Abstract
Soybean residue is the main by-product of making soybean milk and tofu, and there is considerable interest in its recovery, recycling and upgrading. This work was to study the effect of fermentation with lactic acid bacteria and dynamic high pressure microfluidization (DHPM) on fibre fractions, fibre composition, surface topography and X-ray diffraction of dietary fibre in soybean residue. The results show that both fermentation and DHPM increased soluble dietary fibre (6.4–9.7 g/100 g and 6.4–14.0 g/100 g, respectively) and decreased the insoluble:soluble ratio (11.6–7.8 and 11.6–4.5, respectively). The minimum insoluble:soluble ratio (2.5) was obtained in samples with combined fermented and DHPM-treated at 200 MPa. The loss of hemicellulose was observed after fermentation and DHPM, while the cellulose content did not show significant differences. Microstructural and crystal structure analysis indicated that fermentation resulted in the modification of the fibrous structure with reduced crystallinity and DHPM damaged the structure to form rugged surface.
Keywords: Soybean residue, Dietary fibre, Fermentation, Dynamic high pressure microfluidization, Monosaccharide
Introduction
Being widely cultivated in Asia since ancient times (Golbitz 1995), soybean is a healthy food and plays a very important role in Chinese diet. Soybean residue is the main by-product of making soybean milk and tofu. Although soybean residue could be a good resource of nutrients, only a limited portion is consumed, while the surplus is used as animal feed or discarded.
Dietary fibre is considered a health-promoting and valuable food component, which has been reported to prevent obesity, cardiovascular disease and type 2 diabetes (Champ et al. 2003; Kushi et al. 1999; Chandalia et al. 2000). It can be divided into water-soluble and water-insoluble fibre. However, soluble and insoluble fractions behave in different ways. Soluble dietary fibre (SDF) has a potential prebiotic character (Gibson and Roberfroid 1995; Fuller and Gibson 1997), plays an important role in the reduction of cholesterol levels (Lukaczer et al. 2006) and the improvement of glucose tolerance in diabetes (Chandalia et al. 2000; Jenkins et al. 2002). It also has anti-inflammatory and anti-carcinogenic effects on the digestive tract (Gray 2008; Scheppach et al. 2004). While insoluble dietary fibre (IDF) increases the fecal bulk and reduces the gastrointestinal transit time, it may result in prevention of diarrhoea, constipation, diverticulitis and haemorrhoids (Goñi and Martin-Carrón 1998). Therefore, the ratio of soluble to insoluble dietary fibre is important for health properties and also for technological characteristics. It is considered to be a well-balanced proportion that 30–50 % of SDF and 50–70 % of IDF to obtain physiological effects associated with both fractions (Grigelmo-Miguel and Martin-Belloso 1999).
To increase SDF content, different methods, like fermentation, chemical or physical treatments have been used (Lambo et al. 2005; Sangnark and Noomhorm 2004; Raghavendra et al. 2006). Among these methods, fermentation has advantages of simple process, low cost, high production rate and no pollution. Lactic acid bacteria, such as streptococcus thermophilus and lactobacillus bulgaricus being common probioctics, could decrease IDF content in oat and barley after fermentation (Lambo et al. 2005). Our previous studies (unpublished) indicated that the content of SDF was increased after fermentation. Physical treatment breaks through the limits of strict reaction condition and high cost of enzymatic treatment, and it avoids dangerous factor caused by chemical treatment. Dynamic high pressure microfluidization (DHPM) technology is an emerging homogenization technology which uses the combined forces of high-velocity impact, high-frequency vibration, instantaneous pressure drop, powerful shear, cavitation force and ultra-high pressures, up to 200 MPa, with a very short treatment time (less than 5 s) (Liu et al. 2009). It has been applied for microbial reduction (Fantin et al. 1996), preparation of mozzarella cheese (Tunick et al. 2000), nano-emulsions (Jafari et al. 2007) and liposomes (Thompson and Singh 2006) and improvement of whey protein and egg white protein (Iordache and Jelen 2003; Tu et al. 2009). However, few reports exist on the effect of DHPM on dietary fibre. Only Liu et al. (2006) reported the effect of instantaneous high pressure treatment on solubility and rheological properties of soybean dietary fiber. In addition, there were few reports about combined treatment as well.
The aim of the present study was to investigate how lactic acid bacteria (LAB) and DHPM could affect the dietary fibre in soybean residue, which was characterized by the content, composition, surface topography and X-ray diffraction of dietary fibre.
Materials and methods
Raw materials
The by-product of making tofu, soybean residue, was obtained from a local supplier (Qingshan Lake Market, Nanchang, China). Tofu was made by Chinese traditional process, and it was prepared from high quality soybeans of Northeast China. The water content in the residue was 81.5 wt.%. The residue was oven-dried at 55 °C for 20 h, ground, and sieved (0.3 mm).
Fermentation procedure
Streptococcus thermophilus (CICC20391) and Lactobacillus delbrueckii subsp.bulgaricus (CICC20249) were obtained from the China Center of Industrial Culture Collection (China-CICC). The strains were cultured in MRS broth and incubated at 42 °C for 24–48 h; each activated culture was inoculated into 10 mL MRS broth and then incubated at 42 °C for 24 h. The culture was diluted with 0.85 % sterile saline to obtain a preparation containing between 106 and 107 cfu/mL, which served as the inoculum for the soybean residue fermentation. After heating at 105 °C for 15 min, 100 mL of soybean residue concentrates (dry matter contents between 8 % and 10 %) were cooled to the fermentation temperature (42 °C) and then inoculated with 0.2 % of a mixed culture containing equal proportions of both strains. The fermentation was performed over a period of about 20 h, and the final pH of the fermented products was 4.0 ± 0.3. Products were lyophilized for further analysis.
Dynamic high pressure microfluidization treatment
The soybean residue and the fermented products were dispersed to a solid–liquid ratio 1:30. Dynamic high pressure microfluidization experiments were performed on laboratory scale equipment (M-110EH, Microfluidics, Newton, USA). The residue suspensions were treated at room temperature and 0, 50, 100, 150 and 200 MPa for two passes, respectively.
Proximate analysis
The moisture, ash, protein and fat contents were determined according to methods established by AOAC (1995). The elementary composition of the sample was moisture (7.79 ± 0.68 wt.%), ash (2.09 ± 0.14 wt.%), protein (16.03 ± 0.97 wt.%) and fat (2.01 ± 0.09 wt.%).
Determination of dietary fibre contents
Total dietary fibre (TDF), SDF and IDF contents were determined by the enzymatic-gravimetric method according to AOAC 991.43 (1995). The hypothesis in this method is to determine the content of dietary fibre under conditions similar to those found in the human alimentary tract using the following enzymes: thermostable α-amylase (A-3306, Sigma-Aldrich Co. LLC, St. Louis, USA) (100 °C, pH 8.2, 15 min), protease (P-3910, Sigma-Aldrich Co. LLC, St. Louis, USA) (60 °C, pH 8.2, 30 min) and amyloglucosidase (A-9913, Sigma-Aldrich Co. LLC, St. Louis, USA) (60 °C, pH 4.0–4.7, 30 min). Subsequently, IDF was filtered and the residue was washed two times with 10 mL 70 °C distilled water. The filtrate and water used for washing were combined, four volumes of 60 °C 95 % ethanol was added to precipitate SDF. The obtained residue was washed two times with 15 mL portions of 78 % ethanol, 95 % ethanol and acetone, then dried at 105 °C overnight and weighed. Both IDF and SDF residues were corrected for protein, ash and a blank sample for the final calculation of IDF and SDF contents. The blank sample was evaluated along with the real sample to determine any contribution from reagents to the residue. TDF was calculated as the sum of IDF and SDF.
The contents of neutral detergent fibre (NDF) and acid detergent fibre (ADF) were determined according to Van Soest (1967; 1963). Hemicellulose content was calculated by the difference between NDF and ADF contents. Lignin content in ADF was determined by the method of Van Soest (1963), which defined it as the insoluble lignin fraction in 72 % H2SO4.
Chemical analysis of dietary fibre components
The composition of dietary fibre was determined after hydrolysis of trifluoroacetic acid. Sample was hydrolysed with 2 M trifluoroacetic acid at 100 °C for 6 h in a sealed tube. Excess trifluoroacetic acid was removed by evaporation under reduced pressure. The residue was repeatedly dissolved in methanol and evaporated to dryness for three times. The residue was re-dissolved in 2 mL of methanol, and transferred to a glass tube. The solution was taken to dryness with nitrogen. Hydroxylamine hydrochloride (10 mg) and pyridine (0.5 mL) were added in the tube. The sealed tube was immersed in a thermostatic water bath at 90 °C for 30 min. Then, 0.5 mL of acetic anhydride was added in. The mixture was kept at 90 °C for 30 min (Chen et al. 2008). The acid hydrolysis released the different fibre components: neutral sugars and uronic acid. The neutral sugar was determined by GC as alditol acetate. An Agilent gas chromatograph model GC-6890 N equipped with a flame ionization detector (FID) and an automatic injector were used. The column was a Supelco HP-1701 capillary fused silica, 30 m × 0.32 mm id, 0.25 μm film thickness. Nitrogen served as carrier gas, and the split ratio was 1:20. The oven, injector and detector temperatures were 240 °C, 270 °C and 270 °C, respectively. Uronic acid was determined colorimetrically following the methods described by Scott (1979) with 3,5-dimethylphenol as the reagent and galacturonic acid as a standard.
Microstructural evaluation
Samples were prepared by sticking the pectin onto double-sided adhesive tape attached to a circular specimen stub. The samples were viewed using an environmental scanning electron microscope (ESEM) (Quanta200F, FEI Deutschland GmbH, Kassel, Germany) at 10 kV voltage and 1,500 × magnification. High vacuum mode was used while operating the ESEM.
Wide angle X-ray diffraction
Lyophilized samples were analyzed for X-ray diffraction (XRD) patterns. Measurements were carried out using a Bruker AXS D-8 diffractometer (anode Cu-Kα, 40 kV, 40 mA), with a diffraction angle range of 5–50° and resolution of 0.02° at room temperature.
Statistical analysis
The data were analyzed by the analysis of variance (ANOVA) and were expressed as mean values from three replicates with standard deviations. The differences between mean values were established using Duncan’s multiple range tests at the level of P < 0.05. The statistical analysis was performed by SPSS (version 18.0).
Results and discussion
Dietary fibre analysis
The effects of fermentation and DHPM on dietary fibre in soybean residue are presented in Table 1. Soybean residue was enriched in dietary fibre (80.0 g/100 g), and IDF was the main fibre fraction (73.7 g/100 g). The ratio of insoluble to soluble dietary fibre in soybean residue was 11.6. TDF content in soybean residue was higher than those reported previously by Redondo-Cuenca et al. (2008) (55.5 g/100 g), whereas, the ratio of insoluble to soluble dietary fibre was similar to those found in soybean residue (Redondo-Cuenca et al. 2008). The difference could be attributed to growing or climatic conditions, geographic origin or to differences in the processing methods.
Table 1.
Effect of fermentation and DHPM on dietary fibre in soybean residue (g/100 g dry matter)
| DHPM/(MPa) | TDF | IDF | SDF | IDF: SDF | ||||
|---|---|---|---|---|---|---|---|---|
| SR | F-SR | SR | F-SR | SR | F-SR | SR | F-SR | |
| 0 | 80.0 ± 1.5a | 85.1 ± 1.4a | 73.7 ± 1.1a | 75.4 ± 0.8a | 6.4 ± 0.3a | 9.7 ± 0.5a | 11.6 | 7.8 |
| 50 | 79.7 ± 1.1ab | 85.0 ± 1.6a | 71.6 ± 0.8ab | 71.1 ± 0.7b | 8.2 ± 0.4b | 13.9 ± 0.9b | 8.8 | 5.1 |
| 100 | 79.9 ± 1.6a | 85.2 ± 0.4a | 69.2 ± 1.0bc | 66.7 ± 0.6c | 10.7 ± 0.6c | 18.5 ± 1.0c | 6.5 | 3.6 |
| 150 | 79.5 ± 1.2ab | 84.5 ± 1.7a | 66.2 ± 0.8c | 62.1 ± 2.1d | 13.4 ± 0.4d | 22.4 ± 0.5d | 5.1 | 2.9 |
| 200 | 76.3 ± 1.3b | 82.4 ± 2.4a | 62.3 ± 2.0d | 58.8 ± 1.1e | 14.0 ± 0.7d | 23.6 ± 1.3d | 4.5 | 2.5 |
Values are means ± SD, n = 3; Means within a column with the different letters are significantly different (P < 0.05)
DHPM dynamic high pressure microfluidization; TDF total dietary fibre; IDF insoluble dietary fibre; SDF soluble dietary fibre; SR soybean residue; F-SR fermented soybean residue
As shown in Table 1, when soybean residue was fermented with lactic acid bacteria, there was a significant increase in TDF (80.0 to 85.1 g/100 g) and SDF (6.4 to 9.7 g/100 g) content. The ratio of insoluble to soluble dietary fibre was decreased from 11.6 to 7.8.
DHPM treatment didn’t affect TDF content, but it made SDF increase and IDF decrease significantly, and both varied with the differing DHPM pressure. However, SDF values increased slightly but differences were not significant at 150–200 MPa. The increase in SDF (9.7 to 23.6 g/100 g) became more evident for samples DHPM-treated after fermentation, and the most increase was found at 200 MPa in samples with combined fermented and DHPM-treated. In this case, well balanced composition of IDF and SDF (IDF: SDF = 2.5) was obtained for soybean residue. Since SDF and IDF play different roles in human health with SDF being more important than IDF in many health aspects, it is recommended that the ratio of insoluble to soluble dietary fibre should be in the range of 1.0–2.3 (Grigelmo-Miguel and Martin-Belloso 1999). The increase observed in the soluble fibre fraction of soybean residue was consistent with previous reports on the effect of high-pressure micronization in carrot insoluble fibres (Chau et al. 2007).
Therefore, on applying fermentation and DHPM treatment there was a redistribution from insoluble to soluble fibre fractions. The increase in TDF after fermentation with LAB could be due to the nutriment in soybean residue, such as protein and starch metabolized by microorganisms. The general increase of SDF content after fermentation may be a result of a breakdown of dietary fibre polysaccharides by the microorganisms in the fermentation medium. The mechanism behind this is not clear, but it is most likely an enzymatic or acid breakdown. Thus, microorganisms are able to hydrolyse and metabolise insoluble polysaccharides by producing extracellular enzymes (Schwarz 2001) and lactic acid.
Reductions in IDF content could be caused by partial degradation of cellulose and hemicellulose into simple carbohydrates (Zia ur et al. 2003). The combined forces of high-velocity impact, high-frequency vibration, instantaneous pressure drop, intense shear, cavitation and ultra-high pressures caused by DHPM, could bring to the puff of texture and breakage of weak bonds between polysaccharides. Thus, IDF content was reduced and SDF content was increased significantly. As there may be a degradation of dietary fibre polysaccharides to smaller fragments, which could be soluble in ethanol and as a consequence there was not significant increase in SDF at 150–200 MPa (Lambo et al. 2005).
In order to demonstrate whether cellulose, hemicellulose or lignin partially degraded into soluble substances, we discussed the effects of fermentation and DHPM on the content of dietary fibre’s fraction in soybean residue (Table 2). Samples before and after fermentation with LAB contained 56.5 g/100 g and 58.7 g/100 g NDF and 31.7 g/100 g and 36.5 g/100 g ADF, respectively. Since lignin content in samples was negligibly small, cellulose content was approximate ADF content shown in Table 2. DHPM treatment did not affect the amount of cellulose, but affected hemicellulose content significantly. Samples in hemicellulose treated under higher pressure were poorer in the content of fibre than those under lower pressure. At 200 MPa after fermentation, hemicellulose content (10.1 g/100 g) was about half that at 0 MPa (22.2 g/100 g). Hemicelluloses were preferentially solubilized and the resultant fibre was enriched in celluloses. However, samples DHPM-treated after fermentation had a slight decrease in cellulose. It is therefore tempting to speculate that a mechanism similar to that of hemicellulose degradation turned into some small molecular substances was responsible for the reduction of IDF. In addition, looser and more porous fibre structure produced by fermentation made IDF degradation easier.
Table 2.
Effect of fermentation and DHPM on the content of dietary fibre’s fraction in soybean residue (g/100 g dry matter)
| DHPM/(MPa) | NDF | ADF(Cellulose) | Hemicellulose | |||
|---|---|---|---|---|---|---|
| SR | F-SR | SR | F-SR | SR | F-SR | |
| 0 | 56.5 ± 0.7a | 58.7 ± 1.4a | 31.7 ± 0.4a | 36.5 ± 1.5a | 24.8 ± 1.1a | 22.2 ± 0.3a |
| 50 | 55.1 ± 1.0a | 56.5 ± 1.8a | 31.4 ± 0.6a | 37.1 ± 1.2ab | 23.7 ± 0.5a | 19.5 ± 0.7b |
| 100 | 52.4 ± 0.9b | 48.5 ± 1.5b | 31.1 ± 1.2a | 34.2 ± 1.2ab | 21.3 ± 0.4b | 14.3 ± 1.3c |
| 150 | 51.1 ± 0.6bc | 48.6 ± 1.4b | 31.9 ± 1.3a | 36.7 ± 0.8ab | 19.2 ± 0.6c | 11.9 ± 0.6d |
| 200 | 49.4 ± 1.0c | 43.6 ± 0.4c | 32.3 ± 1.9a | 33.5 ± 0.8b | 17.2 ± 0.9d | 10.1 ± 0.7d |
Values are means ± SD, n = 3; Means within a column with the different letters are significantly different (P < 0.05)
DHPM dynamic high pressure microfluidization; NDF neutral detergent fibre; ADF acid detergent fibre; SR soybean residue; F-SR fermented soybean residue
Monosaccharide composition
Tables 3 and 4 show fibre monosaccharide composition in soybean residue. In general, SDF of soybean residue (Table 3) was mainly constituted of uronic acid and galactose, and notable contents of arabinose, with lesser glucose, mannose, rhamnose, fucose and xylose. IDF was characterized by its high content in glucose, followed by galactose and uronic acid, as well as significant amount of arabinose and xylose. The remaining monomers, mannose, rhamnose and fucose, were present in smaller amounts. These results were in concordance with those previously shown for soybean by Redondo-Cuenca et al. (2007). The authors reported that IDF was mainly composed of glucose, uronic acid, galactose, arabinose and xylose and SDF was rich in uronic acid, galactose and arabinose.
Table 3.
Effect of DHPM on monosaccharides of dietary fibre in non-fermented soybean residue (g/100 g dry matter)
| DHPM/(MPa) | 0 | 50 | 100 | 150 | 200 |
|---|---|---|---|---|---|
| SDF | 6.35 ± 0.26a | 8.15 ± 0.37b | 10.70 ± 0.59c | 13.36 ± 0.40d | 14.01 ± 0.67d |
| Rha | 0.40 ± 0.07ab | 0.45 ± 0.04ab | 0.55 ± 0.10b | 0.43 ± 0.03ab | 0.31 ± 0.06a |
| Fuc | 0.13 ± 0.03a | 0.22 ± 0.01b | 0.22 ± 0.04b | 0.35 ± 0.03c | 0.30 ± 0.03c |
| Ara | 1.09 ± 0.13a | 1.50 ± 0.14ab | 1.99 ± 0.28bc | 2.11 ± 0.11bc | 2.52 ± 0.42c |
| Xyl | 0.08 ± 0.01a | 0.09 ± 0.00a | 0.14 ± 0.01b | 0.09 ± 0.01a | 0.07 ± 0.00a |
| Man | 0.37 ± 0.04a | 0.39 ± 0.06a | 0.31 ± 0.04ab | 0.23 ± 0.05b | 0.20 ± 0.03b |
| Glc | 0.26 ± 0.03a | 0.30 ± 0.01a | 0.47 ± 0.07b | 0.65 ± 0.07c | 0.59 ± 0.04bc |
| Gal | 1.98 ± 0.02a | 2.20 ± 0.28a | 3.75 ± 0.35b | 4.67 ± 0.66b | 5.00 ± 0.71b |
| UA | 2.04 ± 0.30a | 3.00 ± 0.29a | 3.27 ± 0.66a | 4.83 ± 0.71b | 5.02 ± 0.14b |
| IDF | 73.68 ± 1.11a | 71.59 ± 0.79ab | 69.22 ± 0.97bc | 66.16 ± 0.80c | 62.32 ± 1.95d |
| Rha | 2.11 ± 0.28a | 2.03 ± 0.04a | 1.98 ± 0.00a | 1.89 ± 0.01a | 1.32 ± 0.29b |
| Fuc | 2.00 ± 0.28ab | 1.79 ± 0.14ab | 2.20 ± 0.27b | 1.67 ± 0.13ab | 1.57 ± 0.10a |
| Ara | 8.98 ± 0.29a | 8.79 ± 0.41a | 7.06 ± 0.34b | 7.11 ± 0.16b | 6.32 ± 0.42b |
| Xyl | 4.42 ± 0.11ab | 4.78 ± 0.15b | 4.45 ± 0.07ab | 4.37 ± 0.38ab | 4.15 ± 0.14a |
| Man | 2.95 ± 0.07a | 3.04 ± 0.27a | 2.48 ± 0.11b | 2.10 ± 0.11c | 2.01 ± 0.06c |
| Glc | 22.17 ± 1.70a | 21.90 ± 1.27a | 21.16 ± 1.07a | 21.17 ± 0.24a | 20.73 ± 1.03a |
| Gal | 16.51 ± 0.71a | 15.63 ± 0.61ab | 15.88 ± 0.54ab | 14.85 ± 0.35bc | 14.13 ± 0.33c |
| UA | 14.54 ± 0.76a | 13.63 ± 0.42ab | 14.01 ± 0.71ab | 13.00 ± 0.42bc | 12.09 ± 0.44c |
| TDF | 80.04 ± 1.46a | 79.54 ± 1.11ab | 79.93 ± 1.55a | 79.53 ± 1.23ab | 76.34 ± 1.26b |
Values are means ± SD, n = 3; Means within a row with the different letters are significantly different (P < 0.05)
DHPM dynamic high pressure microfluidization; SDF soluble dietary fibre; IDF insoluble dietary fibre; TDF total dietary fibre; Rha rhamnose; Fuc fucose; Ara arabinose; Xyl xylose; Man mannose; Glc glucose; Gal galactose; UA uronic acid
Table 4.
Effect of DHPM on monosaccharides of dietary fibre in fermented soybean residue (g/100 g dry matter)
| DHPM/(MPa) | 0 | 50 | 100 | 150 | 200 |
|---|---|---|---|---|---|
| SDF | 9.71 ± 0.52a | 13.85 ± 0.90b | 18.45 ± 0.98c | 22.37 ± 0.47d | 23.56 ± 1.28d |
| Rha | 0.47 ± 0.07a | 0.50 ± 0.06a | 0.61 ± 0.07ab | 0.72 ± 0.03b | 0.80 ± 0.14b |
| Fuc | 0.15 ± 0.01a | 0.22 ± 0.03ab | 0.20 ± 0.03ab | 0.19 ± 0.01ab | 0.24 ± 0.04b |
| Ara | 1.90 ± 0.14a | 2.26 ± 0.33a | 3.47 ± 0.42b | 4.94 ± 0.62c | 5.11 ± 0.16c |
| Xyl | 0.19 ± 0.00a | 0.22 ± 0.03a | 0.20 ± 0.01a | 0.25 ± 0.04a | 0.23 ± 0.01a |
| Man | 0.51 ± 0.08a | 0.59 ± 0.07a | 0.63 ± 0.04a | 0.57 ± 0.01a | 0.50 ± 0.07a |
| Glc | 0.51 ± 0.07a | 0.95 ± 0.07b | 0.98 ± 0.14b | 1.42 ± 0.10c | 1.51 ± 0.16c |
| Gal | 2.50 ± 0.14a | 4.00 ± 0.71b | 5.99 ± 0.20c | 7.41 ± 0.93c | 7.21 ± 0.30c |
| UA | 3.48 ± 0.28a | 5.11 ± 0.16b | 6.37 ± 0.52c | 6.87 ± 0.53cd | 7.96 ± 0.65d |
| IDF | 75.36 ± 0.83a | 71.13 ± 0.74b | 66.72 ± 0.55c | 62.09 ± 2.14d | 58.81 ± 1.09e |
| Rha | 2.99 ± 0.27a | 2.97 ± 0.38a | 2.56 ± 0.14ab | 2.22 ± 0.24b | 2.00 ± 0.28b |
| Fuc | 2.75 ± 0.35a | 2.80 ± 0.28a | 2.52 ± 0.13ab | 2.01 ± 0.42ab | 1.80 ± 0.21b |
| Ara | 8.39 ± 0.69a | 7.37 ± 0.45a | 7.02 ± 0.71ab | 5.66 ± 0.37bc | 4.55 ± 0.78c |
| Xyl | 4.01 ± 0.38a | 3.82 ± 0.45a | 3.44 ± 0.06ab | 3.41 ± 0.25ab | 3.01 ± 0.08b |
| Man | 3.86 ± 0.51a | 3.70 ± 0.34a | 3.95 ± 0.07a | 4.01 ± 0.44a | 3.50 ± 0.14a |
| Glc | 22.55 ± 0.78a | 22.64 ± 0.91a | 21.79 ± 0.41a | 21.57 ± 0.38a | 21.00 ± 0.71a |
| Gal | 16.99 ± 0.98a | 15.79 ± 0.58ab | 14.05 ± 1.13bc | 12.38 ± 0.54cd | 12.01 ± 0.28d |
| UA | 13.82 ± 0.31a | 12.04 ± 0.57b | 11.39 ± 0.55bc | 10.83 ± 0.47c | 10.94 ± 0.06bc |
| TDF | 85.07 ± 1.38a | 84.98 ± 1.61a | 85.16 ± 0.40a | 84.46 ± 1.68a | 82.37 ± 2.43a |
Values are means ± SD, n = 3; Means within a row with the different letters are significantly different (P < 0.05)
DHPM dynamic high pressure microfluidization; SDF soluble dietary fibre; IDF insoluble dietary fibre; TDF total dietary fibre; Rha rhamnose; Fuc fucose; Ara arabinose; Xyl xylose; Man mannose; Glc glucose; Gal galactose; UA uronic acid
In the present study (Tables 3 and 4), arabinose, xylose, mannose, glucose and uronic acid content were increased in SDF after fermentation, meanwhile, arabinose, xylose and uronic acid content were decreased in IDF. The decrease in IDF produced by fermentation was apparently due to a reduction of insoluble polymers containing arabinose, uronic acid and xylose.
The increase in SDF of DHPM-treated samples was mainly due to the rise of fucose, arabinose, glucose, galactose and uronic acid. In general, the higher the pressure used for processing samples, the higher the neutral sugars and uronic acid content of SDF. Moreover, after samples were fermented, rhamnose increased slightly, while fucose did not significantly increase in SDF (Table 4).
With respect to IDF in DHPM-treated samples (Table 3), rhamnose, arabinose, mannose, galactose and uronic acid were diminished. Meanwhile, samples DHPM-treated after fermentation increased in xylose slightly (Table 4).
When SDF content increased sharply it was due to a rise of arabinose, glucose, galactose and uronic acid. A noteworthy concurrent decrease of arabinose, galactose and uronic acid was revealed in IDF. DHPM was of minor importance for other fibre monosaccharides as these were present in very low amounts. Therefore, it seemed that the increase in solubility produced by DHPM was due to a shift of insoluble to soluble fibre, most probably from hemicellulose and pectic polysaccharides containing arabinose, galactose and uronic acid.
Surface topography
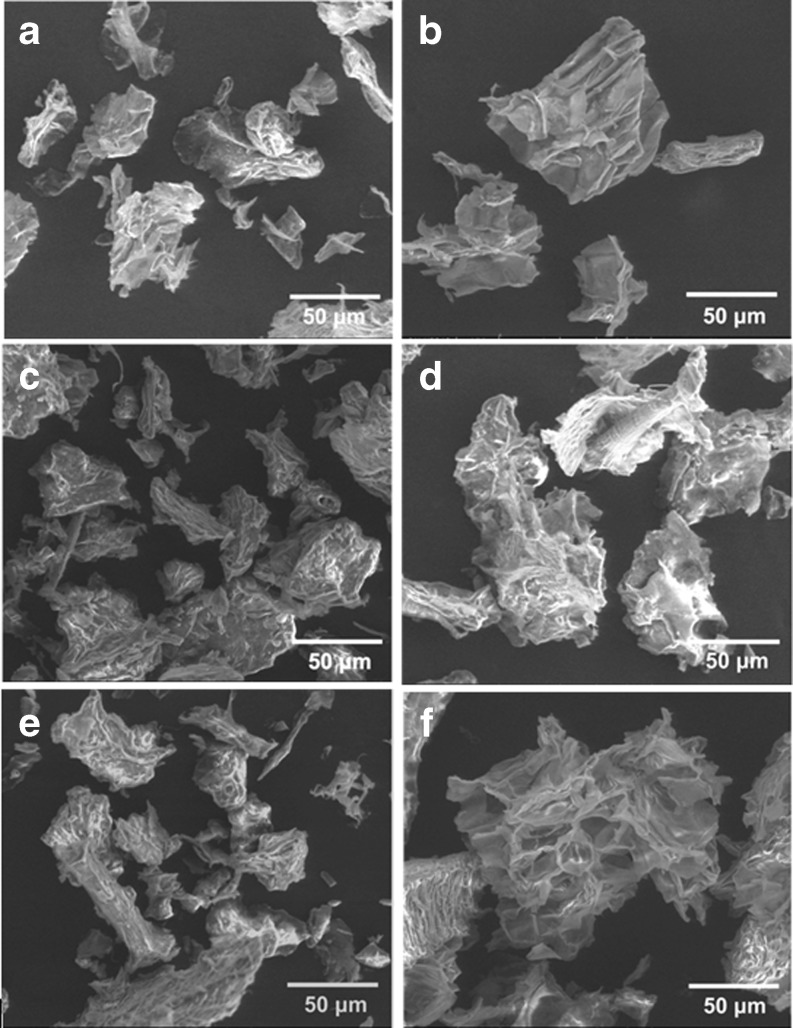
Scanning microscopy analysis was used to characterize the topography modification of dietary fibre in soybean residue. The microstructure of dietary fibre powder prepared by different treatments was observed with ESEM and the images presented in Fig. 1. The tissue of samples was seen as an irregular and massy shape with fibrous structure and amorphous structure (Fig. 1a).
Fig. 1.
Environmental scanning electron micrograph of dietary fibre that fermented and dynamic high pressure microfluidization-treated at different pressures. a – original; b – fermented; c – 50 MPa; d – 100 MPa; e – 150 MPa; f – 200 MPa
As shown by ESEM, the image (Fig. 1b) revealed that fermentation resulted in the modification of the fibrous structure. DHPM damaged the structure and caused it to be rugged. The higher DHPM pressure applied, the more sample’s structure was puffed. The surface of samples DHPM-treated at 100–150 MPa showed rupture and breakage, and inner fibrous structure was seen (Fig. 1d and e). The original structure was totally puffed up into cellular structure after DHPM treatment at 200 MPa (Fig. 1f).
The results indicated that topography modification in samples was mainly caused by the laceration and shear from the outer to inner at relatively low pressure, which were perhaps produced by powerful shear, high-velocity impact and high-frequency vibration of DHPM. In addition, instantaneous pressure drop, cavitation force and expansion from the inside out probably resulted in the modification of tissue at relatively high pressure.
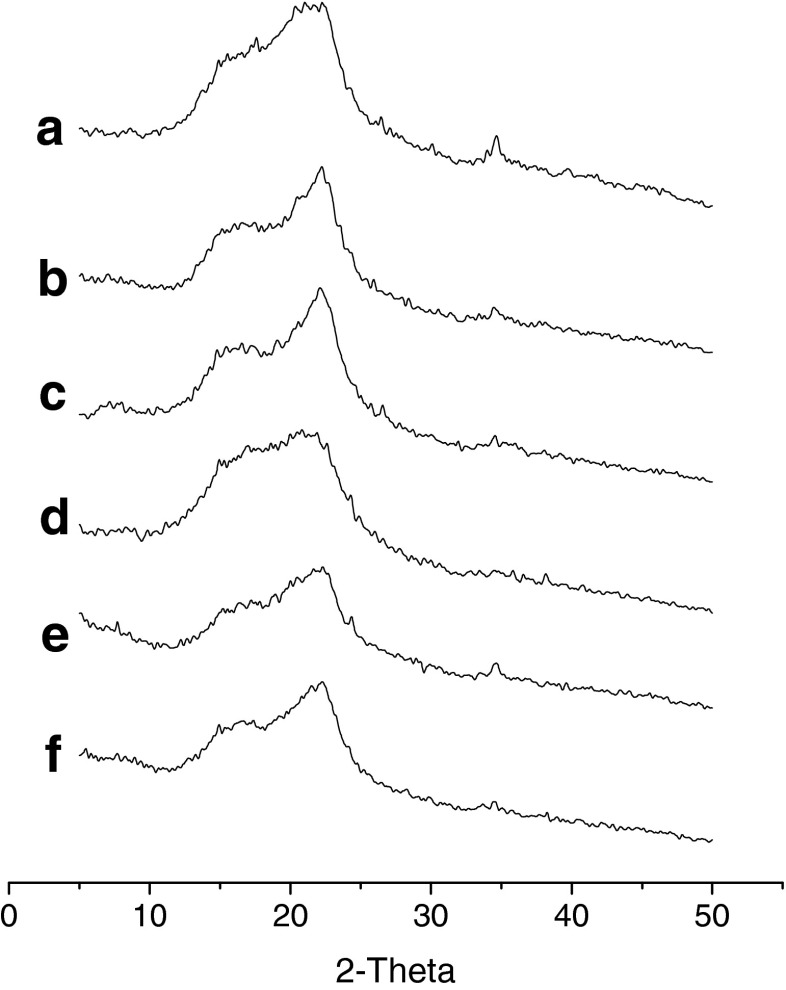
Crystal structure analysis
Figure 2 shows XRD patterns of samples treated differently. Figure 2a, which corresponds to the original sample, shows broad diffraction peaks, with a low relative crystallinity (data not shown), as calculated from the ratio of the areas of the peaks vs. the total area. When samples were DHPM-treated, a relatively stronger crystallinity was observed, suggesting a type I crystalline structure. In addition, crystallinity increased with increasing DHPM pressure. However, it could be observed that fermented samples had a lower relative crystallinity (data not shown) than the original sample and showed an amorphous profile by comparing Fig. 2d to Fig. 2a.
Fig. 2.
X-ray diffraction patterns of dietary fibre that fermented and dynamic high pressure microfluidization-treated at different pressures. a – original; b – 100 MPa; c – 200 MPa; d – fermented; e – 100 MPa after fermentation; f – 200 MPa after fermentation
XRD results showed that the fermented samples presented amorphous structure and showed low overall crystallinity. This meant that fermentation with LAB changed the fibre crystalline regions. The crystalline structures of DHPM-treated samples were not changed, since crystalline regions were not damaged during DHPM treatment. That was, DHPM did not result in the advanced degradation of fibre polymer, and only amorphous regions were damaged preferentially.
Conclusion
On applying fermentation and DHPM treatment there was a redistribution from insoluble to soluble fibre fractions. Fermentation with LAB and DHPM produced a decrease of IDF and an increase of SDF, reducing the ratio of insoluble to soluble dietary fibre. The increase in SDF produced by fermentation was due to a degradation of insoluble polymers containing arabinose, uronic acid and xylose into simple carbohydrates, and the increase in solubility produced by DHPM was due to a shift of insoluble to soluble fibre, most probably from hemicellulose and pectic polysaccharides containing arabinose, galactose and uronic acid. Fermentation and DHPM both damaged the microstructure. XRD results indicated that fermentation changed the fibre crystalline regions, and DHPM resulted in the degradation of amorphous regions. Combined fermentation and DHPM could make soybean residue give more SDF and may be used as more valuable methods to improve the quality of dietary fibre of soybean residue.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Nr 20976078). The authors are very grateful to Dr. Hui Xiao and Dr. Derong Lin with the help of writing assistance.
References
- AOAC . Official methods of analysis of the Association of Analytical Chemists. 16. Washington, DC: Association of Official Analytical Chemists; 1995. [Google Scholar]
- Champ M, Langkilde AM, Brouns F, Kettlitz B, Collet YL. Advances in dietary fibre characterisation. 1. Definition of dietary fibre, physiological relevance, health benefits and analytical aspects. Nutr Res Rev. 2003;16(1):71–82. doi: 10.1079/NRR200254. [DOI] [PubMed] [Google Scholar]
- Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. New Engl J Med. 2000;342(19):1392–1398. doi: 10.1056/NEJM200005113421903. [DOI] [PubMed] [Google Scholar]
- Chau C-F, Wang Y-T, Wen Y-L. Different micronization methods significantly improve the functionality of carrot insoluble fibre. Food Chem. 2007;100(4):1402–1408. doi: 10.1016/j.foodchem.2005.11.034. [DOI] [Google Scholar]
- Chen Y, Xie MY, Nie SP, Li C, Wang YX. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107(1):231–241. doi: 10.1016/j.foodchem.2007.08.021. [DOI] [Google Scholar]
- Fantin G, Fogagnolo M, Guerzoni ME, Lanciotti R, Medici A, Pedrini P, Rossi D. Effect of high hydrostatic pressure and high pressure homogenization on the enantioselectivity of microbial reductions. Tetrahedron Asymmetr. 1996;7(10):2879–2887. doi: 10.1016/0957-4166(96)00379-5. [DOI] [Google Scholar]
- Fuller R, Gibson GR. Modification of the intestinal microflora using probiotics and prebiotics. Scand J Gastroenterol. 1997;32:28–31. doi: 10.1080/00365521.1997.11720714. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Golbitz P. Traditional soyfoods: processing and products. J Nutr. 1995;125(3):S570–S572. doi: 10.1093/jn/125.suppl_3.570S. [DOI] [PubMed] [Google Scholar]
- Goñi I, Martin-Carrón N. In vitro fermentation and hydration properties of commercial dietary fiber-rich supplements. Nutr Res. 1998;18(6):1077–1089. doi: 10.1016/S0271-5317(98)00090-6. [DOI] [Google Scholar]
- Gray J. Dietary fibre - definition, analysis, physiology, health. Agro Food Industry Hi-Tech. 2008;19(2):4–5. [Google Scholar]
- Grigelmo-Miguel N, Martin-Belloso O. Comparison of dietary fibre from by-products of processing fruits and greens and from cereals. LWT Food Sci Technol. 1999;32(8):503–508. doi: 10.1006/fstl.1999.0587. [DOI] [Google Scholar]
- Iordache M, Jelen P. High pressure microfluidization treatment of heat denatured whey proteins for improved functionality. Innov Food Sci Emerg. 2003;4(4):367–376. doi: 10.1016/S1466-8564(03)00061-4. [DOI] [Google Scholar]
- Jafari SM, He Y, Bhandari B. Optimization of nano-emulsions production by microfluidization. Eur Food Res Technol. 2007;225(5–6):733–741. doi: 10.1007/s00217-006-0476-9. [DOI] [Google Scholar]
- Jenkins DJA, Kendall CWC, Augustin LSA, Franceschi S, Hamidi M, Marchie A, Jenkins AL, Axelsen M. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- Kushi LH, Meyer KA, Jacobs DR. Cereals, legumes, and chronic disease risk reduction: evidence from epidemiologic studies. Am J Clin Nutr. 1999;70(3):451S–458S. doi: 10.1093/ajcn/70.3.451s. [DOI] [PubMed] [Google Scholar]
- Lambo AM, Oste R, Nyman M. Dietary fibre in fermented oat and barley beta-glucan rich concentrates. Food Chem. 2005;89(2):283–293. doi: 10.1016/j.foodchem.2004.02.035. [DOI] [Google Scholar]
- Liu C, Liu W, Xiong H, Liang H, Tu Z, Ruan R. Effect of instantaneous high pressure (IHP) treatment on solubility and rheologic properties of soybean dietary fiber. Abstr Paper Am Chem Soc. 2006;232:165–165. [Google Scholar]
- Liu W, Liu J, Xie M, Liu C, Liu W, Wan J. Characterization and high-pressure microfluidization-induced activation of polyphenoloxidase from Chinese pear (Pyrus pyrifolia Nakai) J Agric Food Chem. 2009;57(12):5376–5380. doi: 10.1021/jf9006642. [DOI] [PubMed] [Google Scholar]
- Lukaczer D, Liska DJ, Lerman RH, Darland G, Schiltz B, Tripp M, Bland JS. Effect of a low glycemic index diet with soy protein and phytosterols on CVD risk factors in postmenopausal women. Nutrition. 2006;22(2):104–113. doi: 10.1016/j.nut.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Raghavendra SN, Swamy SRR, Rastogi NK, Raghavarao K, Kumar S, Tharanathan RN. Grinding characteristics and hydration properties of coconut residue: a source of dietary fiber. J Food Eng. 2006;72(3):281–286. doi: 10.1016/j.jfoodeng.2004.12.008. [DOI] [Google Scholar]
- Redondo-Cuenca A, Villanueva-Suarez MJ, Rodriguez-Sevilla MD, Mateos-Aparicio I. Chemical composition and dietary fibre of yellow and green commercial soybeans (Glycine max) Food Chem. 2007;101(3):1216–1222. doi: 10.1016/j.foodchem.2006.03.025. [DOI] [Google Scholar]
- Redondo-Cuenca A, Villanueva-Suarez MJ, Mateos-Aparicio I. Soybean seeds and its by-product okara as sources of dietary fibre. Measurement by AOAC and Englyst methods. Food Chem. 2008;108(3):1099–1105. doi: 10.1016/j.foodchem.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Sangnark A, Noomhorm A. Chemical, physical and baking properties of dietary fiber prepared from rice straw. Food Res Int. 2004;37(1):66–74. doi: 10.1016/j.foodres.2003.09.007. [DOI] [Google Scholar]
- Scheppach W, Luehrs H, Melcher R, Gostner A, Schauber J, Kudlich T, Weiler F, Menzel T. Antiinflammatory and anticarcinogenic effects of dietary fibre. Clin Nutr. 2004;1(2):51–58. [Google Scholar]
- Schwarz WH. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biot. 2001;56(5–6):634–649. doi: 10.1007/s002530100710. [DOI] [PubMed] [Google Scholar]
- Scott RW. Colorimetric determination of hexuronic acids in plant materials. Anal Chem. 1979;51(7):936–941. doi: 10.1021/ac50043a036. [DOI] [Google Scholar]
- Thompson AK, Singh H. Preparation of liposomes from milk fat globule membrane phospholipids using a Microfluidizer. J Dairy Sci. 2006;89(2):410–419. doi: 10.3168/jds.S0022-0302(06)72105-1. [DOI] [PubMed] [Google Scholar]
- Tu Z-c, Wang H, C-m L, Liu G-x, Chen G, Ruan R. Dynamic high pressure micro-fluidization effects on structure and physico-chemical properties of egg white protein. Chem Res Chinese U. 2009;25(3):302–305. [Google Scholar]
- Tunick MH, Van Hekken DL, Cooke PH, Smith PW, Malin EL. Effect of high pressure microfluidization on microstructure of mozzarella cheese. LWT Food Sci Technol. 2000;33(8):538–544. doi: 10.1006/fstl.2000.0716. [DOI] [Google Scholar]
- Van Soest P. Use of detergents in the analysis of fibrous feeds. I. Preparation of fiber residues of low nitrogen content. J Assoc Off Anal Chem. 1963;46(5):825–829. [Google Scholar]
- Van Soest P, Wine R. Use of detergents in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J Assoc Off Anal Chem. 1967;50(50):50–55. [Google Scholar]
- Zia ur R, Islam M, Shah WH. Effect of microwave and conventional cooking on insoluble dietary fibre components of vegetables. Food Chem. 2003;80(2):237–240. doi: 10.1016/S0308-8146(02)00259-5. [DOI] [Google Scholar]