Abstract
Organically grown ginger rhizome (Zingiber officinale Roscoe) SC CO2 extract obtained at 280 bar and 40 °C and its column chromatographic fractions are characterised for its composition. The components in the extract and fractions are identified by HPLC and LC based MS and are used as standard for the estimation of gingerol analogues in the extract. HPLC and mass analysis of the extracts confirmed the various forms of gingerol constituents [4]-, [6]-, [10]-gingerols and [6]-, [8]-, [10]-shogaols in ginger extracts. SC CO2 extract of organic ginger was found to show 6-gingerol around 25.97 % of total extract. The estimation of [6]-gingerol, [6]-shogaols, [4]gingerol, [10]-gingerol and 6-gingediol content of the SC CO2 purified ginger extract was found to be 75.92 ± 1.14, 1.25 ± 0.04, 4.54 ± 0.04, 13.15 ± 0.30 and 0.37 ± 0.00 % respectively. Antioxidant activity was measured by 2, 2-diphenyl-1-pycryl-hydrazyl (DPPH) free radical scavenging and ferric reducing antioxidant power (FRAP) and the assay have shown 652 ± 0.37 mg TE/g and 3.68 ± 0.18 mg TE/100 g respectively, are significantly higher results with SC CO2 organic ginger extract. Paradol analogues are not detected in this study. Small quantities of [4]-, [10]gingediol and [6]-gingediacetate are also found in ginger extract.
Keywords: Organic ginger, SC CO2 ginger extract, [6]-Gingerol, Shogaols, Total phenolic content, Antioxidant activity
Introduction
Ginger (Zingiber Oficinale Rascoe) rhizome has been used since ancient times for its flavouring and medicinal properties, as an aid to digestion and as a carminative. Ginger is a household remedy for dyspepsia, flatulence, colic and diarrhoea (Yai 1991). Gingerols that occurs in the species, has been found to possess various pharmacological and physiological effects including antioxidant (Masuda et al. 2004), anti-inflammatory, analgesic (Young et al. 2005), antipyretic, gastroprotective (Suekawa et al. 1984), cardiotonic (Kobayashi et al. 1987), antihepatotoxic (Hikino et al. 1985), antifungal (Ficker et al. 2003), antinuclear, antiemetic (Kawai et al. 1994) and antischistosomal activities (Chen and Ho 1988). In addition, [6]-gingerol has been known to suppress cytokine formation (Phan et al. 2005) and promote angiogenesis (Kim et al. 2005). The phytochemical, pharmacological and physiological properties of ginger are well reviewed (Ali et al. 2008). A study has confirmed that the spices derived from plants exert their anticancer properties through the suppression of NF-κB (Nuclear Factor-kappa B) (Aggarwal and Shishodia 2006). [6]-gingerol is a natural chemopreventive agent that has been found to be potent inhibitor of NF-κB (Kim et al. 2004).
The gingerols are responsible for the pungency of ginger; they are homologous series of phenolic ketones known as gingerols, shogaols and paradols. Shogaols and paradols are even more pungent than the gingerols is virtually absent in fresh ginger and is derived from gingerols during thermal processing or long term storage (Zhang et al. 1994). Shogaols are gingerol analogues with a 4, 5 double bond, resulting from elimination of 5-hydroxy group. Paradols are formed by hydrogenation of the corresponding shogaol. [6]-Gingerol (5-hydroxy-1-(4′-hydroxy3′-methoxyphenyl)-3-decanone) is one of the most abundant constituent in fresh ginger but it found to decrease during thermal processing and post harvest storage and shogaols even more pungent than gingerols, is virtually absent in fresh ginger and is derived from gingerol during long term post harvest storage (Zhang et al. 1994).
Ginger contains number of different pungent and active ingredients mainly gingerols (Govindarajan 1982a, b) are homologous series of phenolic ketones known as ginger constituents such as [4]-, [6]-, [8]-, [10] -and [12] -gingerols (Kikuzaki et al. 1994). SC CO2 extraction is a best method to extract the gingerol constituents from fresh ginger without the drawbacks of the chemical solvent extraction and SC CO2 extraction can be considered to result in products that are near natural for food applications (Diaz-Reinoso et al. 2006). The pressure and temperature dependent can fractionate rich compositions of non-polar components, essential oils and isoprenoids individually (Udaya Sankar 1989; Yonei et al. 1995). In addition CO2 has a low surface tension, viscosity and high diffusivity (Roy et al. 1996) that help in faster mass transfer rates. Very few reports available on supercritical corbon-dioxide extraction, estimation and characterization of gingerol (Yai 1991).
In the present work, organically grown ginger is used for carbon dioxide extraction. The agriculture practice of organic production results in superior produce/products than that were grown with intervention of fertilizers and there is also a need to characterize the composition of the same (Aldrich 1981; Siderer et al. 2005). In the present study, SC CO2 extracts of organic ginger are fractionated on silica gel column chromatography for the isolation, characterization of compounds; the gingerol constituents estimated by HPLC (Chen et al. 1986; Wood 1987) LC based mass chromatography has been used to analyse the ginger analogues (Hiserodt et al. 1998) and the extracts and the fractions are evaluated for DPPH radical scavenging, ferric reducing antioxidant power (FRAP) to evaluate the antioxidant activity. Total phenolic contents had been determined by the Folin–Ciocalteu method.
Materials and methods
Chemicals
Organically grown ginger SC CO2 extract was obtained from M/s South East Agro India Limited, Mysore and SC CO2 extraction of ginger (origin, Assam, India) was carried out at a pressure of 280 bar at 40 °C in a commercial SCF CO2 extraction plant of capacity 2 × 200 lit (M/s Natex, Austria) on 90 kg of ginger powder. Analytical grade reagents hexane and diethyl ether procured from Merck Ind.Ltd. All the solvents distilled once before use. Folin–Ciocalteu reagent from Loba chemicals Ind Ltd., TPTZ (2,4,6-tripyridyl-S-triazine), DPPH (2,2′-diphenyl1-pycryl-Hydrazyl), Gallic acid and Trolox (6-Hydroxy-2,5,7,8-tetramethyl chroman-2carboxylic acid) from Sigma USA. Sodium carbonate, ferric chloride hexa hydrate and sodium acetate are from Rankem Fine Chemicals India Ltd., glacial acetic acid from SD fine chemicals India Ltd.
Column chromatographic fractionation of ginger extract
Super critical fluid carbon dioxide extract (400 mg) was fractionated on silica gel column to obtain fractions by eluting with different proportions of (successively) n-hexane: diethyl ether (80:20) 77 mg I (19.2 %), (50:50) 158 mg II (39.5 %), (40:60) 138 mg III (34.5 %) and (20:80) 20 mg (5 %) after evaporating to dryness. These fractions were characterized for their compositions by HPLC and LCMS.
High performance liquid chromatography
The ginger fractions which were purified on silica gel column chromatography were subjected to high performance liquid chromatography in both gradient and isocratic mode of elution was used for identification and quantification of gingerols, while gradient was used for identification of components, using HPLC grade (A) water and (B) acetonitrile. The gradient elution had the following profile: 0–8 min, 45–50 % B; 8–15 min, 50-55 % B; 15–40 min, 55–90 % B; 40–45 min, 90–45 % B; 45–55 min, 45–45 % B. For isocratic (A) water and (B) acetonitrile (55:45 v/v), equipped with a reverse phase column C18 (250 × 4.6 mm), flow rate 1 ml/min. UV detector set at 282 nm. The compounds were identified and quantified based on retention time. HPLC measurements will be ± 5 %. HPLC analyzed gingerols fractions were characterized by mass to determine the individual constituents.
Preparative HPLC
Purified fractions from column chromatography were subjected to preparative HPLC, of stationary phase column Pursuite ERS C18 (250 × 21.2 mm) particle size, 10 μm, flow rate 5 ml/min UV detector 282 nm, fractions (10 %) in acetonitrile injected 500–1,000 ml, isocratic mode acetonitrile: water (55:45 v/v), the fractions eluted were collected, separately, pooled and evaporated under N2 to obtain purified components.
Mass spectroscopy
The aliquots were subjected mass analysis directly on the mass head of LCMS system using mass spectrometry (Waters 2996, PDA detector and mass by MS-Q-Tof, Ultima, Mass Lynx 4.0 software) equipped with an ESI (electrospray ionization) positive mode under the following conditions. Capillary, -3.00KV; Cone, 100KV; Source temperature, 120 °C; Dissolvation temperature, 300 °C; Cone gas, 50 L/h; Dissolvation, Gas flow 500 L/h; scan range, m/z 100–800 with a scan speed of 1,000 amu/s. A peak thresh hold of 1 % identity is applied to the mass spectra.
Total phenolic assay
Total phenolic content was determined by the Folin–Ciocalteu method adapted from Swain Hillis (1959). The different concentration of extract was made up to 150 μl using distilled ethanol. To this, 150 μl of 0.25 N Folin–Ciocalteu reagent, 2,400 μl of distilled water was added and then mixed well using vortex. Mixture was allowed to react for 3 min then 300 μl of 1 N Na2CO3 solution is added and mixed well. The solution is incubated at room temperature (23 °C) in the dark for 2 h. The absorbance was measured at 725 nm using a spectrophotometer. And the results were expressed in gallic acid equivalents (GAE; mg/100 g fresh mass) using a standard gallic acid curve. Additional dilution was done if the observed absorbance value was over the linear range of the standard curve. Error in measurements will be ± 5 %.
Antioxidant activity measurements
Antioxidant activity was measured by DPPH method (Blois 1958) with few modifications. Three mililiter of ethanolic solution of ginger extract (35.06 μg/3 ml) and standard trolox solutions (0.1 mM) were taken in different test tubes. To this solution 1 ml of 0.4 mM ethanolic solutions of DPPH was added. The mixture was vigorously mixed in a vortex mixer for a minute and allowed to stand at 27 °C in dark for 20 min. Reagent blank was prepared as above without addition of sample or standard. Then the absorbance was taken at 517 nm. Results are expressed in mg TE/g fresh mass using standard trolox calibration curve. Error in measurements will be ± 5 %.
The FRAP assay was done according to (Guo et al. 2003) with some modifications. The stock solutions included 300 mM acetate buffer pH3.6, 10 mM TPTZ (2,4,6-tripyridyl-Striazine) solution in 40 mM HCl and 20 mM FeCl3.6H2O solution. The fresh FRAP working solution was prepared by mixing 10:1:1 ratio respectively and then warmed at 37 °C before using. Then sample extracts in different concentrations were allowed to react with 2.9 ml of FRAP solution for 30 min in the dark condition. Readings of the colored product (ferrous tripyridyl-triazine complex) were then taken at 593 nm. The standard trolox curve was used to express results in mg TE/100 g fresh mass. Error in measurements will be ± 5 %.
Result and discussion
In this study, the estimation of gingerol analogues from crude SC CO2 organic ginger extract as well as purified fractions was carried out. There are several disadvantages associated with GC and GC-MS methods for analyzing 6-gingerol and its analogues as the column temperature significantly affect the results due to conversion of 6-gingerol to 6-shogaol (Zhang et al. 1994). The crude ginger extract and column chromatographic fractions were subjected to HPLC both isocratic and gradient method to identify the compounds in the samples.
Identification of compounds in ginger extract:
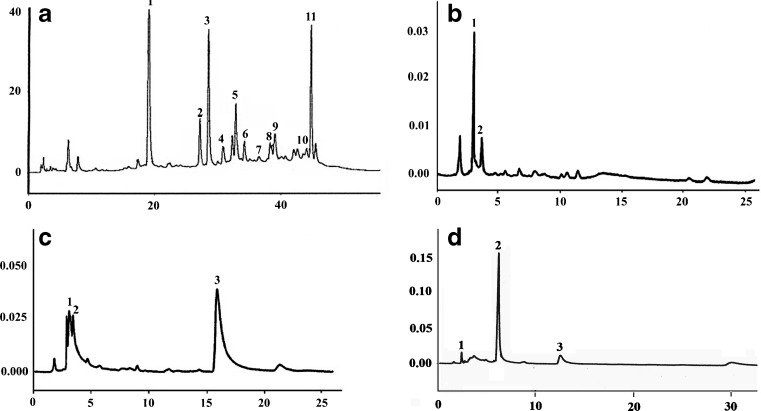
As the gradient HPLC method showed better separation and was used for characterization and identification of the compounds. SC CO2 crude extract of ginger showed fifteen peaks with different retention time varied from 6.2 to 45.5 (Fig. 1a). We could identify the twelve compounds and respective mass obtained using LC-MS by UV diode array detector given in the Table 1.
Fig. 1.
High performance liquid chromatography of super critical carbon dioxide ginger extract and its column chromatographic fractions. a Ginger extract by gradient HPLC. Peak 1-[4]-gingerol, 2-[6]-gingediol, 3-[6]-gingerol, 4-methyl-6-gingerol, 5-[8]-gingerol, 6-[6]-shogaol, 7-[12]-gingediol, 8- methyl-6-shogaol, 9-[10]-gingerol, 10-[1]-gingedione and 11-[8]-gingediol. b HPLC (isocractic) Column chromatographic fraction I of ginger extract. Peak 1-[4]-gingerol and 2-[6]-gingediol. c HPLC (isocractic) Column chromatographic fraction II of ginger extract. Peak 1-[4]-gingerol, 2-[6]-gingediol and 3-[1]-gingedione. d HPLC (isocractic) Column chromatographic fraction III of ginger extract. Peak 1-[4]-gingerol, 2-[6]-gingerol and 3-[10]-gingerol
Table 1.
Components of SCCO2 organic ginger extract identified based on retention time and mass using gradient HPLC program (RT-Retention time, RRT-Relative Retention time with respect to 6-gingerol)
| Sl.No. | RT | RRT | M+ | [M + H]+ | [M + Na]+ | Compound name |
|---|---|---|---|---|---|---|
| 1 | 6.2 | 0.218 | – | – | – | – |
| 2 | 7.737 | 0.272 | – | – | – | – |
| 3 | 17.278 | 0.607 | – | – | – | – |
| 4 | 18.933 | 0.665 | – | 266 | 289 | 4-gingerol |
| 5 | 27.054 | 0.950 | – | 297 | 320 | 6-gingediol |
| 6 | 28.481 | 1.000 | – | 294 | 317 | 6-gingerol |
| 7 | 30.834 | 1.083 | 308 | – | 331 | methyl-6-gingerol |
| 8 | 32.257 | 1.133 | – | – | – | – |
| 9 | 32.804 | 1.152 | – | 322 | – | 8-gingerol |
| 10 | 34.193 | 1.201 | – | 277 | – | 6-shogaol |
| 11 | 36.475 | 1.281 | – | 403 | – | 12-gingediol |
| 12 | 38.258 | 1.343 | – | 291 | 313 | methyl-6-shogaol |
| 13 | 39.074 | 1.372 | – | 351 | 373 | 10-gingerol |
| 14 | 42.034 | 1.476 | – | – | – | – |
| 15 | 44.059 | 1.547 | – | – | – | 1-gingedione |
| 16 | 44.76 | 1.572 | – | – | 347 | 8-gingediol |
| 17 | 45.478 | 1.597 | – | – | – | – |
To aid the identification of the components the SC CO2 crude ginger extract was fractionated by silica gel column chromatography using n-hexane:diethyl ether with increasing polarity of 80:20 I (Fig. 1b), 50:50 II (Fig. 1c) and 40:60 III (Fig. 1d) to obtain three fractions. It has been observed that the HPLC chromatography of the fractions showed three different sets of compounds as observed from the peaks with different retention times in each of the chromatogram (Fig. 1b, c and d). It is found that the fractions eluted with n-hexane: diethyl ether (40:60 III) was found to contain only gingerols.
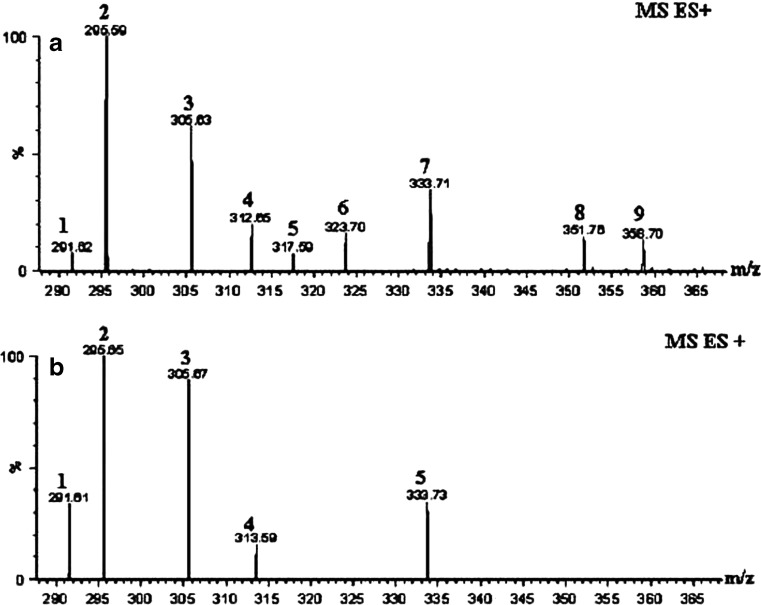
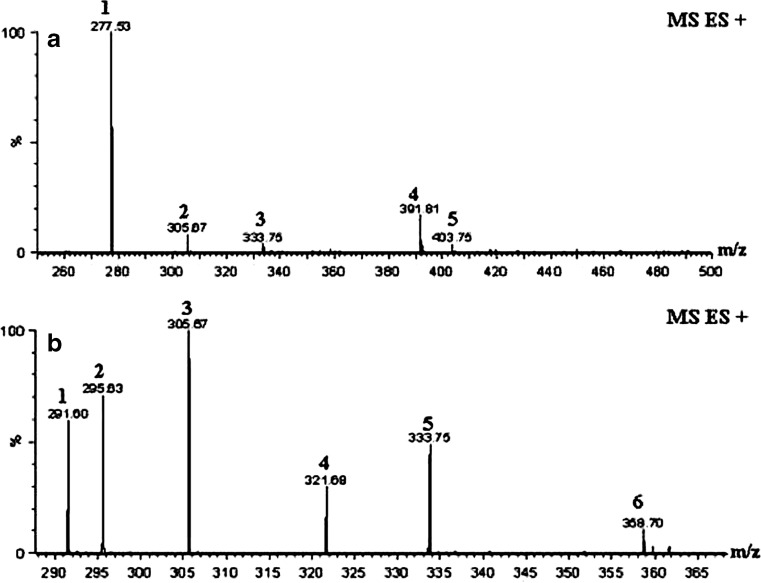
Both the crude SC CO2 and fractionated extract were characterized by ESI + mass spectra to identify the peaks in the HPLC. [6]-gingerol gave major fragment with 295 molecular weight. All the peaks were assigned based on the molecular mass and compounds were given Tables 1, 2 and 3 separately. Crude ginger extract was showed fewer amounts of gingerols and shogaols when compared to purified fraction. Purified fraction showed [M + H]+, [M + Na]+ peaks for [6]-[8]-and [10]-gingerols and [M + H]+ peaks for [10]-, [8]-shogaols with small [M + Na]+ peak for [4]-gingediol (Table 2 and Fig. 2a, b). Crude ginger extract was showed [M + H]+ peak for [6]-, [8]-shogaols and [6]-gingerol, [M + K]+ peak with the molecular weight of 391, which could be [4]gingediacetate or [10]-gingediol, [M + Na]+ peak for [6]-gingediacetate, [10]-shogaol and [4]gingediol, [M-H]+ peak for [8]-gingerol (Table 3 and Fig. 3a, b) respectively. Mass results illustrate that [8]-, [10]-shogaol exists in fresh ginger as measurable constituent. However, the occurrence of [6]-, [8]-and [10]-shogaols in the ginger extract is owing to the conversion from corresponding gingerols but are insignificant quantities as observed by HPLC. Also the mass chromatogram demonstrates that [6]-gingerol was a major constituent in purified fraction III in various forms such as [M + H]+, [M + Na]+ peak with low intensity of [M + H]+ peaks are also observed for [8]-, [10]-gingerols. One of the minor peaks was also identified in mass spectrum with molecular weight of 358 which could not be determined. In this study, we did not find any paradol constituents like [6]-, [8]-and [10]-paradol in both crude and purified ginger extracts.
Table 2.
| Peak no | Mass value | Analysis | Compound | Actual molecular weight | |
|---|---|---|---|---|---|
| Chromatogram in Fig. 2a | Chromatogram in Fig. 2b | ||||
| 1 | 1 | 291 | [M + Na] + | [4]-Gingediol | 268 |
| 2 | 2 | 295 | [M + H] + | [6]-Gingerol | 294 |
| 3 | 3 | 305 | [M + H] + | [8]-Shogaol | 304 |
| 4 | – | 317 | [M + Na] + | [6]-Gingerol | 294 |
| 5 | – | 323 | [M + H] + | [8]-Gingerol | 322 |
| 6 | 4 | 333 | [M + H] + | [10]-Shogaol | 332 |
| 7 | – | 351 | [M + H] + | [10]-Gingerol | 350 |
Table 3.
Components of the crude ginger supercritical carbon dioxide extract as indicated in the mass spectra of the Fig. 3a and b
| Peak no | Mass value | Analysis | Compound | Actual molecular weight | |
|---|---|---|---|---|---|
| Chromatogram in Fig. 3a | Chromatogram in Fig. 3b | ||||
| 1 | – | 277 | [M + H] + | [6]-Shogaol | 276 |
| – | 1 | 291 | [M + Na] + | [4]-Gingediol | 276 |
| – | 2 | 295 | [M + H] + | [6]-Gingerol | 294 |
| 2 | 3 | 305 | [M + H] + | [8]-Shogaol | 304 |
| – | 4 | 321 | [M-H] + | [8]-Gingerol | 322 |
| 3 | 5 | 333 | [M + Na] + | [10]-Shogaol | 332 |
| 4 | – | 391 | [M + K] + | [4]-Gingediacetate Or [10]-Gingediol | 352 |
| 5 | – | 403 | [M + Na] + | [6]-Gingediacetate | 380 |
Fig. 2.
Mass chromatograms of purified ginger extract showing gingerol constituents such as gingerols, shogaols and gingediols with different intensity of peaks
Fig. 3.
Mass chromatogram of the crude ginger supercritical carbon dioxide ginger extract showing gingerol constituents such as gingerols, shogaols, [4]-gingediacetate,, [6]-gingediateacte and gingediols with different intensity of peaks
Based on relative retention times from literature (Schwertner and Rios 2007), mass fragments by ESI-MS spectra the 4-gingerol, 6-gingediol, 6-gingerol, methyl-6-gingerol, 8-gingerol, 6-shogaol, 12-gingediol, methyl-6-shogaol, 10-gingerol, 1-gingedione and 8 gingediol were identified in the HPLC chromatogram (Table 1A).
Quantification:
[4]-gingerol, [6]-gingerol and [10]-gingerol were the major compounds found in 40:60 III fraction. These compounds were collected by preparative HPLC and confirmed by ESI-MS, used for quantification by injecting different concentration in isocratic mode. The standard curve of [4]-gingerol, [6]-gingerol and [10]-gingerol was linear in the range of 500–3,000 ng. The calibration curve of [4]-gingerol, [6]-gingerol and [10]-gingerol was represented by the linear equation Y = 13360X and R2 value 0.889, Y = 22059X and R2 value 0.891 and Y = 21142X and R2 value 0.761. This indicates [6]-gingerol content was abundant than the other constituents. The HPLC percentage area of [6]-gingerol was found to be 30–35 % in crude extract and 75–79 % in purified ginger extract. HPLC analysis also shows abundance of gingerol and shogaols in purified ginger extract. The [6]-gingerol, [6]-shogaol, [4]-gingerol, [10]-gingerol and [6]-gingediol content of the SC CO2 purified ginger extract was found to be 75.92 ± 1.14, 1.25 ± 0.04, 4.54 ± 0.04, 13.15 ± 0.30 and 0.37 ± 0.00 % (n = 3) respectively.
Estimation and biological activity measurements showed best results for purified SC CO2 ginger extract than crude ginger extract, confirming the [6]-Gingerol and it analogues contribute to the activity measurements. [6]-gingerol is the most abundant in the SC CO2 extracts and extraction with SC CO2 was best method for obtaining bioactive compounds like gingerols without transformation to paradols. HPLC analysis of the extracts gave the various forms of gingerol constituents [4]-, [6]-, [10]-gingerols, [6]-shogaol and [6]-gingediol. Mass analysis of the extracts gave the various forms of gingerol constituents [8]-, [6]-, [10]-gingerols and [6]-, [8]-, [10]-shogaols in ginger extracts. [6]-gingerol and [6]-shogaol are found to be in maximum quantity than other gingerols and shogaols in purified ginger extract. Small quantities of [4]-, [10]-gingediol and [6]-gingediacetate were also found in ginger extract.
The carbon dioxide extraction resulted in a yield 4.8 % of oleoresin on the basis of dried ginger rhizome with an essential oil content 20 % in the oleoresin. The gingerol content is found to be around 34.5 % of the total extract, which are much higher than the earlier reports (11–27 %) by Govindarajan et al. (1982a, b).
Ginger extract from SC CO2 as well as purified ginger fraction from column chromatography are taken into account to determine total phenolic content. Gingerol constituents showing significant amount of gallic acid equivalence for the total phenolic activity. Total phenolic content is increased with increasing concentration of gingerol. Purified ginger extract is showing higher phenolic content due to the higher gingerol constituents. Total phenolic content of SC CO2 ginger extract is found to be 46.0 ± 2.3 g GAE/100 g and purified ginger fraction (40:60) was found to be 58.5 ± 2.93 g GAE/100 g.
Antioxidant activity of ginger has been proposed as one of the major possible mechanism for the protective actions of the plant and animal against toxicity and lethality of radiation (Haksar et al. 2006) and a number of toxic agents like carbon tetra chloride and cisplatin (Yemitan and Izegbu 2006) showing higher toxicity. DPPH and FRAP assays have been carried out to measure antioxidant property of ginger with various concentrations of SC CO2 ginger extract and purified fraction ginger extracts. Activity results are an average of all the measurements. DPPH assay was showed 415 ± 0.3 mg TE/g for SC CO2 ginger extract and 652 ± 0.37 mg TE/g for purified fraction ginger (40:60). According to FRAP method, SC CO2 ginger extract exhibits 2.67 ± 0.13 mg TE/100 g and purifide fraction ginger is 3.68 ± 0.18 mg TE/100 g for antioxidant activity. Assay results strongly suggest, gingerol constituents have potent antioxidant power with high content of phenolic compounds. SC CO2 fractionated ginger extract showed the antioxidant activity significantly higher than that was reported earlier (Puengphian and Sirichote 2008). Successfully we could extract high bioactive compounds using SC CO2 with high [6]-gingerol content (> 30 %).
Conclusion
HPLC determination of 6-gingerol and it analogues has confirmed no significant transformation of gingerols occur in the extracts obtained by supercritical carbon dioxide and occurrence of higher amount of gingerols are reported as estimated by HPLC as high as 40 % in oleoresin with 6-gingerol content of 30–35 % in the extract. The 6-gingerol content in the gingerol fraction is found to be 79–82 %. Several different components of gingerols are identified and the antioxidant activity of the ginerols is quite significant as measured by DPPH and FRAP assays.
Acknowledgments
The authors thank the Director, CFTRI, Mysore for providing the facilities and encouragement. M/s South East Agro India is acknowledged for the supply of SC-CO2 extract of organically grown ginger.
References
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Aldrich SR. Organic farming. Science. 1981;213:708–710. doi: 10.1126/science.213.4509.708-b. [DOI] [PubMed] [Google Scholar]
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe)—a review of recent research. Food Chem Toxicol. 2008;46:409–420. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- Blois MH. Antioxidant determination by the use of stable free radicals. Nature. 1958;181:11991200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- Chen CC, Ho CT. Gas chromatographic analysis of volatile components of ginger oil (Zingiber officinale Roscoe) extracted with a liquid carbon dioxide. J Agric Food Chem. 1988;36:322–328. doi: 10.1021/jf00080a020. [DOI] [Google Scholar]
- Chen CC, Kuo MC, Ho CT. High performance liquid chromatographic determination of pungent gingerol compounds of ginger (Zingiber officinale) J of Food Science. 1986;51:1364–1365. doi: 10.1111/j.1365-2621.1986.tb13124.x. [DOI] [Google Scholar]
- Diaz-Reinoso B, Moure A, Dominguez H, Parajo JC. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J Agric Food chem. 2006;54:2441–2469. doi: 10.1021/jf052858j. [DOI] [PubMed] [Google Scholar]
- Ficker C, Smith ML, Akpagana K, Gbeassor M, Zhang J, Durst T, Assabgui R, Arnason JT. Bioassay-guided isolation and identification of antifungal compounds from ginger. Phytother Res. 2003;17:897–902. doi: 10.1002/ptr.1335. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS. Ginger, chemistry, technology and quality evaluation: part 1. CRC Crit Rev Food Sci Nutr. 1982;17(1):1–96. doi: 10.1080/10408398209527343. [DOI] [PubMed] [Google Scholar]
- Govindarajan VS. Ginger, chemistry, technology and quality evaluation: part 2. CRC Crit Rev Food Sci Nutr. 1982;17(3):189–258. doi: 10.1080/10408398209527348. [DOI] [PubMed] [Google Scholar]
- Guo C, Yang J, Wei J, Li Y, Xu J, Jiang Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr Res. 2003;23:1719–1726. doi: 10.1016/j.nutres.2003.08.005. [DOI] [Google Scholar]
- Haksar A, Sharma A, Chawla R, Kumar R, Arora R, Singh S, Prasad J, Gupta M, Tripathi RP, Arora MP, Islam F, Sharma RK. Zingiber offiicinale exhibits behavioral radioprotection against radiation-induced CTA in a gender-specific manner. Pharmacol Biochem Behav. 2006;84(2):179–188. doi: 10.1016/j.pbb.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Hikino H, Kiso Y, Hamada Y. Antihepatotoxic actions of gingerols and diaryheptanoids. J Ethnopharmocol. 1985;14(1):31–39. doi: 10.1016/0378-8741(85)90025-X. [DOI] [PubMed] [Google Scholar]
- Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-,8-and 10-gingerol from ginger rhizome by HPLC and preliminary evaluation of inhibition of Mycobacteruium avium and Mycobacterium tuberculosis. J Agric Food Chem. 1998;46:2504–2508. doi: 10.1021/jf970948l. [DOI] [Google Scholar]
- Kawai T, Kinoshita K, Koyama K, Takahashi K. Anti-emetic principles of magnolia obovata bark and Zingiber officinale rhizome. Plantamedica. 1994;60(1):17–20. doi: 10.1055/s-2006-959399. [DOI] [PubMed] [Google Scholar]
- Kikuzaki H, Kawasaki Y, Nakatani N (1994) Structure of antioxidant compounds in ginger. In: Ho CT, Osawa T, Huang MT, Rosen RT (Eds) Food Phytochemicals for cancer prevention II (ACS Symposium series No. 547). American Chemical Society, Washington, pp 237–243
- Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappa B and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- Kim EC, Min JK, Kim TY, Lee SJ, Yang HO, Han S, Kim YM, Kwon YG. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis invitro and invivo. Biochem Biophys Res Commun. 2005;23:300–308. doi: 10.1016/j.bbrc.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Shoji N, Ohizumi Y. Gingerol, a novel cardiotonic agent, activates the Ca2+-pumping ATPase in skeletal and cardiac sarcoplasmic reticulum. Biochim Biophys Acta. 1987;903:96–102. doi: 10.1016/0005-2736(87)90159-3. [DOI] [PubMed] [Google Scholar]
- Masuda Y, Kikuzaki H, Hisamoto M, Nakatani N. Antioxidant properties of gingerol related compounds from ginger. Biofactors. 2004;21:293–296. doi: 10.1002/biof.552210157. [DOI] [PubMed] [Google Scholar]
- Phan PV, Sohrabi A, Polotsky A, Hungerfold DS, Lindmark L, Frondoza CG. Ginger extract components suppress induction of chemokine expression in luman synoviocytes. J Altern Complement Med. 2005;11(1):149–154. doi: 10.1089/acm.2005.11.149. [DOI] [PubMed] [Google Scholar]
- Puengphian C, Sirichote A (2008) [6]-gingerol content and bioactive properties of ginger (Zingiber Officinale Roscoe) extracts from supercritical CO2 extraction. Asian J Food Ag-Ind 1(1):29–36
- Roy BC, Goto M, Hirose T. Extraction of ginger oil with supercritical carbon dioxide: experiments and modeling. Ind Eng Chem. 1996;35:607–612. doi: 10.1021/ie950357p. [DOI] [Google Scholar]
- Schwertner HA, Rios DC. High-performance liquid chromatographic analysis of 6gingerol, 8-gingerol, 10-gingerol and 6-shogaol in ginger-containing dietary supplements, spices, teas and beverages. J Chromatogr B. 2007;856:41–47. doi: 10.1016/j.jchromb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Siderere Y, Maquet A, Elke A. Need for research to support consumer confidence in the growing organic food market. Trends Food Sci Technol. 2005;16:332–343. doi: 10.1016/j.tifs.2005.02.001. [DOI] [Google Scholar]
- Suekawa M, Ishige A, Yusas K, Sudo K. Pharmacological studies on ginger1. pharmacological actions of pungent constituents, [6]-gingerol and [6]-shogaol. J Pharmacobio-Dyn. 1984;7:836–848. doi: 10.1248/bpb1978.7.836. [DOI] [PubMed] [Google Scholar]
- Swain T, Hillis WE. The phenolic constituents Prunu domestica I-the quantitative analysis of phenolic constituents. J Sci Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- Udaya Sankar K. Studies on Physico-chemical characteristics of volatile oil from pepper (Piper Nigraum) extracted by supercritical carbon dioxide. J SciFood Agric. 1989;48:483–493. doi: 10.1002/jsfa.2740480411. [DOI] [Google Scholar]
- Wood AB. Determination of the pungent principles of chillies and ginge by reversed-phase high performance liquid chromatography with use of a single standard substance. Flavour Fragr J. 1987;2(1):1–12. doi: 10.1002/ffj.2730020102. [DOI] [Google Scholar]
- Yai H. Botany 2000 Asia Zinberacai Workshop. Thailand: Prince of Songkla University; 1991. [6]-gingerol content and bioactive properties of Ginger (Zingiber Officinale Roscoe) extracts from supercritical CO2 extraction; pp. 15–18. [Google Scholar]
- Yemitan OK, Izegbu MC. Protective effects of Zingiber offiicinale (Zingiberaceae) against carbon tetrachloride and acetaminophen-induced hepatotoxicity in rats. Phytotherapy Res. 2006;20:997–1002. doi: 10.1002/ptr.1957. [DOI] [PubMed] [Google Scholar]
- Yonei Y, Ohinata H, Yoshida R, Shimizu Y, Yokoyama C. Extraction of ginger flavor with liquid or supercritical carbon dioxide. J Supercrit Fluids. 1995;8:156–161. doi: 10.1016/0896-8446(95)90028-4. [DOI] [Google Scholar]
- Young HY, Luo YL, Cheng HY, Hsieh WC. Analgesic and anti-inflammatory activities of [6]-gingerol. J Ethnopharmacol. 2005;96:207–210. doi: 10.1016/j.jep.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Zhang X, Iwaoka WT, Huang AS, Nakamoto ST, Wong R. Gingerol decreases after processing and storage of ginger. J Food Sci. 1994;59(6):1338–1343. doi: 10.1111/j.1365-2621.1994.tb14710.x. [DOI] [Google Scholar]