Abstract
The main objective of the present investigation was to develop an improvised method for the preparation of Rhododendron squash, which otherwise had a narrow consumer’s acceptability, despite being rich in antioxidants due to faulty preparation procedure and to compare the superiority of the new method over existing preparation method by examining various antioxidants and total antioxidant capacity. For the preparation of squashes in the present investigation, Rhododendron petals were heated with water at 80 °C for 20 min and left for 3-hour (or 180 min) followed by filtration and addition of sugar with or without ginger juice. Leaving Rhododendron petals with water for 3-hour at room temperature following heating facilitated maximum recovery of anthocyanin in water. Rhododendron squashes, prepared through improvised method, were compared with a Rhododendron squash collected from the market (control) for their physico-chemical characteristics, antioxidants and sensory quality attributes. The improvised Rhododendron squashes registered higher values for most of the parameters than the control.
Keywords: Rhododendron, Anthocyanin, Total phenolics, Total flavonoids, CUPRAC
Introduction
The genus Rhododendron (Greek Rhodon = rose and Dendron = tree) belongs to the family Ericaceae of order Ericales. Three major species (Rhododendron arboreum Smith, R. anthopogon D. Don, and R. campanulatum D. Don) have been reported from western Himalayas (Prakash et al. 2007); however, R. arboreum Smith is the predominating species found in hills of Uttarakhand state of India (Mehta et al. 2010). Leaves and flowers have got various medicinal uses such as treatment of illness, headache, diabetes, rheumatism etc. (Sharma et al. 2010).
The genus Rhododendron has long been regarded as a rich source of secondary metabolites such as phenols, phenolic acids, flavonoids, terpenoids, resins etc. The ethanol soluble portion of the flowers of R. arboretum showed α-glucosidase inhibitor and potent diabetic activity (Sharma et al. 2010). Phenolic acids obtained from its leaves and twigs have shown anti-HIV activity (Kashiwada et al. 2001). Recently, Verma et al. (2010) investigated the anti-inflammatory and antinoniciceptive properties of flowers of R. arboreum.
The flowers of R. arboreum are deep scarlet in colour, which comes in upto twenty blossoms in a single truss. The fresh flowers, which are sweet–sour in taste, are used to make a health promoting beverage i.e. squash by local inhabitants. Therefore, Rhododendron squash fetches relatively higher prices and preferred for medicinal purposes by consumers in this region. However, this squash has a slight bitter taste and consumers did not enjoy consuming it. This is the reason that Rhododendron squash has fewer takers beyond this region, despite its high nutritive value. Moreover, Rhododendron squashes available in the market are not uniform in their organoleptic and quality attributes owing to arbitrary preparation practices adopted by individuals. In this research, we attempted to blend the Rhododendron squash with ginger juice so as to make a more acceptable ginger flavoured Rhododendron squash.
A few studies have focussed on phytochemical composition of Rhododendron (Prakash et al 2007; Silici et al. 2010; Sharma et al. 2010; Verma et al 2010). Until now, no attempt has been made to estimate the total phenolic content, anthocyanin content, total antioxidant capacity and other quality parameters of the prepared product from the fresh flowers of Rhododendron. In the present investigation, above mentioned parameters have been reported for the first time.
Materials and methods
Sample preparation
Flower truss of Rhododendron, grown in the Kumaon hills of Uttarakhand at an altitude of 2300 m above mean sea level, were plucked from trees and brought to the laboratory for further processing and biochemical analyses. Except petals, other floral parts such as sepals, stamen, ovary etc. were discarded. For biochemical analyses, the juice of petals was extracted through an electronic juicer (Philips, India). The extracted sample was centrifuged at 12,000 g for 7 min (Remi, India). Thereafter, the supernatant was filtered through a 0.45 μm cellulose ester filter (Merck, Germany) and transferred into a vial and used for analyses.
Squash preparation
Petals were weighed @ 250 g/ L of finished product and were thoroughly washed with the tap water before squash preparation. The required acidity in squash was adjusted with citric acid. Sodium benzoate was also added @ 600 ppm/ L of finished product (Ranganna 1986). The final squash characteristics were adjusted to 1 % acidity and 45 % TSS. For the preparation of blended squash, 2.5 % fresh ginger juice was added and the Rhododendron petals were reduced to 225 g/L of finished product. The step wise preparation of Rhododendron squash has been presented in Fig. 1.
Fig. 1.
Flow chart of squash preparation
Soluble solid content, titrable acidity, reducing and total sugars and pH
Brix was measured at 20 °C using an Abbe refractometor (Atago, Tokyo, Japan). Titratable acidity, reducing and total sugar were determined according to the method suggested by Ranganna (1986). The pH of the squashes were determined using a pH meter (Inolab pH 730, Merck Specialities Pvt. Ltd., India).
Estimation of ascorbic acid, total carotenoids and total anthocyanin
The ascorbic acid and total carotenoids contents of the samples were determined as per the method suggested by Ranganna (1986). Results were expressed on mg/ 100 ml.
The total monomeric anthocyanin content was determined on a UV-visible spectrophotometer by the pH-differential method (Giusti and Wrolstad 2001). The pigment content was calculated and expressed as mg cyanidin 3-glucoside (Cyd 3-glu) per L, using an extinction coefficient (Є) of 26,900 L/cm/ mol and a molecular weight of 449.2 g mol/L.
Determination of total flavonoids, flavanols and total phenolics content
The quantification of total flavonoids was carried out by means of the aluminium chloride colorimetric method (Chang et al. 2002). Results were expressed as mg of rutin trihydrate equivalents/ 100 ml. The estimation of flavanols was performed according to the method described by Thimmaiah (1999) using the Vanillin reagent. The results were expressed as mg phloroglucinol equivalents/ 100 ml. Total phenolic content was estimated spectrophotometrically using Folin–Ciocalteu reagent and results expressed as gallic acid equivalents (mg GAE/100 mL) (Singleton and Rossi 1965).
Antioxidant activity (Cupric reducing antioxidant capacity, CUPRAC)
CUPRAC assay was performed according to method developed by Apak et al. (2004). To100 μL of sample aliquot, 1 mL each of copper (II) chloride solution (10−2 M), neocuproine solution (7.5 × 10−3 M) and ammonium acetate buffer solution (pH 7) solution, was mixed. The tubes were stoppered and after 1 h and absorbance at 450 nm was recorded against a reagent blank. The antioxidant activity was expressed as m mol Trolox®/ liter, or mM TE.
Organoleptic evaluation for acceptability of the squash
Organoleptic evaluation was performed on squash preparations by a ten-member trained panel. For each sensory parameter, such as colour & appearance, body or texture, flavour, taste and overall acceptability, 100 marks were allotted and the products were given to the panelist in coded form (Attri et al. 1998). The panelists washed their mouths with water intermittently to evaluate samples. Significant differences were determined at the (P < 0.05) level of significance using the Duncan’s multiple range tests.
Statistical analysis
Experiments were laid in complete randomized design with three replications. Duncan’s Multiple Range Test was used to determine significant differences. Significance was determined at P < 0.05. Correlation between total antioxidant capacity and different attributes were computed.
Results and discussion
Reactive oxygen species, by product of respirative cycle of oxidative phosphorylation, pre-disposes cells to several anomalies like mutagenic aberration, cell ageing, diseases, cancerous tumour growth and development of various lethal diseases such as cardio-vascular diseases, cancer, atherosclerosis etc. (Apak et al. 2004; Ramakrishna et al. 2008; Ayman and Rehab 2011). Epidemiological evidences suggests that intake of antioxidants helps in scavenging free radicals, cause of a broad spectrum damages to biological system (Naik et al. 2008; Verma et al. 2010). This has led consumers to look for a diet, derived from natural sources as well as rich in antioxidants, as an strategy to counteract the cellular damages wreak by oxidative stress. Rhododendrons are widely hailed for their aesthetic and ethnic significance. In addition, Rhododendron sp. have found various commercial and medicinal uses like production of honey (Silici et al 2010) and cure for gout, chloretic inflammation, antispasmodic, chronic eczema, diarrhoea, dysentery etc. (Verma et al. 2010). This high medicinal and therapeutic value of Rhododendron is attributed to the presence of several antioxidants phenols, carotenoids, anthocyanins, flavanols, glycosides etc. (Silici et al 2010). Following our research focussed on providing an alternative use for this plant, the design of antioxidant rich beverages prepared through improvised method from the Rhododendron petals supplemented with or without ginger juice was carried out in this study.
The results of our investigation show that Rhododendron flowers possess high content of various antioxidants (Table 1) and so are the squashes prepared (Table 2).
Table 1.
Ascorbic acid, total carotenoids, flavanols, total flavonoids, total phenols, and antioxidant capacities of Rhododendron flower and ginger juice
| Attributes | Rhododendron flower juice | Ginger juice |
|---|---|---|
| Ascorbic acid (mg/100 ml) | 11.5 | 11.7 |
| Total carotenoids (μg/100 ml) | 2685.0 | 648.0 |
| Flavanols (mg/100 ml) | 288.7 | 4.8 |
| Total flavonoids (mg/100 ml) | 1276.5 | 18.6 |
| Total anthocyanins (mg/L) | 154.8 | - |
| Total phenols (mg/100 ml) | 956.5 | 28.2 |
| Total antioxidant capacity (mM Trolox Equivalent (TE)/L) | 70.4 | 1.6 |
(n = 3)
Table 2.
Quality characteristics of different Rhododendron squashes
| Quality attributes | Plain Rhododendron squash | Blended Rhododendron Squash | Control |
|---|---|---|---|
| TSS* (0Brix) | 45.0 ± 1.5 b | 45.0 ±1.5 b | 49.0 ±1.6 a |
| pH | 2.4 ± 0.07 a | 2.4 ±0.07 a | 3.8 ± 0.09 b |
| Reducing sugars (%) | 13.4 ±0.3 c | 13.6 ±0.3 b | 18.3 ±0.3 a |
| Total sugars (%) | 44.5 ±1.8 b | 44.6 ±1.9 b | 47.1 ±1.9 a |
| Acidity (%) | 1.0 ±0.005 a | 0.99 ±0.006 a | 0.27 ±0.006 b |
| TSS: acid ratio | 43.7 ±2.2 a | 45.4 ± 2.3 a | 175.2 ±9.3 b |
| Ascorbic acid (mg/100 ml) | 2.3 ±0.006 b | 2.4 ± 0.007 a | 2.2 ±0.005 c |
| Total carotenoids (μg/100 ml) | 587.5 ±18.4 a | 576.2 ±18.1 b | 475.5 ± 15.4 c |
| Flavanols (mg/100 ml) | 59.3 ±3.2 a | 54.5 ±3.0 b | 48.6 ±2.5 c |
| Total flavonoids (mg/100 ml) | 206.0 ±11.4 a | 187.3 ±9.7 b | 136.1 ±7.5 c |
| Total anthocyanins (mg/L) | 20.6 ±0.4 a | 20.5 ±0.4 b | 12.4 ± 0.3 c |
| Total phenols (mg/100 ml) | 177.6 ±8.7 a | 158.8 ±8.4 b | 94.1 ±10.4 c |
| Total antioxidant capacity (mM Trolox Equivalent (TE)/L) | 12.5 ± 0.3 a | 12.0 ±0.3 b | 8.9 ±0.3 c |
(n = 3) 1Values within the same row with different superscripts are significantly different (P < 0.05)
*Total soluble solids
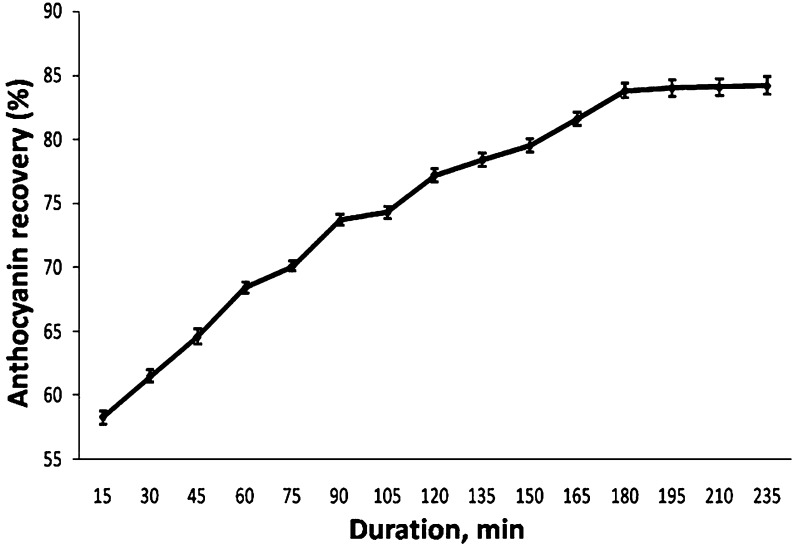
Recovery of anthocyanin from Rhododendron petals
Leaving Rhododendron petals with water for 180 min or 3-hour at room temperature following heating at 80 °C for 20 min facilitated maximum recovery of anthocyanin in water (Fig. 2). Heating for some period is done to achieve proper heating of entire plant samples (Kalt 2005). The heating treatment could have resulted into the splitting of petal tissues, which further rendered the slow leaching of anthocyanin in water. With the increasing time period i.e. upto 180 min, an increase in anthocyanin recovery was noted; however, beyond this period a plateau was observed in recovering anthocyanin from Rhododendron petals (Fig. 2). Kalt (2005) reported that like vitamin C, anthocyanins are water soluble and can be leached from fruit and vegetable tissues by processing in water.
Fig. 2.
Effect of duration on anthocyanin recovery from Rhododendron petals (n = 3)
Ascorbic acid, total carotenoids and anthocyanin content of Rhododendron flowers and squashes
The vitamin C content of Rhododendron flowers and derived squashes varied from 11.5 to 2.2 mg/100 ml (Tables 1 and 2). Rhododendron flowers and squash made from it had vitamin C contents even more than some other fruits like mangosteen, 1.0 μg/g; red jambu, 1.6 μg/g; European plum, 1.8 μg/g and banana, 2.2 μg/g fresh weight of fruits (Isabelle et al. 2010). Heat treatment reduced the ascorbic acid content in all heat-treated Rhododendron squash. The losses of ascorbic acid may be attributed to the thermal treatment applied (Kaushal et al. 2008).
Total carotenoid content in Rhododendron flower and squash varied from a maximum of 2685.0 μg/100 ml in Rhododendron flower to 475.5 μg/100 ml in control. Though, the thermal treatment enhances availability of the carotenoid (Azizah et al. 2009) by promoting the degradation of carotenoid-associated proteinaceous structures; however, the reduced content of total carotenoids in control despite more use of flower petals, in comparison to improvised method, could be attributed to prolong heating (Graziani et al. 2003).
Anthocyanins are the pigments, which are quite unstable during both processing and storage. Light, pH, temperature, oxygen, ascorbic acid and sugar are considered to be important factors in influencing its degradation or stability (Tsai and Huang 2004). Rhododendron was found to have 154.8 mg anthocyanins/L flower juice, while it varied from 20.5 mg/ L in blended squash, 20.6 in plain squash to 12.4 mg/L in control. The lower anthocyanin content in control, despite the use of relatively more Rhododendron petals, could be attributed to degradation of anthocyanin due to prolong boiling (at 100 °C) while preparation of squash. In their study on black currant juice processing, Mikkelsen and Poll (2002) observed decomposition and transformation of anthocyanins and the heating treatments seemed to be the most destructive process steps. Heating may open the structure of anthocyanin to form chalcones, which are degraded further to form brown products (Lin and Chou 2008). This may thus lead to the reduced anthocyanin content noted in the control. Moreover, anthocyanins at 49.0 0Brix are expected to be more susceptible to degradation than that at 45 0Brix. The reason might be that when the juice is concentrated, the reacting molecules (such as oxygen) become closer, thus the rate of chemical reactions accelerates (Wang and Xu 2003).
The other possible reasons could be the presence of ascorbic acid and higher pH of the prepared product, which accelerate anthocyanin degradation. Since traditionally prepared squash (control) involves more flower petals for its preparation; therefore, there could be more ascorbic acid, which during preparation of squash, accelerated degradation of anthocyanins. Furthermore, interaction of ascorbic acid with anthocyanins may result in the degradation of both compounds through a condensation reaction (González-Molina et al. 2009). It has been well documented that pH has a strong influence on the stability of anthocyanins (Tsai and Huang 2004). Previously, low acidity and a pH higher than 3 were shown to considerably reduce anthocyanin stability (Laleh et al. 2006). The pH of the control was 3.8 as compared to 2.4 in ginger blended and plain Rhododendron squash, respectively in this study (Table 2).
In addition, although the effect of sucrose on anthocyanins is still not clear, reducing sugars, particularly fructose, have been shown to indirectly enhance anthocyanin degradation during heating because of the formation of furfural and 5-(hydroxymethyl)furfural, which accelerate the rate of anthocyanin degradation (Cisse et al. 2009). The higher content of reducing sugars in the control (Table 2) with respect to the improvised squash prepared in this study also might have affected anthocyanin stability.
Total flavonoids, flavanols and total phenolic contents
Several studies have emphasized that flavonoids from different botanical sources can act as powerful antioxidants, even more so than can the traditional vitamins. In the present study, the total flavonoid and flavanol contents were found to be significantly higher in plain Rhododendron squash than blended squash or control.
The total phenolic content of Rhododendron flowers was found to be 956.5 mg GAE / 100 ml juice. The squashes blended with and without ginger had 177.6 and 158.8 mg GAE / 100 ml total phenolics, respectively, while control registered the minimum value of 94.1 GAE mg/100 ml. Lower phenolic content of squash blended with ginger as compared to plain squash could be attributed to relatively lesser phenolic content of ginger juice (Table 1). The phenolic content of these squashes are higher than the reported values of some of the common fruits like kiwifruits, 1.20; nectarines, 0.38; papaya, 0.45; mango, 1.54; red grapefruit, 1.30 mg GAE/ g FW (Isabelle et al. 2010) oranges, 126 ± 6 and apples, 109 ± 15 GAE/ g (Tsai et al. 2002). It has been suggested that higher the total polyphenolic content, the greater is the antioxidant activity (Gorinstein et al. 2001). However, these activities tend to degrade during the processing of food products. This degradation is associated with temperature, with the rate of degradation increasing with rising temperature (Lin and Chou 2008). It was observed that heating, irrespective of the heating temperature (80 °C and 100 °C in improvised and traditional methods, respectively), reduced the total phenolic content in the squash. The reduction in total phenolic content caused by heating is in line with that observed in fermented black soybeans by Lin and Chou (2008).
Furthermore, the presence of chelators like citric acid in squash, prepared by improvised method, is highly effective as synergists for both primary antioxidants and oxygen scavengers. Chelating agents are valuable antioxidant synergists since they remove metal ions and that catalyze oxidation and provide an acidic environment in our food systems, which enhance the stability of primary antioxidant (Lindsay 1985).
Total antioxidant capacity
The total antioxidant capacity index, in the present study, was tested using cupric reducing antioxidant capacity “CUPRAC” method, which is an easy, rapid and sensitive way to estimate the antioxidant activity of a specific compound or plant extracts (Apak et al. 2004). The total antioxidant activities of squashes, regardless of their preparation method, were lower than those of the fresh Rhododendron flowers. This is probably attributed to degradation of the bioactive compounds and absorption of water during boiling, resulting in dilution of the active compounds (Azizah et al. 2009). The polyphenols, which are present in the Rhododendron squash, can be destroyed or transformed into other phytochemicals during heat treatment and processing. Transformation of existing structure, oxidation of phenolic compounds during processing steps and interaction of phenolic antioxidant with other food components (Isabelle et al. 2010) may also explain the reduction in the value of antioxidant activity in Rhododendron squashes. The decrease in phenolic total polyphenol is always associated with significant decrease of antioxidant activity (Tsai et al. 2002). The reducing rate in antioxidant activity is also associated with the reduction of ascorbic acid, carotenoid, flavanol and flavonoids contents during heat processing of Rhododendron squash. Antioxidant capacity of different squashes varied widely from each other. The highest antioxidant capacity was noted in plain squash followed by squash blended with ginger juice, while least was noted in control (Table 2).
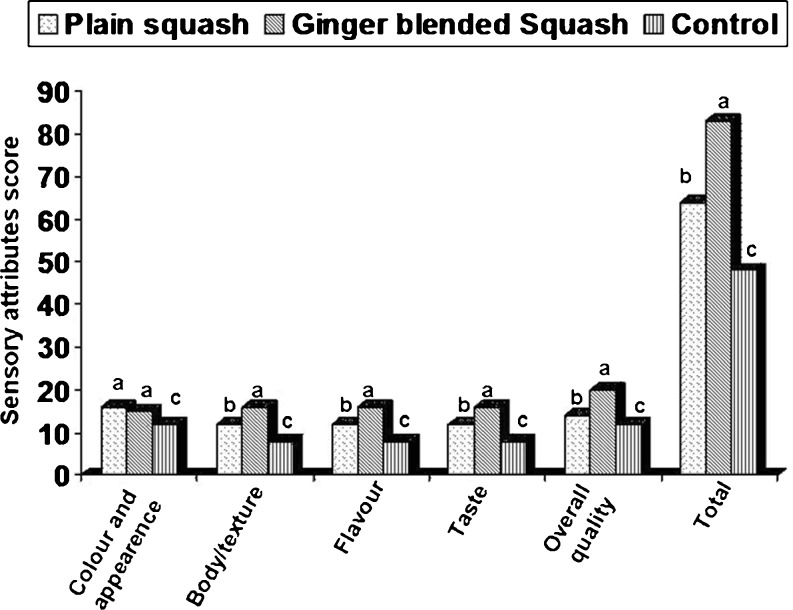
Sensory evaluation
Results of the trained sensory panel are presented in Fig. 3. Feedback collected from the trained panel revealed significant (P < 0.05) differences in appearance, colour, flavour and texture upon sensory evaluation of the squashes. Based on ranking, ginger blended squash was most preferred (P < 0.05) in overall appearance, colour and flavour (Fig. 3). Similarly, González-Molina et al. (2009) found a fruit beverage, prepared from 75 % lemon juice plus 25 % pomegranate juice combination to be rich in vitamin C although it gave the lowest antioxidant capacity. On the other hand, beverage prepared from 50 % lemon juice plus 50 % pomegranate juice, presented better organoleptic properties with an attractive red colour, as well as an acceptable content of bioactive compounds and a moderate antioxidant activity, while fruit beverage prepared from 25 % lemon juice plus 75 % pomegranate juice offered highest antioxidant capacity.
Fig. 3.
Sensory analysis of different Rhododendron squashes (n = 10 panelists). Values followed by the same letter are not significantly different (P < 0.05)
The least values were scored by the control on all sensory attributes in the present study. This could be attributed to the procedural differences. Traditionally, squash is prepared by boiling equal amount of sugar and Rhododendron petals in water till petals are completely bleached. This results in charring of sugar due to prolonged heating. The charring of sugar in turn gives a slight bitter taste and not-so-pleasant flavour to the product. As noted previously, prolong heating might render opening the structure of anthocyanin to form chalcones, which are degraded further to form brown products (Lin and Chou 2008). Therefore, the visual appeal of control was far lower than the squashes prepared in this study. Colour play a very important role in the acceptability of foods as it is one of the principal characteristics perceived by the senses and is used by consumers for the rapid identification and ultimate acceptance of foods (Giusti and Wrolstad 2003). In addition, the TSS: acid ratio was higher than the squashes prepared in this study. In the present method, petals were boiled with water for 20 min followed by filtration and addition of sugar (Fig. 1). In addition, blending with 2.5 % ginger juice improves taste and overall quality of the product, which can be attributed to distinct flavour of ginger. This squash also had a good natural colour appearance and desired TSS: acid ratio (Table 2).
Relations between measured attributes
The correlations between total antioxidant capacity and different attributes such as ascorbic acid, total carotenoids, anthocyanins and total phenolics of different Rhododendron squashes varied significantly. The highest correlation was obtained for anthocyanins (r = 0.998; P < 0.01) followed by total phenolics (r = 0.985; P < 0.01), total flavonoids (r = 0.974; P < 0.01), flavanol (r = 0.966; P < 0.01), total carotenoids (r = 0.754; P < 0.01) and ascorbic acid (r = 0.732; P < 0.01). This indicates that the anthocyanins, phenolics, flavonoids and flavanols are the major components responsible for the antioxidant activity of squashes. Our data are in agreement with those reported by other researchers (Isabelle et al. 2010; Pantelidis et al. 2007).
Conclusion
Results indicate that improvised method involving heating of Rhododendron petals with water at 80 °C for 20 min and leaving for 3 h at room temperature followed by filtration for the preparation of squash (with or without supplementation with ginger juice) can remarkably improve the antioxidant as well as organoleptic attributes of squash. The ginger blended Rhododendron squash registered better organoleptic properties with an appealing red colour and an acceptable content of bioactive compounds and a moderate antioxidant capacity. The lesser antioxidant content of market samples of Rhododendron squash despite more use of flower petals could be due to prolonged boiling of Rhododendron petals with sugar and water involved during their preparation. Exploiting the phytochemical contents of Rhododendron flowers could offer the enormous opportunities for devising better marketing strategies for the sale of squash and other beverages made of it.
References
- Apak R, Guculu K, Ozyurek M, Karademir SE. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agric Food Chem. 2004;52:7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Attri BL, Lal BB, Joshi VK. Physico-chemical characteristics, sensory quality and storage behaviour of sand pear juice blended with temperate fruit juices/pulps. Indian Food Pack. 1998;52:36–42. [Google Scholar]
- Azizah AH, Wee KC, Azizah O, Azizah M. Effect of boiling and stir frying on total phenolics, carotenoids and radical scavenging activity of pumpkin (Cucurbita moschato) Int Food Res J. 2009;16:45–51. [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Cisse M, Vaillant F, Acosta O, Dhuique-Mayer C, Dornier M. Thermal Degradation kinetics of anthocyanins from Blood orange, blackberry, and roselle using the arrhenius, eyring, and ball models. J Agric Food Chem. 2009;57:6285–6291. doi: 10.1021/jf900836b. [DOI] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Anthocyanins. Characterization and measurement with UV-visible spectroscopy. In: Wrolstad RE, Schwartz SJ, editors. Current protocols in food analytical chemistry. New York: Wiley; 2001. pp. 1–13. [Google Scholar]
- Giusti MM, Wrolstad RE. Acylated anthocyanins from edible sources and their applications in food systems. Biochem Eng J. 2003;14:217–225. doi: 10.1016/S1369-703X(02)00221-8. [DOI] [Google Scholar]
- González-Molina E, Moreno DA, García-Viguera C. A new drink rich in healthy bioactives combining lemon and pomegranate juices. Food Chem. 2009;115:1364–1372. doi: 10.1016/j.foodchem.2009.01.056. [DOI] [Google Scholar]
- Gorinstein S, Belloso OM, Park YS, Haruenkit R, Lojek A, Ciz M, Caspi A, Libman I, Trakhtenberg S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001;74:309–315. doi: 10.1016/S0308-8146(01)00157-1. [DOI] [Google Scholar]
- Graziani G, Pernice R, Lanzuise S, Vitaglione P, Anese M, Fogliano V. Effect of peeling and heating on carotenoid content and antioxidant activity of tomato and tomato-virgin olive oil systems. Eur Food Res Technol. 2003;216:116–121. [Google Scholar]
- Isabelle M, Lee BL, Ling MT, Koh WP, Huang D, Ong CN. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010;123:77–84. doi: 10.1016/j.foodchem.2010.04.002. [DOI] [Google Scholar]
- Kalt W. Effects of production and processing factors on major fruit and vegetable antioxidants. J Food Sci. 2005;70:11–19. doi: 10.1111/j.1365-2621.2005.tb09053.x. [DOI] [Google Scholar]
- Kashiwada Y, Yamazaki K, Ikeshiro Y, Yamagishi T, Fujioka T, Mihashi K, Mizuki K, Cosentino LM, Fowke K, Morris-Natschke SL, Lee KH. Isolation of rhododaurichromanic acid B and the anti-HIV principles rhododaurichromanic acid A and rhododaurichromenic acid from Rhododendron dauricum. Tetrahed. 2001;57:1559–1563. doi: 10.1016/S0040-4020(00)01144-3. [DOI] [Google Scholar]
- Kaushal M, Sharma PC, Kaushal BB, Lal SAK. Standardization of methods for the preparation of appetizer and ready-to-serve beverage from seabuckthorn (Hippophae sp.) berries. J Food Sci Technol. 2008;45(2):139–142. [Google Scholar]
- Laleh GH, Frydoonfar H, Heidary R, Jameei R, Zare S. The effect of light, temperature, pH and species on stability of anthocyanin pigments in four Berberis species. Pak J Nutr. 2006;5:90–92. doi: 10.3923/pjn.2006.90.92. [DOI] [Google Scholar]
- Lin YC, Chou CC. Effect of heat treatment on total phenolic and anthocyanin contents as well as antioxidant activity of the extract from Aspergillus awamori-fermented black soybeans, a healthy food ingredient. Int J Food Sci Nutr. 2008;1:1–10. doi: 10.1080/09637480801992492. [DOI] [PubMed] [Google Scholar]
- Lindsay RC. Food Additives. In: Fennema OR, editor. Food cehmistry. New York: Marcel Dekker; 1985. pp. 629–688. [Google Scholar]
- Mehta PS, Negi KS, Ojha SN. Native plant genetic resources and traditional foods of Uttarakhand Himalaya for sustainable food security and livelihood. Indian J Natur Prod Reso. 2010;1(1):89–96. [Google Scholar]
- Mikkelsen BB, Poll L. Decomposition and transformation of aroma compounds and anthocyanins during black currant (Ribes nigrum L.) juice processing. J Food Sci. 2002;67(9):3447–3455. doi: 10.1111/j.1365-2621.2002.tb09604.x. [DOI] [Google Scholar]
- Naik S, Jayaprakasha GK, Singh RP. Antioxidant activity of custard apple (Annona squamosa) peel and seed extracts. J Food Sci Technol. 2008;45(4):349–352. [Google Scholar]
- Pantelidis GE, Vasilakakis M, Manganaris GA, Gr D. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007;102:777–783. doi: 10.1016/j.foodchem.2006.06.021. [DOI] [Google Scholar]
- Prakash D, Upadhyay G, Singh BN, Dhakarey R, Kumar S, Singh KK. Free-radical scavenging activities of Himalayan rhododendrons. Curr Sci. 2007;92(4):526–532. [Google Scholar]
- Ramakrishna BV, Jayaprakasha GK, Jena BS, Singh RP. Antioxidant activities of roselle (Hibiscus sabdariffa) calyces and fruit extracts. J Food Sci Technol. 2008;45(3):223–227. [Google Scholar]
- Ranganna S. Manual for analysis of fruit and vegetable products. New Delhi: Tata McGraw Hill; 1986. [Google Scholar]
- Ayman M El-Anany and Rehab FMA (2011) Biochemical and histopathological effects of administration various levels of Pomposia (Syzygium cumini) fruit juice as natural antioxidant on rat health. doi:10.1007/s13197-011-0372-6 [DOI] [PMC free article] [PubMed]
- Sharma N, Sharma UK, Gupta AP, Sinha AK. Simultaneous determination of epicatechin, syringic acid, quercetin-3-O-galactoside and quercitrin in the leaves of Rhododendron species by using a validated HPTLC method. J Food Comp Anal. 2010;23:214–219. doi: 10.1016/j.jfca.2009.11.003. [DOI] [Google Scholar]
- Silici S, Sagdic O, Ekici L. Total phenolic content, antiradical, antioxidant and antimicrobial activities of Rhododendron honeys. Food Chem. 2010;121:238–243. doi: 10.1016/j.foodchem.2009.11.078. [DOI] [Google Scholar]
- Singleton VI, Rossi JA., Jr Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;10:149–158. [Google Scholar]
- Thimmaiah SK (1999) Pigments. In: Standard methods of biochemical analysis, Kalyani Publishers, Ludhiana, India. pp 309-310
- Tsai PJ, Huang HP. Effect of polymerization on the antioxidant capacity of anthocyanins in Roselle. Food Res Int. 2004;37:313–318. doi: 10.1016/j.foodres.2003.12.007. [DOI] [Google Scholar]
- Tsai P, McIntosh J, Pearce P, Camden B, Jordon BR. Anthocyanin and antioxidant capacity in Roselle (Hibiscus Sabdariffa) extract. Food Res Int. 2002;35(4):351–356. doi: 10.1016/S0963-9969(01)00129-6. [DOI] [Google Scholar]
- Verma NP, Singh AP, Amresh G, Sahu PK, Rao CV. Anti-inflammatory and anti-nociceptive activity of Rhododendron arboreum. J Pharma Res. 2010;3(6):1376–1380. [Google Scholar]
- Wang WD, Xu SY. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J Food Engr. 2003;82:271–275. doi: 10.1016/j.jfoodeng.2007.01.018. [DOI] [Google Scholar]