Abstract
Modified atmosphere packaging (MAP) is a dynamic system during which respiration and permeation occur simultaneously. Hence factors affecting both respiration and permeation were considered for designing a package. In the design of MA packages for guava (cv. Baruipur) a total of 13 variables were considered. The independent variables includes: weight of fruits, surface area of packaging film, free volume of the package, thickness of the film and permeabilities of film to O2 and CO2 gas. The fixed variables considered were: the surrounding gas composition and temperature, the respiration rates for O2 consumption and CO2 evolution, and the equilibrium gas compositions to be attained in the package so that the fruit’s shelf-life is extended. Two types of MA packages, having package size of 19 cm × 19 cm for a fill weight of 1,000 ± 100 g were developed. Packages were designed to accommodate a fill weight range of 0.90–1.10 kg. Various package parameters were optimized to facilitate establishment of dynamic equilibrium at target levels of O2 and CO2 concentration in the package. The storage study of MA packages was performed at 10, 15, 20 and 25 °C temperatures. The performance of film packages was evaluated for their ability to establish equilibrium at target levels and to extend the shelf life of the packaged fruit. The MA packaging system increased the shelf life of guava by 128–200 % compared to the unpacked fruits at various storage temperatures with a quality comparable with the freshly harvested commodity.
Keywords: Guava, Modified atmosphere packaging, Modeling, Respiration rate, Dynamic equilibrium, Quality attributes, Storage, Gas composition
Introduction
India accounts for 12.7 % of the total world production of fruits and ranks second with the production of 63.50 million tonnes (Singhal 2009). Guava is considered as common man’s fruits liked by both the rich as well as the poor and is rightly called as the apple of the tropics (Adsule and Kadam 1995). The world production of guava (Psidium guajava L.) is estimated to be 5.0 MT. Guava is an important tropical fruits grown extensively in India (Morton 1987; Adsule and Kadam 1995). India leads the world in guava production with annual production of 1.80 MT with the production area of 0.15 Mha. It is the fourth most widely grown fruit crop in India. The popular varieties of guava grown in India are Allahabad Safeda, Lucknoe-49, Baraipur, Nagpur Seedless, Dharwar etc. (Singh and Pal 2008). Guava has gained considerable importance because of its commercial and high nutritive value, availability at moderate price, a pleasant aroma, good flavor, delicious taste and remunerative nature of crops. Guava fruit is an excellent source of ascorbic acid (vitamin C), dietary fiber, pectin and good source of vitamin A, phosphorus, calcium and iron as well as thiamine, niacin, riboflavin and carotene (Adsule and Kadam 1995).
The physicochemical characteristics of guava fruits changes significantly with maturity (Mukherjee and Datta 1967; Teotia et al. 1970). The main objective of storage of guava is to prolong its availability on the market throughout year. Certain post harvest constraints like short shelf life, chilling sensitivity and susceptibility to diseases limit its long duration storage and transportation. The development of peel colour i.e. faster fruit ripening, weight loss, decay incidence, loss of firmness (fruit softening) and off flavor etc. are main challenges during the preservation and storage of guava (Paul et al. 2002; Pereira et al. 2004). In general Guavas fruits were stored at 10–13 °C at 85–90 % RH. (Jacomino et al. 2001a). The need of postharvest management of guava fruit in a scientific way like CA/MAP storage was realized to extend its period of availability in market and also to harness the export opportunities (Mangaraj and Goswami 2011a). MAP technology has the potential to reduce the weight loss, maintained firmness, preserve colour and alleviated chilling injury of guava fruits by controlling the overall metabolic activities (Jacomino et al. 2001a; Mohamed et al. 1994; Sunjka et al. 2003).
MAP technology has a great advantage in developing countries because it can economically be done by hand saving the high cost of new machinery. Additionally the need there for such a technique is much greater because of the dearth of refrigerated storage. Modified atmosphere packaging utilizes only the natural components of air, has achieved public acceptance due to these two trends. MAP has the advantages that synthetic chemicals are not used, no toxic residue is left, and there is little environmental impact, particularly if the plastic films used can be recycled (Kader et al. 1989; Sivakumar and Korsten 2006; Mangaraj and Goswami 2009a; Mangaraj et al. 2009)
Paul et al. (2002) observed that CA storage of guava reduced the rate of respiration and ethylene evolution to variable extent and the fruit could be stored well in unripe condition for 1 month. Multilayer co-extruded polyolephinic film with selective permeability (PSP) prolonged storage of guava up to 3 weeks, while LDPE and PVC film package was suitable for guava storage to 14 days at 10 °C and 85–90 % relative humidity (Jacomino et al. 2001a). The PSP film and LDPE film with mineral incorporation provided an atmosphere of 3 % O2 and 4.5 % CO2 inside the packages, which kept the fruit with good sensorial characteristics for 28 and 14 days, respectively. Guava packed with PVC, LDPE or PET and stored for 2 and 3 weeks at 5 and 8 °C hindered the development of peel colour and the loss of firmness (Gaspar et al. 1997; Mohamed et al. 1994; Jacomino et al. 2001b; Pereira et al. 2004). MA packaging of fresh guava in PET film had a strong influence on color preservation and weight loss of the guavas (Sunjka et al. 2003; Pereira et al. 2004). Combrink et al. (2004) reported that non-perforated polyethylene bags maintained guava fruit quality better than perforated bags.
From the above mentioned reviews it can be inferred that most of the MAP studies are under taken with trial and error basis at lower temperatures. There is no particular standard for MA packaging of a commodity. Respiration of commodity and permeation of gases are two important component of MA packaging system. The respiration rate varies among climatic conditions, growing pattern, commodities, cultivar and the varieties of same commodity. The permeabilities of films vary with production process, company and even with batch of production (Mangaraj and Goswami 2009b). The designed package at a particular temperature when exposed to another temperature, the purpose of MAP may be defeated. Hence a systematic design of MAP system is essential for guava (cv. Baruipur) in particular, at various simulated condition of MAP storage to enhance shelf life and propagate the developed technology to the producer/processor/industry.
Materials and methods
Raw material
The fruits guava (cv. Baruipur) was procured from the orchard at Baruipur farm for their experimental study. The fruits were harvested at the commercial maturity based on subjective evaluation. Usually, this stage is determined by visual observation of its ground colour, size and the days after full bloom (Kadam and Deshpande 1995). Medium size fruit free from visual injury were sorted out. It was ensured to maintain uniformity in terms of the size and weight of individual fruits in the whole lot of samples. The harvested fruits were washed thoroughly using tap water to remove any adhering dirt and wiped with a clean dry cloth and kept under the fan for about 30 min to remove adhered moisture. Thereafter, the fruits were used for the experiments
Determination of maturity indices of fruits
The physico-chemical properties were determined objectively in the laboratory with a view to specify harvest maturity levels of the fruits. The fruits color and firmness was determined using colorimeter and Texture analyzer, respectively (Mangaraj et al. 2005, 2006; Mangaraj and Goswami 2009c). The size and weight of fruit was measured using a vernier caliper having least count of 0.05 mm and a precision balance (Essae Teraoka, Japan) having an accuracy of 0.01 g. True volume, density, ascorbic acid, total soluble solids and titratable acidity etc. of fruits were determined as per the methods described by Ranganna (1995). The chlorophyll content and solubilization of pectin of guava was estimated according to Chittara et al. (2002) and Simpson et al. (1984).
Measurement of respiration rate
The experimental respiration rate in terms of O2 and CO2 at a given temperature were calculated using Eqs. (1) and (2) as given by Kays (1991).
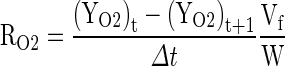 |
1 |
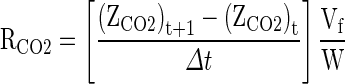 |
2 |
Where: RO2 is the respiration rate, ml [O2] kg−1 h−1, RCO2 is the respiration rate, ml [CO2] kg−1 h−1, YO2 and ZCO2 are the gas concentrations for O2 and CO2 respectively, t is the storage time in h, Δt is the time difference between two gas measurements, Vf is the free volume of the respiration chamber in ml and W is the weight of the fruit in kg.
Modeling of respiration rate
The Michaelis-Menten type equation based on principle of enzyme kinetics with uncompetitive type of inhibition, wherein CO2 does not bind with the enzyme but reacts with enzyme substrate complex, was the model fitted to the experimental respiration data (Lee et al. 1991, 1996; Peppelenbos and Leven 1996; Mahajan and Goswami 2001; Menon and Goswami 2008; Mangaraj and Goswami 2011a, b). The relevant respiration rate models are shown in Eqs. (3) and (4). The model parameters were determined using the experimental respiration data.
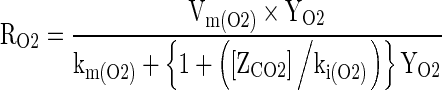 |
3 |
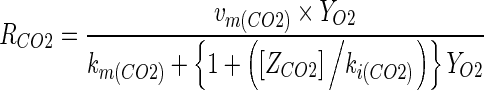 |
4 |
Where: RO2 is the respiration rate, ml [O2] kg−1 h−1, RCO2 is the respiration rate, ml [CO2] kg−1 h−1, YO2 and ZCO2 are the gas concentrations for O2 and CO2 respectively, %, Vm(O2) and Vm (CO2) are the maximum respiration rate for O2 consumption and CO2 evolution, respectively, km(O2) and km(CO2) are the Michaels-Menten constant for O2 consumption and CO2 evolution, % O2 respectively, ki(O2) and ki(CO2) are the inhibition constants for O2 consumption, CO2 evolution, % CO2 respectively.
The temperature dependence of the model parameters of the above Michaelis-Menten equations were quantified using an Arhhenius type equation (Lakakul et al. 1999) of the following form:
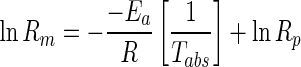 |
5 |
Where, Rm is the model parameter of respiration rate, Rp is the respiration pre-exponential factor, Ea is the activation energy, kJ/g-mole, Tabs is the storage temperature, K, and R is the Universal gas constant (8.314 kJ/kg-mole-K).
Modeling of gas transmission rate (GTR) of selected films
The gas transmission rates (OTR/CTR) of polymeric film are temperature dependent and hence Arrhenius-equations (Eq. 6) was fitted to the experimental data to depict the relationship of GTR with temperature.
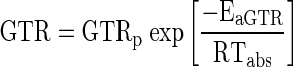 |
6 |
Where, GTR is the gas transmissions rates of films (cm3/m2 h ΔC) at temperature Tabs, GTRp is the gas transmission rates pre-exponential factor for O2 and CO2 (cm3/m2 h ΔC), Eap is the activation energy of gas transmission rates for O2 and CO2 (kJ/kg-mole), R is the universal gas constant (8.314 kJ/kg-mole-K) and Tabs is the absolute temperature in K.
Design of MA packages
MA packaging of fresh fruits constitutes a dynamic system during which fruit respiration and gas permeation occur simultaneously at a given packaging environment surrounding the product. Hence factors affecting respiration and permeation as well as the environmental conditions were considered while designing MA packages (Cameron et al. 1989; Yam and Lee 1995; Mannapperuma et al. 1989; Jacxsens et al. 2000). The variables involved in the MA package design are: the surrounding gases composition (YaO2 and ZaCO2), temperature (T), O2 consumption rate and CO2 production rate (RO2 and RCO2), the optimum gas composition to be attained in the package (YeqO2 and ZeqCO2), weight of the fruits in package (Wp), surface area of the packaging film (Ap), free volume of the package (Vfp), thickness of the film (x), gas transmission rates of film to O2 and CO2 (OTR and CTR). It was observed that some of these variables are inter-dependent, e.g. once the packaging film is selected both OTR and CTR are fixed. The ultimate aim of this design was to select suitable films and the package surface area required for O2 and CO2 gas transmission for a given fruits, fill weight, optimum O2 and CO2 concentration, temperature, and environmental gas composition, so that the equilibrium concentrations of O2 and CO2 are reached within shortest possible time and these concentrations lie within the range required for maximum shelf life of stored fruits (Das 2005; Mangaraj et al. 2011). Here O2, CO2, and temperatures are the important external factors that could influence the respiration and gas permeation significantly.
Fresh fruits are still living and their respiration continue even after harvest from the parent plant and detached from their normal nutrient supplies. In a passive MA packaging system, fresh fruits are sealed in suitable polymeric film packages. Initially, the O2 and CO2 concentrations in the MA package are same as that of the external atmosphere. Due to respiration of the packaged fruits, O2 starts depleting and CO2 starts accumulating within the package because of the consumption of O2 and the production of CO2 in the metabolic process. Consequently, respiration begins to decrease while O2 and CO2 concentration gradients between package and ambient atmosphere begin to develop. The development of concentration gradients induced ingress of O2 and egress of CO2 through the packaging material i.e. polymeric films. Simultaneously respiration rate decreases with decrease in O2 level and increase in CO2 level, provided the variations in O2 and CO2 levels are within safe limits. The decrease in respiration rate decreases the rate of increase of concentration gradient. Transmission of O2 and CO2 through the film further reduces concentration gradient. As the rate of increase in concentration gradient retards, respiration tend to retrieve which again increases the gradients. The increase in concentration gradient again decreases respiration and increases gas transmission. Thus the cyclic process continues until a steady state is established (Chau and Talasila 1994; Renault et al. 1994; Cameron et al. 1989; Mahajan et al. 2007; Mangaraj and Goswami 2008).
In a properly designed MAP, after a period of transient state (the state at which the O2 and CO2 concentration changes continuously within the package with time) an equilibrium state is established. At equilibrium, the amount of O2 entering (ingress) into the package and that of CO2 permeating out (egress) of the package become equal to the amount of O2 consumed and that of CO2 evolved by the packaged fruit, respectively (Jacxsens et al. 2000; Del Nobile et al. 2007). The package atmosphere is then considered to be in dynamic equilibrium with external atmosphere. Hence, Package equilibrium or steady state is defined as the point at which the commodity O2 consumption and CO2 production rates (respiration rates) are equal to the permeation rates of the respective gases (O2 and CO2) through a package at a given temperature. Once established, the equilibrium gas concentrations remained nearly constant throughout the stipulated period of storage unless there is considerable variation in ambient conditions. The period from sealing of fruits in the packaged to the establishment of steady state or equilibrium state is called transient period or equilibrium time. The O2 and CO2 concentration levels of package atmosphere at which dynamic equilibrium establish are called as O2 equilibrium concentration and CO2 equilibrium concentration, respectively. An ideal package system should equilibrate and maintain at the levels of O2 and CO2 are known to be optimal for storage, transport and handling through out the market chain for a specific commodity (Jacxsens et al. 1999; Paul and Clarke 2002; Mahajan et al. 2007; Fonseca et al. 2000; Goswami and Mangaraj 2011).
Mathematical modeling of gaseous exchange in MAP system
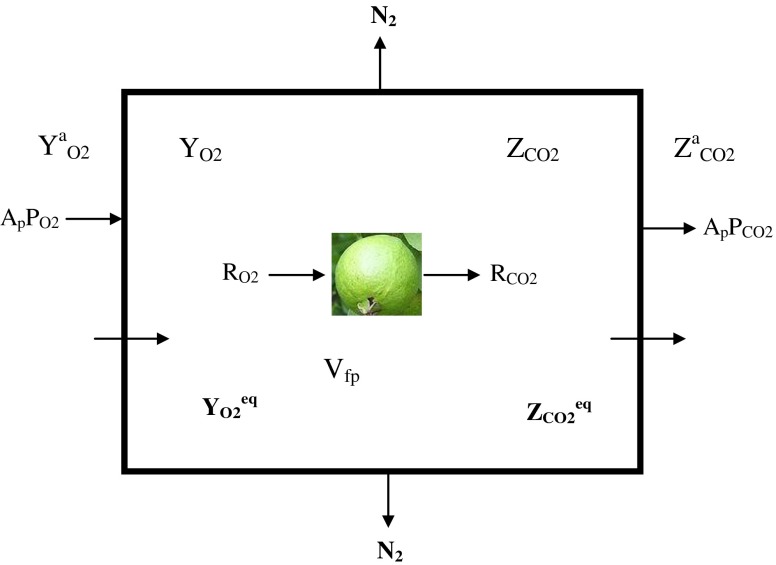
A systematic theoretical design and modeling is needed to establish conditions for the success and benefit of MAP for a particular produce (Exama et al. 1993; Mahajan et al. 2007). Such a design and analysis could provide closely the characteristic of the commodity, film properties and optimized packaging parameters. It helps in minimizing the number of experimental trials: as the trial and error approach is extremely time consuming procedure. Simulation of a MAP system is the most appropriate method to allow a correct MAP design and consequently obtain a successful commercial product (Cameron et al. 1989; Geeson 1989; Makino and Iwasaki 1997; Del Nobile et al. 2007). When fresh fruits is sealed in a selected polymeric film packages, respiration of the product and the gas permeation through the packaging film takes place altogether. In the respiration process O2 is consumed and the fruits evolve CO2. In general, the relative humidity in the internal package atmosphere is higher than the external atmosphere. Hence some amount of water vapor may permeate out of the package, depending upon the WVTR of the polymeric film. However, the mathematical modeling of gaseous exchange for respiratory gases (O2 and CO2) has been attempted here. The diagrammatic representation of the gaseous exchange in MAP of guava is shown in Fig. 1.
Fig. 1.
Gaseous exchange in modified atmosphere packaging system of guava
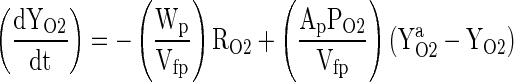
The idea is that once the fruit is sealed inside the package the O2 and CO2 concentration gradients develop due to the fruit respiration and the polymeric film serves as the regulator of O2 flow into the package and the flow of CO2 out of the package. At a given temperature and for a considerably small length of transient period, the rates of O2 consumption (RO2) and the rate of CO2 evolution (RCO2) of the packaged fruits depend greatly on O2 concentration (YO2) and CO2 concentration (ZCO2). Considering that there is no gas stratification inside the packages and that the total pressure is constant, the differential mass balance equations that describe the O2 concentration changes in a package containing respiring product are:
Rate of O2 entry into package space – Rate of O2 consumed by product = Rate of O2 accumulation inside package space
That is,
 |
7 |
Similarly, the CO2 concentration changes in a package can be written as,
Rate of CO2 generated by the fruits – Rate of CO2 leaving out of the package space = Rate of accumulation CO2 inside package space
That is,
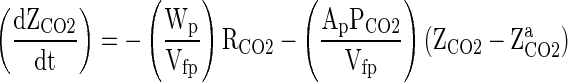 |
8 |
Where Ap is the Area of the package through which the O2 and CO2 permeates (m2), YaO2 and ZaCO2 are the O2 and CO2 concentration in the atmospheric air (cm3 per cm3 of air), respectively, YO2 and ZCO2 are the O2 and CO2 concentration in side the package (cm3 per cm3 of air), respectively, PO2 and PCO2 are the O2 and CO2 permeability of packaging material (cm3. m−2 . h−1. [Conc. diff. of O2 in volume fraction]−1), respectively, Wp is the weight of the fruit kept inside the package (kg), RO2 and RCO2are the respiration rate for O2 consumption and CO2 evolution by the fruits (cm3. kg−1. h−1), respectively, Vfp is the free volume in the package (cm3), t is the storage time (h) and dYO2/dt and dZCO2/dt are the rate of change of O2 concentration ‘YO2’ and CO2 concentration ‘ZCO2’ within the package at time ‘t’ of storage (cm3 per cm3 of air. h−1), respectively.
The Eqs. (7) & (8) coupled to the models that describes the dependence of respiration rate on gas composition, temperature and time (i.e. Eqs. 3, 4 and 5) and models that describes the dependence of packaging film on temperature (Eq. 6) constitute the basic of MAP design (Chau and Talasila 1994; Exama et al. 1993; Jacxsens et al. 2000; Mahajan et al. 2007; Del Nobile et al. 2007).
Numerical analysis
Simultaneous solution of above differential equations would give variation of O2 concentration and CO2 concentration in volume fraction as a function of time, t (h). Using Adam’s numerical method, which is based on Taylor’s formula, the above simultaneous differential equations were solved (Piskunov 1981). A MATLAB programme was developed for modelling the gaseous exchange in MAP system.
Optimization of package parameters
The differential mass balance Eqs. (7) and (8) and results of preliminary investigation were used for the optimization of package parameters. The various parameters well thought-out for optimization of MAP system are as follows:
Fill weight and surface area of the package
Keeping in view the requirements of a consumer carry-bag, family size and eating habit of fruits the MA package was finalized for 1 kg fill weight of guava consisting of four medium sized fruits. For the fixed amount of fruits, the actual area of the film in package (Ap) was finalized in such a way that it is a well-fit package. Also, for the define quantity of fruits to be packed and target level of O2 and CO2 concentrations, the area required for gas exchange was calculated employing Eq. (9).
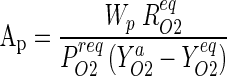 |
9 |
The package size of 19 cm × 19 cm (Ap = 0.0722 m2) was found to be appropriate for packaging of 1.00 kg ± 100 g guava (cv. Baruipur). Packages were designed to accommodate a fill weight (Wp) range of 0.90–1.10 kg. It suggests an optimal range of Wp: Ap ratio of 12.47–15.23 for guava.
GTR requirement of MAP
The GTR requirement of the packaging films are estimated, from basic mass balance Eqs. (7) and (8). Oxygen transmission rate requirement (OTRreq) of the packaging film is calculated employing the following Eq. (10). The target values of equilibrium concentrations of O2 and CO2 for guava were specified for calculating the OTRreq of the packaging film (Mangaraj et al. 2011).
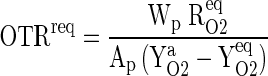 |
10 |
Similarly, carbon dioxide transmission rate requirement (CTRreq) of the packaging film was calculated as follows:
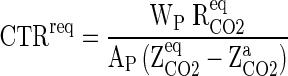 |
11 |
The required CO2 and O2 transmission ratio for the packaging film (TRreqpf) was calculated employing Eq. (12).
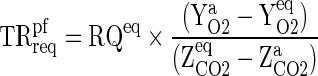 |
12 |
Where,
 |
Equilibrium gas concentration inside the package
For a fixed fruit weight as well as for both the extreme of fill weight range, film area of the package and gas transmission rate requirement (GTRreq) of the MAP, the equilibrium concentration of O2 and CO2 inside the package was calculated employing Eqs. (13) and (14).
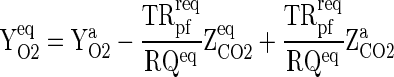 |
13 |
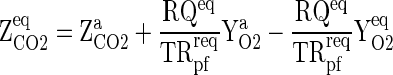 |
14 |
The calculated equilibrium concentration of O2 and CO2 was compared with the target level of O2 and CO2 concentrations in MAP design. For extreme conditions the filling weight range of 900–1,100 g was considered for MA packaging of fruits. During the preliminary design calculation the average fill weight of 1.00 kg was used. Substituting the average fill-weight values of fruits and the target level of equilibrium concentration of O2 and CO2 in Eqs. (10) and (11) the required value of OTR and CTR were calculated. Using these values of OTR and CTR in Eqs. (13) and (14) the YeqO2 and ZeqCO2 was again calculated for both the extreme fill weight range. It was found that YeqO2 and ZeqCO2 values deviated considerably from the target levels for these extreme values of fill weight range. However, it was ensured that the calculated values of YeqO2 and ZeqCO2 have not deviated far from the target levels. Thus the entire fill weight range (900–1,100 g) was separated in to five section i.e. 900, 950, 1,000, 1,050 and 1,100 g and designated as a, b, c, d and e, respectively. For each fill weight section the OTR and CTR and subsequently the YeqO2 and ZeqCO2 were determined. It was observed that the deviations between the calculated values and target levels YeqO2 and ZeqCO2 are small and the deviated values are well within the optimal ranges of O2 and CO2 concentration. Hence the MAP design is considered to be appropriate for guava.
Target level of gas composition in MAP of guava
The recommended level of gas concentration in CA/MA storage of guava for maintaining quality and extending shelf-life is 2–5 % and 2–5 % O2 and CO2, respectively (Singh and Pal 2008; Mangaraj et al. 2009) with nitrogen. However, incase of MAP, it is quite possible that a package design for optimal level of O2 and CO2, may develop deleterious levels when exposed to temperature fluctuations during transportation, marketing and distribution chain. Unlike CA storage system, the external means are not employed in the MAP system to precisely control and monitor package air composition, temperature and relative humidity etc. Thus, a preliminary investigation study with various designs of optimum combinations of O2 and CO2 for MA packaging of guava was carried out at the laboratory. Also, the MAP was designed and evaluated for sub-optimal air compositions with a view to provide a factor of safety against the development of deleterious levels of O2 and CO2 in MAP at any stage, through out the distribution chain. On the basis of preliminary investigations and the sub-optimal package air composition it was found appropriate for designing the optimal MA packages for guava with target air composition of 5 % O2 and 4 % CO2 with nitrogen.
Film laminates for MA packaging
The gas transmission rates of the selected films were compared with the gas transmission requirement of MAP for guava (cv. Baruipur). Not a single film could meet the gas permeability requirement for MAP, satisfactorily. Thus two different films were combined through the tailoring of film laminates to bring the gas transmission requirement of the laminates close to the required values. The area of the two individual films was optimized and the films were adhesive laminated.
Optimization of the area of films OTRreq of MAP
The area of the film combinations and the un-perforated film was optimized to match the OTRreq of MAP by employing the following equations (Mangaraj et al. 2011).
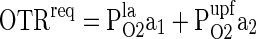 |
15 |
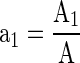 |
16 |
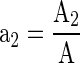 |
17 |
Where, OTRreq is the oxygen transmission rate requirements of film laminate for MAP, PlaO2 is the OTR of the combined laminated film, PupfO2 is the OTR of un-perforated film, a1 is the fractional area of laminated portion, a2 is the effective fractional area of un-perforated film, A1 is the area of laminated portion of the film laminate and A2 is the effective area of un-perforated film of the laminate.
Calculation of PlaO2
The value of OTR of combined film (PlaO2) was estimated by employing the following equation.
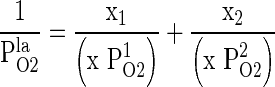 |
18 |
Where, P1O2 and P2CO2 are the OTR of individual films, x1 and x2 are the thickness of individual films, and x is the thickness of the film laminate.
Calculation of size and nos. of circular disc for removal from film
For the development of film laminates, an area equal to A2 was removed in the form of circular disc of same or different diameter from the film piece. The numbers of discs as well as their sizes required to be removed from film was calculated by employing Eq. (19).
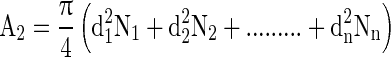 |
19 |
Where, d1, d2, d3…dn are the diameter of the circular disc to be removed, and N1, N2, N3…Nn are the numbers of discs to be removed from the film.
Design parameters for MA packages of guava
The combination of BOPP and PVC as well as that of LDPE and PVC was considered for development of film laminates and MA packaging of guava. Based on the design considerations illustrated above, various parameters of film laminates LFR-3 (A, B, C, D, E) and LFR-4 (A, B, C, D, E) were calculated using appropriate equations and presented in Tables 1 and 2 for development of GTR tailored MA packages for guava precisely.
Table 1.
Design parameters of film laminate LFR-3 for modified atmosphere packaging of guava
| Temp. | OTRreq | CTRreq | Nc (d) | Na (d) | A1 | A2 | a1 | a2 | OTRfl | CTRfl |
|---|---|---|---|---|---|---|---|---|---|---|
| Film laminate: A (Wp = 0.90 kg (a), Vfp = 740 ml) | ||||||||||
| 10 | 587.4 | 2259.4 | 32.8(5) | 32(5) + 2(3) + 3(1) | 77.5 | 644.5 | 0.11 | 0.89 | 585.2 | 3353.4 |
| 15 | 659.7 | 2512.3 | 24.9(5) | 25(5) | 231.3 | 490.6 | 0.32 | 0.68 | 659.8 | 3817.1 |
| 20 | 763.1 | 2854.5 | 20.1(5) | 20(5) + 4(1) | 326.3 | 395.6 | 0.45 | 0.55 | 762.2 | 4436.5 |
| 25 | 889.2 | 3263.1 | 15.9(5) | 15(5) + 2(3) + 1(2) | 408.8 | 313.2 | 0.56 | 0.43 | 889.8 | 5233.1 |
| Film laminate: B (Wp = 0.95 kg (b), Vfp = 685 ml) | ||||||||||
| 10 | 620.1 | 2384.9 | 34.8(5) | 34(5) + 1(3) + 3(2) | 38.2 | 683.7 | 0.05 | 0.94 | 618.5 | 3544.0 |
| 15 | 696.4 | 2651.9 | 26.5(5) | 26(5) + 2(2) + 5(1) | 201.5 | 520.4 | 0.28 | 0.72 | 696.4 | 4028.6 |
| 20 | 805.5 | 3013.1 | 21.4(5) | 21(5) + 1(3) + 2(1) | 301.2 | 420.7 | 0.42 | 0.58 | 806.4 | 4694.0 |
| 25 | 938.7 | 3444.4 | 16.9(5) | 17(5) | 388.4 | 333.6 | 0.54 | 0.46 | 942.5 | 5528.1 |
| Film laminate: C (Wp = 1.00 kg (c), Vfp = 618 ml) | ||||||||||
| 10 | 652.7 | 2510.4 | 36.7(5) | 36(5) + 2(3) + 1(1) | 0.6 | 721.4 | 0.00 | 0.99 | 650.4 | 3826.9 |
| 15 | 733.1 | 2791.5 | 28.0(5) | 28(5) + 1(1) | 171.7 | 550.3 | 0.24 | 0.76 | 732.9 | 4240.1 |
| 20 | 847.9 | 3171.7 | 22.7(5) | 22(5) + 1(3) + 2(2) | 276.9 | 445.1 | 0.38 | 0.61 | 848.0 | 4935.9 |
| 25 | 988.1 | 3625.7 | 18.0(5) | 18(5) | 368.7 | 353.2 | 0.51 | 0.49 | 988.2 | 5811.6 |
| Film laminate: D (Wp = 1.05 kg (d), Vfp = 547 ml) | ||||||||||
| 10 | 685.4 | 2635.9 | 38.7(5) | 36(5) + 2(3) + 2(1) | 0.0 | 722.0 | 0.00 | 1.00 | 683.9 | 3968.8 |
| 15 | 769.7 | 2931.1 | 29.5(5) | 29(5) + 1(3) + 1(2) | 141.9 | 580.1 | 0.19 | 0.80 | 772.5 | 4451.5 |
| 20 | 890.3 | 3330.3 | 23.9(5) | 24(5) | 251.0 | 471.0 | 0.35 | 0.65 | 892.2 | 5193.3 |
| 25 | 1037.5 | 3806.9 | 19.0(5) | 19(5) | 349.1 | 372.8 | 0.48 | 0.51 | 1033.4 | 6095.2 |
| Film laminate: E (Wp = 1.10 kg (e), Vfp = 490 ml) | ||||||||||
| 10 | 718.0 | 2761.5 | 40.7(5) | 36(5) + 1(3) + 2(2) | 0.1 | 721.8 | 0.00 | 0.99 | 716.7 | 3968.8 |
| 15 | 806.3 | 3070.6 | 31.1(5) | 31(5) + 3(1) | 111.3 | 610.7 | 0.15 | 0.84 | 810.0 | 4668.6 |
| 20 | 932.7 | 3488.9 | 25.2(5) | 25(5) + 1(2) + 1(1) | 227.4 | 494.5 | 0.31 | 0.68 | 932.4 | 5427.4 |
| 25 | 1086.9 | 3988.2 | 20.0(5) | 20(5) + 1(1) | 328.7 | 393.3 | 0.45 | 0.54 | 1087.6 | 6390.1 |
Table 2.
Design parameters of film laminate LFR-4 for modified atmosphere packaging of guava
| Temp. | OTRreq | CTRreq | Nc (d) | Na (d) | A1 | A2 | a1 | a2 | OTRfl | CTRfl |
|---|---|---|---|---|---|---|---|---|---|---|
| Film laminate: LFR-4-A (Wp = 0.90 kg (a), Vfp = 753 ml) | ||||||||||
| 10 | 587.4 | 2259.4 | 31.1(5) | 31(5) + 2(1) | 112.0 | 609.9 | 0.15 | 0.84 | 585.7 | 3554.0 |
| 15 | 659.7 | 2512.3 | 19.5(5) | 19(5) + 1(3) + 1(2) | 338.1 | 383.8 | 0.47 | 0.53 | 659.9 | 4007.1 |
| 20 | 763.1 | 2854.5 | 12.6(5) | 12(5) + 1(3) + 2(2) | 474.7 | 247.3 | 0.66 | 0.34 | 763.3 | 4640.6 |
| 25 | 889.3 | 3263.1 | 5.8(5) | 5(5) + 2(3) + 2(1) | 608.2 | 113.8 | 0.84 | 0.16 | 888.8 | 5370.9 |
| Film laminate: LFR-4-B (Wp = 0.95 kg (b), Vfp = 697 ml) | ||||||||||
| 10 | 620.1 | 2384.9 | 33.9(5) | 33(5) + 2(3) + 1(2) | 56.3 | 665.7 | 0.08 | 0.92 | 618.1 | 3760.3 |
| 15 | 696.4 | 2651.9 | 21.7(5) | 21(5) + 2(3) + 1(1) | 294.9 | 427.0 | 0.40 | 0.59 | 696.1 | 4239.9 |
| 20 | 805.5 | 3013.1 | 14.4(5) | 14(5) + 2(2) + 3(1) | 438.6 | 283.4 | 0.60 | 0.39 | 805.7 | 4916.2 |
| 25 | 938.7 | 3444.4 | 7.3(5) | 7(5) + 2(2) | 578.3 | 143.6 | 0.80 | 0.19 | 938.2 | 5695.7 |
| Film laminate: LFR-4-C (Wp = 1.00 kg (c), Vfp = 625 ml) | ||||||||||
| 10 | 652.7 | 2510.4 | 36.7(5) | 36(5) + 2(3) + 1(1) | 0.6 | 721.4 | 0.00 | 0.99 | 650.5 | 3966.6 |
| 15 | 733.1 | 2791.5 | 24.0(5) | 24(5) | 251.0 | 471.0 | 0.34 | 0.65 | 733.0 | 4476.9 |
| 20 | 847.9 | 3171.7 | 16.2(5) | 16(5) + 1(2) + 3(1) | 402.5 | 319.5 | 0.55 | 0.44 | 848.1 | 5191.7 |
| 25 | 988.1 | 3625.7 | 8.8(5) | 8 + 2(3) + 3(1) | 548.5 | 173.5 | 0.76 | 0.24 | 987.5 | 6020.5 |
| Film laminate: LFR-4-D (Wp = 1.05 kg (d), Vfp = 558 ml) | ||||||||||
| 10 | 685.3 | 2636.0 | 39.6(5) | 36(5) + 2(3) + 2(1) | 0.0 | 722.0 | 0.00 | 1.00 | 650.9 | 3970.2 |
| 15 | 769.7 | 2931.1 | 26.2(5) | 26(5) + 1(2) + 2(1) | 207.0 | 514.9 | 0.28 | 0.71 | 769.8 | 4713.9 |
| 20 | 890.3 | 3330.3 | 18.1(5) | 18(5) + 3(1) | 366.4 | 355.6 | 0.50 | 0.49 | 890.5 | 5467.3 |
| 25 | 1037.5 | 3806.9 | 10.4(5) | 10(5) + 1(3) | 518.7 | 203.3 | 0.72 | 0.28 | 1036.8 | 6345.3 |
| Film laminate: LFR-4-E (Wp = 1.10 kg (e), Vfp = 504 ml) | ||||||||||
| 10 | 718.0 | 2761.5 | 42.4(5) | 36(5) + 2(3) + 2(1) | 0.0 | 722.0 | 0.00 | 1.00 | 714.7 | 3969.1 |
| 15 | 806.3 | 3070.6 | 28.4(5) | 28(5) + 1(3) + 3(1) | 163.1 | 558.9 | 0.22 | 0.77 | 806.7 | 4950.9 |
| 20 | 932.7 | 3488.9 | 19.9(5) | 19(5) + 1(3) + 4(2) | 330.3 | 391.7 | 0.45 | 0.54 | 932.9 | 5742.9 |
| 25 | 1086.9 | 3988.2 | 11.9(5) | 11(5) + 2(3) + 5(1) | 488.1 | 233.9 | 0.67 | 0.32 | 1087.4 | 6678.6 |
Design parameters for film laminate
Film type: Laminates of BOPP-45 μ + PVC-25 μ
Film code: LFR-3 (A, B, C, D, E)
Package type: PCG-LFR-3 (A, B, C, D, E)
Film type: Laminates of LDPE-40 μ + PVC-25 μ
Film code: LFR-4 (A, B, C, D, E)
Package type: PCG-LFR-4 (A, B, C, D, E)
Fruit weight (Wp): 1.00 kg ± 100 g (a, b, c, d, e)
Package surface area (Ap): 19 cm × 19 cm (0.0722 m2)
YaO2 = 21 % (i.e. 0.21)
ZaCO2 = 0.03 % (i.e. 0.0003)
Target level of YeqO2 = 5 % (i.e. 0.05)
Target level of ZeqCO2 = 4 % (i.e. 0.04)
Selection of polymeric films
With the objective of meeting MAP requirements the polymeric films namely LDPE, BOPP, PVC, PVDC were procured considering various film characteristics such as gas transmission rates for O2 and CO2, water vapor transmission rates, sealing reliability, clarity, strength, durability, printability and cost effectiveness (Kader et al. 1989; Exama et al. 1993; Costa et al. 2011). The films were hydrophilic in nature and their gas transmittance properties were not affected significantly by relative humidity. The film properties viz. thickness, haze, tensile strength, elongation strength, seal strength, tear strength, WVTR and GTR were measured using standard methods and given in Table 3. The GTR of films was determined employing equal pressure method since it facilitated similar condition under which gas transmission takes place in MAP (Karel et al. 1975; Mangaraj et al. 2009).
Table 3.
Properties of the selected polymeric films
| Film Properties | Units | Procedure | Types of films with code | ||
|---|---|---|---|---|---|
| BOPP | PVC | LDPE | |||
| Thickness | μ | ASTM D37 | 45 | 25 | 40 |
| Tensile strength at yield | MPa | MD/TD | 14.3/16.4 | 52.0/58.0 | 9.2/9.9 |
| Tensile at break | MPa | MD/TD | 14.9/16.3 | 52.0/53.0 | 18.5/20.1 |
| Elongation at yield | % | MD/TD | 4.7/3.0 | 2.2/2.0 | 7.5/5.4 |
| Elongation at break | % | MD/TD | 177/428 | 307/486 | 593/817 |
| Tear strength | Gms/microns | MD/TD | 0.81/3.79 | 0.47/0.60 | 1.93/18.6 |
| Haze | % | ASTM D1003 | 1.35(0.09) | 1.38(0.06) | 23.57(0.31) |
| Dart impact | Gms/microns | ASTM D2457 | 0.78(0.03) | 0.76(0.05) | 2.6(0.12) |
| Seal temperature at 2 kg/cm2 | °C | ASTM F88 | 180–200 | 125–180 | 142–180 |
| WVTR at 38 °C and 90 % RH | g/m2. day | ASTM E96 | 4.91(0.13) | 34.8(0.34) | 11.67(0.23) |
| OTR (10 °C, 90 % RH) | (Cm3 (m2.h. ΔC)−1) | ASTM D3985 | 25.7(0.47) | 650.9(22.3) | 164.7(10.7) |
| OTR (25 °C, 70 % RH) | (Cm3 (m2.h. ΔC)−1) | ASTM D3985 | 79.1(6.5) | 1894.3(41.9) | 502.7(18.7) |
| CTR (10 °C, 90 % RH) | (Cm3 (m2.h. ΔC)−1) | ASTM D3985 | 87.2(6.2) | 3968.8(53.8) | 912.3(22.5) |
| CTR (25 °C, 70 % RH) | (Cm3 (m2.h. ΔC)−1) | ASTM D3985 | 368.7(10.6) | 11992.6(65.8) | 2930.9(29.6) |
(Values inside parentheses are the standard deviation values of three replicate experiments)
MD machine direction, TD transverse direction
Tailoring of film laminates for MA packaging
The gas transmission rates for O2, CO2 as well as the transmission ratio of any of the selected films could not match the gas transmission requirements of MAP for guava for 1.00 kg ± 100 g packages. Thus two combinations of PVC and BOPP as well as that of PVC and LDPE films were considered for preparing film laminates, so as to bring the gas transmission characteristics of laminates as close to the gas transmission requirement of MAP as possible. The un-perforated and perforated films as per design calculations (Tables 1 and 2) were taken for development of laminated films. Film roll with out perforation was passed through the rubber roller where adhesive was applied and the drying tunnel, which consists of cooling chamber, heating chamber and normal chamber. Subsequent heating and cooling was given for the bonding of the adhesive with the film. Then it was passed through the laminating nip through the idle roller. The secondary substrate (film with perforation) coming from another idle roller also passed through the laminating nip. Both the films were pressed in the laminating nip along with the application of heat. Finally the desired laminated film roll comes out through the idle roller. Two types of film laminates i.e. LFR-3 (BOPP-45 μ + PVC-25 μ) and LFR-4 (LDPE-40 μ + PVC-25 μ) were developed to meet the GTR requirements of the MAP precisely. Each type of film laminates was further customized into five categories i.e. A, B, C, D, and E to convene the GTR requirement of entire five section of package fill-weight range of fruits.
MA packages for guava storage
The guava fruits of medium size were number-labeled for MA packaging. Weight and volume of each fruit was measured. The representative samples were taken from the fruit lot and the physico-chemical attributes were determined employing standard techniques and procedures. Using film laminates LFR-3 and LFR-4 two types of packages (PCGKG-LFR3, PCG-LFR4) of required size (19 cm × 19 cm) were developed. Four guavas were inserted in each package and the packages were heat-sealed. Silicon rubber septums were glued to the packages to facilitate gas sampling. The MA packages were labeled marked for subsequent storage study.
Performance evaluation of MA packages
The MA packages were kept at 10 °C, 15 °C, 20 °C and 25 °C storage temperatures inside the incubator for their evaluation. The performance of various packages was evaluated for their, (i) ability to establish/achieve equilibrium at target levels and (ii) ability to extend the shelf-life of the packaged fruit with quality perseverance.
-
(i)
Establishment of package equilibrium condition
Sample of air were drawn from the package with the help of gas tight syringe through self-sealing septums, which were glued, to the package surface for this purpose. The samples of package air were analyzed on a Gas Chromatograph for determining the variation of O2 and CO2 concentration in the package with time (Mangaraj et al. 2011). The equilibrium concentration of O2, and CO2 (YeqO2, ZeqCO2) and equilibrium time (teq) were subsequently determined. From the mathematical model of gaseous exchange in MAP, the values of YO2, ZCO2, YeqO2, ZeqCO2 and teq were predicted by employing MATLAB Programme. The predicted and the experimental values of YO2, ZCO2, YeqO2, ZeqCO2 and teq were compared for validation of the developed model.
-
(ii)
Variation in quality parameters of fruits during storage
The variation in the quality attributes such as weight loss, volume, firmness, TSS, TA, chlorophyll content, pectin solubilization, color parameters (l*, a*, b*, hue angle, chroma, total color difference) of MA packed as well as unpacked guava were determined at 10 °C, 15 °C, 20 °C and 25 °C storage temperatures at a regular interval (Ranganna 1995; Mangaraj and Goswami 2009c).
Statistical methodology
Three-factor analysis of variance was also carried out to find the direct two-factor and three-factor interaction effect of temperature, storage system and storage period on the quality parameters of guava (Das and Giri 1986). Response surface methodology was employed to evaluate the effect of temperature, storage periods and their interaction on the quality parameters of MA packed and unpacked fruits.
Mathematically this can be represented as
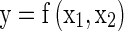 |
20 |
Where ‘f’ is the response surface function, ‘y’ is dependent variable (quality parameters) and input factors: temperatures (x1) and storage periods (x2).
To approximate the function ‘f’, second order polynomial equation of the following form was assumed (Mangaraj and Singh 2011).
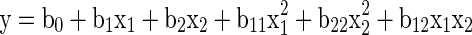 |
21 |
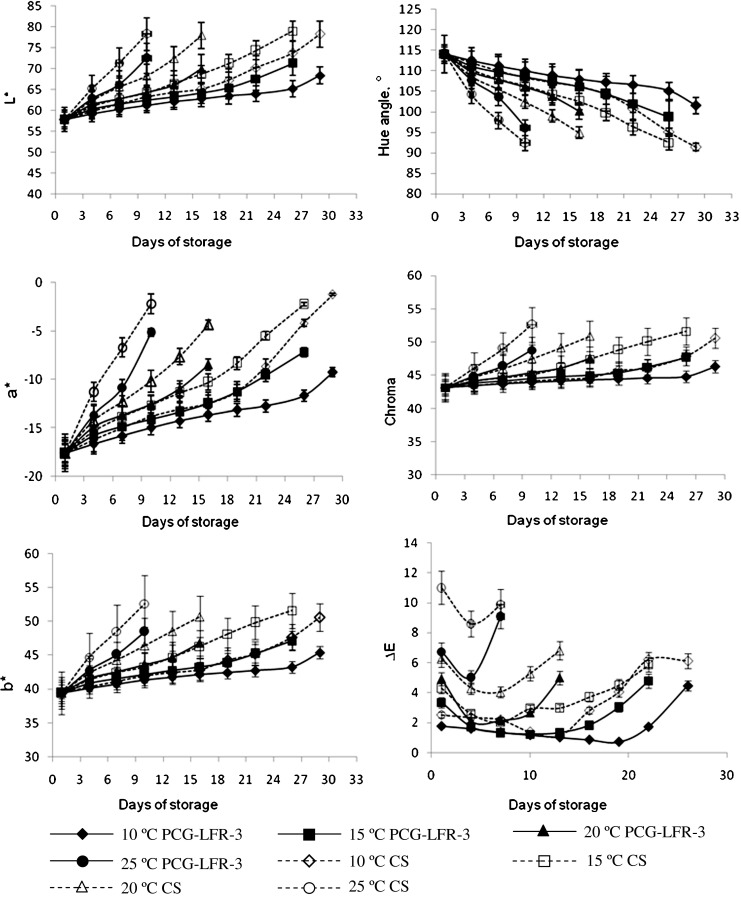
Where, y, is the predicted response (dependent variables); b0, b1, b2, b11, b12 and b22 are the regression coefficients; and x1, x2 are the coded value of independent variables (factors) i.e. the temperature and storage period, respectively which are linearly related by the following equation with the original values (Khuri and Cornell 1987; Das 2005).
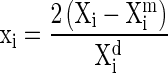 |
22 |
Where, Xi is actual value in original units, Xmi is mean of maximum and minimum values/levels of Xi and Xdi is difference between maximum and minimum values/levels of Xi and xi is the coded values of independent variables.
Results and discussions
Maturity indices of fruits
The maturity indices of guava (cv.Baruipur) was determined based on the evaluation of physico-chemical properties. The average unit weight, flesh firmness, specific gravity and total soluble solids of good medium size guava (cv. Baruipur) were found to be 250 g, 9527.40 g, 1.1 and 8.50, respectively which indicated that the fruits were matured. Similar results were obtained by Kumar and Honda (1974), Palaniswamy and Shanmugavelu (1974), Wilson (1980), Morton (1987), Adsule and Kadam (1995). The color values namely L* a* b*, hue angle and chroma were measured to be 57.85, -17.58, 39.41, 114.00 and 43.15, respectively and these values are in agreement with Kumar and Honda (1974), Jacomino et al. (2001a) and Singh and Pal (2008) during harvest maturity for achieving good quality fruits for retaling and storage. The negative value of ‘a’ implied the greenness of guava at harvest maturity.
Respiration of fruits for transient state of MAP
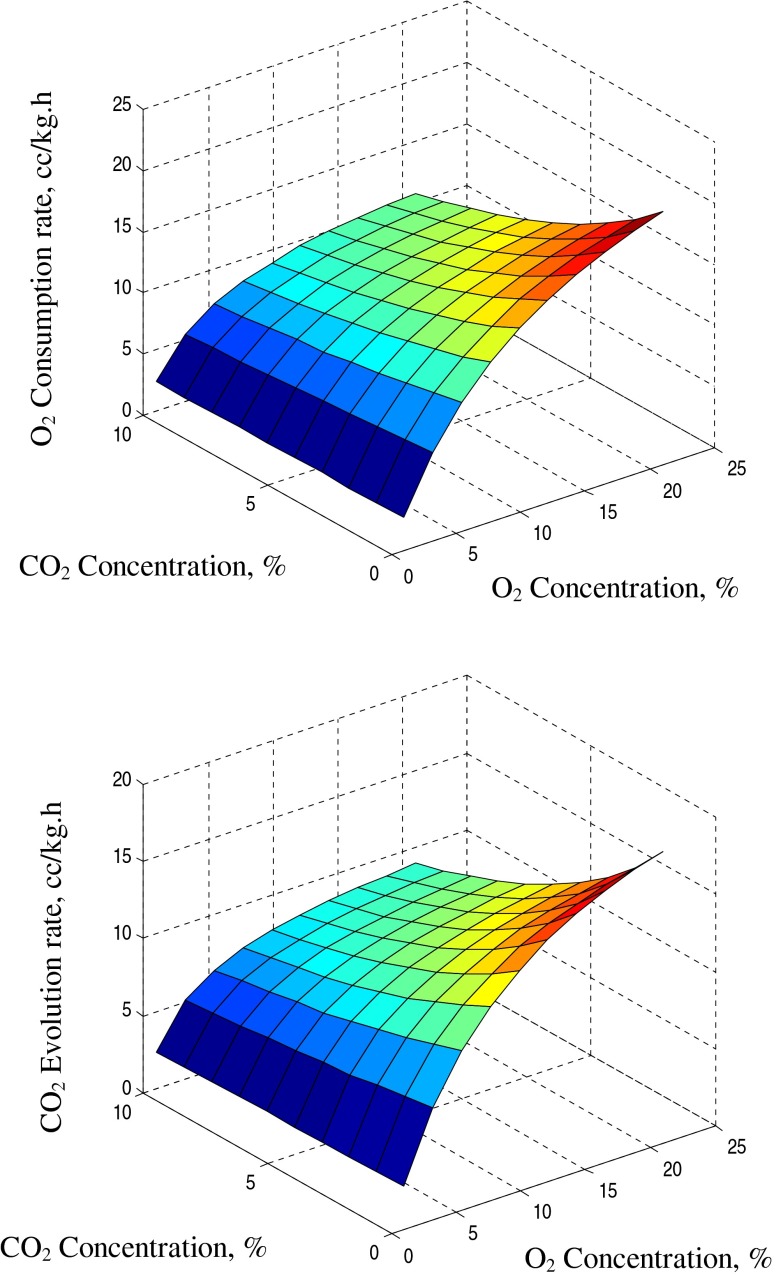
In MAP, during transient period, the package air composition changes continuously due to the respiration of the fruits and permeation of gaseous through the packaging film. Hence, the transient state of MAP of guava was simulated at various storage temperatures as closely as possible and respiration profile of guava at 15 °C has been shown in Fig. 2. The effect of O2 and CO2 concentration on respiration rates at different storage temperature under simulated transient state of MAP was observed in this study.
Fig. 2.
Respiration profile of guava under simulated transient state of modified atmosphere packaging at 15 °C
The decrease in O2 concentration from 21 % to 4 % reduced RO2 and RCO2 from 14.5 to 2.3 and 13.0 to 2.2 cc/kg-h, respectively for guava at 2.0 % CO2 concentration level and 10 °C storage temperature. For similar reduction in the O2 concentration at 5 % CO2 level, RO2 and RCO2 were found to have reduced from 11.0 to 2.1 and 9.5 to 1.8 cc/kg-h, respectively. At 5 % O2 and 5 % CO2, the respective values of RO2 and RCO2 were found to be around 3.2 and 2.5 cc/kg-h, and those for normal air were 14.0 and 12.7 cc/kg-h, respectively indicating 77 to 80 % reduction in respiration rate. Similarly at 15 °C, the percent reduction of RO2 and RCO2 from normal air to modified atmosphere of 5 % O2 and 5 % CO2 was found to be 80–84 % (Fig. 2). At 20 °C storage temperature, the decrease in O2 concentration from 21 % to 4 % reduced RO2 and RCO2 from 21.5 to 3.2 and 18.5 to 3.0 cc/kg-h, respectively at 2.0 % CO2 concentration level. At 5 % O2 and 5 % CO2, the respective values of RO2 and RCO2 were found to be 3.5 and 2.8 cc/kg-h, and those for normal air were 21.2 and 18.3 cc/kg-h, respectively indicated 83.5 to 85 % reduction in respiration rate. Thus, the percentage reduction in RO2 and RCO2 were found to be higher at higher levels of temperature.
At 15 °C and at a O2 level of 21 %, the increase in CO2 concentration from 2.0 % to 10.0 %, reduced RO2 and RCO2 from 17.2 to 11.10 and 15.8 to 9.5 cc/kg-h, respectively; whereas for similar increase in CO2 at 5 % O2 level, the RO2 and RCO2 were found to have reduced from 3.2 to 2.9 and 2.8 to 2.2 cc/kg-h (Fig. 2). Thus, it can be inferred that the increase in CO2 reduces respiration rates at all the level of O2. The effect of variation in O2 on respiration rates is much higher than CO2. The effect of CO2 level on RO2 and RCO2 was found more pronounced at higher level of O2 concentration (Fig. 2). These results are in close agreement with those obtained by Talasila et al. (1992), Mangaraj and Goswami (2011a).
Overall, at all the reference temperatures, RO2 decreased by decreasing the O2 and increasing the CO2 levels and this effect was more pronounced at higher temperatures. Similar trends were observed for CO2 production rates (RCO2). Therefore, low O2 and high CO2 atmospheres imposed a decreasing trend in the respiration rate, which should benefit the shelf life of guava.
MA packages for storage of guava
Two types of MA packages viz. PCG-LFR-3 and PCG-LFR-4 were developed for MA packaging of guava with package surface area (Ap) of 0.0722 m2. Packages were designed to accommodate a fill weight (Wp) range of 0.90–1.10 kg. It suggests an optimal range of Wp: Ap ratio of 12.47–15.23 for guava. The shape and size of the fruits was found to have affected Wp: Ap ratio as well as package free volume (Vfp). The Vfp was found to have varied between 490 and 625 ml for the entire fill weight range. The Vfp was found to have varied inversely with the Wp: Ap ratio. The performance of the MA packages was evaluated at various storage temperatures and discussed as follows.
Equilibrium concentrations of O2 and CO2 in MA packages
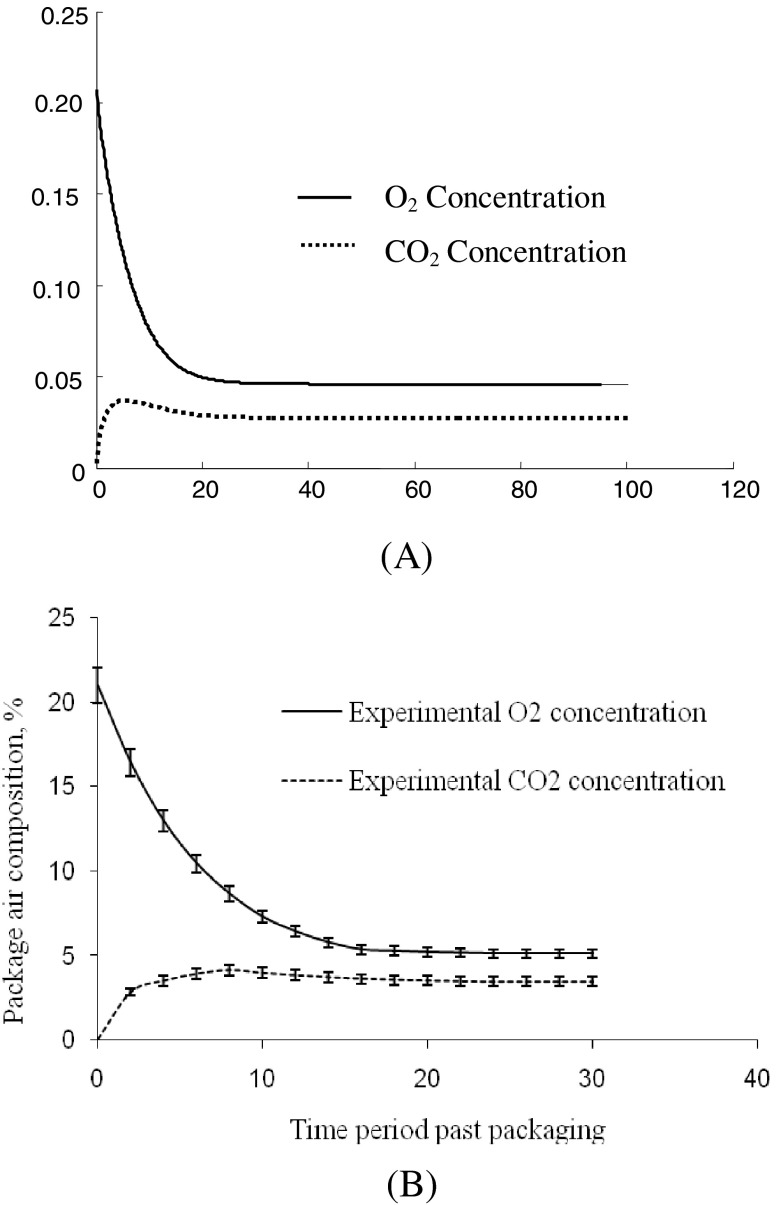
The predicted as well as experimental values of YeqO2, ZeqCO2 and teq for various MA packages at different storage temperatures have been presented in Table 4. The profile of package air composition with time predicted by the modeling of gaseous exchange in MAP for these packages at 15 °C has been depicted in Fig. 3(a). Most of the packages have established equilibrium at such levels of O2 and CO2, which were fairly close to the target levels. The predicted and experimental values of YeqO2, ZeqCO2 were found to be higher than the target levels for all the MA packages. There was good agreement between predicted as well as experimental values of YeqO2 and ZeqCO2. The experimental values of YeqO2 varied between 5.00 and 5.37 % whereas those of ZeqCO2 varied between 3.14 and 3.72 for PCG-LFR-3 and PCG-LFR-4 packages. The experimental variation of O2 and CO2 levels in PCG-LFR-3 MA packages at15 °C storage temperatures has been shown in Fig. 3(b). During steady state period, the experimental values of O2 and CO2 were found to be nearly constant for an extended period of storage. By and large all types of MA packages have accommodated varying fill weight between 0.90 and 1.10 kg have established dynamic equilibrium state with out causing any unfavorable deviation from the target levels of O2 and CO2 at all the reference storage temperatures.
Table 4.
Equilibrium concentration of O2 and CO2 predicted by the model and experimental observations for modified atmosphere packaged guava
| Temperature (°C) | Yeq-preO2 (%) | Yeq-expO2 (%) | Zeq-preCO2 (%) | Zeq-expCO2 (%) | teq-pre (h) | teq-exp (h) |
|---|---|---|---|---|---|---|
| MA package fill weight: 1.00 kg; Free volume of the package: 618 ml | ||||||
| 10 | 4.97 | 5.20 | 2.91 | 3.34 | 24.0 | 26.0 |
| 15 | 4.86 | 5.13 | 2.97 | 3.45 | 22.0 | 24.0 |
| 20 | 4.82 | 5.00 | 2.93 | 3.39 | 18.0 | 20.0 |
| 25 | 5.11 | 5.27 | 2.86 | 3.27 | 16.0 | 16.0 |
| MA package fill weight: 0.90 kg; Free volume of the package: 740 ml | ||||||
| 10 | 4.95 | 5.26 | 3.05 | 3.58 | 30.0 | 34.0 |
| 15 | 4.90 | 5.28 | 3.00 | 3.39 | 26.0 | 28.0 |
| 20 | 5.05 | 5.37 | 2.94 | 3.31 | 24.0 | 24.0 |
| 25 | 5.03 | 5.31 | 2.87 | 3.26 | 20.0 | 18.0 |
| MA package fill weight: 0.95 kg; Free volume of the package: 685 ml | ||||||
| 10 | 4.92 | 5.24 | 3.07 | 3.65 | 28.0 | 30.0 |
| 15 | 4.88 | 5.17 | 3.03 | 3.38 | 24.0 | 24.0 |
| 20 | 5.01 | 5.26 | 2.95 | 3.30 | 20.0 | 22.0 |
| 25 | 5.07 | 5.33 | 2.86 | 3.25 | 18.0 | 18.0 |
| MA package fill weight: 1.05 kg; Free volume of the package: 547 ml | ||||||
| 10 | 4.91 | 5.16 | 2.97 | 3.36 | 22.0 | 24.0 |
| 15 | 4.84 | 4.98 | 2.93 | 3.30 | 20.0 | 22.0 |
| 20 | 4.88 | 5.17 | 2.87 | 3.32 | 16.0 | 18.0 |
| 25 | 4.95 | 5.21 | 2.82 | 3.14 | 14.0 | 14.0 |
| MA package fill weight: 1.10 kg; Free volume of the package: 490 ml | ||||||
| 10 | 4.85 | 5.14 | 3.10 | 3.72 | 20.0 | 20.0 |
| 15 | 4.81 | 5.00 | 2.95 | 3.36 | 16.0 | 18.0 |
| 20 | 4.89 | 5.20 | 2.90 | 3.29 | 14.0 | 16.0 |
| 25 | 5.03 | 5.26 | 2.80 | 3.17 | 12.0 | 10.0 |
Fig. 3.
Variation of O2 and CO2 with time predicted by the model in modified atmosphere packed guava at 10 °C (a); and Experimental variation in package air composition with time for pcg-lfr-3c at 15 °C storage temperature (b)
Equilibrium time
The length of time taken by the MA package to establish dynamic equilibrium from the time of packaging is considered as equilibrium time. For MA packages, the predicted values of equilibrium time were found to have varied between 12 and 30 h, whereas those of experimental values varied between 10 and 34 h at all the reference storage temperatures as given in Table 4. The experimental values some what deviated from the predicted ones. The development of quasi-equilibrium conditions and the variations in the free volume in the package (Vfp), because of the varying fill weight, were probably the cause of such deviations in equilibrium time (teq) values. In fact, small variations in Vfp are always possible in a flexible package. Hence, it is unrealistic to expect a constant value of Vfp in the flexible packages.
It has been seen that the variation in O2 affects both RO2 and RCO2 significantly. With the variation in RO2 and RCO2, the O2 consumption as well as the CO2 evolution of the package varies which in turn affects O2 and CO2 level in the internal atmosphere of the package. Thus, as O2 decreases, RCO2 reduces which in turn reduces CO2 in the internal atmosphere of the package. Reduction in CO2 level tends to retrieve RCO2 slightly. However, the amount of reduction in CO2 due to decrease in O2 is greater than the amount of retrieved. As such, with the decrease in O2 level CO2 level also decreases, though by small amounts. Thus, though the equilibrium condition for CO2 level appears to be approaching earlier than that of O2 level but in true sense, CO2 level becomes stable only when O2 level attains equilibrium (Fig. 3). It implies that the equilibrium for both, O2 and CO2 is attained simultaneously in MA packaging. Also, in view of the fact that O2 level has more pronounced effect on respiration rates than CO2 level, the equilibrium time (teq) for O2 level assume greater importance. The single equilibrium time (teq) approach observed here is in agreeance with the study of Torrieri et al. (2007); Rocculi et al. (2006); Rai and Paul (2007); González-Buesa et al. (2009); Iqbal et al. (2009).
Quality assessment of MA packaged and unpacked guava fruits
The performance of the developed MA packages for their ability to extend the shelf life of fruits has been evaluated at 10, 15, 20 and 25 °C storage temperatures along with the unpacked fruits, which served as control samples. The variation in physico-chemical quality attributes of guava fruits under MA packaged (PCG-LFR-3, PCG-LFR-4) with those of unpackaged fruits (control) were comprehensively compared. The results of the variation in quality parameters namely, physical loss in weight (PLW), reduction in volume, flesh firmness, puncture resistance, TSS, TA, ascorbic acids, chlorophyll content, solubilization in pectin and the colour parameters like l*, a*, b*, hue angle, chroma and total colour difference of guava samples are analyzed and elaborated.
Data on different quality attributes of MA packaged and unpacked (control) guava stored at 15 °C is given in Table 5 and their variation with storage temperature are shown in Fig. 4. The % PLW of MA packaged guava was found to be much smaller than that of unpackaged fruits. The PLW in MA packaged was 4–5 times lower than the control storage guava during different period of storage at 10–25 °C. It implies the laminated MAP system was effective in preventing loss of water from guava. The rate of water loss was small at lower temperatures even in control fruits. It was observed that the water loss of 10–12 % was not enough to promote visual shrinkage of the fruit skin during 26 days of storage at 10 °C. However, at elevated temperature the reserve carbohydrate utilization increased (Auda et al. 1966), hence the PLW is more and so also the reduction in volume. The loss of water is one of the main causes of deterioration of fruits, since it not only results in direct quantitative losses (PLW), but also causes losses in appearance, texture (softening) and nutritional quality (hydrolysis of starch to sugars). MA does not directly influence the rate of water loss, but the polymeric films packaging results in significantly higher RH around the commodity and consequently reduced water loss compared to air storage (Kader 1986; Singh and Pal 2008).
Table 5.
Variation in physico-chemical properties of modified atmosphere packed and unpacked guava at 15 °C and 90 % RH
| Physico-chemical properties | Storage/Packaging condition | Days after storage | |||||
|---|---|---|---|---|---|---|---|
| 1 | 4 | 10 | 16 | 22 | 26 | ||
| PLW (%) | PCG-LFR-3 | 0 | 0.3(0.05) | 0.7(0.06) | 1.4(0.08) | 2.2 (0.13) | 2.5(0.11) |
| PCG-LFR-4 | 0 | 0.3(0.04) | 0.7(0.05) | 1.4(0.09) | 2.3(0.11) | 2.6(0.16) | |
| CS | 0 | 1.8(0.11) | 4.1(0.14) | 9.6(0.32) | 18.9(0.62) | 24.3(0.95) | |
| Firmness (g) | PCG-LFR-3 | 9527(34.3) | 9362(28.6) | 8455(30.1) | 6836(27.3) | 5094(22.1) | 3824(16.6) |
| PCG-LFR-4 | 9527(34.3) | 9350(31.4) | 8429(27.9) | 6795(28.8) | 4865 (21.9) | 3619(15.8) | |
| CS | 9527(34.3) | 9283(33.7) | 8032(29.8) | 6027(25.6) | 4058(18.6) | 2867(12.6) | |
| TSS (°Brix) | PCG-LFR-3 | 8.5(0.61) | 8.7 (0.52) | 9.1(0.61) | 9.4(0.54) | 10.5(0.65) | 11.4(0.87) |
| PCG-LFR-4 | 8.5(0.61) | 8.7(0.54) | 9.1(0.67) | 9.5(0.63) | 10.6(0.53) | 11.5(0.72) | |
| CS | 8.5(0.61) | 8.9(0.62) | 9.8(0.72) | 11.8(0.78) | 9.8(0.68) | 8.6(0.74) | |
| TA (% citric acid) | PCG-LFR-3 | 0.67(0.04) | 0.66(0.02) | 0.61(0.05) | 0.56(0.08) | 0.47(0.03) | 0.38(0.03) |
| PCG-LFR-4 | 0.67(0.04) | 0.66(0.04) | 0.60(0.03) | 0.55(0.04) | 0.44(0.07) | 0.35(0.02) | |
| CS | 0.67(0.04) | 0.64(0.07) | 0.56(0.04) | 0.47(0.05) | 0.36(0.07) | 0.31(0.04) | |
| Ascorbic acid (mg/100 ml of juice) | PCG-LFR-3 | 284(12.5) | 278(12.8) | 263(10.5) | 247(11.9) | 225(10.5) | 190(8.8) |
| PCG-LFR-4 | 284(12.5) | 277(12.2) | 261(13.1) | 245(12.3) | 220(9.7) | 183(9.7) | |
| CS | 284(12.5) | 266(13.5) | 240(12.7) | 212(10.8) | 164(11.5) | 139(9.4) | |
| Chlorophyll-content | PCG-LFR-3 | 19.1(0.25) | 17.3(0.21) | 15.5(0.22) | 14.5(0.19) | 13.6(0.17) | 13.2(0.17) |
| PCG-LFR-4 | 19.1(0.25) | 17.2(0.24) | 15.4(0.18) | 14.3(0.16) | 13.5(0.21) | 13.0(0.15) | |
| CS | 19.1(0.25) | 15.7(0.20) | 13.5(0.21) | 12.2(0.19) | 10.8(0.24) | 10.2(0.14) | |
| Solubilization (% of total pectin) | PCG-LFR-3 | 6.6(0.17) | 10.7(0.32) | 13.5(0.47) | 19.4(0.52) | 31.5(0.67) | 24.7(0.44) |
| PCG-LFR-4 | 6.6(0.17) | 10.9(0.29) | 13.6(0.47) | 20.7(0.48) | 32.1(0.52) | 25.7(0.47) | |
| CS | 6.6(0.17) | 12.6(0.34) | 17.2(0.53) | 29.2(0.69) | 31.8(0.54) | 23.3(0.34) | |
| L* | PCG-LFR-3 | 57.8(0.32) | 60.3(0.25) | 62.4(0.28) | 64.1(0.33) | 67.5(0.28) | 71.2(0.34) |
| PCG-LFR-4 | 57.8(0.32) | 60.2(0.31) | 62.5(0.23) | 64.4(0.39) | 67.9(0.35) | 71.8(0.34) | |
| CS | 57.8(0.32) | 60.9(0.38) | 64.2(0.31) | 68.7(0.42) | 74.4(0.32) | 78.9(0.37) | |
| a* | PCG-LFR-3 | −17.6(0.26) | −15.8(0.22) | −14.1(0.24) | −12.4(0.21) | −9.5(0.18) | −7.2(0.15) |
| PCG-LFR-4 | −17.6(0.26) | −15.8(0.21) | −13.9(0.25) | −12.3(0.20) | −9.3(0.15) | −6.8(0.11) | |
| CS | −17.6(0.26) | −15.3(0.27) | −12.6(0.21) | −10.2(0.26) | −5.5(0.14) | −2.2(0.12) | |
| b* | PCG-LFR-3 | 39.4(0.24) | 40.8(0.24) | 42.2(0.27) | 43.2(0.34) | 45.2(0.27) | 47.1(0.25) |
| PCG-LFR-4 | 39.4(0.24) | 40.9(0.26) | 42.3(0.22) | 43.6(0.36) | 45.6(0.31) | 47.5(0.29) | |
| CS | 39.4(0.24) | 41.4(0.31) | 43.3(0.27) | 46.3(0.40) | 49.8(0.35) | 51.5(0.32) | |
| Hue angle | PCG-LFR-3 | 114.1(3.2) | 111.1(3.4) | 108.5(3.2) | 106.1(2.8) | 101.9(2.7) | 98.7(2.2) |
| PCG-LFR-4 | 114.1(3.2) | 111.1(3.5) | 108.2(2.8) | 105.7(2.5) | 101.6(3.4) | 98.2(2.4) | |
| CS | 114.1(3.2) | 110.3(3.7) | 106.3(3.3) | 102.5(2.2) | 96.3(2.8) | 92.5(1.9) | |
| Chroma | PCG-LFR-3 | 43.1(0.4) | 43.8(0.4) | 44.4(0.3) | 44.9(0.5) | 46.2(0.3) | 47.6(0.5) |
| PCG-LFR-4 | 43.1(0.4) | 43.8(0.4) | 44.5(0.3) | 45.3(0.4) | 46.5(0.6) | 48.0(0.5) | |
| CS | 43.1(0.4) | 44.1(0.5) | 45.1(0.6) | 47.4(0.8) | 50.1(0.7) | 51.6(0.7) | |
| ΔE | PCG-LFR-3 | 3.3(0.04) | 1.7(0.03) | 1.2(0.02) | 1.8(0.01) | 4.8(0.06) | 5.6(0.05) |
| PCG-LFR-4 | 3.3(0.04) | 1.8(0.02) | 1.3(0.02) | 1.8(0.01) | 5.0(0.05) | 5.8(0.05) | |
| CS | 3.3(0.04) | 2.5(0.02) | 2.9(0.04) | 3.7(0.02) | 5.8(0.06) | 6.9(0.08) | |
(Values inside parentheses are the standard deviation values of three replicate experiments)
Fig. 4.
Variation in firmness, total soluble solids and solubilization of pectin of modified atmosphere packaged and control guava stored at different temperature (each observation is a mean ± SD of three replicate experiments)
The % reduction in volume (Vr) of guava for MA packaged as well as unpackaged guava at all the temperature increased with storage periods. Initially, Vr was slow and it increased rapidly later specially in the fruits stored under control. In MAP, the evaporation of water from the fruit surface is drastically reduced due to low WVTR of the packaging film creates high RH around the fruits. Hence, the %Vr was found to be much smaller in MA packaged guava than the unpackaged fruits (Table 5).
There was a decline in the firmness and puncture strength of guava stored in MA package as well as in control. Fruit firmness declined slowly at the initial storage periods however, at later part, the values decreased sharply, mainly in the control fruits (Fig. 4). MA packaging maintained the firmness of guava for a longer period; however, it could not provided a consistent maximum retention of firmness during storage for shelf life extension. Nevertheless, the fruit held in control softened to a greater extent during storage than fruit stored in MA package. The softening is accompanied by solubilization of pectin, involving the action of enzymes pectin esterase (PE), polygalacturonase (PG) and pectate lyases (PL) (White 2002). The increase in pectin solubilization and disruption of the xyloglucan–cellulose microfibril networks of guava fruit moderated by increases in the activities of exo-polygalacturonase (PG), pectin methylesterase, (1 → 4)-glucanase, and galactosidase has been proposed to be associated with the rapid softening of fruit (Ali et al. 2004). The loss of firmness of guava during storage is due to the degradation of the cell walls, which have as their basic composition 90 to 95 % of polymers and carbohydrates (cellulose, hemi-cellulose and pectins). Two main enzymes, polygalacturonase and pectinametylesterase, act during the evolution of ripeness, being responsible for the degradation of the cell walls. Therefore, the modified atmosphere in the film package promoted good results possibly by affecting the activity of these two enzymes (Jacomino et al. 2001a; Chitarra et al. 2002). According to Mohamed et al. (1994), the general decrease in firmness of guava during storage is primarily due to loss of water from the surface, promoting a loss of cell turgidity. The decline in the water potential adversely affects cell membranes and increases the activity of hydrolases, resulting in the solubilization of cell wall compounds (Chitarra et al. 2002). The loss of firmness in the control fruits was probably a consequence of the high mass loss during storage. The decline in firmness observed was about eight to ten fold from the hard mature green stage to the final soft ripe stage.
The TSS content of MA packaged guava was found to have increased slowly and gradually with storage period. However, TSS of fruits stored under air has increased at a higher rate, which declined towards later part of the storage (Fig. 4). The increase in TSS and sugar content during the earlier part of storage may be due to the hydrolysis of insoluble polysaccharides in to simple sugar. TSS which were fairly low at harvest, increased during storage due to hydrolysis of starch in to sugar and after the completion of hydrolysis of starch, further increase in TSS content did not occurred and hence a decline in this attributes are predictable. This trend is similar to the study reported by Rodriguez et al. (1971), Smith et al. (1988) and Wills et al. (1981). TA of guava was found to have decreased as time progressed and the rate of decline in MA packages was slower as compared to control samples even under low temperature. The titratable acidity of PCG-LFR-3 and control stored guava were found to be 0.56, 0.42; 0.53, 0.41; 0.52, 0.36 and 0.53, 0.34 % at 10 °C, 15 °C, 20 °C and 25 °C for 26, 19, 13 and 07 days of storage, respectively. The experimental variations in TA values corresponded closely with those of Sunjaka et al. (2003).
The ascorbic acid content in guava fruit at mature green stage was 284-mg/100 ml of juice and decline rapidly in control storage as the fruit ripens. The high oxygen atmosphere might be responsible for increased oxidation by ascorbate oxidase, causing acceleration of more ascorbic acid loss. The MA packaging influenced the ascorbic acid content positively and was found effective in preventing the losses. The beneficial effects of low O2 atmospheres in reducing ascorbic acid losses in fruit are well documented by Lee and Kader (2000); Singh and Pal (2008).
The extent of chlorophyll degradation was approximately 30 % less in MA packed guava as compared to control samples. The MA packaged fruits, presented higher chlorophyll levels than the control because the degradation of chloroplast was reduced. Chlorophyllase is considered as the first enzyme in the pathway of chlorophyll degradation. The modified atmosphere delayed the increases in the activities of cell wall hydrolytic enzymes and chlorophyllase activity (Ahmed and Labavitch 1980). At lower temperature, the fruit metabolism was slowed down, mainly due to the cold temperature of the environment.
During storage of guava the solubilization of total pectin increased in all storage system (Fig. 4). The rate of pectin solubilization was slower under modified atmosphere than in air. After 22 days of storage the solubilization of pectins was 4.5 times higher in the control than the PCG-LFR-3 package guava at 10 °C. Pectolytic enzyme activity, PME and PG, might have rose during storage, when the pectin began to solubilize and the fruits started loosing their firmness. The enzymic breakdown of cell wall polymers parallels the formation of water-soluble pectins and tissue softening (Ahmed and Labavitch 1980; Besford and Hobson 1972). The high solubilzation may be attributed to the PME activity, remaining high up to the end of the storage period. PME catalyses the hydrolysis of the methyl ester in the pectin chain and it precedes the degradation of pectin by polygalacturonase (Brett and Waldron 1990). In the MA packaged guava due to change in atmospheric composition, the activities of pectic enzymes was reduce in compare to control.
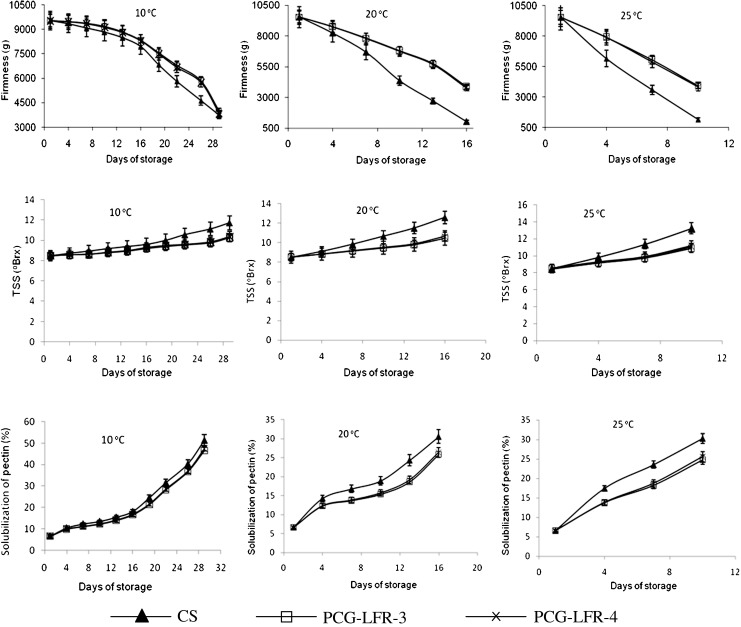
It was observed that the fruits stored under MA packaging and at lower temperature preserved better color than air-storage. Air-stored fruit had a drastic change in skin colour as it changed from light green to greenish-yellow. The L*, a* and b* values of MA packaged and unpackaged guava increased, decreased and increased, respectively during storage periods (Fig. 5). The negative a* value at harvest maturity indicated the green color of guava. When the storage period progressed the greenness decreased and yellowness increased (Table 5). The skin of the fruits kept its green color in the film packages till 26, 19, 13 and 7 days of storage at 10, 15, 20 and 25 °C, respectively and the color of guava were comparable with that of harvested one. This indicated the extension of shelf life of MA packaged guava by two times as compared to air-stored guava. Similar results was reported by Jacomino et al. (2001b); Chitarra et al. (2002); Singh and Pal (2008) during MA/CA storage of guava at different temperatures. At lower temperatures significant retardation of ripening was observed. The increase in storage temperature (particularly in control) accelerated the loss of green colour in guava. The fruit stored under MAP did not undergo any conspicuous change in the skin colour especially during cold storage. The inhibition of loss of green colour in guava held in low oxygen and elevated carbon dioxide can be primarily due to the effects of modified atmosphere on slowing down the metabolic activities leading to retarded ethylene sensitivity and biosynthesis, lower ripening changes and delayed senescence. (Beaudry 1993; Bron et al. 2005; Jacomino et al. 2001a).
Fig. 5.
Variation in color parameters of modified atmosphere packaged and control stored guava at various storage temperatures (each observation is a mean ± SD of three replicate experiments)
The derived color parameters, such as Hue, chroma and ΔE of guava stored under MAP was 5–8º higher, 7–10 % less brighter and 20–27 % less change in color as compared to control during different storage periods at the reference temperatures (Fig. 5). The decreased in hue angle of guava stored under air, reflecting the shift from green to yellow spectrum due to ripening. However, preservance of color pigment under modified atmosphere retarded the spectral changes during storage. The chroma value, which indicates the relative amount of color remains in the fruit, revealed that all samples lost colour during storage. The rate of colour lost under MAP was lower but similar with the air stored. There was increased in the chroma value during storage. It is reported that the increase in chroma value during storage indicates the shifting of intensity of color from dullness to brightness. It was observed that ΔE of guava increased sharply at the beginning and at later part of storage. However, extended storage revealed that in the long run, MAP preserved/presented the colour of the guava better than the control as evident from the lower L*, high hue angle, lower a* and lower ΔE values.
It was observed that guava is a climacteric fruits that ripens rapidly, highly perishable, and shelf-life period’s ranges from 2 to 3 days at room temperatures at 25 °C and 1 week at 20 °C (Bassetto et al. 2005; Jacomino et al. 2001b; Bron et al. 2005; Chitarra et al. 2002). Reyes and Paull (1995) observed that mature green guavas attained full yellow colour in 11 days of storage at 12 and 15 °C. Ripening delayed at 10 °C for matured green guava like at 8–10 °C guava may be stored for up to 2–3 weeks (Gaspar et al. 1997; Chitarra et al. 2002). Fruits stored at 31 °C and 41 °C presented incidence of anthracnosis, a disease caused by Colletotrichum gloeosporioides (Bron et al. 2005). At 5 °C the fruit does not ripen properly and develop a skin disorder after 2 week (Reyes and Paull 1995; Chitarra et al. 2002). The failure in ripening is a common symptom of chilling injury in tropical fruit (Couey 1982). Storage temperature below 10 °C causes serious chilling injury (CI) symptoms in guava fruits (Singh and Pal 2008). As a matter of fact, Osman and Ayub (1998) verified that guavas stored at 3–5 °C did not ripen satisfactorily, and that was related to chilling injury. The chilling injury includes abnormal ripening, skin browning or discoloration and increase in the incidence of decay (Wang 1982; Bron et al. 2005; Chitarra et al. 2002; Singh and Pal 2008; Jacomino et al. 2001a). According to Kader (1995), the ideal storage temperature for guava fruits can vary from 10 to 15 °C, depending on species and maturity stage. These discussions support the storage temperature range (10–25 °C) chosen in the present study.
These results of the present storage study of guava are in agreement with those found with several other researchers. Jacomino et al. (2001b) reported that LDPE film with mineral incorporation and multilayer co-extruded polyolephinic film with selective permeability (PSP) prolonged storage of guava (cv. Kumagai) up to 2–3 weeks, at 10 °C with 85–90 % relative humidity. MA packaging with PSP provided an atmosphere of 3–4 % O2 and 4–4.5 % CO2 inside the packages, which kept the fruit with good sensorial characteristics for 28 days. Gaspar et al. (1997) reveals that Kumagai guava wrapped either in PVC or LDPE hindered the development of peel colour and the loss of firmness. The skin color, firmness and weight of guava were best preserved/maintained by LDPE packaging along with sucrose ester and palm oil emulsion dips (Mohamed et al. 1994). Sunjka et al. (2003) reported that MA storage with silicon membrane at 11 °C had the best overall market quality after storage and after ripening. MA packaging of fresh guava in PET containers had a strong influence on color preservation and weight loss of the guavas (Pereira et al. 2004). Chitarra et al. (2002) revealed that the major beneficial effect of MA packaging of Kumagai guava (both tight and perforated) was the reduction of mass loss. The packaging of guava in LDPE bags contributed to a reduction in pectin solubilization, as compared to control fruits. A greater suppression of respiration and ethylene production was observed in guava stored in low O2 (5 %) atmospheres compared to normal storage (Singh and Pal 2008). CA storage retarded the changes in TSS, TA, ascorbic acid and was effective in reducing weight loss, and maintaining firmness of fruit.
ANOVA of quality parameters during storage
Three-factor Analysis of Variance (ANOVA) reveals that direct effect as well as two and three factor interaction effects of temperature, storage system and storage periods found to have significant effect on quality parameters of guava fruits at 1 % level of significance. Table 6 shows the ANOVA for quality attributes viz. PLW, firmness and a* value of guava during storage.
Table 6.
ANOVA for quality parameters of modified atmosphere packed and unpacked guava
| Sources of variation | DF | MSS | F-Value |
|---|---|---|---|
| PLW (%) | |||
| Model | 49 | 80.698274 | 1246.17** |
| Replication | 2 | 0.055526 | 0.86 |
| Days | 3 | 297.440572 | 4593.19** |
| Temp | 3 | 491.473661 | 7589.52** |
| Storage system | 2 | 115.722772 | 1787.03** |
| Days*Temp | 9 | 118.283799 | 1826.58** |
| Days*Storage system | 6 | 22.198552 | 342.80** |
| Temp*Storage system | 6 | 15.224022 | 235.09** |
| Days*Temp*Storage system | 18 | 3.712582 | 57.33** |
| Error | 94 | 0.064757 | --- |
| Firmness | |||
| Model | 49 | 11093775.8 | 1198.58** |
| Replication | 2 | 2455.2 | 0.27 |
| Days | 3 | 58245457.0 | 6292.89** |
| Temp | 3 | 69035076.8 | 7458.61** |
| Storage system | 2 | 10005921.0 | 1081.05** |
| Days*Temp | 9 | 12391178.7 | 1338.75** |
| Days*Storage system | 6 | 1605930.4 | 173.51** |
| Temp*Storage system | 6 | 2297301.8 | 248.20** |
| Days*Temp*Storage system | 18 | 377592.1 | 40.80** |
| Error | 94 | 9255.8 | --- |
| a* | |||
| Model | 49 | 57.653201 | 440.88** |
| Replication | 2 | 0.047713 | 0.36 |
| Days | 3 | 465.875982 | 3562.64** |
| Temp | 3 | 252.501158 | 1930.92** |
| Storage system | 2 | 49.903888 | 381.62** |
| Days*Temp | 9 | 52.397098 | 400.69** |
| Days*Storage system | 6 | 8.844920 | 67.64** |
| Temp*Storage system | 6 | 5.048696 | 38.61** |
| Days*Temp*Storage system | 18 | 0.835370 | 6.39** |
| Error | 94 | 0.130767 | --- |
** = Significant at 1 %
Response surface analysis of fruits
Second order response surface regression was fitted to the experimental data on different quality parameters of guava. The regression coefficients of all the quality parameters obtained from response surface regression analysis was presented in Tables 7, 8 and 9. It was found that the linear, quadratic and interaction regression coefficients of temperature and days of storage were significant at 1 % level of significance for all the variables except PLW and Reduction in Volume, for which significance was at 5 % level of significance. The following mathematical models were obtained for the quality parameter ‘firmness’ under different storage system of guava.
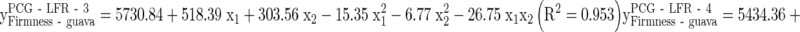 |
Table 7.
Regression parameters of response surface analysis for guava stored under PCG-LFR-3
| Variables | b0 | b1 | b2 | b11 | b22 | b12 | R2 |
|---|---|---|---|---|---|---|---|
| PLW | 17.4803 | −2.3891 | −0.5473 | 0.0711 | −0.0033 | 0.0570 | 0.7742 |
| Red.vol | 6.6774 | −0.9106 | −0.2041 | 0.0270 | −0.0013 | 0.0218 | 0.7866 |
| Firmness | 5730.84 | 518.3981 | 303.5595 | −15.3477 | −6.7736 | −26.7484 | 0.9537 |
| Puncture | 454.597 | 39.8106 | 19.1565 | −1.1222 | −0.2992 | −1.9743 | 0.9688 |
| TSS | 10.2700 | −0.2365 | −0.0862 | 0.0068 | 0.0013 | 0.0098 | 0.9426 |
| Acidity | 0.4495 | 0.0274 | 0.0182 | −0.0007 | −0.0002 | −0.0015 | 0.9559 |
| Ascacid | 223.0740 | 8.0199 | 2.8374 | −0.2283 | −0.0439 | −0.3046 | 0.9455 |
| Clrophyl | 17.1335 | 0.3422 | −0.2004 | −0.0123 | 0.0085 | −0.0182 | 0.9210 |
| Pectin | 21.7066 | −1.7877 | −0.7926 | 0.0533 | 0.0481 | 0.0579 | 0.9687 |
| L* | 68.2980 | −1.3376 | −0.3639 | 0.0386 | 0.0027 | 0.0558 | 0.9323 |
| a* | −8.6853 | −1.1582 | −0.2925 | 0.0337 | 0.0020 | 0.0451 | 0.9282 |
| b* | 46.0572 | −0.8621 | −0.2542 | 0.0252 | 0.0012 | 0.0365 | 0.9333 |
| Hue ang | 101.8534 | 1.6090 | 0.3417 | −0.0475 | −0.0011 | −0.0641 | 0.9343 |
| Chroma | 47.9447 | −0.5975 | −0.2693 | 0.0169 | 0.0026 | 0.0260 | 0.9214 |
| ΔE | 16.7909 | −1.9278 | −0.8663 | 0.0618 | 0.0176 | 0.0404 | 0.8241 |
Table 8.
Regression parameters of response surface analysis for guava stored under PCG-LFR-4 package
| Variables | b0 | b1 | b2 | b11 | b22 | b12 | R2 |
|---|---|---|---|---|---|---|---|
| PLW | 18.3088 | −2.5048 | −0.5650 | 0.0746 | −0.0034 | 0.0592 | 0.7776 |
| Red.vol | 7.0301 | −0.9595 | −0.2130 | 0.0285 | −0.0014 | 0.0231 | 0.7881 |
| Firmness | 5434.36 | 557.4429 | 316.3071 | −16.4184 | −6.7264 | −28.3076 | 0.9652 |
| Puncture | 451.9149 | 40.5424 | 18.5444 | −1.1480 | −0.2783 | −2.0241 | 0.9688 |
| TSS | 10.5323 | −0.2720 | −0.0900 | 0.0079 | 0.0013 | 0.0105 | 0.9406 |
| Acidity | 0.4400 | 0.0286 | 0.0198 | −0.0007 | −0.0002 | −0.0017 | 0.9683 |
| Ascacid | 218.2955 | 8.5211 | 3.5110 | −0.2401 | −0.0524 | −0.3587 | 0.9503 |
| Clrophyl | 16.4105 | 0.4333 | −0.1884 | −0.0149 | 0.0085 | −0.0197 | 0.9223 |
| Pectin | 22.7918 | −1.9101 | −0.8729 | 0.0565 | 0.0498 | 0.0632 | 0.9687 |
| L* | 68.7118 | −1.3888 | −0.3823 | 0.0400 | 0.0031 | 0.0574 | 0.9330 |
| a* | −8.2281 | −1.2166 | −0.2950 | 0.0353 | 0.0022 | 0.0460 | 0.9243 |
| b* | 46.0780 | −0.8710 | −0.2535 | 0.0254 | 0.0008 | 0.0384 | 0.9418 |
| Hue ang | 101.4578 | 1.6616 | 0.3339 | −0.0490 | −0.0010 | −0.0652 | 0.9335 |
| Chroma | 48.0446 | −0.6167 | −0.2778 | 0.0174 | 0.0023 | 0.0281 | 0.9360 |
| ΔE | 17.7947 | −2.0388 | −0.9178 | 0.0650 | 0.0184 | 0.0432 | 0.8178 |
Table 9.
Regression parameters of response surface analysis for guava stored under control condition
| Variables | b0 | b1 | b2 | b11 | b22 | b12 | R2 |
|---|---|---|---|---|---|---|---|
| PLW | 25.3737 | −3.3023 | −1.2712 | 0.0946 | 0.0161 | 0.1186 | 0.9312 |
| Red.vol | 14.7518 | −2.0292 | −0.4121 | 0.0606 | −0.0030 | 0.0489 | 0.7935 |
| Firmness | 3414.93 | 894.8920 | 321.6529 | −27.5348 | −3.4523 | −39.3256 | 0.9352 |
| Puncture | 424.5199 | 51.0764 | 15.2750 | −1.5781 | −0.0235 | −2.7368 | 0.9629 |
| TSS | 12.5362 | −0.5583 | −0.1216 | 0.0166 | 0.0009 | 0.0180 | 0.9042 |
| Acidity | 0.3406 | 0.0474 | 0.0160 | −0.0014 | −0.0001 | −0.0020 | 0.9415 |
| Ascacid | 164.9696 | 16.5453 | 4.1133 | −0.5003 | −0.0598 | −0.5048 | 0.9133 |
| Clrophyl | 15.2254 | 0.6243 | −0.4296 | −0.0220 | 0.0123 | −0.0185 | 0.8995 |
| Pectin | 24.7603 | −2.2232 | −0.8944 | 0.0679 | 0.0491 | 0.0792 | 0.9616 |
| L* | 72.3913 | −1.9446 | −0.4700 | 0.0577 | 0.0090 | 0.0785 | 0.9567 |
| a* | −5.4533 | −1.6240 | −0.3844 | 0.0496 | 0.0106 | 0.0534 | 0.9377 |
| b* | 47.2026 | −1.0647 | −0.3873 | 0.0324 | 0.0054 | 0.0534 | 0.9565 |
| Hue ang | 99.6394 | 2.0017 | 0.3158 | −0.0629 | −0.0090 | −0.0705 | 0.9405 |
| Chroma | 49.6845 | −0.8454 | −0.5008 | 0.0245 | 0.0079 | 0.0456 | 0.9548 |
| ΔE | 14.9432 | −1.8064 | −0.4929 | 0.0653 | 0.0136 | 0.0231 | 0.8542 |
The quality attributes of guava like PLW, reduction in volume, TSS, solubilization of pectin, color values i.e. l*, b*, chroma, and total color difference etc. were increased whereas firmness, puncture strength, TA, ascorbic acid, chlorophyll content, color values i.e. a* and hue angle etc. were found to be decreased during MAP as well as control storage. The rate of change of all the quality attributes was slower in MA packages as compared to control storage (Tables 7, 8 and 9). The linear regression coefficients of firmness indicate that the mean change in the firmness was minimum under PCG-LFR-3 (518.11, 303.55) followed by PCG-LFR-4 (557.44, 316.30) and maximum under control storage (894.89, 321.65) guava.
Conclusions
Most of the packages have established equilibrium at such levels of O2 and CO2, which were fairly close to the target levels. The modified atmosphere of 5 % O2 and 4 % CO2 was found to be suitable for preservation of guava (cv. Baruipur) till 26, 19, 13 and 7 days of storage at 10, 15, 20 and 25 °C, respectively. The packaging of guava with selective permeability was more efficient in maintaining the skin color or chlorophyll content, texture, smell, and overall market quality with least amount of defects after storage and ripening. The major beneficial effect was the reduction of weight loss, pectin solubilization, retention of color and firmness as compared to control fruits. Mathematical model equations were obtained with their multiple regression coefficients for the prediction of quality parameters of fruits under various MAP systems and control storage conditions. The comparison of experimental and predicted quality parameters yielded the mean relative deviation moduli (E) value of less than 5, which indicated that the developed regression models have good agreements for predicting the quality attributes of MA packaged and unpackaged fruits during storage.
References
- Adsule RN, Kadam SS. Guava. In: Salunkhe DD, Kadam SS, editors. Handbook of fruit science and technology. New York: Marcel Dekker; 1995. pp. 419–434. [Google Scholar]
- Ahmed AE, Labavitch JM. Cell wall metabolism in ripening fruits. II. Changes in carbohydrate-degrading enzymes in ripening “Bartlett” pears. Plant Physiol. 1980;65:1014–1016. doi: 10.1104/pp.65.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali ZM, Chin LH, Lazan H (2004) A comparative study on wall degrading enzymes, pectin modifications and softening during ripening of selected tropical fruits. Plant Sci 167:317–327
- Auda H, Blaser RE, Brown RH. Tillering and carbohydrate content of orchard grass as influenced by environmental factors. Crop Sci. 1966;6(2):139–142. doi: 10.2135/cropsci1966.0011183X000600020010x. [DOI] [Google Scholar]
- Bassetto E, Jacomino AP, Pinheiro AL, Kluge RA. Delay of ripening of ‘Pedro Sato’ guava with 1-methylcyclopropene. Postharvest Biol Technol. 2005;35:303–308. doi: 10.1016/j.postharvbio.2004.08.003. [DOI] [Google Scholar]
- Beaudry RM. Effect of carbon dioxide partial pressure on blueberry fruit respiration and respiratory quotient. Postharvest Biol Technol. 1993;3:249–258. doi: 10.1016/0925-5214(93)90060-G. [DOI] [Google Scholar]
- Besford RI, Hobson GE. Pectic enzymes associated with softening of tomato fruits. Adv Food Res. 1972;10:293–354. [Google Scholar]
- Brett C, Waldron K. Physiology and biochemistry of plant cell walls. In: Black M, Chapman J, editors. Topics in plant physiology. London: Unwin Hyman; 1990. [Google Scholar]
- Bron IU, Ribeiro RV, Cavalini FC, Jacomino AP, Trevisan MJ. Temperature - related changes in respiration and Q10 co-efficient of guava. Sci Agric. 2005;62(5):458–463. doi: 10.1590/S0103-90162005000500008. [DOI] [Google Scholar]
- Cameron AC, Boylan-Pett W, Lee J. Design of modified atmosphere packaging systems: modeling oxygen concentrations within sealed packages of tomato fruits. J Food Sci. 1989;54:1413–1421. doi: 10.1111/j.1365-2621.1989.tb05123.x. [DOI] [Google Scholar]
- Chau KV, Talasila PC. Design of modified atmosphere packages for fresh fruits and vegetables. In: Singh RP, Oliveira FAR, editors. Minimal processing of foods and process optimization. Boca Raton: CRC Press; 1994. pp. 407–416. [Google Scholar]
- Chitarra MIF, Chitarra AB, Carvalho HA. Use of LDPE packaging for guava fruit under cold storage: changes in some sensory characteristics and cell wall compounds. Braz J Food Technol. 2002;5:157–166. [Google Scholar]
- Combrink JC, De-Kock SL, Van-Ecden CJ. Effect of postharvest treatment and packaging on the keeping quality of fresh guava fruit. Acta Hort. 2004;275:539–645. [Google Scholar]
- Costa C, Lucera A, Conte A, Mastromatteo M, Speranza B, Antonacci A, Del Nobile MA. Effects of passive and active modified atmosphere packaging conditions on ready-to-eat table grape. J Food Eng. 2011;102:115–121. doi: 10.1016/j.jfoodeng.2010.08.001. [DOI] [Google Scholar]
- Couey HM. Chilling injury of crops of tropical and subtropical origin. Hort Sci. 1982;17:162–165. [Google Scholar]
- Das H. Food processing operations analysis. New Delhi: Asian Books Private Limited; 2005. [Google Scholar]
- Das MN, Giri NC. Design and analysis of experiments. New Delhi: Wiley Eastern Ltd.; 1986. [Google Scholar]
- Del Nobile ME, Licciardello F, Scrocco C, Muratore G, Zappa M. Design of plastic packages for minimally processed fruits. J Food Eng. 2007;79:217–224. doi: 10.1016/j.jfoodeng.2006.01.062. [DOI] [Google Scholar]
- Exama A, Arul J, Lencki RW, Lee LZ, Toupin C. Suitability of plastic films for modified atmosphere packaging of fruits and vegetables. J Food Sci. 1993;58:1365–1370. doi: 10.1111/j.1365-2621.1993.tb06184.x. [DOI] [Google Scholar]
- Fonseca SC, Oliveira FAR, Lino IBM, Brecht JK, Chau KV. Modeling O2 and CO2 exchange for development of perforation mediated modified atmosphere packaging. J Food Eng. 2000;43(1):9–15. doi: 10.1016/S0260-8774(99)00122-3. [DOI] [Google Scholar]
- Gaspar JW, Couto FAA, Salomao LC. Effect of low temperature and plastic films on postharvest life of guava (Psidium Guajava L.) Acta Hort. 1997;452:47–53. [Google Scholar]
- Geeson JD. Modified atmosphere packaging of fruits and vegetables. Acta Hort. 1989;258:143–150. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Buesa J, Ferrer-Mairal A, Oria R, Salvador M. A mathematical model for packaging with microperforated films of fresh-cut fruits and vegetables. J Food Eng. 2009;95:158–165. doi: 10.1016/j.jfoodeng.2009.04.025. [DOI] [Google Scholar]
- Goswami TK, Mangaraj S. Advances in polymeric materials for modified atmosphere packaging (MAP) In: Lagaron JM, editor. Multifunctional and nanoreinforced polymers for food packaging. Cambridge: Woodhead Publishing Limited; 2011. pp. 163–242. [Google Scholar]
- Iqbal T, Rodrigues FAS, Mahajan PV, Kerry JP. Mathematical modeling of the influence of temperature and gas composition on the respiration rate of shredded carrots. J Food Eng. 2009;91:325–332. doi: 10.1016/j.jfoodeng.2008.09.012. [DOI] [Google Scholar]
- Jacomin PA, Sarantopoulo CIGL, Sigrist JMM, Kluge RA, Minami K. Sensorial characteristics of “Kumagai” guavas submitted to passive modified atmosphere in plastic packages. J Plastic Film Sheet. 2001;17(1):6–21. [Google Scholar]
- Jacomino AP, Kluge RA, Sarantopoulos CIGL, Sigrist JMM. Evaluation of plastic packages for guava refrigerated preservation. Packag Technol Sci. 2001;14:11–19. doi: 10.1002/pts.522. [DOI] [Google Scholar]
- Jacxsens L, Devlieghere F, Debevere J (1999) Validation of a systematic approach to design Equilibrium Modified Atmosphere packages for freshcut produce. Lebensm-Wiss Technol 32:425–432
- Jacxsens L, Frank DE, Tom DR, Johan D. Designing equilibrium modified atmosphere packages for fresh-cut vegetables subjected to changes in temperature. Lebensm-Wiss u.-Technol. 2000;33:178–187. doi: 10.1006/fstl.2000.0639. [DOI] [Google Scholar]
- Kadam SS, Deshpande SS. Lychee. In: Salunkhe DD, Kadam SS, editors. Handbook of fruit science and technology. New York: Marcel Dekker; 1995. pp. 435–443. [Google Scholar]
- Kader AA. Biochemical and physiological basis for effects of controlled and modified atmospheres on fruits and vegetables. Food Technol. 1986;40(5):99–104. [Google Scholar]
- Kader AA. Regulation of fruits physiology by controlled and modified atmosphere. Acta Hort. 1995;398:59–70. [Google Scholar]
- Kader AA, Zagory D, Kerbel EL. Modified atmosphere packaging of fruits and vegetables. CRC Crit Rev Food Sci Nutr. 1989;28:1–30. doi: 10.1080/10408398909527490. [DOI] [PubMed] [Google Scholar]
- Karel M, Fennema OW, Lund DB. Protective packaging of foods. In: Fennema OW, editor. Principle of food science. USA: Marcel Dekker Inc; 1975. p. 474. [Google Scholar]
- Kays SJ. Postharvest physiology of perishable plant products. New York: Van Nostrand Reinhold Publication; 1991. Metabolic processes in harvested products respiration; p. 532. [Google Scholar]
- Khuri AI, Cornell JA. Response surfaces. New York: Marcel Dekker; 1987. [Google Scholar]
- Kumar BP, Honda MN. Fixation of maturity standars of guava (Psidium guajava L.) Indian J Hort. 1974;31:140–144. [Google Scholar]
- Lakakul R, Beaudry RM, Hernandez RJ. Modelling respiration of apple slices in modified atmosphere packages. J Food Sci. 1999;64(1):105–110. doi: 10.1111/j.1365-2621.1999.tb09870.x. [DOI] [Google Scholar]
- Lee SK, Kader AA. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol Technol. 2000;20:207–220. doi: 10.1016/S0925-5214(00)00133-2. [DOI] [Google Scholar]
- Lee DS, Hagger PE, Lee J, Yam KL. Model for fresh produce respiration in modified atmosphere based on principles of enzyme kinetics. J Food Sci. 1991;56:1580–1585. doi: 10.1111/j.1365-2621.1991.tb08645.x. [DOI] [Google Scholar]
- Lee DS, Song Y, Yam KL. Application of an enzyme kinetics based respiration model to permeable system experiment of fresh produce. J Food Eng. 1996;27:297–310. doi: 10.1016/0260-8774(95)00012-7. [DOI] [Google Scholar]
- Mahajan PV, Goswami TK. Enzyme kinetics based modeling of respiration rate for apple. J Agric Eng Res. 2001;79:399–406. doi: 10.1006/jaer.2001.0718. [DOI] [Google Scholar]
- Mahajan PV, Oliveira FAR, Montanez JC, Frias J. Development of user-friendly software for design of modified atmosphere packaging for fresh and fresh-cut produce. Innov Food Sci Emerg Technol. 2007;8:84–92. doi: 10.1016/j.ifset.2006.07.005. [DOI] [Google Scholar]
- Makino YK, Iwasaki TH. Application of transition state theory in model development for temperature dependence of fresh produce. J Agric Eng Res. 1997;67:43–52. doi: 10.1006/jaer.1997.0152. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK. Respiration rate modelling of royal delicious apple at different temperature. Fresh Produce. 2008;2(2):72–80. [Google Scholar]
- Mangaraj S, Goswami TK. Modified atmosphere packaging—An ideal food preservation technique. J Food Sci Technol. 2009;46(5):399–410. [Google Scholar]
- Mangaraj S, Goswami TK. Modified atmosphere packaging of fruits and vegetables for extending shelf-life-A review. Fresh Produce. 2009;3(1):1–31. [Google Scholar]
- Mangaraj S, Goswami TK. Determination of maturity indices of fruits based on physico-chemical properties. Indian Food Packers. 2009;63(1):67–79. [Google Scholar]
- Mangaraj S, Goswami TK. Measurement and modelling of respirtion rates of guava (cv. Baruipur) for modfied atmosphere packaging. Int J Food Prop. 2011;14(3):609–628. doi: 10.1080/10942910903312403. [DOI] [Google Scholar]
- Mangaraj S, Goswami TK. Modelling of respiration rates of litchi fruit under aerobic condition. Food Bioprocess Technol. 2011;4:272–281. doi: 10.1007/s11947-008-0145-z. [DOI] [Google Scholar]
- Mangaraj S, Singh KP. Optimisation of machine parameters for milling of pigeon pea using RSM. Food Bioprocess Technol. 2011;4:762–769. doi: 10.1007/s11947-009-0215-x. [DOI] [Google Scholar]
- Mangaraj S, Agrawal S, Gandhi AP. Studies on physico-chemical changes in selected fruits during storage. Beverage Food World. 2005;32(11):72–75. [Google Scholar]
- Mangaraj S, Singh R, Singh SP. Studies on measurement and analysis of colors of fruits during storage. Indian Food Packers. 2006;60(6):133–140. [Google Scholar]
- Mangaraj S, Goswami TK, Mahajan PV. Application of plastic films in modified atmosphere packaging of fruits and vegetables—A review. Food Eng Rev. 2009;1:133–158. doi: 10.1007/s12393-009-9007-3. [DOI] [Google Scholar]
- Mangaraj S, Sadawat IJ, Prasad S. Assessment of quality of pears stored under laminated modified atmosphere packages. Int J Food Prop. 2011;14:1–14. doi: 10.1080/10942910903580900. [DOI] [Google Scholar]
- Mannapperuma JD, Zagory D, Singh RP, Kader AA. Design of polymeric packages for modified atmosphere storage of fresh produce. In: Fellman JK, editor. Proceedings of the 5th international controlled atmosphere research conference. Washington: Wenatchee; 1989. pp. 225–233. [Google Scholar]
- Menon RR, Goswami TK. Modelling of respiration rates of green matures mango under aerobic conditions. Biosys Eng. 2008;99:239–248. doi: 10.1016/j.biosystemseng.2007.10.005. [DOI] [Google Scholar]
- Mohamed S, Mamakyi K, Yusof S. Effects of various surface treatments on the storage life of guava (Psidium Guajava L.) at 10 °C. J Sci Food Agric. 1994;66:9–11. doi: 10.1002/jsfa.2740660103. [DOI] [Google Scholar]
- Morton JF. Fruits of warm climates. Miami: Julia F. Morton; 1987. Guava; pp. 356–363. [Google Scholar]
- Mukherjee SK, Datta MN. Physico-chemical changes in Indian Guava during fruit development. Curr Sci. 1967;36:671–677. [Google Scholar]
- Osman A, Ayub MNA. Effects of different post harvest treatments on the respiration patterns of guava (Psidium guajava L.) Acta Hort. 1998;464:502. [Google Scholar]
- Palaniswamy KP, Shanmugavelu Physico-chemical characteristics of some guava varieties. South Indian Hort. 1974;22:5–10. [Google Scholar]
- Paul DR, Clarke R. Modeling of modified atmosphere packaging based on designs with a membrane and perforations. J Membr Sci. 2002;208:269–283. doi: 10.1016/S0376-7388(02)00303-4. [DOI] [Google Scholar]
- Paul RK, Singh SP, Singh CP, Asre R. Response of guava fruits (Psidium guava L. cv. Lucknow-49) to control atmosphere storage. Acta Hort. 2002;735:68–74. [Google Scholar]
- Peppelenbos HW, Leven J. Evaluation of four types of inhibition for modelling the influence of carbon dioxide on oxygen consumption fruits and vegetables. Postharvest Biol Technol. 1996;7:27–40. doi: 10.1016/0925-5214(96)80995-1. [DOI] [Google Scholar]
- Pereira LM, Rodrigues ACC, Sarantopoulos CIGL, Junqueira VCA, Cunha RL, Hubinger MD. Influence of modified atmosphere packaging and osmotic dehydration on the quality maintenance of minimally processed guavas. J Food Sci. 2004;69(4):1107–1111. [Google Scholar]
- Piskunov . Differential calculus. Moscow: Mir Publishers; 1981. [Google Scholar]
- Rai DR, Paul S. Transient state in-pack respiration rates of mushroom under modified atmosphere packaging based on enzyme kinetics. Biosys Eng. 2007;98:319–326. doi: 10.1016/j.biosystemseng.2007.07.012. [DOI] [Google Scholar]
- Ranganna S. Handbook of analysis and quality control for fruits and vegetable products. New Delhi: Tata McGraw Pub. Co; 1995. [Google Scholar]
- Renault P, Souty M, Chambroy Y. Gas exchange in modified atmosphere packaging. 1. A new theoretical approach for micro-perforated packs. Int J Food Sci Technol. 1994;29:365–378. doi: 10.1111/j.1365-2621.1994.tb02079.x. [DOI] [Google Scholar]
- Reyes MU, Paull RE. Effect of storage temperatures and ethylene treatment on guava (Psidium guajava L.) fruit ripening. Postharvest Biol Technol. 1995;6:357–365. doi: 10.1016/0925-5214(95)00007-S. [DOI] [Google Scholar]
- Rocculi P, Del Nobile MA, Romani S, Baiano A, Dalla Rosa M. Use of a simple mathematical model to evaluate dipping and MAP effects on aerobic respiration of minimally processed apples. J Food Eng. 2006;76:334–340. doi: 10.1016/j.jfoodeng.2005.05.034. [DOI] [Google Scholar]
- Rodriguez R, Agarwal PC, Saha NK. Biochemical changes during development of “Safeda” guava fruit. Indian Food Packer. 1971;25(1):5–20. [Google Scholar]
- Simpson BK, Egyankor KB, Martin AM. Extraction, purification and determination of pectin in tropical fruits. J Food Proc Preserv. 1984;8(2):63–72. doi: 10.1111/j.1745-4549.1984.tb00688.x. [DOI] [Google Scholar]
- Singh SP, Pal RK. Controlled atmosphere storage of guava (Psidium guajava L.) fruit. Postharvest Biol Technol. 2008;47:296–306. doi: 10.1016/j.postharvbio.2007.08.009. [DOI] [Google Scholar]
- Singhal V. Agriculture research data book, National Horticulture Board. New Delhi: Govt. of India; 2009. Indian horticulture database-2008. [Google Scholar]
- Sivakumar D, Korsten L. Influence of modified atmosphere packaging and post harvest treatments on quality retention of litchi cv. Mauritius. Postharvest Biol Technol. 2006;41:135–142. doi: 10.1016/j.postharvbio.2006.03.007. [DOI] [Google Scholar]
- Smith SM, Geeson JD, Genge PM. Effects of harvest date on the responses of discovery apples to modified atmosphere retail packaging. Int J Food Sci Technol. 1988;23:78–86. [Google Scholar]
- Sunjka PS, Nieuwenhof F, Raghavan GSV (2003) Extension of storage life of guava using silicon membrane system. Written for presentation at the CSAE/SCGR 2003 Meeting Montreal, Quebec
- Talasila PC, Chau KV, Brecht JK. Effects of gas concentrations and temperature on O2 consumption of strawberries. Trans ASAE. 1992;35:221–224. doi: 10.13031/2013.28591. [DOI] [Google Scholar]
- Teotia SS, Tripathy RS, Bhan S. Evaluation of indices for determination of maturity of Guava (Psidium guajava L.) Hort Adv. 1970;7:97–103. [Google Scholar]
- Torrieri E, Cavella S, Masi P. Modelling the respiration rate of fresh-cut Annurca apples to develop modified atmosphere packaging. Int J Food Sci Technol. 2007;46:1–10. [Google Scholar]
- Wang CY. Physiological and biochemical responses of plants to chilling stress. Hort Sci. 1982;17:173–186. [Google Scholar]
- White PJ. Recent advances in fruit development and ripening: an overview. J Expt Bot. 2002;53:1995–2000. doi: 10.1093/jxb/erf105. [DOI] [PubMed] [Google Scholar]
- Wills RHH, Lee TH, Graham D, Mcglasson WB, Hall FG. Postharvest: an introduction to the physiology and handling of fruit and vegetables. Westport: AVE Publ. Co.; 1981. [Google Scholar]
- Wilson WC. Guava. In: Nagy, Shaw, editors. Tropical and subtropical fruits. Westport: AVE Publ. Co; 1980. pp. 142–167. [Google Scholar]
- Yam KL, Lee DS. Design of modified atmosphere packaging for fresh produce. In: Rooney ML, editor. Active food packaging. New Zealand: Blackie Academic and Professional; 1995. pp. 55–105. [Google Scholar]