. Antioxidant functions of vitamins. Ann N Y Acad Sci. 1992;669:7–20. doi: 10.1111/j.1749-6632.1992.tb17085.x. [DOI] [PubMed] [Google Scholar]
- Singh RP, Chidamdara M, Jayaprakasha GK. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin ciocalteu reagent. Meth Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sun J, Chu Yf WX, Liu RH. Antioxidant and antiproliferative activities of common fruits. J Agric Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Vendemiale G, Grattagliano E, Altomare E. An update on the role of free radicals and antioxidant defense in human diseases. Int J Clin Lab Res. 1999;29:49–55. doi: 10.1007/s005990050063. [DOI] [PubMed] [Google Scholar]
- Wang SY, Lin HS. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stages. J Agric Food Chem. 2000;48:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44:701–705. doi: 10.1021/jf950579y. [DOI] [Google Scholar]
- Weber AA, Perkins-Veazie Moore PP, Howard L (2008) Variability of antioxidant contents in raspberry germplasm. Proc. IXth Intl. Rubus and Ribes Symp. P Banados and A Dale (eds). Acta 777
- You BR, Moon HJ, Han YH, Park WH. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and or necrosis. Food and Chem Toxicol. 2010;48:1334–1340. doi: 10.1016/j.fct.2010.02.034. [DOI] [PubMed] [Google Scholar]