Abstract
Native microflora in raw milk cheeses, including the Mexican variety Queso Chihuahua, contribute to flavor development through degradation of milk proteins. The effects of proteolysis were studied in four different brands of Mexican Queso Chihuahua made from raw milk. All of the cheeses were analyzed for chemical and sensory characteristics. Sensory testing revealed that the fresh cheeses elicited flavors of young, basic cheeses, with slight bitter notes. Analysis by gel electrophoresis and reverse phase-high performance liquid chromatography (RP-HPLC) revealed that the Queseria Blumen (X) and Queseria Super Fino (Z) cheeses show little protein degradation over time while the Queseria America (W) and Queseria Lago Grande (Y) samples are degraded extensively when aged at 4 °C for 8 weeks. Analysis of the mixture of water-soluble cheese proteins by mass spectrometry revealed the presence of short, hydrophobic peptides in quantities correlating with bitterness. All cheese samples contained enterococcal strains known to produce enterocins. The W and Y cheese samples had the highest number of bacteria and exhibited greater protein degradation than that observed for the X and Z cheeses.
Keywords: Queso Chihuahua, Cheese microbiology, RP-HPLC, Protein profiling, Mass spectrometry
Introduction
Hispanic foods in the United States are gaining popularity as the number of immigrants originating from Mexico, Latin and South America are on the rise. This growing population has led to an increase in demand for authentic Hispanic foods. These foods include Mexican artisanal cheeses with unique taste and functional properties, not associated with pasteurized varieties available in the American market. In many instances, such raw milk cheeses may contain contaminant bacteria that are not permitted in foods in the U.S. Understanding the distinct characteristics of these cheeses, with specific attention to texture, flavors and the factors that influence cooking properties, will aid in addressing the needs of the Hispanic population in the U.S. Characterization of the peptides resulting from proteolysis and sensory analyses can help in selecting processes that may impart distinctive flavor and texture properties to these foods. These cheese-making processes in combination with pasteurization may lead to the development of Quesos Chihuahua suitable for sale in the U.S.
Queso Chihuahua is a semi-hard young cheese made from raw cows’ milk, consumed by the Mennonite population in the Mexican state of Chihuahua. It has a flavor similar to mild Cheddar cheese (Kosikowski and Mistry 1997). Queso Chihuahua is usually sent to the marketplace within 3 weeks of manufacture and is typically consumed within 30 days of production. This, however, violates the U.S. standard for selling raw milk cheeses, which requires a holding period of 60 days (Code of Federal Regulations 2004). Additionally, Mexican regulations (Dirección General de Normas 1994) require that cheeses sold within 100 days of manufacture (i.e., fresh cheeses) should be made from pasteurized milk only. Therefore, in order to prepare a Queso Chihuahua from pasteurized milk that conforms to both U.S. and Mexican regulations, it is necessary to identify factors within the cheese-making process and overall characteristics of the final product that give Queso Chihuahua its distinct properties.
A major factor determining the flavor and texture of a cheese product is in the bacterial cultures that are used in its production. Starter cultures are intentionally added to pasteurized milk in order to develop a specific type of cheese. In raw milk artisanal cheeses, the bacteria involved are indigenous to the milk used in cheese-making (Hayaloglu et al. 2005). Microbial fermentation in the cheese matrix influences texture as well as flavor during cheese ripening. Bacterial proteinase and peptidase enzymes degrade milk proteins, resulting in smaller peptides and free amino acids that significantly contribute to the flavor of the cheese (Urbach 1997). Such proteolysis causes softening of the cheese and changes the overall functional properties and sensory characteristics (Sousa et al. 2001). Substantial work has been directed on study of hard and semi-hard cheeses to understand the protein degradation, especially during cheese ripening (Lau et al. 1991; Fallico et al. 2006; Ferreira et al. 2006; De Simone et al. 2009). However, characterization of Hispanic-style cheeses, like Queso Chihuahua, has been minimally addressed. Therefore, monitoring proteolysis in Quesos Chihuahua during the ripening process may yield valuable information which would help in understanding the flavor and textural changes that may occur in cheese with time (Sousa et al. 2001).
Understanding how the native microflora of raw milk influences cheese will aid in developing Queso Chihuahua from pasteurized milk with desired characteristics. Generally, flavor components in cheese are small, hydrophilic molecules and can therefore be isolated through water extraction (Salles et al. 1995; Tunick 2007a and b). There is no single compound or class of compounds responsible for the full flavor of cheeses (Engels et al. 1997). Peptides and proteins found in the water-soluble portion of cheese vary tremendously in their overall hydrophobicity. Numerous studies on flavor peptides isolated from a variety of cheeses have shown that the hydrophobicity of peptides in cheese matrices can have a significant influence on overall flavor (Gomez et al. 1997).
The objective of this work is to characterize four varieties of Queso Chihuahua that are made from raw milk and understand the changes that occur in milk proteins during cheese ripening. It was attempted to identify if any correlation existed between proteolysis and the flavor of cheese.
Materials and methods
Materials
Two 1-kg packaged blocks of Queso Chihuahua were obtained within 24 h after manufacture from four different commercial cheese plants in the Chihuahua region of Mexico (Queseria America labeled W, Queseria Blumen labeled X, Queseria Lago Grande labeled Y, and Queseria Super Fino labeled Z). Samples were packed in a cooler with ice packs to keep cheeses cool (below 15 °C) during its overnight shipment to Wyndmoor, PA. The cheese samples were subsequently stored at 4 °C until assayed.
Methods
Composition and physical properties
Moisture content was determined on triplicate samples from each block of cheese using the forced-air oven method 948.12 (AOAC 2000). The fat content was determined on duplicate samples from each block of cheese using the modified Babcock procedure (Kosikowski and Mistry 1997). Total nitrogen levels were determined on duplicate samples from each block of cheese using a Nitrogen Analyzer (model FP-2000, LECO Corp., St. Joseph, MI) with the chamber set at 1050 °C. Total protein content was calculated by multiplying total nitrogen with 6.38 (AOAC 2000; method 920.123). Sodium chloride content was determined on duplicate samples from each block of cheese using Quantab Chloride Titrators (Hach Co., Loveland, CO) (AOAC 2000; method 971.19). Lactose was determined on triplicate samples of warm water filtrates from each cheese block using a lactose analyzer (Application Note # 320, model YSL 2700 Select, YSI US, Yellow Springs, OH). All the Mexican cheeses were assayed for alkaline phosphatase activity using the Charm Pas Lite test (Charm Sciences, Inc., Lawrence, MA) and confirmed that all four samples were raw milk cheeses.
Sensory evaluation of cheese
Flavor evaluation of each brand of cheese was conducted as suggested by Van Hekken et al. (2006). Portions from each block of cheese were sent to a certified microbiology laboratory prior to any sensory testing to ensure product safety. Cheeses were tested for the presence of Campylobacter, Escherichia coli O157:H7, Listeria monocytogenes, Salmonella, Staphylococcal enterotoxin, Yersinia using PCR- or fluorescence- based assays (Van Hekken et al. 2006). Within 14 days of manufacture, six trained descriptive analysis panelists (minimum 40 h of cheese training) used the universal Spectrum TM 15-point intensity scale to identify and score the cheese flavor. Interior portions of the cheeses were cubed, placed in a capped 2-oz souffle cup, and brought to room temperature for at least 1 h prior to evaluation. Each panelist evaluated all cheeses on two consecutive days.
Protein extraction
For each sample of Queso Chihuahua (W, X, Y, and Z), water-soluble proteins were extracted in the following manner: 5 mL of extraction buffer (0.166 M Tris, 1 mM ethylenediaminetetraacetic acid, pH 8.0) was added to 2 g of cheese. This mixture was homogenized by sonication for 6 min. To this mixture, 5 mL of 7 % sodium dodecyl sulfate was added and further sonicated for 3 min. While on ice, 2 mL of 10 mm dithiothreitol was added and the solution stirred for 15 min. The resulting solution was centrifuged at 30,000 g for 1 h at 4 °C. The supernatant was filtered and lyophilized to yield the water-soluble protein extract (Tunick et al. 1995).
Peptide separation
Peptide separation was carried out by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), high performance liquid chromatography (HPLC) and ultra performance liquid chromatography (UPLC). Water-soluble protein extracts of all four Quesos Chihuahua were dissolved in water to obtain 10 mg/mL concentration. Protein extract samples (900 μL) were injected and analyzed on an Agilent 1200 series HPLC instrument using a Vydac C18 reverse phase chromatography peptide column (5 μm, 4.6 mm × 250 mm). Peptides were eluted using a gradient of 5 to 100 % B, 95 % to 0 % A over 55 min (where A is 0.1 % trifluoroacetic acid (TFA) in water and B is 0.086 % TFA in acetonitrile). Fractions (1.5 mL) were collected between 15 and 30 min retention times, and analyzed further by mass spectrometry (MS).
The proteins were also separated by SDS-PAGE, using a 12 % bis-tris acrylamide NuPAGE® Novex gel (Invitrogen) and the XCell SureLock™ Mini-Cell vertical gel electrophoresis system (Invitrogen). The gels were stained using SimplyBlue™ SafeStain (Invitrogen) and destained in water. The relevant bands were excised using a razor blade, destained and digested with trypsin. These smaller protein fragments were subsequently analyzed by matrix assisted laser desorption ionization-tandem time of flight mass spectrometry (MALDI-TOF/TOF MS) to obtain sequence data and identify larger proteins of origin.
Additionally, protein mixtures (1 mL total volume) extracted from the cheeses (20 mg/mL) were filtered using 10 kDa molecular weight cutoff (MWCO) Amicon Ultra® centrifugal filtration devices (Millipore, Billerica, MA). The filtrate was then passed through a 3 kDa MWCO Amicon concentrator to isolate material with mass less than 3 kDa. The second filtrate was collected and concentrated further using a Savant SpeedVac® concentrator (ThermoFisher Scientific Inc., Waltham, MA). This concentrated peptide mixture was separated by reversed-phase UPLC (RP-UPLC) using a nano-Acquity UPLC (Waters Co., Milford, MA). Samples (3–4 μl) were injected onto a Symetry C18 trap column (5 μm, 180 μm × 20 mm, Waters) for salt removal, concentration, and subsequently separated on a Waters C18 RP nano-column (1.7 μm, 75 μm × 10 cm). Elution of the columns was achieved using a 40-min aqueous gradient of 5 % to 80 % acetonitrile (0.1 % TFA) at a flow rate of 400 nL/min. The chromatographic profile was obtained with a tunable UV (TUV) detector (Waters) equipped with a nano-flow cell, and set to monitor at 214 nm with a frequency of 20 Hz. After detection, the flow was directed to a spotting Probot robot (Dionex, Sunnyvale, Ca.), where fractions were collected every 30 s onto a 144-well plate with simultaneous mixing with 1 μL of matrix (5 mg/mL, α-cyano-4-hydroxycinamic acid in 40 % acetonitrile, 0.1 % TFA) for MS analysis.
Mass Spectrometric (MS) analysis
Peptides resulting from trypsin digestion of the gel bands and UPLC spotted plates were subjected to analysis using a MALDI-TOF/TOF with a 4700 Proteomics Analyzer mass spectrometer (Applied Biosystems, Framingham, MA) in the positive reflectron mode. Spectra were obtained by averaging 1000 acquired spectra in the MS mode in the mass range of 800 to 4000 Da, and 2500 spectra in the MS/MS mode. Post source decay with the instrument operating at 1 keV acceleration voltage was used for obtaining the MS/MS spectra of the selected 15 peptides with the highest signal-to-noise ratio. Conversion of time of flight to mass (Da) for the monoisotopic ions, [M + H]+, was based on calibration of the instrument with a peptide standard calibration kit (Applied Biosystems).
All MS/MS of peptides were searched against the Swiss-Prot database using Mascot (Matrix Science, Inc. Boston, MA) search engine through GPS Explorer Software (Applied Biosystems) with a 50 ppm for MS and 0.1 Da for MS/MS as error tolerance. Further search criteria included oxidation of methionine, formation of N-terminal pyro-glutamic acid as a variable modification, non-digestion enzyme for the UPLC fractions, and trypsin as the digestive enzyme for the gel analysis. In both analyses, the taxonomy was not specified. The signal to noise ratio for peak filtering was set at 20 for MS and 10 for MS/MS. Protein identification from gel bands was based on the combined MS and MS/MS spectra database search, while peptide identification leading to protein identification from the UPLC fractions were based on MS/MS spectra. Protein and peptides reported are with confidence of at least 95 %.
Results and discussion
Monitoring protein breakdown
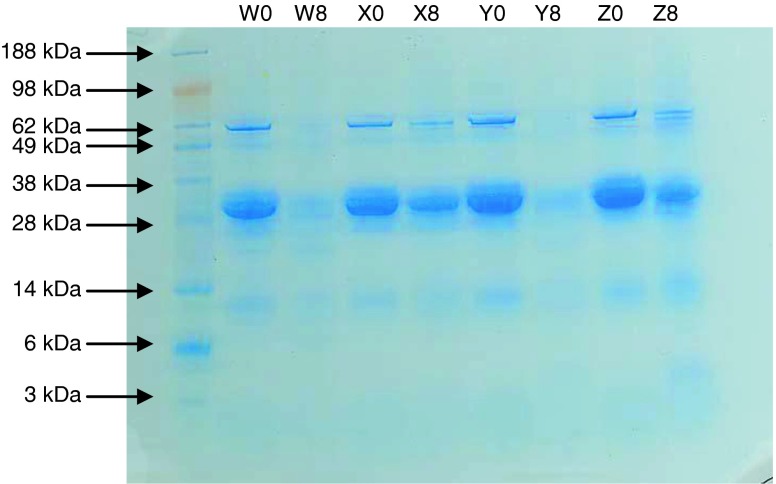
Water-soluble proteins extracted from all four cheese samples (W, X, Y, and Z) prior to aging (fresh cheeses) and at 8 weeks of aging were analyzed by SDS-PAGE whose results are shown in Fig. 1. Comparison of the fresh and 8 week samples within the gel for samples X and Z showed the presence of intact major protein bands in both sets of lanes, indicating limited protein degradation during aging of 8 weeks. However, cheese samples W and Y showed marked proteolysis with aging up to 8 weeks, as indicated by multiple casein subunits bands (thick bands between 25 and 40 kDa) that are clearly visible in the fresh samples and barely discernible after 8 weeks.
Fig. 1.
SDS-PAGE analysis of W, X, Y, and Z samples of unaged (fresh) and after 8 weeks of aging. Bands associated with caseins are between 25 and 40 kDa. Lower molecular weight bands correspond to degradation products. Samples labeled 0 (e.g., W0) are fresh cheeses and those labeled 8 (e.g., W8) are aged 8 weeks
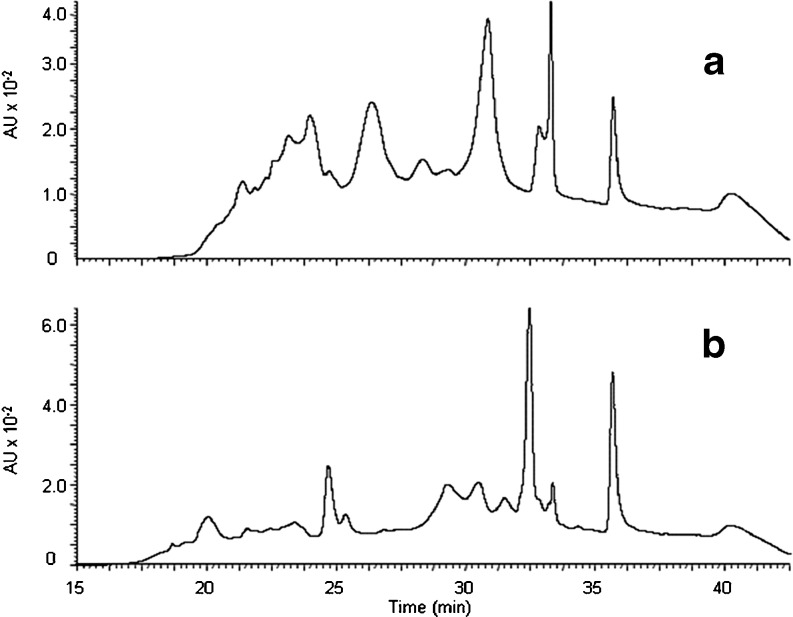
Further analysis by C18 RP-HPLC confirmed the results of SDS-PAGE, indicating limited proteolysis for samples X and Z (M. Paul, unpublished results), while significant protein degradation for samples W (Fig. 2) and Y (M. Paul, unpublished results). In the case of the X and Z samples, the majority of peaks eluted at higher retention times for fresh cheeses, corresponding to larger, intact proteins, with few peaks observed at shorter elution times suggesting few degradation products. Similar results were observed with the X and Z cheeses at 8 weeks, indicating minimal proteolysis. The W and Y elution patterns of fresh cheeses were similar to those of the X and Z cheeses, with a greater proportion (larger peak height) of intact or higher molecular weight proteins (Fig. 2a). The 8 week aged samples of W and Y cheeses showed a decrease of material with longer retention times, and a marked increase in compounds with shorter retention times, consistent with the formation of small peptides as a result of protein degradation (Fig. 2b). Concentrated samples of the W and X (fresh or 8 week aged) Queso Chihuahua samples were analyzed by MALDI-TOF/TOF-MS mass spectrometric methods to identify short peptides that possibly contributed to flavor and/or texture of product. The fresh W sample contained peptides corresponded to derivatives of bovine β-casein and αS1-casein (Table 1), while the X sample showed much larger proportion of peptides corresponding to κ-casein as well as fragments corresponding to αS1-casein breakdown (Table 2). The remaining band(s) at 25–30 kDa in the SDS-PAGE gel of the X sample at 8 weeks also supports this observation, corresponding to intact β-casein (molecular weight 25 kDa), and other degraded casein subunits. Overall bitterness can be correlated to hydrophobicity of peptides generated in cheese matrices and, specifically, the breakdown of αs1-casein and β-casein into small hydrophobic peptides has been shown to influence the bitterness in many cheeses (Gomez et al. 1997). Therefore, the protein fragments identified in the W sample (corresponding to αs1-casein and β-casein proteolysis) relative to the H71X sample (corresponding to κ-casein and αs1-casein proteolysis) are likely to play a role in the overall flavor profiles of these cheeses, particularly the fragments derived from κ- or β-casein.
Fig. 2.
RP-HPLC chromatograms of a unaged (fresh) and b at 8 weeks aged W Queso Chihuahua. AU stands for Absorbance Units
Table 1.
Mass spectrometric data for the fresh W sample showing masses and sequences of peptides and the whole protein from which they are derived
| Calc. mass (Da) | Obs. Mass (Da) | Peptide Sequence | Origin Protein |
|---|---|---|---|
| 878.46 | 878.47 | VPLGTQYT | αs1-casein |
| 885.44 | 885.44 | YLGYLEQ | αs1-casein |
| 905.48 | 905.52 | NENLLRF | αs1-casein |
| 965.57 | 965.58 | IPPLTQTPV a | β-casein |
| 969.53 | 969.52 | LNVPGEIVE | β-casein |
| 998.52 | 998.54 | YLGYLEQL | αs1-casein |
| 1001.53 | 1001.53 | YQEPVLGPV | β-casein |
| 1006.52 | 1006.52 | IGVNQELAY | αs1-casein |
| 1033.48 | 1033.49 | PVEPFTESQ | β-casein |
| 1079.61 | 1079.61 | NIPPLTQTPV | β-casein |
| 1120.52 | 1120.53 | PVEPFTESQS | β-casein |
| 1122.54 | 1122.57 | KEDVPSERY | αs1-casein |
| 1126.56 | 1126.57 | QKEPMIGVNQ | αs1-casein |
| 1142.53 | 1142.57 | SDIPNPIGSEN | αs1-casein |
| 1175.56 | 1175.57 | FSDIPNPIGSE | αs1-casein |
| 1196.55 | 1196.56 | YPVEPFTESQ | β-casein |
| 1233.60 | 1233.60 | PVEPFTESQSL | β-casein |
| 1252.59 | 1252.63 | QEQNQEQPIR | κ-casein |
| 1283.58 | 1283.59 | YPVEPFTESQS | β-casein |
| 1373.61 | 1373.65 | EQNQEQPIRCE | κ-casein |
| 1422.66 | 1422.71 | SENSEKTTMPLW | αs1-casein |
| 1438.65 | 1438.71 | SENSEKTTMPLW | αs1-casein |
| 1482.71 | 1482.71 | VPLGTQYTDAPSFS | αs1-casein |
| 1633.77 | 1633.81 | FSDIPNPIGSENSEK | αs1-casein |
| 1734.82 | 1734.84 | FSDIPNPIGSENSEKT | αs1-casein |
| 1916.89 | 1916.91 | SDIPNPIGSENSEKTTMP | αs1-casein |
aItalicized sequences correspond to known peptides contributing to bitter flavors while underlined sequences impart umami flavors
Table 2.
Mass spectrometric data for the fresh X sample showing masses and sequences of peptides and the whole protein from which they are derived
| Calc. mass (Da) | Obs. mass (Da) | Peptide Sequence | Origin Protein |
|---|---|---|---|
| 863.43 | 863.42 | APFPEVFG | αs1-casein |
| 905.48 | 905.49 | NENLLRF | αs1-casein |
| 1020.55 | 1020.55 | HQGLPQEVL | αs1-casein |
| 1099.61 | 1099.62 | EVLNENLLR | αs1-casein |
| 1130.56 | 1130.55 | QEQPIRCEK | κ-casein |
| 1200.62 | 1200.62 | IQKEDVPSER a | αs1-casein |
| 1252.59 | 1252.61 | QEQNQEQPIR | κ-casein |
| 1355.60 | 1355.61 | QEQNQEQPIRC | κ-casein |
| 1373.61 | 1373.62 | EQNQEQPIRCE | κ-casein |
| 1612.74 | 1612.74 | QEQNQEQPIRCEK | κ-casein |
| 2012.91 | 2012.91 | QEQNQEQPIRCEKDER | κ-casein |
aThe underlined sequence corresponds to known peptides imparting umami flavors
Quesos Chihuahua are typically consumed within 30 days. Monitoring the proteolysis up to 8 weeks of aging provides insight into the degradation process that may take place within the cheese matrix. Unraveling this may help in determining which components involved in flavor and texture generation of cheeses. Several peptides that influence flavor have been identified and reported (Ney 1971; Clegg et al. 1974). A number of short glutamate-rich peptides, with molecular weights (MW) less than 1000 Da, were found in Manchego cheese varieties to impart umami tastes (Gomez-Ruiz et al. 2007). A series of peptides that contribute to kokumi (heartiness or mouthfulness) flavors associated with aged steaks and cheeses (Toelstede et al. 2009) and peptides originating from αs1-casein and β-casein that are associated with bitter flavors (Toelstede and Hofmann 2008) have been isolated from Gouda cheeses.
Interestingly, some bitter peptide sequences observed in Gouda cheese have also been identified in the present study. Specifically, fragments containing IPPL within the sequences, identified in Gouda as slightly bitter tasting peptides, were observed in the fresh W cheese. A number of bitter flavor sequences were observed in the aged W samples (M. Paul, unpublished results). It is possible that such peptides were present in fresh cheese, but in quantities that are too small to be analyzed. One of the peptides in the W cheese containing the IPPL sequence has a mass of 965.6 Da. The spectral window for sample analysis in this study ranged from 800 to 4000 Da, and the ability to detect very short peptides like IPPL is limited. Peptide fragments smaller than 800 Da containing the IPPL or other similar sequences may be present that could not be detected. The presence of the IPPL sequence in the larger peptides identified suggests that further study of smaller MW fragments in these samples may reveal the presence of dipeptides, tripeptides or other short peptides that may also contribute to flavor.
Lactic acid bacteria (LAB) are used as starter and adjunct cultures in cheese making for controlled acidification and the development of organoleptic qualities through their proteolytic and/or lipolytic activities. A number of bacterial strains were identified from the Quesos Chihuahua used in this study; the W and Y cheeses possessing higher bacterial load than the X and Z samples (Renye et al. 2011). In the present study, there seems to be a correlation between numbers of bacterial isolates found in these raw milk cheeses with the degree of proteolysis observed. This is supported by the extent of protein degradation seen in the X and Z samples (Fig. 1) compared to those of the W and Y cheeses. The latter two cheeses (W and Y) were associated with higher bacterial counts.
Traditional cultures such as Lactococcus lactis ssp. lactis and Streptococcus thermophilus were isolated along with other bacterial species like Leuconostoc mesenteroides (Rondinini and Bortolussi 1994) and Streptococcus macedonius (Georgalaki et al. 2000), which can be used as adjunct cultures for flavor development. Enterococci, known to be highly proteolytic and lipolytic (Centeno et al. 1996) were observed in all four cheese samples. Enterococci can produce enterocins, antibacterial peptides (bacteriocins) that are active against LAB and/or food borne pathogens (Foulquie Moreno et al. 2003). Bacteriocin-producing LAB may influence the taste and texture of cheese and may accelerate the rate of ripening through the lysis of nearby bacterial cells, releasing intracellular proteases (Morgan et al. 1997; Oumer et al. 2001; Garde et al. 2006). Interestingly, the enterococcal strains detected in the cheeses studied in the present study were enterocin-producing (Renye et al. 2009). Although there is potential for using enterococci in food (Giraffa 1995), currently they are not candidates for use as cheese cultures because they are opportunistic pathogens and resistant to antibacterial treatment (Klein et al. 1998). Further studies on the LAB strains identified in Queso Chihuahua may divulge strains of enterococci that will potentially serve as food grade starter cultures in the production of pasteurized Queso Chihuahua.
Composition of Queso Chihuahua varieties
The composition and protein content of W, X, and Z Chihuahua cheeses were similar ranging from 40.39–40.75 % for moisture and 23.2 to 24.2 % for protein, while the Y cheese had higher moisture (43.02 %) as well as protein (30.2 %) content (Table 3). However, the fat content of the W, X and Z cheeses samples were higher (32.1–34.5 %) than the Y cheese (27.9 %). The lactose content was highest (0.945 g/L) for X cheese, medium for W and Z samples (0.456 and 0.402 g/L, respectively), and least (0.158 g/L) for the Y cheese. The salt content of the cheeses ranged from 0.65 to 1.79 % NaCl. None of these measurements is drastically different from those values previously published for the moisture, protein, salt and fat content of Queso Chihuahua varieties (Olson et al. 2011).
Table 3.
Composition measurements for W, X, Y, and Z samples of commercial raw milk Queso Chihuahua
| Sample | Moisture% | Protein% | Fat% | Lactose (%) | NaCl% |
|---|---|---|---|---|---|
| W | 40.75 | 24.17 | 32.0 | 0.456 | 1.16 |
| X | 40.39 | 23.45 | 32.1 | 0.945 | 1.79 |
| Y | 42.64 | 25.17 | 30.1 | 0.158 | 0.65 |
| Z | 40.42 | 23.16 | 34.0 | 0.402 | 0.80 |
Sensory analysis of Quesos Chihuahua
The flavor profile assessments were carried out for fresh cheeses only, corresponding with the time frame for normal consumption, and are presented in Table 4. With respect to individual flavors (Drake et al. 2001), overall the cheeses displayed consistent basic and young cheese flavors. The only aged flavor that appeared in all four cheeses was a free fatty acid taste. The panelists identified young flavors like cooked, whey, milk fat and diacetyl consistently in all four samples. The basic flavors, i.e., sour, bitter, salty and astringent, were also identified in all four cheeses. As expected, the X cheese, which displayed the highest salt content, was associated with maximum salty tast. These results confirmed that the cheese flavor profiles were consistent with previous published data on Queso Chihuahua (Van Hekken et al. 2006).
Table 4.
Intensity scores for flavors (maximum score 15) identified in Queso Chihuahua samples
| Flavor | W | X | Y | Z | Overall | |
|---|---|---|---|---|---|---|
| Cooked | 1.29 | 1.07 | 1.07 | 1.36 | 1.20 | |
| Whey | 1.79 | 1.29 | 1.29 | 1.43 | 1.45 | young |
| Milkfat | 1.07 | 1.50 | 1.07 | 1.43 | 1.27 | |
| Diacetyl | 1.93 | 1.43 | 1.29 | 1.07 | 1.43 | |
| Sour | 2.14 | 1.07 | 1.43 | 1.93 | 1.64 | |
| Bitter | 1.93 | 0.93 | 0.71 | 1.21 | 1.20 | basic |
| Salty | 1.86 | 2.07 | 1.43 | 1.64 | 1.75 | |
| Astringent | 1.00 | 0.43 | 0.21 | 0.36 | 0.50 | |
| Free fatty | 1.21 | 0.43 | 0.50 | 0.50 | 0.66 | aged |
Conclusion
This study has provided general characteristics of raw milk Quesos Chihuahua, as well as presented information pertaining to their overall flavor including the identification of milk protein degradation products that may potentially contribute. Future work with these cheeses will address the presence and identification of smaller molecular weight peptides (less than 800 Da) that may affect the cheese flavor. Production of Queso Chihuahua varieties using a single microbial culture will provide information as to how each strain affects proteolysis contributing to the desired taste and texture. These microbes will also be tested for the production of bacteriocins that may influence proteolytic patterns in cheeses, affecting its flavor. All of this information will aid in the development of a pasteurized milk cheese using food grade bacterial starter that mimics the taste and functionality of the Queso Chihuahua, permitting it to be sold and consumed in the U.S.
Acknowledgments
We would like to acknowledge Laurie Fortis, Susan Iandola, Brien Sullivan, Jim Shieh, and Danielle Tilman (USDA, ARS, ERRC) for their technical assistance.
Footnotes
Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
References
- AOAC (2000). Gaithersburg, MD: Association of Official Analytical Chemists International, page nos. 920.123, 948.12, 971.19
- Centeno JA, Menendez S, Rodriguez-Otero JL. Main microbial flora present as natural starters in Cebreiro raw cow’s-milk cheese (northwest Spain) Int J Food Microbiol. 1996;33:307–313. doi: 10.1016/0168-1605(96)01165-8. [DOI] [PubMed] [Google Scholar]
- Clegg KM, Lim CL, Manson W. The structure of a bitter peptide derived from casein by digestion with papain. J Dairy Res. 1974;41:283–287. doi: 10.1017/S0022029900019695. [DOI] [Google Scholar]
- Code of Federal Regulations (2004). Cheeses and related cheese products. United States Code of Federal Regulations. Cheeses and related cheese products, Vol. 2. Part 133
- De Simone C, Picariello G, Mamone G, Stiuso P, Dicitore A, Vanacore D, Chianese L, Addeo F, Ferranti P. Characterisation and cytomodulatory properties of peptides from Mozzarella di Bufala Campana cheese whey. J Pept Sci. 2009;15:251–258. doi: 10.1002/psc.1093. [DOI] [PubMed] [Google Scholar]
- Dirección General de Normas . Queso Madurados. Norma Official Mexicanna. 121-SSA1-1994. Mexico City: Secretaria de Salud; 1994. [Google Scholar]
- Drake MA, McIngvale SC, Gerard PD, Cadwallader KR, Civille GV. Development of a descriptive language for Cheddar cheese. J Food Sci. 2001;66:1422–1427. doi: 10.1111/j.1365-2621.2001.tb15225.x. [DOI] [Google Scholar]
- Engels WJM, Dekker R, de Jong C, Neeter R, Vissera S. A comparative study of volatile compounds in the water-soluble fraction of various types of ripened cheese. Int Dairy J. 1997;7:255–263. doi: 10.1016/S0958-6946(97)00003-4. [DOI] [Google Scholar]
- Fallico V, Tuminello L, Pediliggieri C, Horne J, Carpino S, Licitra G. Proteolysis and microstructure of Piacentinu Ennese cheese made using different farm technologies. J Dairy Sci. 2006;89:37–48. doi: 10.3168/jds.S0022-0302(06)72067-7. [DOI] [PubMed] [Google Scholar]
- Ferreira IM, Veiros C, Pinho O, Veloso AC, Peres AM, Mendonca A. Casein breakdown in terrincho ovine cheese: comparison with bovine cheese and with bovine/ovine cheeses. J Dairy Sci. 2006;89:2397–2407. doi: 10.3168/jds.S0022-0302(06)72312-8. [DOI] [PubMed] [Google Scholar]
- Foulquie Moreno MR, Callewaert R, Devreese B, Van Beeumen J, De Vuyst L. Isolation and biochemical characterisation of enterocins produced by enterococci from different sources. J Appl Microbiol. 2003;94:214–229. doi: 10.1046/j.1365-2672.2003.01823.x. [DOI] [PubMed] [Google Scholar]
- Garde S, Avila M, Gaya P, Medina M, Nunez M. Proteolysis of Hispanico cheese manufactured using lacticin 481-producing Lactococcus lactis ssp. lactis INIA 639. J Dairy Sci. 2006;89:840–849. doi: 10.3168/jds.S0022-0302(06)72147-6. [DOI] [PubMed] [Google Scholar]
- Georgalaki MD, Sarantinopoulos P, Ferreira ES, De Vuyst L, Kalantzopoulos G, Tsakalidou E. Biochemical properties of Streptococcus macedonicus strains isolated from Greek Kasseri cheese. J Appl Microbiol. 2000;88:817–825. doi: 10.1046/j.1365-2672.2000.01055.x. [DOI] [PubMed] [Google Scholar]
- Giraffa G. Enterococcal bacteriocins: their potential as antilisteria factors in dairy technology. Food Microbiol. 1995;12:291–299. doi: 10.1016/S0740-0020(95)80109-X. [DOI] [Google Scholar]
- Gomez MJ, Garde S, Gaya P, Medina M, Nunez M. Relationship between level of hydrophobic peptides and bitterness in cheese made from pasteurized and raw milk. J Dairy Res. 1997;64:289–297. doi: 10.1017/S0022029996002129. [DOI] [Google Scholar]
- Gomez-Ruiz JA, Taborda G, Amigo L, Ramos M, Molina E. Sensory and mass spectrometric analysis of the peptidic fraction lower than one thousand daltons in Manchego cheese. J Dairy Sci. 2007;90:4966–4973. doi: 10.3168/jds.2007-0350. [DOI] [PubMed] [Google Scholar]
- Hayaloglu AA, Guven M, Fox PF, McSweeney PL. Influence of starters on chemical, biochemical, and sensory changes in Turkish White-brined cheese during ripening. J Dairy Sci. 2005;88:3460–3474. doi: 10.3168/jds.S0022-0302(05)73030-7. [DOI] [PubMed] [Google Scholar]
- Klein G, Pack A, Reuter G. Antibiotic resistance patterns of enterococci and occurrence of vancomycin-resistant enterococci in raw minced beef and pork in Germany. Appl Environ Microbiol. 1998;64:1825–1830. doi: 10.1128/aem.64.5.1825-1830.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosikowski FV, Mistry VV (1997). Cheese and Fermented Milk Foods. Volume 1: Origins and Principles. Westport, CT: F. V. Kosikowski L.L.C
- Lau KY, Barbano DM, Rasmussen RR. Influence of pasteurization of milk on protein breakdown in Cheddar cheese during aging. J Dairy Sci. 1991;74:727–740. doi: 10.3168/jds.S0022-0302(91)78218-0. [DOI] [Google Scholar]
- Morgan S, Ross RP, Hill C. Increasing starter cell lysis in Cheddar cheese using a bacteriocin-producing adjunct. J Dairy Sci. 1997;80:1–10. doi: 10.3168/jds.S0022-0302(97)75906-X. [DOI] [Google Scholar]
- Ney K. Prediction of bitterness of peptides from their amino acid composition. Lebensm Forsch. 1971;147:64–68. doi: 10.1007/BF01879606. [DOI] [Google Scholar]
- Olson DW, Van Hekken DL, Tunick MH, Tomasula PM, Molina-Corral FJ, Gardea AA. Mexican Queso Chihuahua: functional properties of aging cheese. J Dairy Sci. 2011;94:4292–4299. doi: 10.3168/jds.2010-3884. [DOI] [PubMed] [Google Scholar]
- Oumer BA, Gaya P, Fernandez-Garcia E, Marciaca R, Garde S, Medina M, Nunez M. Proteolysis and formation of volatile compounds in cheese manufactured with a bacteriocin-producing adjunct culture. J Dairy Res. 2001;68:117–129. doi: 10.1017/S0022029900004568. [DOI] [PubMed] [Google Scholar]
- Renye JA, Jr, Somkuti GA, Paul M, Van Hekken DL. Characterization of antilisterial bacteriocins produced by Enterococcus faecium and Enterococcus durans isolates from Hispanic-style cheeses. J Ind Microbiol Biotechnol. 2009;36:261–268. doi: 10.1007/s10295-008-0494-7. [DOI] [PubMed] [Google Scholar]
- Renye JA, Somkuti GA, Van Hekken DL, Guerrero Prieto VM. Characterization of microflora in Mexican Chihuahua cheese. J Dairy Sci. 2011;94:3311–3315. doi: 10.3168/jds.2011-4177. [DOI] [PubMed] [Google Scholar]
- Rondinini G, Bortolussi G. Importance of Leuconostoc in Montasio cheesemaking and setting up of a quantification rapid method in milk. Ann Microbiol Enzymol. 1994;44:277–281. [Google Scholar]
- Salles C, Septier C, Roudot-Algaron F, Guillot A, Etievant PX. Sensory and chemical analysis of fractions obtained by gel permeation of water-soluble Comte cheese extracts. J Agric Food Chem. 1995;43:1659–1668. doi: 10.1021/jf00054a046. [DOI] [Google Scholar]
- Sousa MJ, Ardö Y, McSweeney PLH. Advances in the study of proteolysis during cheese ripening. Int Dairy J. 2001;11:327–345. doi: 10.1016/S0958-6946(01)00062-0. [DOI] [Google Scholar]
- Toelstede S, Hofmann T. Sensomics mapping and identification of the key bitter metabolites in Gouda cheese. J Agric Food Chem. 2008;56:2795–2804. doi: 10.1021/jf7036533. [DOI] [PubMed] [Google Scholar]
- Toelstede S, Dunkel A, Hofmann T. A series of kokumi peptides impart the long-lasting mouthfulness of matured Gouda cheese. J Agric Food Chem. 2009;57:1440–1448. doi: 10.1021/jf803376d. [DOI] [PubMed] [Google Scholar]
- Tunick MH. Hispanic Dairy Products. Hispanic Foods. Washington, D.C: American Chemical Society; 2007. p. 33. [Google Scholar]
- Tunick MH. Origins of Cheese Flavor. Flavor of Dairy Products. Washington, D.C: American Chemical Society; 2007. p. 155. [Google Scholar]
- Tunick MH, Malin EL, Smith PW, Holsinger VH. Effects of skim milk homogenization on proteolysis and rheology of Mozzerella cheese. Int Dairy J. 1995;5:483–491. doi: 10.1016/0958-6946(95)00026-Y. [DOI] [Google Scholar]
- Urbach G. The flavour of milk and dairy products. II. Cheese: Contribution of volatile compounds. Int J Dairy Technol. 1997;50:79–89. doi: 10.1111/j.1471-0307.1997.tb01743.x. [DOI] [Google Scholar]
- Van Hekken DL, Drake MA, Corral FJ, Prieto VM, Gardea AA. Mexican chihuahua cheese: sensory profiles of young cheese. J Dairy Sci. 2006;89:3729–3738. doi: 10.3168/jds.S0022-0302(06)72414-6. [DOI] [PubMed] [Google Scholar]