Abstract
Fruit ripening is associated with many hydrolase activities involved in the softening of the fruit during the maturation. This study investigates the relationship between the loss of firmness along with the changes of sugar content and the enzymatic activities in Carica papaya L.var solo 8 during post-harvest storage. Three maturation stages (green immature: the fruit is entirely green, green mature: the fruit shows 1/32 yellow skin and fully mature: the fruit shows 1/8 yellow skin) have been selected and stored at 15, 22 and 28 °C. The reduction of fruit firmness, total sugar contents, refractive index (% Brix) and enzymatic activities were measured. Low enzymatic activities (0.035 μmol/min/mg) were recorded in fruit harvested at the green immature stage with no significant (p ≥ 0.05) effect on the softening while fruit harvested at the green mature and fully mature stages showed enzymatic activities 7 times as high as those of the green immature stage. These high enzymatic activities were responsible for the loss of firmness of the fruit. Accordingly, papayas at the green mature and fully mature stages displayed higher maxima of sugar content (4.8 g/100 g at 28 °C at day 12, and 10.2 g/100 g at 22 °C at day 8, respectively) at higher temperatures. Meanwhile in green immature papayas, the maximum was only 4.3 g/100 g at 22 °C and day 12 of storage. The results show that the loss of firmness of the papaya was highly related to the hydrolytic enzyme activities and the sweet taste to the presence of simple sugars such as galactose liberated from the polysaccharide complexes.
Keywords: Hydrolases, Firmness, Sugar content, Maturation, Post-harvest, Papaya
Introduction
Fruit maturation is a complex process characterized by a set of physiological, biochemical, and organoleptic changes such as anthocyanins biosynthesis, chlorophyll degradation, increase of enzymatic degradation in the cell wall, sugar content, respiratory activities, ethylene production, and changes in aromatic compounds (Paliyath and Murr 2006). During the maturation of starch-rich fruit, the starch is converted into sugar by a catabolic degradation while the organic acids are converted into sugar by the process of gluconeogenesis (Sharma et al. 2008). The maturation is associated with the softening of the fruit, which leads to a modification of the texture (Jha et al. 2011). The softening of the fruit after harvesting could be due to physical damages during handling and transportation. These damages could increase the susceptibility of the fruit to microbial contamination (Manrique and Lajolo 2004).
The softening of the fruit during maturation is due to the activity of hydrolyses on the fruit cell wall polysaccharides, which leads to a modification of the cell wall composition. According to Prasanna et al. (2007) and Tucker (1993), this enzymatic activity is low during the first stage of the fruit development, then increases and reaches a maximum at the climacteric stage of the maturation process. For Soh et al. (2006), the activity of an isoform of α-galactosidase increases as the papaya fruit loses its firmness. Payasi et al. (2009) indicated that the activity of glycosidases such as β-hexosaminidase, α-mannosidase, and α-galactosidase increases in tomato when it is harvested at the fully mature stage. Moreover, Yashoda et al. (2007) attributed the softening of ripened mango to the activities of endomannosidase and α-mannosidase. Ohtani and Misaki (1983) showed that the seeds of Carica papaya L. exhibited a high level of α-D-galactosidase and α-D-mannosidase activities. Other studies indicated that cellulase activities increase during the maturation of avocados, peaches, strawberries, tomatoes, and papayas (Ignacio et al. 2011; Awad and Young 1979; Hobson 1981; Paull and Chen 1983).
Accordingly, an increase of solubilization of pectic substances, a progressive loss of firmness of fruit tissues, and a rapid increase of polygalacturonase (PG) activities occur during the maturation of many fruit (Pressey 1986; Brady 1987; Fisher and Bennett 1991; Tucker 1993). Furthermore, Paull and Chen (1983) showed a link between polygalaturonase and xylanase activities, an increase of the climateric respiration, the ethylene production, and the softening of Carica papaya L. Likewise, Karakurt and Huber (2003) demonstrated that the loss of firmness of Carica papaya L. was facilitated by the hydrolysis of the methyl groups on the galacturonic acid by pectin methylesterase. The development of the sweet taste in ripened fruit is the result of the hydrolysis of polysaccharides, particularly from starch-rich fruit, into simple sugars (glucose, fructose…) during the gluconeogenesis (Prasanna et al. 2007; Taiz and Zeiger 2002). Fructose is 1.8 sweeter than sucrose while glucose represents 0.6 folds the sweetness of sucrose (Wang and Zheng 2005). However, the relative concentrations of these sugars vary according to the type of fruit, the species, the cultivar, and the stage of maturity. In apples, pears, strawberries and grapes, the main soluble sugars at maturity are glucose and fructose while the one in bananas, pineapples, peaches, and melons, is sucrose (Harold et al. 2011).
In general, Climacteric fruit such as bananas and kiwi have a high starch content, which can be metabolized into soluble sugars after harvesting, giving the sweet taste to the fruit. On the other hand, papaya, which is also a climacteric fruit does not accumulate starch during it development (Gomez et al. 2002). The respiration which implies the use of a considerable amount of sugar as substrates for several metabolic processes increases during the storage of the fruit at ambient temperature. For Carica papaya L. var solo 8, a substantial amount of sugar remains at the end of the maturation process (Padmanaban et al. 2011; Nunes et al. 2006; Chen et al. 2006). However, we have no knowledge concerning an increase of sweetness in Carica papaya L. var solo 8. The objective of this study was to investigate the relationship between the enzymatic activities related to the loss of firmness of Carica papaya L. var solo 8 along with the changes in sugar content during the storage after harvesting.
Materials and methods
Collection of fruit and sampling
The papayas (Carica papaya L. var solo 8) were harvested from a farm near Tomassé (Azaguié), a village located at about 50 km north of Felix Houphouet Boigny Airport, Abidjan, Cote d’Ivoire. The fruit were transported directly to the Laboratory of Food Biochemistry and Tropical Products Technology, Abobo-Adjamé University. Three maturity stages: the green immature stage (the fruit is entirely green), the green mature stage (the fruit shows 1/32 of yellow skin), and the fully mature stage (the fruit shows 1/8 of yellow skin) were selected for this study. The fruit were washed with water, sorted according to the shape, the size and the weight, and packed in boxes of 12 fruit each. Twelve boxes of each maturity stage were then stored immediately at 15, 22, and 28 °C (for a total of 108 boxes) for 12 days. On days 0, 4, 8, and 12, three boxes (36 fruit) of each maturity stage and storage temperature were pulled out for testing. We recorded the firmness, the reducing and total sugars content, the refractive index, and the enzymatic activities (α-mannosidase, α-galactosidase, β-galactosidase, cellulase, pectin methylesterase, polygalacturonase and xylanase activities) of each fruit.
Measurement of the physico-chemical parameters of the fruit
Firmness
Using a penetrometer (a device for testing the firmness of the fruit, model FT 327, EFFEGI, Milan, Italy) equipped with stress indicator, the tip of the device is pressed on the middle of the papaya until it penetrates the pulp of the fruit to a depth of 8 mm. The value indicated by the device represents the maximum stress expressed in Newton (N) required for the pulp to be penetrated by the tip of the penetrometer; that value represents the firmness of the fruit (Tano et al. 2007).
Reducing and total sugars
One gram of papaya pulp was ground (Moulinex Masterchef 750, France) in 10 mL of ethanol in order to measure the ethanol-soluble sugars. The mixture was centrifuged (Centrifuge Jouan Multifunction B4i-BR4i, Germany) at 3000 rpm for 30 min. The supernatant was used to determine the reducing sugars according to the method described by Bernfeld (1955) using 3,5-dinitrosalicylic acid (DNS). 0.5 mL of DNS was added to 0.1 mL of the supernatant diluted in 0.9 mL of distilled water. The mixture was heated in a water bath at 100 °C for 5 min and let to cool down for 5 min at room temperature (28 ± 2 °C); then, 3.5 mL of distilled water were added. The absorbance was determined by a spectrophotometer (Spectronic Genesys 5, Madison, USA) at 540 nm against the blank containing all the reagents except the supernatant. The determination of the total sugars was performed using the method of Dubois et al. (1956). 1 mL of phenol 5 % (w/v) was added to 0.1 mL of the supernatant diluted in 0.9 mL of distilled water. The mixture was homogenized, heated in a water bath at 100 °C for 5 min, and let cool down at room temperature for 5 min. Then 2 mL of concentrated sulfuric acid was added to the mixture. The optical density (O.D) was read at 490 nm against the blank on a spectrophotometer (Spectronic Genesys 5, Madison, USA).
Refractive Index (°Brix)
The refractive Index, expressed in °Brix, was measured with a refractometer (model N-20E, ATAGO, Tokyo, Japan) equipped with a temperature corrector. A drop of papaya juice obtained after grinding was placed on the prism of the refractometer and the refractive index was directly read under sun light.
Measurement of the enzymatic activities
Extraction of enzymes
In order to obtain the enzymatic extract, we ground 10 g of papaya pulp in 10 ml of 0.9 % (w/v) NaCl. The mixture was centrifuged (centrifuge Jouan multifunction B4i-BR4i, Germany) at 6000 rpm for 30 min at 4 °C and the pellet discarded. The supernatant obtained contained the enzymes.
pNP-glycosidase activity
The p-nitrophenol (pNP)-glycosidase activity was obtained by mixing 50 μl of enzyme extract and 125 μl of 100 mM sodium acetate buffer (pH 5.6), 75 μl of 5 mM pNP-α- or β-D-glycoside (Amersham Pharmacia Biotech RPN 1064, Paris, France) as substrate of the enzyme. After the incubation of the mixture at 37 °C for 10 min, the reaction was stopped by adding 2 ml of 2 % (w/v) sodium carbonate. The appearance of a yellow color means the presence of an enzymatic activity, which is the result of the hydrolysis of p-nitrophenyl-α- or β-glycoside by the enzyme. The quantification of p-nitrophenol (pNP) produced was obtained through a spectrophotometer (Spectronic Genesys 5, Madison, USA) at 410 nm with a negative control (reagents without the enzyme). The optical density was converted into micromole of pNP/min. The specific activity is expressed in micromole of p-nitrophenol per min or per mg of protein (μmol/min/mg).
Xylanase and carboxymethylcellulase activities
Xylanase and carboxymethylcellulase activities were determined by measuring the content of reducing sugars freed during the hydrolysis of xylan and carboxymethycellulose polysaccharides by xylanase and carboxymethylcellulase present in the papaya enzymatic extract using Bernfeld method (1995). Forty eight milligrams (0.16 %) of enzyme substrate and 50 μl of enzymatic extract were mixed together with a final volume of 300 μl of 100 mM sodium acetate buffer pH 5.6. The mixture was incubated at 37 °C for 30 min. Then 300 μl of 3,5dinitrosalycilic (DNS) were added to it in order to stop the reaction. After the homogenization and heating of the mixture to 100 °C in a water bath, the reaction was let to cool down at ambient temperature for 10 min, then 2 ml of distilled water were added to it. The optical density was read using a spectrophotometer (Spectronic Genesys 5, Madison, USA) at 540 nm in order to measure the reducing sugars. The enzymatic activity was expressed in micromole of reducing sugars per min or per mg of protein (enzymes) using a standard curve (serial dilutions of 2 mg/ml of glucose used as standard).
Polygalacturonase activity
The polygalacturonase (PG) activity was determined using the method of Gross (1982). We mixed the subtract (1 % of polygalacturonic acid washed with 80 % ethanol) and 100 mM of acetate buffer (pH 5.6) containing 0.1 M NaCl and 50 μl of enzymatic extract for a total of 200 μl. Then the mixture was incubated at 37 °C for 2 h under continuous agitation. The reaction was stopped by adding 1 ml of sodium borate (pH 9). Then 200 μl of 0.1 % 2-cyanoacetamide were added before the whole mixture was placed in a boiling water bath for 10 min. Finally, the mixture was let to cool down at ambient temperature and the absorbance was read at 276 nm using a blank (mixture without subtract) and D-galacturonic acid for the standard curve. The galacturonase activity was expressed as the equivalent of galacturonic acid produced per milligram of protein per minute (μmoL/mg/min).
Pectin methylesterase activity
The enzymatic activity was determined using the method of Mehri-Kamoun (2001). The principle of the reaction consists in removing the specific methoxyl groups located on the C6 of some galacturonyl groups using a pectin methylesterase (PME) enzyme. The measurement of the activity relies on the pH variation due to the removal of the carboxylic group, which leads to the acidification of the medium. The method used in this experiment involved the mixing of 2 ml of subtract (1 % pectin in 0.15 % NaCl solution pH 7.0) and 1 ml of enzymatic solution. Then the mixture was incubated at 37 °C for 2 h in a water bath. The pH of the mixture was measured at the beginning of the incubation then 2 h after. One unit of PME activity corresponded to 10 fold the volume (μl) of 0.01 M NaOH added to the mixture to bring the pH value back to initial value at 37 °C. The PME activity was then expressed in unit/mg of protein (U/mg).
Determination of proteins
The proteins concentration of the different enzymatic samples was measured using the Lowry method (Lowry et al. 1951) with serum albumin bovine (SAB) as protein standard.
Statistical analysis
The statistical analysis was performed on the results using SPSS (version 10.0) software. The comparison of the variables measured during this study was done using the analysis of variance (ANOVA) and Duncan test. The differences were considered significant if p ≤ 0.05. All the experiments were conducted in triplicate.
Results and discussion
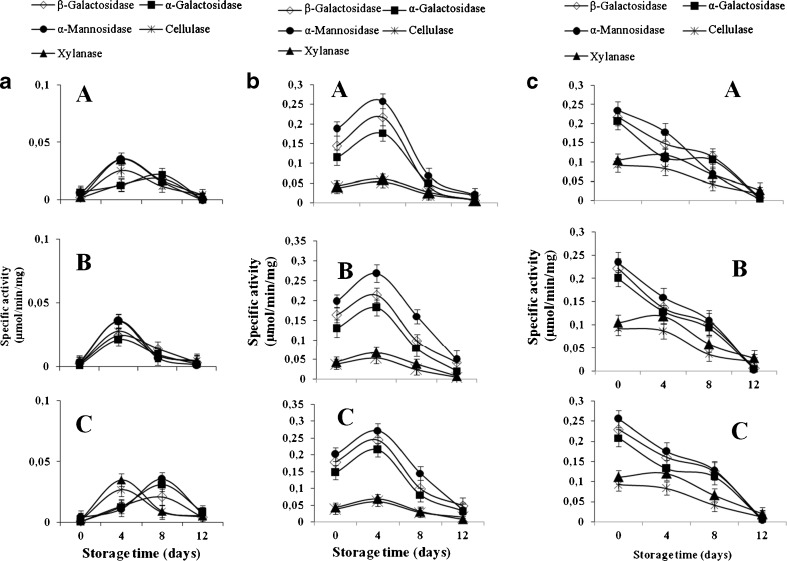
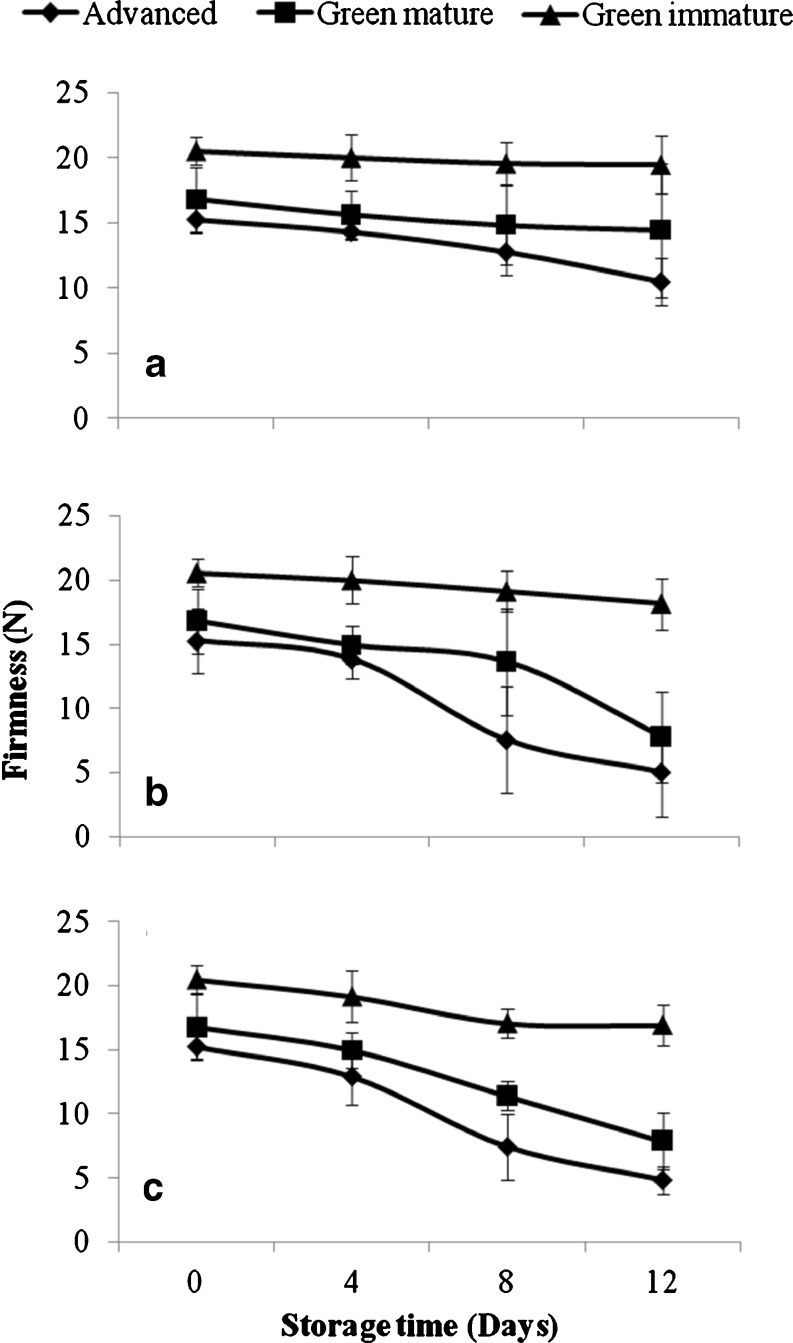
The enzymatic activities were very low in the pulp of papayas harvested at the green immature stage and stored at 15, 22, and 28 °C (Fig. 1). The maximum enzyme activity was 0.035 μmol/min/mg on day 4, about 1/7 of the green mature stage for the same storage day (Fig. 1). The enzyme activity increases as the papaya loses its firmness (Fig. 2). However, no significant difference in the firmness (p ≥ 0.05) was noted during the storage of the fruit harvested at green immature stage (Fig. 2). These results agree with those of Thumdee et al. (2010) who showed that hydrolase activities were weak in papayas harvested at the immature stage. It should be noted that the loss of firmness of the fruit increases as the enzyme activity does in the fruit cell wall (Sancho et al. 2010). This assertion supports the fact that the degradation of the major polysaccharides of the cell wall (cellulose, hemicellulose, and pectin) was responsible for the loss of firmness of the fruit (Mbeguie 2000).
Fig. 1.
Hydrolases activity in Carica papaya L.var solo 8 harvested at green immature (a), green mature (b) and fully mature (c) stored at 15(A), 22 (B), and 28 °C (C) for 12 days (n = 3)
Fig. 2.
Change of firmness of papaya (Carica papaya L. var solo 8) harvested at green immature, green mature and fully mature stages and stored at 15 (a), 22 (b) and 28 (c) for 12 days (n = 3)
Let’s remind that Paull and Chen (1983) showed a relation among the maturity stage, the respiratory activity, the ethylene production, the color of the fruit skin, the loss of firmness, and the enzyme activities responsible for the degradation of the cell wall during the maturation of Carica papaya L. This correlation could explain the weak enzyme activity of the immature papayas, which leads to a non-significant (p ≥ 0.05) degradation of the cell wall polysaccharides and loss of firmness during storage. Moreover, other studies have indicated that the ethylene was the main trigger of climacteric fruit maturation (Vendrell and McGlasson 1971; Zeroni et al. 1976; Pech et al. 1994; Jaimes-Miranda 2006).
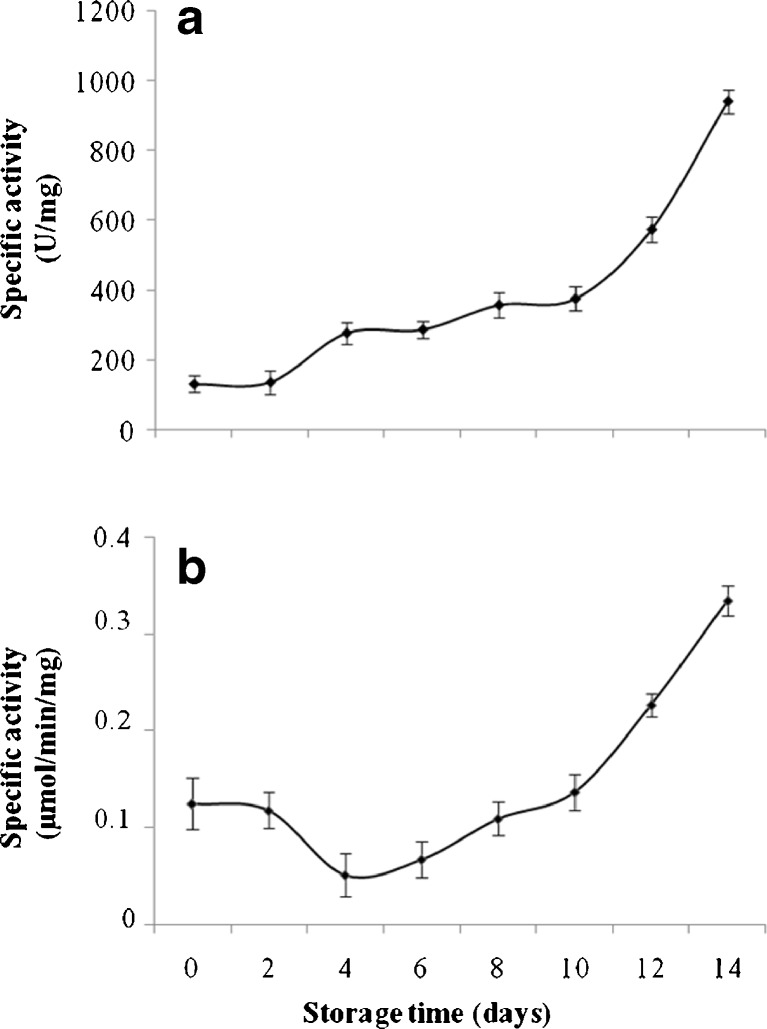
The enzyme activities in papayas harvested at the green mature stage and stored at 22 and 28 °C were higher than those stored at 15 °C, with a greater loss of firmness. This result indicates that higher temperatures contribute to the softening of the fruit during storage at 22 and 28 °C through enzymatic reactions. In fact, fruit at the green mature stage exhibited higher enzymatic activities during storage with a maximum of 0.25 μmol/min/mg for α-mannosidase, α-galactosidaseet β-galactosidase (Fig. 1), while those of xylanase and cellulase were at about 0.060 μmol/min/mg on day 4. In addition, pectin methylesterase (Fig. 3a) and polygalacturonase (Fig. 3b) activities increased during the storage and resulted in the loss of firmness of the fruit (Fig. 2). These results are supported by several studies (Brummell et al. 2004; Manenoi and Paull 2007; Sanudo-Barajas et al. 2009), which indicated that the softening of Carica papaya L. resulted from the modification of cell wall polysaccharides by enzymes such as polygalacturonase, β-galactosidase (β-gal), and pectin methylesterase. Other authors (Paull and Chen 1983; Lazan et al. 1995) suggested that enzymes found in papayas during maturation included exo- and endo-polygalacturonase, pectin methylesterase, β-1-4-glucanase, galactosidase, and xylanase. Their activities were also highly correlated to the changes in polysaccharides concentrations in the cell wall (Paull et al. 1999).
Fig. 3.
Change of pectine methylesterase (a) and polygalacturonase (b) activity on papaya (Carica papaya L. var solo 8) harvested at green mature stage and stored at 15 °C for 14 days (n = 3)
On the other hand, low storage temperatures slowed the enzymatic activities down, which in turn slowed the softening of the fruit down during storage at 15 °C. These results are in agreement with those of Bron and Jacomino (2009) who indicated that storage at low temperature reduces the respiratory and senescence enzymatic reactions, and minimizes the loss of quality of the fruit. Kader (2002) showed that the respiratory activity of papayas decreases approximately from 15 to 35 mL CO2 kg−1 h−1 at 20 °C to 4–6 mL CO2 kg−1 h−1 when stored at 10 °C, suggesting that lower temperature decreased enzymatic reactions and consequently the softening of the fruit.
For papayas harvested at the fully mature stage, enzyme activities decreased during storage regardless of the storage temperature (Fig. 1). This result indicates a depletion of the substrates necessary for these enzymatic reactions. Moreover, sugars were the first substrate used for respiratory metabolism in papaya (Gomez et al. 2002; Sharma et al. 2008) and in peach (Chen et al. (2006). Indeed, Harold et al. (2011) indicated that respiration is a property of all living cells tissues and is the center of the metabolic processes of development, maturation and senescence of fruit. The respiratory activity is an indicator of the metabolic activity thus, it indicates the speed at which metabolic changes are taking place. Myhara et al. (2000) showed that the decrease of pectin in fruit during the ripening was accompanied by a decrease of the hydrolytic enzymes activities responsible for their degradation. Koslanund et al. (2005) also observed that the activities of the enzymes responsible for degradation of the cell wall were low during the pre-climacteric phase, increased rapidly during the climacteric, then decreased during the post-climacteric phases.
Thus, papayas harvested at the fully mature stage may have already passed the climacteric phase. Paull et al. (1999) indicated that the solubilization of pectin and hemicelluloses, and the loss of firmness in papayas happen simultaneously and increase with the storage temperature. We agree with these authors that the loss of firmness in the papayas is due to the enzymatic degradation of the cell wall, in association with the respiratory activity, the synthesis and the action of ethylene. The increase of the respiratory activity is accompanied by the increase of sugar as substrate for several metabolic processes (Gomez et al. 2002; Sharma et al. 2008). In general, the amount of reducing sugars and dry soluble extracts (Table 1) of Carica papaya L. var solo 8 increases during storage. However, the total sugars concentration (Table 1) increases initially, then, decreases slightly during the maturation. This reduction of total sugars content is due to the increasing use of the saccharose during the climacteric respiration as indicated by Gomez et al. (2002) in their work on the evolution of soluble sugars throughout the maturation of papaya solo. They also showed that the accumulation of soluble sugars in papayas was mostly done when the fruit was still attached to the plant.
Table 1.
sugars content and Refractive Index in Carica papaya L.var solo 8 harvested at three stages of maturity and stored at three different temperatures for 12 days
| Total sugars (g/100 g) | Reducing sugars (g/100 g) | Refractive Index (°Brix) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day | 15 °C | 22 °C | 28 °C | 15 °C | 22 °C | 28 °C | 15 °C | 22 °C | 28 °C | |
| Green immature | 0 | 1.6 ± 0.20a, J | 1.6 ± 1.20a, J | 2.0 ± 1.30a, J | 0.4 ± 0.19a, J | 0.4 ± 0.19a, J | 0.4 ± 0.19a, J | 5.2 ± 0.06a, O | 5.2 ± 0.06a, O | 5.2 ± 0.06a, O |
| 4 | 2.1 ± 0.49a, K | 2.1 ± 0.49ab, K | 2.1 ± 0.50a, K | 0.8 ± 0.24a, K | 0.6 ± 0.39ab, K | 0.5 ± 0.03ab, K | 5.9 ± 0.10b, P | 5.6 ± 0.12b, Q | 5.6 ± 0.12b, Q | |
| 8 | 3.1 ± 0.75a, L | 3.5 ± 1.09ab, L | 3.9 ± 1.37a, L | 0.8 ± 0.07a, L | 0.9 ± 0.24b, L | 0.7 ± 0.07b, L | 6.0 ± 0.00b, R | 6.0 ± 0.06c, R | 6.0 ± 0.06c, R | |
| 12 | 3.2 ± 1.74a, M | 4.3 ± 1.79b, M | 2.6 ± 0.55a, M | 0.9 ± 0.37a, M | 1.0 ± 0.22b, M | 1.0 ± 0.13c, M | 6.6 ± 0.41c, S | 6.3 ± 0.12d, S | 6.3 ± 0.12d, S | |
| Green mature | 0 | 3.4 ± 0.93a, E | 3.0 ± 0.89a, E | 3.4 ± 0.93a, E | 2.5 ± 1.25a, F | 2.5 ± 1.25a, F | 2.3 ± 0.14a, F | 8.5 ± 0.50a, I | 8.1 ± 0.21a, I | 8.1 ± 0.21a, I |
| 4 | 3.5 ± 0.41a, F | 3.4 ± 0.93ab, F | 7.2 ± 1.20b, G | 2.5 ± 1.02a, G | 2.5 ± 0.82a, G | 2.4 ± 0.50a, G | 8.1 ± 0.21a, J | 8.8 ± 0.21b, K | 8.8 ± 0.21b, K | |
| 8 | 4.5 ± 1.17a, H | 4.9 ± 0.47b, H | 5.7 ± 0.70ab, H | 2.6 ± 0.16a, H | 2.7 ± 0.05a, H | 2.5 ± 1.25a, H | 9.2 ± 0.15b, L | 9.1 ± 0.12b, M | 9.0 ± 0.00b, M | |
| 12 | 4.3 ± 1.00a, I | 4.5 ± 0.65b, I | 4.8 ± 0.93ab, I | 2.7 ± 0.02a, I | 2.7 ± 0.92a, I | 2.7 ± 0.65a, I | 9.9 ± 0.12c, N | 10.0 ± 0.00c, N | 9.9 ± 0.12c, N | |
| Fully mature | 0 | 6.4 ± 1.65a, A | 6.4 ± 1.65a, A | 6.4 ± 1.65a, A | 2.6 ± 0.87a, A | 2.6 ± 0.87a, A | 2.6 ± 0.87a, A | 10.0 ± 0.00a, A | 10.0 ± 0.00a, A | 10.0 ± 0.00a, A |
| 4 | 6.8 ± 1.57a, B | 5.4 ± 2.14a, B | 9.3 ± 2.25a, B | 4.2 ± 1.21ab, B | 3.8 ± 0.27b, B | 3.2 ± 0.12ab, B | 11.1 ± 0.17 b, B | 10.4 ± 0.15ab, C | 10.4 ± 0.15b, C | |
| 8 | 8.6 ± 2.88a, C | 10.2 ± 2.78a, C | 8.5 ± 1.97a, C | 4.3 ± 1.06ab, C | 4.0 ± 0.59b, C | 4.1 ± 0.54bc, C | 11.4 ± 0.15b, D | 10.6 ± 0.36b, E | 11.0 ± 0.00c, E | |
| 12 | 6.6 ± 0.26a, D | 8.4 ± 3.44a, D | 6.9 ± 0.62a, D | 5.3 ± 0.27b, D | 4.4 ± 0.09b, E | 4.8 ± 0.61c, E | 12.4 ± 0.17c, F | 11.2 ± 0.25c, G | 11.2 ± 0.29c, G | |
Each observation is a mean ± SD of three replicate experiments (n = 3). Values in a column with the same lower case letter or in a row with the same upper case letter were not significantly different (p ≤ 0.05)
However, after harvesting, there was a synthesis of sucrose indicated by a highly correlation of the sucrose-phosphate synthesis activity and the content of sucrose in the fruit. The synthesized sucrose was later converted into glucose and fructose. According to the same authors, the carbon necessary for the synthesis of sucrose originated from the cell wall (30 % cellulose, 30 % hemycellulose, 25 % pectin, and 5 % proteins). Other studies showed a decrease of galactose and an increase of glucose due to the degalactosylation of polysaccharide chains in apples, strawberries, tomatoes, and in germinating seeds (Ignacio et al. 2011; Brett and Waldron 1996; Pressey 1983). This rapid metabolization of galactose explains why Gomez et al. (2002) did not find free galactose in ripped papayas. In fact, they determined that galactose was the main source of carbon during the synthesis of sucrose and that the resulting product was greatly used in the climacteric respiration.
Conclusion
The results of this study show that the loss of firmness of Carica papaya L. var solo 8 is highly (p ≤ 0.05) related to the activities of several enzymes such as pectin methylesterase, polygalacturonase, α-mannosidase, α-galactosidase, β-galactosidase, xylanase, and cellulase. The loss of firmness is greater in fruit harvested at green mature and fully mature stages and stored at 22 and 28 °C where the enzymatic activities are high. On the other hand, it is very low in fruit stored at 15 °C where respiration is low because of the low temperature leading to a reduced enzymatic activity. The high level of α-mannosidase, α-galactosidase and β-galactosidase activities seem to play an important role in the sweet taste of the fruit by liberating the simple sugars such as galactose and mannose.
References
- Awad M, Young ER. Postharvest variation in cellulase, polygalacturonase, and pectinmethylesterase in avocado (Persea americana Mill. cv. Fuerte) fruits in relation to respiration and ethylene production. Plant Physiol. 1979;64:306–308. doi: 10.1104/pp.64.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernfeld P. Amylase β and α (Assay method) In: Colowick SP, Kaplan N, editors. Methods in enzymology I. New York: Academic; 1955. pp. 149–154. [Google Scholar]
- Brady CJ. Fruit ripening. Annu Rev Plant Physiol. 1987;38:155–178. doi: 10.1146/annurev.pp.38.060187.001103. [DOI] [Google Scholar]
- Brett C, Waldron K. Physiology and biochemistry of plant cell walls. London: Unwin Hyman; 1996. [Google Scholar]
- Bron IU, Jacomino AP. Ethylene action blockade and cold storage affect Ripening of ‘Golden’ papaya fruit. Acta Physiol Plant. 2009;31:1165–1173. doi: 10.1007/s11738-009-0336-x. [DOI] [Google Scholar]
- Brummell DA, Dal-Cin V, Crisoto CH, Labavitch JM. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J Exp Bot. 2004;55:2029–2039. doi: 10.1093/jxb/erh227. [DOI] [PubMed] [Google Scholar]
- Chen JL, Wu JH, Wang Q, Deng H, Hu XS. Changes in the volatile compounds and chemical and physical properties of Kuerle fragrant pear (Pyrusserotina Reld) during storage. J Agric Food Chem. 2006;54:8842–8847. doi: 10.1021/jf061089g. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Ann Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Fisher R, Bennett A. Role of cell wall hydrolases in fruit ripening. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:675–703. doi: 10.1146/annurev.pp.42.060191.003331. [DOI] [Google Scholar]
- Gomez M, Lajalo F, Cordenunsi B. Evolution of soluble sugars during ripening of papaya fruit and its relation to sweet taste. J Food Sci. 2002;67:442–447. doi: 10.1111/j.1365-2621.2002.tb11426.x. [DOI] [Google Scholar]
- Gross KC. A rapid and sensitive spectrophotometric method for assaying polygalacturonase using 2- cyanoacetamide. HortScience. 1982;17:933–934. [Google Scholar]
- Harold CP, Karapanos IC, Alexopoulos AA. The biological basis of fruit quality. In: Matthew AJ, Penelope JB, editors. Breeding for fruit quality. New York: Wiley; 2011. pp. 5–38. [Google Scholar]
- Hobson GE. Enzymes and texture changes during ripening. In: Friend J, Rhodes MJC, editors. Recent advances in the biochemistry of fruits and vegetables. London: Academic; 1981. pp. 123–132. [Google Scholar]
- Ignacio CS, Armando CL, Misael VG, Elhadi MY (2011) The effect of antifungal hot-water treatments on papaya postharvest quality and activity of pectinmethylesterase and polygalacturonase. J Food Sci Technol. doi:10.1007/s13197-011-0228-0 [DOI] [PMC free article] [PubMed]
- Jaimes-Miranda F (2006) La régulation transcriptionnelle dépendant de l’éthylène. Caractérisation fonctionnelle d’un cofacteur transcriptionnel du type MBF1 et d’un facteur de transcription de la famille des ERF chez la tomate. PhD thesis, Sciences Agronomiques, France, p153. http://ethesis.inp-toulouse.fr/archive/00000242/01/jaimesmiranda.pdf. Accessed 19 May 2011
- Jha SN, Jaiswal P, Narsaiah K, Kaur PP, Singh AK, Kumar R (2011) Textural properties of mango cultivars during ripening. J Food Sci Technol. doi:10.1007/s13197-011-0431-z [DOI] [PMC free article] [PubMed]
- Kader AA. Postharvest biology and technology: an overview. In: Kader AA, editor. Postharvest technology of horticultural crops. Oakland: University of California Agriculture and Natural Resources Publication 3311; 2002. pp. 39–47. [Google Scholar]
- Karakurt Y, Huber DJ. Activities of several membrane and cell-wall hydrolase, ethylene biosynthetic enzymes and cell wall polyuronide degradation during low-temperature storage of intact and fresh-cut papaya (Carica papaya) fruit. Postharvest Biol Technol. 2003;28:219–229. doi: 10.1016/S0925-5214(02)00177-1. [DOI] [Google Scholar]
- Koslanund R, Archbold DD, Pomper WK. Papaw [Asimina triloba (L.) Dunal] fruit ripening II. Activity of selected cell-wall degrading enzymes. J Am Soc Hortic Sci. 2005;130(4):643–648. [Google Scholar]
- Lazan H, Selamat MK, Ali ZM. β-Galactosidase, polygalacturonase and pectinesterase in differential softening and cell wall modification during papaya softening. Physiol Plant. 1995;95:106–112. doi: 10.1111/j.1399-3054.1995.tb00815.x. [DOI] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farra L, Randall RJ. Protein measurement with folin- phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manenoi A, Paull RE. Papaya fruit softening, endoxylanase gene expression, protein and activity. Physiol Plant. 2007;131:470–480. doi: 10.1111/j.1399-3054.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Manrique GD, Lajolo FM. Cell-wall polysaccharide modifications during postharvest ripening of papaya fruit (Carica papaya L.) Postharvest Biol Technol. 2004;33(1):11–26. doi: 10.1016/j.postharvbio.2004.01.007. [DOI] [Google Scholar]
- Mbeguie AMD (2000) Isolement, identification et caractérisation de gènes impliqués dans la maturation de l’abricot (Prunus armeniaca L.). PhD thesis, Université de Droit, d’Economie et des Sciences, France, Marseille, p 196. Available at: http://www.prodinra.inra.fr/prodinra/pinra/data/2010/06/PUB000002508808261_20100602043151509.pdf. Accessed 12 May 2011
- Mehri-Kamoun R. Effet de la pectolyase Y-23 et de la cellulase RS sur le rendement en protoplastes viables de Prunus cerasus L. “Montmorency”. Biotechnol Agron Soc Environ. 2001;5(2):99–104. [Google Scholar]
- Myhara RM, Al-Alawi A, Karkarlas J, Taylor MS. Sensory and textural changes in maturing Omani dates. J Sci Food Agric. 2000;80:2181–2185. doi: 10.1002/1097-0010(200012)80:15<2181::AID-JSFA765>3.0.CO;2-C. [DOI] [Google Scholar]
- Nunes MCN, Emond JP, Brecht JK. Brief deviations from set point temperatures during normal airport handling operations negatively affect the quality of papaya (Carica papaya) fruit. J Food Sci Technol. 2006;41:328–340. [Google Scholar]
- Ohtani K, Misaki A. Purification and characterization of β-D-galactosidase and α-D-Mannosidase from papaya (Carica papaya L.) Seeds. Agric Biol Chem. 1983;47(ll):2441–2451. doi: 10.1271/bbb1961.47.2441. [DOI] [Google Scholar]
- Padmanaban G, Singaravelu K, Annavi ST (2011) Increasing the shelf-life of papaya through vacuum packing. J Food Sci Technol. doi:10.1007/s13197-011-0468-z [DOI] [PMC free article] [PubMed]
- Paliyath G, Murr DP. Biochemistry of fruits. In: Hui YH, editor. Food biochemistry and food processing. Oxford: Blackwell Publishing; 2006. pp. 487–514. [Google Scholar]
- Paull RE, Chen NJ. Postharvest variation in cell wall-degrading enzymes of papaya (Carica papaya L.) during fruit ripening. Plant Physiol. 1983;72(2):382–385. doi: 10.1104/pp.72.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull RE, Gross K, Qiu YX. Changes in papaya cell walls during fruit ripening. Postharvest Biol Technol. 1999;16:79–89. doi: 10.1016/S0925-5214(98)00100-8. [DOI] [Google Scholar]
- Payasi A, Mishra NN, Soares-Chaves AL. Biochemistry of fruit softening: an overview. Physiol Mol Biol Plants. 2009;15(2):103–113. doi: 10.1007/s12298-009-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech JC, Balagué C, Latché A, Bouzayen M. Postharvest physiology of climacteric fruits: recent developments in the biosynthesis and action of ethylene. Sci Aliments. 1994;14:3–15. [Google Scholar]
- Prasanna V, Prabha TN, Tharanathan RN. Fruit ripening phenomena—an overview. Crit Rev Food Sci Nutr. 2007;47(1):1–19. doi: 10.1080/10408390600976841. [DOI] [PubMed] [Google Scholar]
- Pressey R. α-galactosidases in ripening tomatoes. Plant Physiol. 1983;71:132–135. doi: 10.1104/pp.71.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. Changes in polygalacturonase isoenzymes and converter in tomatoes during ripening. HortSci. 1986;21:1183–1185. [Google Scholar]
- Sancho GGLE, Yahia ME, Martinez-Téllez MA, Gonzalez Aguillar GA. Effect of maturity stage of papaya Maradol on Physiological and Biochemical parameters. Am J Agric Biol Sci. 2010;5(2):194–203. doi: 10.3844/ajabssp.2010.194.203. [DOI] [Google Scholar]
- Sanudo-Barajas JA, Labavitch J, Greve C, Osuna-Enciso T, Muy-Rangel D, Siller-Cepeda J. Cell wall disassembly during papaya softening: role of ethylene in changes in composition, pectinderived oligomers (PDOs) production and wall hydrolases. Postharvest Biol Technol. 2009;51:158–167. doi: 10.1016/j.postharvbio.2008.07.018. [DOI] [Google Scholar]
- Sharma M, Sitbon C, Subramanian J, Paliyath G (2008) Changes in nutritional quality of fruits and vegetables during storage. In: Wiley J, Sons (ed) postharvest biology and technology of fruits, vegetables, and flowers. Wiley-Blackwell Publishing, New York, pp 443–466. ISBN-10: 0813804086
- Soh CP, Ali ZM, Lazan H. Characterisation of an alpha-galactosidase with potential relevance to ripening related texture changes. Phytochemistry. 2006;67(3):242–254. doi: 10.1016/j.phytochem.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Taiz L, Zeiger E. Plant physiology. 3. Sunderland: Sinauer Associates; 2002. p. 690. [Google Scholar]
- Tano K, Oulé MK, Doyon G, Lencki RW, Arul J. Comparative evaluation of the effect of storage temperature fluctuation of modified atmosphere packages of selected fruit and vegetables. Postharvest Biol Technol. 2007;46:212–221. doi: 10.1016/j.postharvbio.2007.05.008. [DOI] [Google Scholar]
- Thumdee S, Manenoi A, Chen NJ, Paull RE. Papaya fruit softening: role of hydrolases. Trop Plant Biol. 2010;3:98–109. doi: 10.1007/s12042-010-9048-z. [DOI] [Google Scholar]
- Tucker GA. Introduction. In: Seymour GB, Taylor JE, Tucker GD, editors. Biochemistry of fruit ripening. 1rst. London: Chapman and Hall; 1993. pp. 1–42. [Google Scholar]
- Vendrell M, McGlasson WB. Inhibition of ethylene production in banana fruit tissue by ethylene treatment. Aust J Biol Sci. 1971;24:885–895. [Google Scholar]
- Wang SY, Zheng W. Preharvest application of methyl jasmonate increases fruit quality and antioxidant capacity in raspberries. Int J Food Sci Technol. 2005;40:187–195. doi: 10.1111/j.1365-2621.2004.00930.x. [DOI] [Google Scholar]
- Yashoda HM, Prabha TN, Tharanathan RN. Mango ripening, role of carbohydrases in tissue softening. Food Chem. 2007;102(3):691–698. doi: 10.1016/j.foodchem.2006.06.001. [DOI] [Google Scholar]
- Zeroni M, Galil J, Ben-Yehoshua S. Auto-inhibition of ethylene formation in non ripening stages of the fruit of sycamore fig (Ficus sycomorus L.) Plant Physiol. 1976;57:647–650. doi: 10.1104/pp.57.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]