Abstract
Extracts of brown lead (Leucaena leucocephala) seed prepared using different extraction solvents were determined for antioxidative activities using different assays. The highest yield (3.4–4.0%) was obtained when water was used as an extraction solvent, compared with all ethanolic extracts used (1.2–2.0 %) (P < 0.05). Much lower chlorophyll content was found in the water extract. When hot water was used, the resulting extract contained lower total phenolic and mimosine contents (P < 0.05). In general, 60–80 % ethanolic extracts had higher 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activities, ferric reducing antioxidant power (FRAP) and metal chelating activity than water extracts (P < 0.05). When brown lead seed was dechlorophyllised prior to extraction, the water extract had slightly increased yield with lower chlorophyll content. Nevertheless, prior chlorophyll removal resulted in the increase in antioxidative activities but lower total phenolic and mimosine contents (P < 0.05). Generally, phenolic compounds and mimosine were more released when water was used as the extraction solvent, while the lower amount of chlorophyll was extracted. Oven-drying exhibited the negative effect on antioxidative activities and mimosine content. The higher antioxidative activities with concomitant higher total phenolic and mimosine contents were found in water extract dried by freeze drying. Thus, extraction solvent, dechlorophyllisation and drying methods directly influenced the yield and antioxidative activity of lead seed extract.
Keywords: Lead seed (Leucaena leucocephala), Mimosine, Phenolic compound, Antioxidative activity, Drying, Dechlorophyllisation
Introduction
Lead tree, Leucaena leucocephala, belongs to a tropical and subtropical legume family. It has been used as livestock feed due to their high contents of protein, carotenoids, vitamin-K, xanthophylls and minerals (Nirmal and Benjakul 2011; Kamada et al. 1997). Additionally, seeds and leaves of lead tree have been consumed as human foods (Sahlu et al. 1995). The seeds of guaje (L.esculenta) are eaten with salt in Mexico. Phenolic compounds found in different parts of Leucaena were condensed tannins (Echeverria et al. 2002), quercetin and myricetin glycosides (Lowery et al. 1984), gallocatechin, epigallocatechin and epicatechin (Erickson et al. 2000). Additionally, lead contains a non-protein amino acid called mimosine, (β-(3-hydroxy-4-pyridon-1-yl)-L-alanine) (Lalitha and Kulothungan 2006). Mimosine is chemically similar to dihydroxyphenylalanine with a 3-hydroxy-4-pyridone ring instead of a 3, 4- dihydroxyphenyl ring (Soedarjo et al. 1994). The presence of 2–10 % mimosine in the dry matter of leaves and seeds has restricted its use as fodder, since ingestion of mimosine resulted in hair loss, goitre, reproductive disorders, epithelial damage and ultimately death of animals. However, no toxicity for human consumption has been reported (Poonam and Pushpa 1995). Recently, lead seed extracts were found to lower melanosis of Pacific white shrimp during ice storage (Nirmal and Benjakul 2011). Mimosine has been reported to inhibit polyphenoloxidase from Pacific white shrimp with mixed type inhibition kinetic (Nirmal and Benjakul 2011).
Natural phenolic extracts with antioxidant activity such as rosemary extract, tea catechin, tannins, etc. have been gaining increasing attention due to their safety (Frankel 1998). Phenolic extracts from H. isora fruits and C. pentandra seeds also showed the antioxidant activities (Loganayaki et al. 2011). Phenolic compounds vary in structure and the number of hydroxyl groups, leading to the variation in their antioxidative activity. In general, phenolic compounds play a role as antioxidants through different mechanisms of action, such as scavenging of free radicals (Antolovich et al. 2002), quenching of reactive oxygen species, inhibition of oxidative enzymes (Edenharder and Grunhage 2003), chelation of transition metals or through interaction with biomembranes (Liao and Yin 2000). A wide range of low and high molecular weight plant polyphenolics possessing antioxidative activity has shown to prevent lipid oxidation in different food systems (Maqsood and Benjakul 2010; Hagerman et al. 1998). Therefore, these compounds have been considered as promising potential protectors against lipid oxidation and biological aging of tissues. Different phenolic compounds may act as antioxidants at varying degrees in different food systems, depending on the polarity and molecular characteristics (Maqsood and Benjakul 2010).
Green lead seeds are generally consumed as fresh side dish in Thailand, but the mature brown seeds have not been exploited. Therefore, brown lead seed can be a source of novel natural antioxidants, apart from melanosis inhibitor. The present study aimed to elucidate the effects of extraction solvent, prior dechlorophyllisation and different drying methods on total phenolic, mimosine and chlorophyll contents and antioxidative activity of brown lead seed extract.
Materials and methods
Chemicals
L-(3,4-dihydroxylphenyl) alanine (L-DOPA), Brij-35, L-mimosine, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,4,6-tripyridyl-s-triazine (TPTZ) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Orthophosphoric acid, Folin–Ciocalteu, ρ-nitroaniline and sodium nitrite were obtained from Merck (Darmstadt, Germany). Chloroform was purchased from Lab-Scan (Bangkok, Thailand).
Collection and preparation of lead seed
Brown lead seeds were collected from lead trees at Prince of Songkla University, Hat Yai. Seeds were dried at 60 °C using a dryer (Mextech, Seoul, South Korea) to obtain a final moisture content of 7–8 % with drying time of approximately 12 h. Dry brown lead seeds were ground into a fine powder using a blender (Phillips, Guangzhou, China) and sieved through a stainless steel sieve of 40 mesh (Frilsch, Oberstein, Germany). The powder was placed in a polyethylene bag and stored in a refrigerator until use.
Effect of extraction solvent on yield and antioxidative activity of brown lead seed extracts
Seed powder (2 g) was mixed with 40 ml of distilled water (26–28 °C), hot water (100 °C), 60, 80 or 100 % (v/v) ethanol. For the mixture containing hot water, it was shaken continuously in a temperature controlled shaker (LMS, Ogawa seiki, Tokyo, Japan) for 1 h. Thereafter, the mixture was stirred continuously up to 12 h at room temperature. For other samples, the mixtures were stirred continuously for totally 12 h at room temperature. After 12 h, all mixtures were centrifuged at 8000 g for 20 min at 20 °C using a Beckman Coulter centrifuge (Avanti J-E Centrifuge, Fullerton, CA, USA). The supernatant was collected and subjected to analyses.
Analyses
Determination of total phenolic content
Total phenolic content in the extracts was determined using Folin–Ciocalteu reagent according to the method of Slinkkard and Singleton (1977). Appropriately 1 ml of diluted solution (20–50 fold dilution) was added with 0.2 ml of two-fold diluted Folin–Ciocalteu reagent and mixed thoroughly. After 3 min, 3 ml of 2 % (w/v) sodium carbonate solution were added. After the solution was left for 30 min at room temperature, an absorbance was measured at 760 nm using a UV-1601 spectrophotometer (Shimadzu, Kyoto, Japan). The concentration of total phenolic compounds was calculated from the standard curve of gallic acid with the range of 0–0.05 mg/ml and expressed as g GAE/100 g extract.
Determination of mimosine content
Mimosine content in the extracts was determined spectrophotometrically according to the method of Lalitha et al. (1993). Extracts with the solid content of 3.6 mg/ml, adjusted with corresponding solvent, (3.5 ml) was mixed with 1 ml of sodium phosphate buffer (0.25 M, pH 7). Then, 0.5 ml of diazotised ρ-nitroaniline reagent was added to the reaction mixture and mixed well. The reaction mixture was incubated at room temperature for 15 min and the developed colour was measured spectrophotometrically at 400 nm. Diazotised ρ-nitroaniline reagent was freshly prepared by mixing an equal volume of ρ-nitroaniline solution (0.05 % in 0.033 M H3PO4) and sodium nitrite solution (0.1 % in distilled water). The concentration of mimosine in the extract was calculated from the standard curve of mimosine with the range of 2–10 M and was expressed as mmol mimosine/100 g extract.
Mimosine content in extracts was also determined by HPLC as per the method of Soedarjo et al. (1994). The separation system consisted of an Agilent 1100 series HPLC equipped with a hypersil ODS 4.0*250 mm, 5 μm column (Agilent, Boblingen, Germany) and a UV detector (Rheodyne, Cotati, CA, USA). The temperature of the column was maintained at 25 °C and the injection volume was 20 μl. Mimosine was eluted by using 0.2 % orthophosphoric acid (v/v) at a flow rate of 1 ml/min and was detected at 280 nm. Standard stock solutions (1,000 mg/l) were diluted with 0.1 N HCl to obtain concentrations of 50–500 mg/l. Standard solutions were then injected into the column and the elution was performed in the same manner with the samples. Mimosine content was quantified using a standard curve.
Determination of chlorophyll content
Total chlorophyll content was determined spectrophotometrically according to the method of AOAC (2000) with the analytical method No. of 942.04. All extracts were adjusted to obtain the concentration of 3.6 mg/ml using the corresponding solvent. Absorbance of solutions were measured spectrophotometrically at 660 and 642 nm. For the blank, the corresponding solvents were used instead of extracts. Total chlorophyll content (TCC) was calculated after blank substraction using the following equation
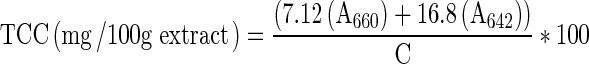 |
The chlorophyll content was expressed as mg/100 g extract.
Determination of antioxidative activities
All extracts were adjusted with corresponding solvent to obtain the final concentration of 3.6 mg/ml before assays for antioxidative activity.
DPPH radical scavenging activity
DPPH radical scavenging activity was determined as described by Wu et al. (2003) with a slight modification. Sample (1.5 ml) was added with 1.5 ml of 0.15 mM 2,2-diphenyl-1-picrylhydrazyl (DPPH) in 95 % ethanol. The mixture was mixed vigorously and allowed to stand at room temperature in dark for 30 min. The absorbance of the resulting solution was measured at 517 nm using a spectrophotometer. Sample blank was prepared in the same manner except that corresponding solvents were used instead of DPPH solution. A standard curve was prepared using Trolox in the range of 10–60 μM. The activity was calculated after the sample blank substraction and expressed as μmol Trolox equivalents (TE)/100 g extract.
ABTS radical scavenging activity
ABTS radical scavenging activity was assayed as per the method of Arnao et al. (2001) with a slight modification. The stock solutions included 7.4 mM ABTS solution and 2.6 mM potassium persulphate solution. The working solution was prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12 h at room temperature in dark. The solution was then diluted by mixing 1 ml ABTS solution with 50 ml of methanol in order to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using spectrophotometer. Fresh ABTS solution was prepared for each assay. Sample (150 μl) was mixed with 2,850 μl of ABTS solution and the mixture was left at room temperature for 2 h in dark. The absorbance was then measured at 734 nm using a spectrophotometer. Sample blank was prepared in the same manner except that methanol was used instead of ABTS solution. A standard curve of Trolox ranging from 50 to 600 μM was prepared. The activity was calculated after sample blank subtraction and was expressed as μmol Trolox equivalents (TE)/100 g extract.
Ferric reducing antioxidant power (FRAP)
FRAP was assayed according to Benzie and Strain (1996). Stock solutions included 300 mM acetate buffer (pH 3.6), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution in 40 mM HCl, and 20 mM FeCl3·6H2O solution. A working solution was prepared freshly by mixing 25 ml of acetate buffer, 2.5 ml of TPTZ solution and 2.5 ml of FeCl3 · 6H2O solution. The mixed solution was incubated at 37 °C for 30 min in a water bath (Memmert, D-91126, Schwabach, Germany) and was referred to as FRAP solution. A sample (150 μl) was mixed with 2,850 μl of FRAP solution and kept for 30 min in dark at room temperature. The ferrous tripyridyltriazine complex (coloured product) was measured by reading the absorbance at 593 nm. Sample blank was prepared by omitting FeCl3 from FRAP solution and distilled water was used instead. The standard curve was prepared using Trolox ranging from 50 to 600 μM. The activity was calculated after sample blank subtraction and was expressed as μmol Trolox equivalents (TE)/100 g extract.
Chelating activity on ferrous ions (Fe2+)
The chelating activity towards Fe2+ was measured by the method of Boyer and McCleary (1987) with a slight modification. Sample (940 μl) was mixed with 20 μl of 2 mM FeCl2 and 40 μl of 5 mM ferrozine. The reaction mixture was allowed to stand for 20 min at room temperature. The absorbance was then read at 562 nm. The blank was prepared in the same manner except that distilled water was used instead of the sample. For sample blank, FeCl2 solution was excluded and distilled water was used instead. The standard curve was prepared using EDTA ranging from 10 to 60 μm. The chelating activity after sample blank substraction was calculated and expressed as EDTA equivalent/100 g extract.
Effect of prior chlorophyll removal on antioxidant activity of brown lead seed extracts
Lead seed powder was separated into two portions, with and without prior chlorophyll removal. To remove chlorophyll, the powder was mixed with chloroform using a powder/ solvent ratio of 1:20 (w/v) (Row and Jin 2006). The mixture was stirred for 30 min, followed by filtration using a Whatman filter paper No.1 (Schleicher and Schuell, Maidstone, England).
The retentate was air dried until chloroform was completely removed. The powder obtained was refered to as dechlorophylled powder. Brown lead seed powder, both with and without prior chlorophyll removal, was subjected to extraction using the selected extraction solvent rendering the highest yield with pronounced antioxidative activity. The resulting extracts were analysed as previously mentioned.
Effect of drying methods on antioxidative activity of brown lead seed extracts
Extracts from brown lead seed without and with prior chlorophyll removal were dried using oven drying and freeze drying. For oven drying, the extracts were dried in an oven (Memmert, Beschickung, Germany) at 60 °C for 12 h. To prepare freeze-dried sample, the extracts were dried using a freeze dryer (Model Coolsafe 55, Scanvac, Lynge, Denmark). Resulting powder was dissolved in distilled water to obtain the concentration of 3.6 mg/ml. All solutions were analysed as previously described.
Statistical analysis
All experiments were run in triplicate. The experimental data were subjected to One-way Analysis of Variance (ANOVA) and the differences between means were evaluated by Duncan’s Multiple Range Test. For pair comparison, T-test was used (Steel and Torrie 1980). Data analysis was performed using a SPSS package (SPSS 14.0 for Windows, SPSS Inc, Chicago, IL, USA).
Results and discussion
Effect of extraction solvent on yield, compositions and antioxidative activities
Yield, total phenolic, mimosine and chlorophyll contents
Lead seed extracts obtained using various extraction solvents showed different yields (1.2–4.0 %) (Table 1). Higher yield (3.4–4.0 %) was obtained when water, especially hot water, was used for extraction, compared with ethanol at all concentrations used (P < 0.05). It was noted that non significantly higher yield was found in extract using hot water, compared with water (P > 0.05). For ethanolic extracts, those using ethanol at lower concentrations had the higher yield (P < 0.05). The result indicated that the compounds with high polarity were dominant in brown lead seed. As a result, water was shown as the potential extraction solvent.
Table 1.
Extraction yield, total phenolic, mimosine and chlorophyll contents of different extracts from brown lead seed
| Extraction media | Extraction yield (%) | Total phenolic content (g GA equivalent/100 g sampleb) | Mimosine content (mmol mimosine/100 g sampleb) | Total chlorophyll content (mg/100 g sampleb) |
|---|---|---|---|---|
| Hot water | 4.0 ± 0.12aa* | 54.5 ± 4.68c (2.2 ± 0.19A**) | 53.0 ± 1.33c (2.1 ± 0.05A) | 4.9 ± 0.00e (0.2 ± 0.00E) |
| Water | 3.4 ± 0.56a | 66.8 ± 2.67b (2.3 ± 0.09A) | 62.4 ± 5.92b (2.1 ± 0.20A) | 15.5 ± 0.01d (0.5 ± 0.00D) |
| 100 % EtOH | 1.2 ± 0.29c | 78.8 ± 0.95a (0.9 ± 0.01C) | 69.2 ± 5.35b (0.8 ± 0.06D) | 64.2 ± 0.04b (0.8 ± 0.00C) |
| 80 % EtOH | 1.5 ± 0.66c | 68.9 ± 1.00b (1.0 ± 0.02C) | 72.5 ± 4.06a (1.1 ± 0.06C) | 74.6 ± 0.01a (1.1 ± 0.00B) |
| 60 % EtOH | 2.0 ± 0.05b | 77.2 ± 1.88a (1.5 ± 0.04B) | 73.7 ± 5.31a (1.5 ± 0.11B) | 57.1 ± 0.04c (1.2 ± 0.00A) |
*Different lowercase letters within the same column denote significant differences (P < 0.05)
**Different uppercase letters in the parenthesis within the same column denote significant differences (P < 0.05)
aValues are mean ± standard deviation (n = 3)
bValues without and in the parenthesis were calculated with reference to the extract and brown lead seed (dry weight), respectively
The extracts using 60 % or 100 % ethanol as the extraction solvent had the highest total phenolic content (78.8 g GA equivalent/100 g extract), compared with other samples (P < 0.05). Hot water extract possessed the lowest total phenolic content (54.5 g GA equivalent/100 g extract) (P < 0.05). High temperature of hot water might cause the destruction of some phenolic compounds. Phenolic compounds are phytochemicals abundant in plants (Cartea et al. 2011). Type and amount of phenolic compounds varied with plant, maturation, season, etc. (Sultana et al. 2007). When calculated with reference to lead seed, the highest amount of phenolic compounds (2.2–2.3 g GA equivalent/100 g lead seed) were obtained (P < 0.05) when hot water and water were used as extraction solvents. The lowest phenolic compounds (0.9 g GA equivalent/100 g lead seed) were released when 100 % ethanol was used (P < 0.05). The result was in agreement with the extraction yield.
Mimosine content was higher in the ethanolic extracts (P > 0.05), especially those extracted with 60 and 80 % ethanol. Similar mimosine content was found between water extract and 100 % ethanolic extract (P > 0.05). Mimosine content was lowest in hot water extract (53.0 mmol mimosine/100 g extract) (P < 0.05). It was reported that mimosine was not stable at high temperature (Wills and Tangendjaja 1981). Different parts of the Leucaena contain varying amounts of mimosine, 2–10 % dry leaf, 2–5 % dry seed (Lalitha et al. 1993) and 1–1.5 % root (Soedarjo et al. 1994). L. leucocephala leaves and seeds had high content of mimosine (10–40 g/kg dry matter) (Puchala et al. 1996). Mimosine in seed (3.46 % dry matter) was 6-fold higher than that found in leaves (0.56 % dry matter) (Kamada et al. 1997). Water could be used to extract mimosine from the brown lead seed powder and spectrophotometric method could be used as rapid and sensitive method for detection of mimosine (Nirmal and Benjakul 2011). The highest content of minosine ( 2.1 mmol mimosine/ 100 g lead seed) was released and recovered when hot water and water were used, while 100 % ethanol yielded the lowest amount of minosine extracted from lead seed (0.8 mmol mimosine/ 100 g lead seed). The result was concomitant with both extraction yield and total phenolic content.
Different extracts from brown lead seed contained varying chlorophyll contents (4.9–74.6 mg/100 g extract). Much lower chlorophyll content was found in hot water extracts (4.9 mg/100 g extract), while chlorophyll contents ranged from 57.1 to 74.6 mg/100 g extract in all ethanolic extracts (Table 1). Ethanol was reported to be the effective solvent for chlorophyll extraction (Sartory and Grobbelaar 1984). Thus, water extracts consisted of chlorophyll at a lower level. This was evidenced by the lighter or less green colour of both water and hot water extracts. When calculated based on the weight of lead seed, the lowest chlorophyll content (0.2 mg/ 100 g lead seed) was obtained as hot water was used as the extraction solvent (P < 0.05), whereas 60 % ethanol was able to extract a highest amount of chlorophyll (1.2 mg/100 g lead seed) from the seed (P < 0.05). In general, phenolic compounds and mimosine could be released effectively when the water was used as the extraction solvent, while the lower amount of chlorophyll was extracted. Therefore, water could be a potential extraction solvent, which rendered the higher yield with lower content of chlorophylls. Green colour of extract might be an obstacle for further application since it more likely results in discolouration and unacceptability of product.
Antioxidative activities
Different extracts using varying extraction solvents showed different antioxidant activities when assayed using different methods
DPPH radical scavenging activity
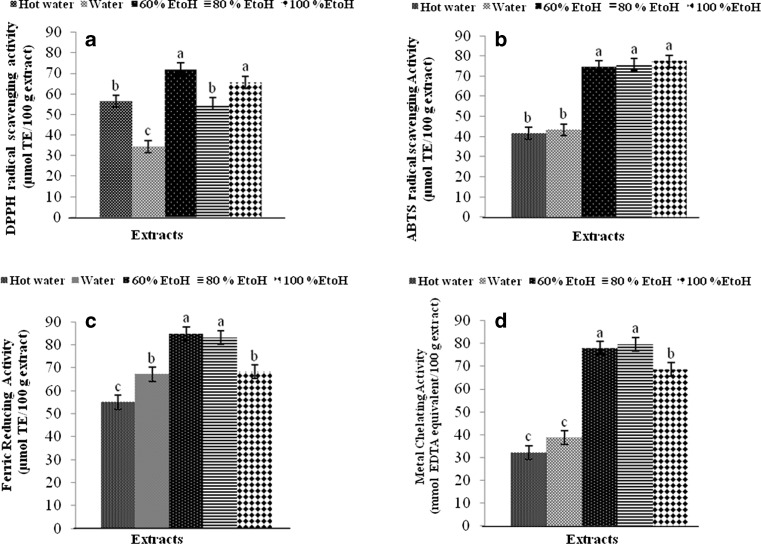
DPPH radical scavenging activity of brown lead seed extracts varied with extraction solvents. DPPH radical scavenging activities of ethanolic extracts using 60 % or 100 % ethanol as the solvent were highest as shown in Fig. 1a (P < 0.05). Extract using water as the extraction solvent showed the lowest DPPH radical scavenging activity (P < 0.05), while those extracted using hot water and 80 % ethanol showed the similar DPPH radical scavenging activity (P > 0.05). DPPH is used as a free radical to evaluate antioxidative activity of some natural compounds and the degree of colour changes is attributed to hydrogen donating ability of test compounds, which is indicative of their scavenging potential (Shimada et al. 1992). Compound in the ethanolic extracts might have the appropriate polarity or hydrophilic-hydrophobic balance, which could localise close to DPPH radicals, in which hydrogen could be donated to DPPH radicals easily.
Fig. 1.
Antioxidative activities of different brown lead seed extracts using various extraction solvents as determined by DPPH radical scavenging (a), ABTS radical scavenging (b), FRAP (c) and metal chelating (d) assays. The extracts with a concentration of 3.6 mg/ml were used for activity assays. Bars represent the standard deviation (n = 3). Different letters on the bars indicate significant differences (P < 0.05)
ABTS radical scavenging activity
ABTS radical scavenging activity of different extracts using various extraction solvents is presented in Fig. 1b. In general, ABTS radical scavenging activity of ethanolic extracts was higher than that of water extracts (P < 0.05). However, ethanol concentrations (60–100 %) had no impact on ABTS radical scavenging activity of resulting extracts. For water extracts, the extraction temperature had no influence on ABTS radical scavenging activity. The result indicated that antioxidant compounds with high polarity exhibited the lower activity in ABTS radical scavenging than those with less polarity found in ethanolic extracts. ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) can be oxidised to generate a radical cation, ABTS+, that is green in colour and can be measured by absorbance at 734 nm. Antioxidants suppress this reaction by electron donation or radical scavenging, thereby inhibiting the formation of the coloured ABTS radical. The concentration of antioxidant in the test sample is inversely proportional to the ABTS radical formation and absorbance at 734 nm (Arnao et al. 2001). Hagerman et al. (1998) reported that the high molecular weight phenolics such as tannic acid have more ability to quench ABTS radical and the effectiveness depends on the molecular weight, the number of aromatic rings and nature of hydroxyl groups’ substitution than the specific functional groups. Phenolic compounds capable of donating hydrogen atom were more effective in scavenging ABTS radical (Leong and Shui 2002). The results suggested that different extracts had the varying capacity of scavenging ABTS radicals.
Ferric reducing antioxidant power (FRAP)
Different extracts showed their ability to reduce TPTZ–Fe (III) complex to TPTZ–Fe (II) complex differently as shown in Fig. 1c. Among all extracts tested, those using 60 % or 80 % ethanol as extraction solvent showed the highest FRAP (P < 0.05). The result indicated that the extract could easily donate the electron to Fe (III) most effectively, thus reducing it to Fe (II). The reducing capacity measures the ease of the compounds in donating electrons (Medina et al. 2007). Absolute ethanol (100 %) exhibited the similar capacity of extracting antioxidative compound with FRAP to water (P < 0.05). Nevertheless, FRAP of extract using hot water was lower than that of water extract (P < 0.05). Hot water might cause the destruction of compounds with FRAP to some degree. Thus, extraction solvent and extraction temperature had the profound impact on FRAP of resulting extracts.
Metal chelating activity
Metal chelating activity of different extracts is depicted in Fig. 1d. Ethanolic extracts showed the higher metal chelating activity than water extracts (P < 0.05). However, the extracts using 100 % ethanol had the lower metal chelating activity than those having 60 % or 80 % ethanol as the extraction solvent (P < 0.05). It was noted that both water and hot water extracts showed similar metal chelating activity (P > 0.05). Metal has been known to act as pro-oxidant, which can induce lipid oxidation (Yasuko et al. 2002). Thus, brown lead seed extracts were able to chelate Fe ions, thereby lowering the oxidation of lipid containing foods.
Due to the higher yield of water extracts, almost 2-fold higher, compared with ethanolic extracts, water was selected as the appropriate solvent to prepare brown lead seed extract with antioxidative activities. Furthermore, much higher chlorophyll content was found in ethanolic extracts, leading to dark green in colour. This might be a drawback for further application as the natural additive. Additionally, it was found that hot water rendered the extract with lower total phenolic and mimosine contents than water extract. Although antioxidative activity of all ethanolic extracts was higher than water extracts, based on the same concentration, total antioxidative activity was higher in water extracts, based on total volume. Additionally, water is cheap, available and safe for consumption. Therefore, water was used as an extraction solvent for further study.
Effect of prior chlorophyll removal on yield, compositions and antioxidative activities
Yield, total phenolic, mimosine and chlorophyll contents
Brown lead seed extract prepared using distilled water as a extraction solvent had the higher yield (20.1 g/100 g dry seed powder) when chlorophyll removal was implemented prior to extraction (Table 2). The extract with prior chlorophyll removal had the much lower chlorophyll content (5.8 mg/100 g extract), compared with that without prior chlorophyll removal (24.9 mg/100 g extract). The result was in accordance with the higher chlorophyll content released from the lead seed without prior chlorophyll removal (4.3 mg/ 100 g lead seed), compared with that from lead seed with prior chlorophyll removal (1.2 mg/ 100 g lead seed) (P < 0.05). Using chloroform to remove the chlorophyll might also eliminate fat from seed. As a result, water could penetrate and extract phenolic compounds more effectively. Water soluble components in the water extract might include proteins, phenolic compounds, mimosine, etc. Prior chlorophyll removal could reduce phenolic content of extracts from 56.2 to 43.1 g GA equivalent/100 g extract. When calculated with reference to the weight of lead seed, the higher phenolic compounds were extracted (9.7 g GA equivalent/ 100 g lead seed) from seed without prior chlorophyll removal, compared with those from non-prior chlorophyll removal counterpart (8.7 g GA equivalent/ 100 g lead seed) (P < 0.05). Total phenolics in shoot tip of Leucaena leucocephala grown in Thailand were 405 and 60.6 mg/100 g dry matter, respectively (Chanwitheesuk et al. 2005). Phenolics are distributed non-uniformly at the tissue, cellular and subcellular levels (Naczk and Shahidi 2004). The result suggested that chloroform used for chlorophyll removal might remove some phenolic compounds, especially those with low polarity. As a consequence, the lower phenolic content was obtained.
Table 2.
Extraction yield, total phenolic, mimosine, chlorophyll contents and antioxidant activity of different extracts from brown lead seed with and without prior chlorophyll removal
| Treatment | Extraction yield (%) | Total phenolic content (g GA equivalent/100 g samplec) | Mimosine content (mmol mimosine/100 g samplec) | Chlorophyll content (mg/100 g samplec) | DPPH ( mmol TE/100 g extract) | ABTS (mmol TE/100 g extract) | Metal Chelating (mmol EDTA equivalent/100 g extract) | FRAP (mmol TE/100 g extract) |
|---|---|---|---|---|---|---|---|---|
| Without PCRb | 17.3 ± 0.00ab* | 56.2 ± 0.01a (9.7 ± 0.00A**) | 76.6 ± 1.66a (13.3 ± 0.29A) | 24.9 ± 0.34a (4.3 ± 0.06A) | 57.8 ± 2.66b | 38.8 ± 1.59b | 43.2 ± 4.47b | 56.4 ± 0.14b |
| With PCR | 20.1 ± 0.00a | 43.1 ± 0.06b (8.7 ± 0.01B) | 58.0 ± 1.47b (11.7 ± 0.30B) | 5.8 ± 0.00b 1.2 ± 0.00B) | 64.3 ± 0.12a | 63.1 ± 0.06a | 56.8 ± 0.03a | 62.6 ± 0.47a |
*Different lowercase letters within the same column denote significant differences (P < 0.05)
**Different uppercase letters in the parenthesis within the same column denote significant differences (P < 0.05)
aValues are mean ± standard deviation (n = 3)
bPrior chlorophyll removal
cValues without and in the parenthesis were calculated with reference to the extract and brown lead seed (dry weight), respectively
Mimosine content was 58.0 and 76.6 mmol mimosine/100 g extract for the extracts with and without prior chlorophyll removal, respectively. This was in agreement with the higher mimosine released from the lead seed without prior chlorophyll removal (13.3 mmol mimosine/ 100 g), in comparison with that found for seed with prior chlorophyll removal (11.7 mmol mimosine/ 100 g) (P < 0.05). Therefore, chlorophyll removal before extraction had the marked impact on total phenolic and mimosine contents in the extract. When chlorophyll content was determined in the extracts, it was lower in the extracts with prior chlorophyll removal (P < 0.05). Chlorophyll was decreased from 24.9 to 5.8 mg/100 g extract (Table 2), when chlorophyll was removed prior to extraction.
Antioxidative activities
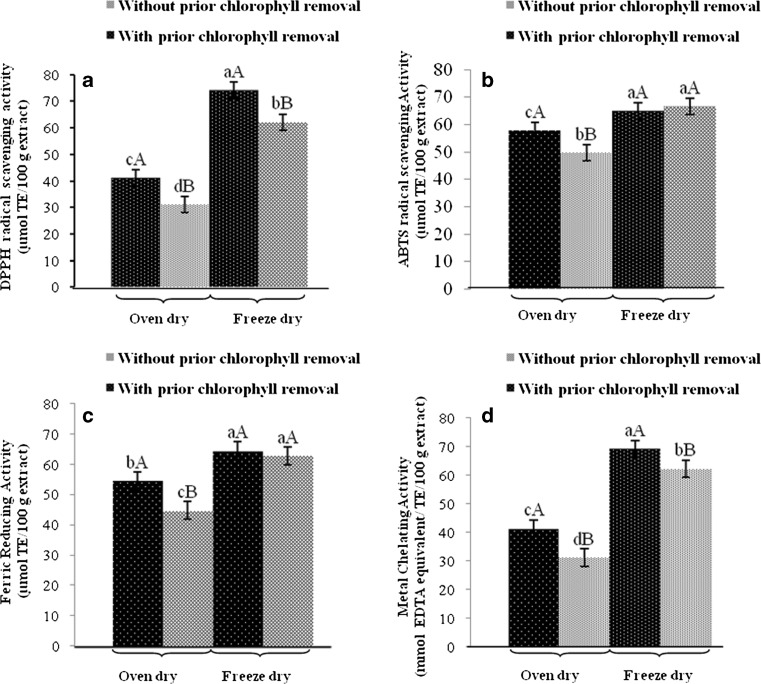
For DPPH and ABTS radical scavenging activities, it was found that both activities increased when prior chlorophyll removal was performed (P < 0.05) (Table 2). DPPH radical scavenging activity of both extracts reflected their hydrogen donating ability (Siddhuraju and Becker 2007). In general, phenolic compounds capable of donating hydrogen atom were more effective in scavenging ABTS radical (Leong and Shui 2002). The results suggested that prior removal of chlorophyll might facilitate the extraction of compounds with DPPH and ABTS radical scavenging activities to a higher degree. Higher FRAP was also found in the extract with prior chlorophyll removal (62.6 mmol TE/100 g extract), compared with that without dechlorophyllisation (56.4 mmol TE/100 g extract) (P < 0.05). The result indicated the higher reducing power of the former. Electron donating property is important mode of action for antioxidant, in which radical chain reaction can be terminated (Prior et al. 2005). It has been reported that chlorophyll had in vitro antioxidative activity as determined by DPPH and ABTS radical scavenging activities. Activity was governed by form or derivatives of chlorophylls (Ferruzzi et al. 2002). Also, chelated metal in porphyrin ring was a prime factor determining antioxidative activity (Lanfer-Marquez et al. 2005). Thus, it was postulated that the phenolic compounds with higher antioxidative activity were more concentrated after chlorophyll removal. As a consequence, the higher antioxidative activity was obtained in the extract with prior chlorophyll removal. In the extracts without prior chlorophyll removal, the lower metal chelating activity was observed. It was also suggested that compounds with capability of metal chelation became concentrated after chlorophyll removal. The efficiency in metal chelation varied with the type of phenolic compounds and did not relate with radical scavenging activity and reducing power (Danilewicz 2003). Therefore, prior dechlorophyllisation of brown seed powder before extraction had the impact on antioxidative activities of the resulting extracts.
Effect of drying methods on the composition and antioxidant activities
Extraction yield, total phenolic, mimosine and chlorophyll contents
Higher yields were found in the extracts with prior chlorophyll removal (10.0 %), compared with those obtained in the extracts without dechlorophyllisation (8.4–8.7 %). Nevertheless, drying methods had no effect on yield, regardless of prior chlorophyll removal (P > 0.05). Total phenolic content of brown lead seed extracts with and without prior chlorophyll removal subjected to two drying methods is presented in Table 3. Higher total phenolic content (62.6–72.9 g GA equivalent/100 g extract) was obtained when freeze drying was used (P < 0.05), regardless of prior chlorophyll removal. The result was concomitant with the total amount of phenolic compounds released with the reference to the weight of lead seed. The highest content of phenolic compounds extracted from the seed with prior chlorophyll removal was obtained when freeze-drying was implemented (7.3 g GA equivalent/ 100 g lead seed) (P < 0.05). The findings indicated that phenolic compounds were sensitive to heat treatment. Some phenolic compounds might undergo destruction or polymerisation during heating at high temperature. Pinela et al. (2011) also reported that freeze drying was able to maintain the antioxidant activity and the concentrations of hydrophilic (phenolics, ascorbic acid and sugars) and lipophilic (tocopherols, chlorophylls and lycopene) compounds in shruby plants including Cytisus genus and Genista genus. Freeze-dried Pterospartum tridentatum had the highest antioxidant properties (EC50 values ≤0.15 mg/ml), whilst the sample with shade-drying had the decreases in antioxidant capacity and phenolic content (Pinela et al. 2011). The results obtained in the present study were also in accordance with Kahkonen et al. (1999) who found that total phenolic contents of vegetable extract prepared by oven drying were very low, compared with that of freeze-dried extract (P < 0.05). Velioglu et al. (1998) reported a strong relationship between total phenolic content and antioxidant activity in selected fruits, vegetables and grain products.
Table 3.
Total phenolic, mimosine and chlorophyll contents of water extracts from brown lead seed as affected by drying methods
| Yield / compositions | Oven drying | Freeze drying | ||
|---|---|---|---|---|
| Without PCRb | With PCR | Without PCR | With PCR | |
| Extraction yield (%) | 8.7 ± 0.00abB* | 10.0 ± 0.00aA | 8.4 ± 0.00bB | 10.0 ± .0.00aA |
| Total phenolic content (g GA equivalent /100 g samplec) | 45.2 ± 0.03dB (3.9 ± 0.00dB)** | 57.4 ± 0.02cA (5.7 ± 0.00bA) | 62.6 ± 0.00bB (5.3 ± 0.00cB) | 72.9 ± 0.00aA (7.3 ± 0.00aA) |
| Mimosine content (mmol mimosine / 100 g samplec) (spectrophotometric method) | 26.9 ± 0.25cA (2.3 ± 0.02dB) | 25.6 ± 0.21cA (2.6 ± 0.02cA) | 258.2 ± 0.00aA (21.7 ± 0.00bB) | 221.5 ± 0.00bB (22.2 ± 0.00aA) |
| Mimosine content (mmol mimosine / 100 g samplec) (HPLC method) | 25.4 ± 0.07cA (2.2 ± 0.01dB) | 24.1 ± 0.02cA (2.4 ± 0.00cA) | 251.3 ± 0.09aA (21.1 ± 0.01bB) | 216.7 ± 0.04bB (21.7 ± 0.00aA) |
| Chlorophyll content (mg/100 g extract) | 11.5 ± 0.00bA (1.0 ± 0.00bA) | 3.2 ± 0.03dB (0.3 ± 0.00dB) | 23.8 ± 0.03aA (2.0 ± 0.00aA) | 9.2 ± 0.03cB (0.9 ± 0.00cB) |
*Different lowercase and uppercase letters within the same row and the same drying method, respectively, indicate significant differences (P < 0.05)
**Different lowercase and uppercase letters in the parenthesis within the same row and the same drying method, respectively, indicate significant differences (P < 0.05)
aValue are mean ± standard deviation (n = 3)
bPrior chlorophyll removal
cValues without and in the parenthesis were calculated with reference to the extract and brown lead seed (dry weight), respectively
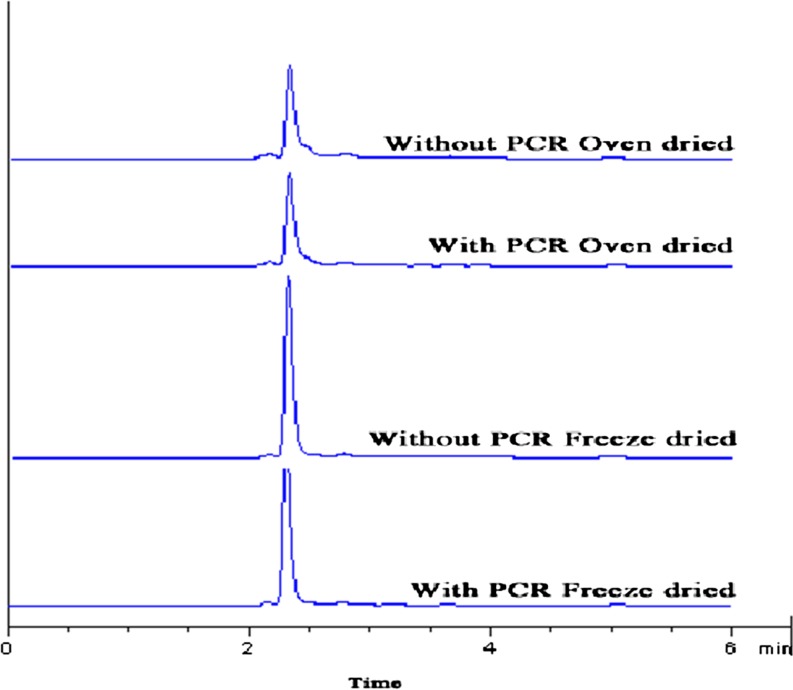
Mimosine content in the extract was lower when oven drying at 60 °C was implemented. The highest mimosine content (258.2 mmol mimosine/100 g extract) was found in the freeze-dried extract without prior chlorophyll removal (Table 3). Mimosine contents in all samples were confirmed by HPLC method. Mimosine content of brown seed extract powder prepared by freeze-drying (216.7–251.3 mmol mimosine/100 g extract) was higher than that of oven dried sample (24.1–25.4 mmol mimosine/100 g extract) when determined by HPLC (Fig. 2). In general, the contents of mimosine in the extract were in accordance with those expressed with the reference to the weight of lead seed. The highest mimosine content was found when lead seed with prior chlorophyll removal was used as material and freeze-drying was applied for drying (21.7–22.2 mmol mimosine/ 100 g lead seed) (P < 0.05). The result indicated that heating or drying affected mimosine content of extracts. Heating could destroy mimosine, resulting in the marked decrease in mimosine content. Budi et al. (2006) reported that the rate of degradation of mimosine to 3-hydroxy-4(1H)-pyridone was optimum at 70 °C. Mimosine and 3-hydroxy-4(1H)-pyridone were degraded in solutions when held at temperatures of 80–120 °C for 1–2 h (Wills and Tangendjaja 1981). Although spectrophotometeric method was claimed to be a rapid, sensitive and specific for routine mimosine determination (Lalitha et al. 1993), HPLC method was used to reconfirm and compare the result with spectrophotometric method in the present study. Nirmal and Benjakul (2011) has recently reported that mimosine content determined by HPLC in brown lead seed extract which was similar to that determined by spectrophotometric method.
Fig. 2.
Antioxidative activities of brown lead seed extracts with and without prior chlorophyll removal dried using oven drier at 60 °C and freeze-drier as determined by DPPH radical scavenging (a), ABTS radical scavenging (b), FRAP (c) and metal chelating (d) assays. Extracts with a concentration of 3.6 mg/ml were used for all the assays. Bars represent the standard deviation (n = 3). Different uppercase letters on the bars within the same drying method indicate the significant differences (P < 0.05). The different lowercase letters on the bars indicate significant differences (P < 0.05)
Chlorophyll content of water extracts dried with both drying methods was different (3.2–23.8 mg/100 g extract) (P < 0.05). Chlorophyll content of lead seed extract was higher in freeze-dried extracts (9.2–23.8 mg/100 g extract), compared with those dried at 60 °C (3.2–11.5 mg/100 g extract) (P < 0.05). This was in agreement with the lowest content of chlorophyll calculated based on the weight of lead seed, when the extract from seed with prior chlorophyll removal was dried in oven (0.3 mg/100 g lead seed) (P < 0.05). Thus, chlorophyll was more likely destroyed or destructed at temperature higher than 60 °C, especially with longer drying time. Chlorophyll levels in spinach and kale leaf were affected by drying temperature (Lefsrud et al. 2008)
Antioxidant activities tested by all assays decreased when dried at 60 °C, compared with those found in extracts dried by freeze drying (P < 0.05) (Fig. 3), irrespective of prior chlorophyll removal. The result was in agreement with the decrease in total phenolic and mimosine contents (Table 3). Heating or drying at higher temperature more likely caused the changes in antioxidant compounds. Vellingiri et al. (2011) reported that heating at 50–90 °C had the detrimental effect on phenolic compounds. The result revealed that drying method played an essential role in antioxidant activity of extracts from brown lead seed. Freeze drying was more proper for drying the water extract of brown lead seed, in which antioxidant activity could be maintained after being dried. ABTS and DPPH radical scavenging activities, FRAP and metal chelating capacity of extracts with prior chlorophyll removal was higher than those found in extracts without prior chlorophyll removal (P < 0.05) for both drying methods except for ABTS radical scavenging activity where similar activity was found between freeze-dried samples with and without prior chlorophyll removal (P > 0.05). The results indicated that freeze-dried samples had higher antioxidative activity in different assays than those dried in oven at 60 °C. This result was in agreement with Sang et al. (2011) who reported that freeze drying could retain antioxidant activity and phenolic content of forgae legume leaves more effectively than oven drying. Freeze-drying is generally better in preserving the quality of medicinal plants during processing (Abascal et al. 2005) but the drying cost is considerably higher than hot air-drying (Lin et al. 2011). Lin et al. (2011) found that vacuum freeze drying (VFD) was the best drying method for preserving caffeic acid derivatives and total phenolics in the fresh flowers, leaves, stems and roots of Echinacea purpurea . Drying conditions also had the impact on total phenolic content and antioxidant activity of roselle (Hibiscus sabdariffa L.) (Daniel et al. 2012). Microwave drying was shown to retain phenolic content and was able to enhance antioxidant activity of methanol extact of Salvia officinails (Hamrouni-Sellami et al. 2012).
Fig. 3.
Antioxidative activities of brown lead seed extracts with and without prior chlorophyll removal dried using oven drier at 60 °C and freeze-drier as determined by DPPH radical scavenging (a), ABTS radical scavenging (b), FRAP (c) and metal chelating (d) assays. Extracts with a concentration of 3.6 mg/ml were used for all the assays. Bars represent the standard deviation (n = 3). Different uppercase letters on the bars within the same drying method indicate the significant differences (P < 0.05). The different lowercase letters on the bars indicate significant differences (P < 0.05)
Conclusions
Lead brown seed extract showed the varying antioxidative activity, depending upon the type of extraction solvent and method of drying used. Ethanolic extracts exhibited the higher antioxidative activity but had the lower extraction yield. Water showed the highest extraction yield with lower chlorophyll content. Chlorophyll removal prior to extraction resulted in the decrease in antioxidative activities and lowered total phenolic and mimosine contents. Compared to the oven drying at 60 °C, freeze drying was found to be an appropriate method, which could maintain the higher antioxidative activity of the extract. Therefore, the lead brown seed extract obtained by using a suitable solvent and drying method could serve as an alternative natural antioxidant to prevent lipid oxidation in different food systems.
Acknowledgments
Authors would like to thank the Graduate School, Prince of Songkla University for the financial support.
References
- Abascal K, Ganora L, Yarnell E. The effect of freeze drying and its implications for botanical medicines: a review. Phytother Res. 2005;19:655–660. doi: 10.1002/ptr.1651. [DOI] [PubMed] [Google Scholar]
- Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127:183–198. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- AOAC . Official methods of analysis. 17. Gaithersberg: Association of Official Analytical Chemists; 2000. [Google Scholar]
- Arnao MB, Cano A, Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Boyer RF, McCleary CJ. Superoxide ion as a primary reductant in ascorbate-mediated ferritin iron release. Free Radic Biol Med. 1987;3:389–395. doi: 10.1016/0891-5849(87)90017-7. [DOI] [PubMed] [Google Scholar]
- Budi T, Brian LB, Ron BHW. Optimisation of conditions for the degradation of mimosine in Leucaena leucocephala leaf. J Sci Food Agric. 2006;35:63–616. [Google Scholar]
- Cartea ME, Marta F, Pilar S, Pablo V. Phenolic compounds in Brassica vegetables. Molecules. 2011;16:251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanwitheesuk A, Teerawutgulrag A, Rakariyatham N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005;92:491–497. doi: 10.1016/j.foodchem.2004.07.035. [DOI] [Google Scholar]
- Daniel DL, Huerta BEB, Sosa IA, Mendoza MGV. Effect of fixed bed drying on the retention of phenolic compounds, anthocyanins and antioxidant activity of roselle (Hibiscus sabdariffa L.) Ind Crops Prod. 2012;40:268–276. doi: 10.1016/j.indcrop.2012.03.015. [DOI] [Google Scholar]
- Danilewicz JC. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: central role of iron and copper. Am J Enol Vitic. 2003;54:73–85. [Google Scholar]
- Echeverria V, Belmar R, Ly J, Santos-Ricalde RH. Effect of Leucaena leucocephala leaf meal treated with acetic acid or sodium hydroxide on apparent digestibility and nitrogen retention in pig diets. Anim Feed Sci Technol. 2002;101:151–159. doi: 10.1016/S0377-8401(02)00082-2. [DOI] [Google Scholar]
- Edenharder R, Grunhage D. Free radical scavenging abilities of flavonoidsas mechanism of protection against mutagenicity induced by ter-butyl hydroperoxide or cumene hydroxide in Salmonella typhimurium TA102. Mutat Res Genet Toxicol Environ Mutagen. 2003;504:1–18. doi: 10.1016/S1383-5718(03)00114-1. [DOI] [PubMed] [Google Scholar]
- Erickson AJ, Ramsewak RS, Smucker AJ, Nair MG. Nitrification inhibitors from the roots of Leucaena leucocephala. J Agric Food Chem. 2000;48:6174–6177. doi: 10.1021/jf991382z. [DOI] [PubMed] [Google Scholar]
- Ferruzzi MG, Bohm V, Courtney PD, Schwartz SJ. Antioxidant and antimutagenic of dietary chlorophyll derivatives determination by radical scavenging and bacterial reverse mutagenesis assays. J Food Sci. 2002;67:2589–2595. doi: 10.1111/j.1365-2621.2002.tb08782.x. [DOI] [Google Scholar]
- Frankel EN (1998) Antioxidants. In: Lipid oxidation, 1st edn. The Oily Press, Dundee, p 303
- Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–1892. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- Hamrouni-Sellami I, Rahali FZ, Rebey IB, Bourgou S, Limam F, Marzouk B (2012) Total phenolics, flavonoids, and antioxidant activity of sage (Salvia officinails L.) plants as affected by different drying methods. Food Bioprocess Technol. doi:10.1007/s11947-012-0877-7
- Kahkonen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kamada Y, Oshiro N, Oku H, Hongo F, Chinen I. Mimosine toxicity in broiler chicks fed Leucaena leucocephala seed powder. Anim Sci Technol. 1997;68:121–130. [Google Scholar]
- Lalitha K, Kulothungan SR. Selective determination of mimosine and its dihydroxypyridinyl derivative in plant systems. Amino Acids. 2006;31:279–287. doi: 10.1007/s00726-005-0226-5. [DOI] [PubMed] [Google Scholar]
- Lalitha K, Vargheese CM, Balasubramanian N. Spectrophotometric determination of mimosine and 3-nydroxy-4-(1H)-pyridone—the toxic principles of Leucaena leucocephala. Anal Biochem. 1993;213:57–62. doi: 10.1006/abio.1993.1385. [DOI] [PubMed] [Google Scholar]
- Lanfer-Marquez UM, Barros RMC, Sinnecker P. Antioxidant activity of chlorophylls and their derivatives. Food Res Int. 2005;38:885–891. doi: 10.1016/j.foodres.2005.02.012. [DOI] [Google Scholar]
- Lefsrud M, Kopsell D, Sams C, Wills J, Both AJ. Dry matter content and stability of carotenoids in kale and spinach during drying. Horticulture. 2008;43:1731–1736. [Google Scholar]
- Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76:69–75. doi: 10.1016/S0308-8146(01)00251-5. [DOI] [Google Scholar]
- Liao K, Yin M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: importance of the partition coefficient. J Agric Food Chem. 2000;48:2266–2270. doi: 10.1021/jf990946w. [DOI] [PubMed] [Google Scholar]
- Lin SD, Sung JM, Chen CL. Effect of drying and storage conditions on caffeic acid derivatives and total phenolics of Echinacea Purpurea grown in Taiwan. Food Chem. 2011;125:226–231. doi: 10.1016/j.foodchem.2010.09.006. [DOI] [Google Scholar]
- Loganayaki N, Siddhuraju P, Manian S (2011) Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J Food Sci Technol. doi:10.1007/s13197-011-0389-x [DOI] [PMC free article] [PubMed]
- Lowery JB, Cook N, Wilson RD. Flavonol glycoside distribution in cultivars and hybrids of Leucaena leucocephala. J Sci Food Agric. 1984;35:401–407. doi: 10.1002/jsfa.2740350407. [DOI] [Google Scholar]
- Maqsood S, Benjakul S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010;119:123–132. doi: 10.1016/j.foodchem.2009.06.004. [DOI] [Google Scholar]
- Medina I, Gallardo JM, Gonzaalez MJ, Lois S, Hedges N. Effect of molecular structure of phenolic families as hydroxycinnamic acids and catechins on their antioxidant effectiveness in minced fish muscle. J Agric Food Chem. 2007;55:3889–3895. doi: 10.1021/jf063498i. [DOI] [PubMed] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. J Chromatogr A. 2004;1054:95–111. doi: 10.1016/j.chroma.2004.08.059. [DOI] [PubMed] [Google Scholar]
- Nirmal NP, Benjakul S. Inhibition of melanosis formation in Pacific white shrimp by the extract of lead (Leucaena leucocephala) seed. Food Chem. 2011;128:427–432. doi: 10.1016/j.foodchem.2011.03.048. [DOI] [PubMed] [Google Scholar]
- Pinela J, Barros L, Carvalho AM, Ferreira ICFR. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem Toxicol. 2011;50:829–834. doi: 10.1016/j.fct.2011.11.045. [DOI] [PubMed] [Google Scholar]
- Poonam S, Pushpa RK. Leucaena leucocephala A nutrition profile. Food Nutr Bull. 1995;16:234–237. [Google Scholar]
- Prior RL, Wu XL, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in food and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Puchala R, Davis JJ, Sahlu T. Determination of mimosine and 3,4-dihydroxypyridine in milk and plasma of goats. J Chromatogr B Biomed Appl. 1996;685:375–378. doi: 10.1016/S0378-4347(96)00221-6. [DOI] [PubMed] [Google Scholar]
- Row KH, Jin Y. Recovery of catechin compounds from Korean tea by solvent extraction. Bioresour Technol. 2006;97:790–793. doi: 10.1016/j.biortech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Sahlu T, Puchala R, Reis PJ, Davis JJ, Tesfai K, Fernandez JM, Millamena AA. Technical note: tissue residues of mimosine and 2, 3-dihdroxypyridine after intravenous infusion in goats. J Anim Sci. 1995;73:172–176. doi: 10.2527/1995.731172x. [DOI] [PubMed] [Google Scholar]
- Sang SY, Jamharee F, Prasad KN, Azlan A, Maliki N (2011) Influence of drying treatments on antioxidant capacity of forage legume leaves. J Food Sci Technol. doi:10.1007/s13197-001-0596-5 [DOI] [PMC free article] [PubMed]
- Sartory DP, Grobbelaa JU. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia. 1984;14:177–187. doi: 10.1007/BF00031869. [DOI] [Google Scholar]
- Shimada K, Fijikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Siddhuraju P, Becker K. The antioxidant and free radical scavenging activities of processed cowpea (Vigna unguiculata L.) seed extracts. Food Chem. 2007;101:10–19. doi: 10.1016/j.foodchem.2006.01.004. [DOI] [Google Scholar]
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparision with manual methods. Am J Enol Vitic. 1977;28:49–55. [Google Scholar]
- Soedarjo M, Hemscheidt TK, Borthakur D. Mimosine, a toxin present in leguminous trees (Leucaena spp.), induces a mimosine-degrading enzyme activity in some Rhizobium strains. Appl Environ Microbiol. 1994;60:4268–4272. doi: 10.1128/aem.60.12.4268-4272.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel RGD, Torrie JH (1980) Principle and procedures of statistics: A biometrical approach. McGraw-Hill, New York.
- Sultana B, Anwar F, Przybylski R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. trees. Food Chem. 2007;104:1106–1114. doi: 10.1016/j.foodchem.2007.01.019. [DOI] [Google Scholar]
- Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- Vellingiri M, Deiva M, Teepica P, Jagathala MS (2011) Effects of processing conditions on the stability of polyphenolic contents and antioxidant capacity of Dolichos lablab L. J Food Sci Technol. doi:10.1007/s13197-011-0387-z [DOI] [PMC free article] [PubMed]
- Wills RBH, Tangendjaja B. Effect of pH and temperature on the degradation of mimosine and 3-hydroxy-4(1H)-pyridone. Phytochem. 1981;20:2017–2018. doi: 10.1016/0031-9422(81)84055-1. [DOI] [Google Scholar]
- Wu HC, Chen HM, Shiau CY. Free amino acid and peptide as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res Int. 2003;36:949–957. doi: 10.1016/S0963-9969(03)00104-2. [DOI] [Google Scholar]
- Yasuko S, Michael FC, Stephen CG, Hideo Y. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicol. 2002;177:67–80. doi: 10.1016/S0300-483X(02)00196-8. [DOI] [PubMed] [Google Scholar]