Abstract
Effects of controlled-temperature fermentation on several physico-chemical properties, lactic acid bacteria (LAB) counts and aroma of durian pulp were examined by storing fresh durian pulp was mixed with 2 % (w/w) salt and stored at 15 °C, 27 °C and 40 °C for 10 days. Storage at 15 °C did not affect the properties of the pulp much. However, at 27 °C and 40 °C, pH and total soluble solids decreased up to 60 % and 52 %, respectively, with greater losses at 27 °C. Titratable acidity, which increased at 27 °C, was due to lactic and acetic acids formation. Loss of sucrose and increases in glucose were greater at 27 °C. LAB population increased up to Day 3 of storage, and then decreased slightly. Principal component analysis based on aroma examination using a zNoseTM showed better retention of aroma profile at 27 °C. Overall, durian fermented at 27 °C was more acceptable than the one prepared at 40 °C, and it is ready to be consumed between Day 4 and 6.
Keywords: Physico-chemical properties, Durio zibethinus, Durian, Aroma profile, Fermentation temperature, Fermented food
Introduction
Natural fermentation has been applied for a long time as a mean of extending the shelf life of food. Basically, this preservation method is based on the interaction between naturally occurring microorganisms present in the food material and the food material itself (Steinkraus 2002). In most cases, fruit and vegetable fermentation including in the production of fermented cauliflower and cucumber results from the process of lactic acid bacterial fermentation (Paramithiotis et al. 2010; McFeeters and Perez-Diaz 2010). These bacteria utilize fermentable substrates in the food material to produce the major fermentable product of lactic acid and also other acids, leading to increase acidity (lower pH). The lactic acid bacterial fermentation process relies on the fermentative composition in the food. Sugars present serve as carbon sources for different microbes to produce a variety of products including acids and aromatic compounds (Kandler 1983). The key function of this sugar metabolism is to produce the energy required for microbial growth and for development of intracellular pH during fermentation (Hugenholtz and Kleerebezem 1999). Some organic acids are generally thought to exert their antimicrobial effect by interfering with the maintenance of cell membrane potential, inhibiting active transport, reducing pH and inhibiting a variety of metabolic functions (Doores 1993). The developed acidity, low pH and complete sugar removal are the determining factors for the success and safety of the fermentation product (Caplice and Fitzgerald 1999).
Durian (Durio zibethinus Murr.) is an exotic fruit grown in Southeast Asia. It belongs to the Bombacacaea family and is widely cultivated in the tropical countries such as Malaysia, Thailand, Brunei and Indonesia (Subhadrabandhu and Ketsa 2001). During fruiting season, the abundant supply of durian in the market has caused the usually high price of durian to drop quite significantly. At present, there have been some efforts at commercializing durian pulp in producing countries, such as a dried powder form (Chin et al. 2008) durian leather (Jaswir et al. 2008) and durian chip (Jamradloedluk et al. 2007). Apart from the alternative methods, fermentation has been applied due to the simple and low energy preservation process. The fermentation process of durian’s pulp has produced a product called acid fermented durian or tempoyak as is commonly known among the locals. It is prepared by placing a mixture of durian pulp with salt (1.3–3.0 % w/v) into a covered jar for several days to let fermentation to take place (Amin et al. 2004; Merican 1977). Fermented durian has characteristics of strong aromatic odor of fresh durian but at the same time it has a sour smell and taste, yellow in color, and soft in texture (Merican 1977). Usually, freshly fermented durian pulp is eaten with rice as a condiment or used as a flavoring ingredient in cooking dishes.
The production of fermented durian pulp is characterized by the dominance of lactic acid bacterial population. Leisner et al. (2001) reported that 64 LAB isolates could be found fermented durian pulp including homo and heterofermentative bacteria. Majority of these isolates were homofermentative bacteria and were identified as Lactobacillus plantarum, Lb. pentosus, Lb. paraplantarum and Lb. mali . Leisner et al. (2002) reported a new species of lactobacillus known as Lb. durionis. The heterofermentative bacteria consisted of Lb. brevis, Lb. fermentum and Leuconostoc mesenteroides. Yuliana and Dizon (2011) identified the presence of Weissella paramesenteroides and Pediococcus acidilactiti in fermented durian.
One of the several factors that influence the activity of microorganisms in foods is temperature, as it is able to influence fermentative composition (Battcock and Ali 1998). This is because temperature is able to control the number of microbial population, select microbial group dominance, select the prevailing microbial and also control the development of end product (Tassou et al. 2002). LAB can grow between 10 to 50 °C (Carr et al. 2002). Several studies have demonstrated the suitability of different incubation temperature in vegetable fermentation to produce desired organoleptic properties. A previous study on the ability of LAB to ferment cauliflower at temperatures more than 40 °C prior to storage at 7 °C was better than using preservatives such as benzoic and sorbic acid showed that organisms with high fermenting capacity were able to inhibit the spoilage and pathogenic microorganisms (Bonestroo et al. 1992). Another study (Holzapfel et al. 2008), noted that the range of temperature for sauerkraut fermentation is between 15 and 20 °C.
Previous studies on fermented durian pulp have been heavily focused on microbiological aspects (Amiza et al. 2006; Amin et al. 2004; Leisner et al. 2002, 2001) and flavor constituents (Neti et al. 2011; Yuliana and Garcia 2009), however, its physico-chemical properties during fermentation have not been investigated widely. A previous study reported that microorganisms in durian pulp were susceptible to heat treatment (Amiza et al. 2006). They observed no microbial growth including LAB, yeast and mould on durian pulp pasteurized at temperature more than 50 °C. Lee et al. (2012) recently reported that fermentation with mono- and mixed-cultures of Saccharomyces cerevisiae and Williopsis saturnus was capable of modulating the volatile compounds of durian pulp in the production of an alcoholic beverage, thus affecting its sensory properties. Since the LAB at different temperature may produce different organoleptic properties, thus, this study was aimed at examining the accompanying changes in some physico-chemical properties, lactic acid bacterial count and aroma of durian during controlled-temperature fermentation.
Materials and methods
Fruit material
Durian fruits were purchased from a local market in Sri Serdang, Selangor, Malaysia, during the fruiting seasons. These fruits were classified as ‘durian kampung’, with no known or specific variety. Only fruits that were intact showing no signs of dehiscence were chosen and each weighed between 3–4 kg.
Sample preparation
The fruits were dehisced using a pair of knife by cutting along the suture in the skin. Normally, each fruit has 4–5 locules with 3–4 fruit bulbs inside each locule. Each bulb comprised a soft custard-like pulp and a seed. The seed was separated from the pulp and a total of 1 kg of unseeded durian pulp was mixed thoroughly with 2 % (w/w) salt (Amin et al. 2004). A total of 21 glass bottles (5.5 cm in diameter × 8.0 cm in height) were filled with 45 g of durian pulp mixture and capped to exclude air. All samples were stored separately at three different incubation temperatures, 15 ± 1 °C, 27 ± 1 °C and 40 ± 1 °C, for up to 10 days. At Day 1, 2, 3, 4, 6 and 10 of fermentation, samples were withdrawn for the analysis of the physico-chemical changes including pH, titratable acidity, total soluble solid, organic acid content, sugar content, LAB count and aroma profile. Salted durian pulp on the first day of preparation (Day 0) acted as the control. Samples that remained in bottles that have been used for analysis were discarded. Other properties that were observed without experimental devices were the colour and odor. Three batches of durian pulp were fermented in this study and all determinations were done in triplicate.
Determination of pH, titratable acidity and total soluble solid
pH and titratable acidity were determined according to previous methods (Ranganna 1979). Ten grams of sample were mixed thoroughly with 50 mL of freshly distilled water and then centrifuged at 2,000 rpm at room temperature. After 10 min, the sample was filtered through a Whatman No. 1 filter paper using an aspirator pump and the residue was washed with distilled water. The filtrate was transferred into a 100 mL volumetric flask and topped up with distilled water. Fifty mL of the sample solution was used to measure the pH value using a pH meter (Delta 320, Mettler Toledo) which has been calibrated against standard buffer solutions at pH 4.0 and 7.0. Titratable acidity was determined by titrating 50 mL of the sample solution against 0.1 N NaOH up to pH 8.1 as the end point of phenolphthalein. Finally, a drop of sample was pipetted on the glass surface of a hand refractometer, (Brix 0~32 %, Atago, Japan) to measure the total soluble solid. The reading was multiplied by ten as the dilution factor.
Determination of organic acid content
The organic acid composition and contents of durian pulp samples before and after fermentation were analyzed using HPLC based on a previous method (Sturm et al. 2003). The HPLC (Waters Corporation, Milford, Massachusetts) used consisted of an autosampler system (Waters 2695 Separation) integrated with a Waters Empower 2002 software and a wavelength detector (Waters 2487 Dual λ Absorbance) set at 210 nm. The separation column was a Purospher® Star RP-18 end capped column (250 cm × 4.6 μm, particle size of 5 μm, Merck, Darmstard, Germany) equipped with RP-18 guard column (Merck, Darmstard, Germany). The separation column was placed in an oven maintained at 35 °C. Organic acids were isocratically separated at a flow rate 0.8 mL/min using 0.004 N H2SO4 as the mobile phase.
Samples for analysis were prepared by mixing 10 grams of durian pulp either before or following fermentation were thoroughly mixed with 100 mL of double-distilled deionized water and centrifuged at 4 °C, 8,000 rpm (Beckman, J2-21 M/E Centrifuge, Fullerton, CA, USA) for 20 min (Sturm et al. 2003). The supernatant was filtered through a 0.45 μm Minisart NY membrane filter (Sortorius Stedim Biotech GmbH, Germany). Ten microliters of the filtered sample solution were injected into the separation column for analysis. Chromatographic peaks were identified by comparing the retention times of samples with those of standard organic acid mixtures while the quantification was carried out using external standards. Malic, lactic, acetic, citric and succinic acids (Fisher Scientific, UK Ltd) were used as the standards.
Determination of sugar composition and content
The sugar composition and contents were analyzed using an HPLC (Shimadzu, Shimadzu Corporation, Tokyo, Japan) equipped with an autoinjector (Shimadzu SIL-10ADVP), a refractive index detector (Shimadzu RID-10A) and a system controller (Shimadzu SLC-10A). The analysis was carried out based on a previous method (Hunt et al. 1977) with some modification. Sugar in the samples were isocratically separated at a flow rate of 1.5 mL/min using a LiChroCART®-NH2 (250 mm × 4.6 mm, particle size of 5 μm, Merck, Darmstard, Germany) separation column attached to a LiChroCART-NH2 guard column set at 40 °C. The mobile phase was composed of HPLC-grade acetonitrile and double distilled deionized water (80:20 v/v).
Extraction of sugar from samples was carried out according to Wills et al. (1980). Ten grams of sample were mixed with 100 mL of 85 % methanol and heated in a water bath at 80 °C. After 30 min, the mixture was filtered through a Whatman No.1 filter paper using an aspirator pump. The residue was re-extracted using 75 mL of 85 % methanol under the same conditions as described above. Pooled filtrates were concentrated under vacuum at 50 °C using a rotary evaporator and topped up to 10 mL with double distilled deionized water. Before injection, the sample solution was filtered through a Sep-Pak® Classic C-18 cartridge (Waters Associates, Milford, MA, USA) and a 0.45 μm Minisart NY membrane filter (Sortorius Stedim Biotech GmbH, Germany). Ten μL of the filtered sample solution was injected into the separation column and sugars were detected using the refractive index detector. Peaks data were collected with the LC Solution Software (Shimadzu Corp.) and quantification was carried out using external standards comprising fructose, glucose, sucrose, maltose and lactose (Fisher Scientific, UK Ltd). The calibration/standard curves for each sugar were prepared using a series of standard solutions with concentration from 1 to 3 g/100 ml.
Determination of lactic acid bacterial count
LAB counts were determined using the standard microbial method (Kang et al. 2003). All apparatus, 0.1 % (w/v) peptone solution (Difco, Becton Dickson and Company, USA) and De Man Rogosa Sharpe (MRS) (Difco, Becton Dickson and Company, USA) agar were sterilized by autoclaving at 121 °C for 15 min. One gram of samples was homogenized in 9.0 mL of sterile 0.1 % (w/v) peptone water and 1.0 mL of dilution was aseptically transferred into another 9.0 mL of sterile 0.1 % (w/v) peptone water up to 10-7 dilution. Then, 0.1 mL aliquots from appropriate dilutions were spread on the MRS agar before incubation in an oven set at 30 °C. The viable cell counts were determined and expressed as log colony forming units (cfu) after 48 hours.
Measurement of aroma profile
The qualitative measurement of aroma profile was performed using a zNoseTM, Model 7100 (Electronic Sensor Technology (EST) Company, Newbury Park, CA, USA), an ultrafast gas chromatograph (GC) vapor analyzer system. The zNoseTM system was equipped with a surface acoustic wave sensor (SAW) detector and a specific column (Model DB-5, EST Company, Newbury Park, CA, USA). zNoseTM operates in two steps including sampling and analysis process (Staples 2003). During the sampling process, the vapors are absorbed onto the capillary loop trap. In the second step, the loop trap is heated and released the trapped vapor to the GC capillary column and then to the uncoated SAW sensors for analysis. Analysis was done based on the method described previously (Lammertyn et al. 2004) with slight modification. For the measurement, 10 grams of sample were placed in a screw-thread poly cap Kimble vial (28 mm diameter × 98 mm length, 40 mL, Kimble, Gerresheimer Inc., Vineland, USA). Then, the vial was heated in a water bath at 60 °C for 5 min to allow the generation and equilibration of aroma compounds just prior to analysis. All samples were analyzed via the following set-up temperatures: sensor temperature 30 °C; column temperature 40 °C; valve temperature 165 °C; inlet temperature 200 °C, and trap temperature 250 °C. The injection pump was set to 5 s and the flow rate of helium as the carrier gas was 3.0 cm3/cm. Before and in between each sample measurement, 100 % methanol (HPLC grade, Merck) as a blank was run until baseline peaks were all under 200 counts. The sampling time was carried out within 20 s. All data was analyzed using Microsense software (version 4.89, EST).
Statistical analysis
Data were expressed as mean ± standard deviation from triplicate measurements. One way analysis of variance (ANOVA) from MINITAB software version 14 (State College, PA, USA) was applied to exhibit the significant difference (p<0.05) with Tukey’s multiple comparison method for data set. For the aromatic analysis, the data that obtained from the software (Microsense 4.89, EST, USA) were processed with Principal Component Analysis (PCA) using UNSCRAMBLER software version 10 (CAMO AS, Trondheim, Norway).
Results and discussion
Many changes take place when suitable foods are fermented. In the case of durian pulp, fermentation led to changes in the physico-chemical properties, LAB counts and aroma when durian pulp was incubated at different temperatures. The product, fermented durian or tempoyak, is used as a condiment or an ingredient in many local dishes in Malaysia and elsewhere where durian is grown and consumed.
Table 1 shows the pH of durian pulp before and during fermentation at three different incubation temperatures. The pH at Day 0 (control) of fermentation was pH 6.92, and was slightly higher compared to previous findings which reported that the pH of fresh durian ranged pH between 6.63–6.83 (Leisner et al. 2001) and 6.70 (Amiza et al. 2006). There was no significant difference (p>0.05) in the pH (pH 6.92–7.19) when incubation was done at 15 °C temperature for 10 days, and the aroma profile of fresh durian pulp was still retained. However, the pH at 27 and 40 °C decreased rapidly during the first 2 days and thereafter declined gradually until pH 4.13 and 4.30, respectively, on Day 10, with no significant difference (p>0.05) between these values. In comparison, the pH values of fermented durian obtained from the local market in Malaysia were between pH 4.0 and 4.2 (Leisner et al. 2001), indicating a long fermentation time. The decrease in pH caused the pulp to acquire a sour taste. Amiza et al. (2006) reported that durian pulp which had been subjected to heat treatment at 50 °C before fermentation had a final pH of 5.14 and 0.758 % acidity on Day 6 without the noticeable sour taste. Besides that, they also reported that microbial growth including LAB, yeasts and moulds also decreased with 30 min of heating. This indicates that at temperatures higher than 40 °C, microbial activity leading to acid production was reduced significantly.
Table 1.
Changes in pH, titratable acidity and total soluble solid of durian pulp during fermentation at different temperature
| Day | pH | Titratable acidity (% lactic acid) | Total soluble solid (°Brix) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 15 ± 1 °C | 27 ± 1 °C | 40 ± 1 °C | 15 ± 1 °C | 27 ± 1 °C | 40 ± 1 ° C | 15 ± 1 °C | 27 ± 1 °C | 40 ± 1 °C | |
| 0 | 6.92 ± 0.03a | 6.92 ± 0.03a | 6.92 ± 0.03a | 0.07 ± 0.01a | 0.07 ± 0.01e | 0.07 ± 0.01c | 25.0 ± 0.00a | 25.0 ± 0.00a | 25.0 ± 0.00a |
| 1 | 6.93 ± 0.25a | 5.35 ± 0.36b | 5.09 ± 0.26b | 0.09 ± 0.02a | 0.30 ± 0.05d | 0.44 ± 0.06b | 24.0 ± 0.00a | 21.0 ± 1.15b | 24.7 ± 1.15a |
| 2 | 7.10 ± 0.18a | 4.77 ± 0.22c | 5.93 ± 0.23bc | 0.08 ± 0.02a | 0.46 ± 0.03d | 0.38 ± 0.05b | 20.0 ± 0.00b | 16.7 ± 1.15c | 19.3 ± 1.15bc |
| 3 | 7.07 ± 0.23a | 4.64 ± 0.11c | 4.96 ± 0.13bc | 0.07± 0.01a | 0.55± 0.07c | 0.40± 0.04b | 20.7 ± 1.15b | 22.0 ± 2.00ab | 20.7 ± 1.15b |
| 4 | 7.10 ± 0.07a | 4.50 ± 0.01cd | 4.86 ± 0.10bc | 0.05 ± 0.01a | 0.66 ± 0.05b | 0.47 ± 0.05b | 19.0 ± 0.00b | 23.3 ± 1.15ab | 18.0 ± 0.00cd |
| 6 | 6.93 ± 0.18a | 4.24 ± 0.13de | 4.51 ± 0.13cd | 0.07 ± 0.00a | 1.15 ± 0.04a | 0.79 ± 0.03a | 20.0 ± 0.00b | 20.7 ± 1.15b | 17.3 ± 1.15cd |
| 10 | 7.19 ± 0.15a | 4.13 ± 0.23d | 4.30 ± 0.1bd | 0.05 ± 0.02a | 1.10 ± 0.02a | 0.86 ± 0.06a | 19.3 ± 1.15b | 13.4 ± 1.15c | 15.3 ± 1.15d |
Each observation is a mean ± standard deviation of three replications. Means within each column of each individual temperature with different superscripts are significantly different (p<0.05)
The initial titratable acidity (TA) of durian pulp, expressed as percent lactic acid, was 0.07 % (Table 1), and was high compared to the previous study (0.03 %) reported by Leisner et al. (2001) but was low (0.71–0.89 %) to that reported by Amin et al. (2004). Differences in the findings may be due to different variety of fruit being used. In this study, changes in TA were found to coincide with changes in pH. The TA of durian pulp fermented at 15 °C remained relatively unchanged (about 0.05–0.09 %, p>0.05) throughout the fermentation period. However, the TA at 27 and 40 °C increased significantly (p<0.05) up to Day 6, and then remained unchanged. TA was greater at 27 °C (p<0.05). The high levels of acidity by Day 6 until at the end of fermentation indicated an acidic product, compared to durian pulp fermented between 3–4 days. A TA of 3.64 % was reported in durian pulp fermented for 10 days at 27 °C (Amin et al. 2004).
Total soluble solid (TSS) (25 % in fresh durian pulp) was another property that was affected by incubation temperature (Table 1). This value is similar to the value (23–25 %) reported by Ketsa and Pangkool (1994) but lower than the range (32–41 %) reported by Voon et al. (2007). A high TSS value is important for fermentation to succeed. For example, lactic acid fermentation of onion (Allium cepa) which has an initial low TSS content ranging between 4.9–8.6 % (Galdon et al. 2008) had required the addition of sugar (Roberts and Kidd 2005). In general, fermentation of durian pulp for 10 days at 27 and 40 °C resulted in a decrease in the TSS content. In concert with the negligible changes in pH and titratable acidity, there was also no significant difference (p<0.05) in TSS content after Day 1 when durian pulp was fermented at 15 °C.
The organic acids detected in fresh durian pulp were succinic (0.86 g/100 g), citric (0.47 g/100 g), malic acid (0.18 g/100 g), acetic (0.12 g/100 g) and lactic acids (0.03 g/100 g) (Table 2). Voon et al. (2007) identified organic acids in different durian cultivars (D2, D24, D101, MDUR78 and Chuk) namely malic (0.17–1.29 g/100 g), citric (0.02–0.26 g/100 g), succinic (0.08–0.31 g g/100 g) and tartaric acids (0.08 g/100 g). Lactic (0.03 g/100 g) and acetic (0.02 g/100 g) acids have also been reported in durian pulp (Leisner et al. 2001). Most of the organic acid contents in durian pulp incubated at 15 °C showed no significant changes (p<0.05) except for a slight increase in lactic and acetic acids (Table 2) indicating low microbial activity. The result obtained is consistent with the unchanging pH and titratable acid of durian pulp incubated at 15 °C. When the temperature and period of fermentation were increased, the changes in organic acid content of durian pulp became more obvious (Table 2). A significant reduction (p<0.05) in succinic acid and malic acid contents was observed at 27 and 40 °C with a greater decrease in the content observed at 40 °C. The decrease in malic acid content was possibly due to degradation to carbon dioxide (CO2) and lactic acid by the malolactic enzyme as reported previously for sauerkraut fermentation (Johanningsmeier et al. 2004) and cucumber fermentation (McFeeters et al. 1984). Malolactic reaction has been found to be active in early fermentation of sauerkraut. This reaction is not preferable for cucumber fermentation because of the bloater formation through the production of CO2.
Table 2.
Changes in organic acid content during fermentation
| Fermentation temperature (°C) | Day | Organic acid concentration (g/100 g) | ||||
|---|---|---|---|---|---|---|
| Malic | Lactic | Acetic | Citric | Succinic | ||
| 15 ± 1 | 0 | 0.18 ± 0.06a | 0.03 ± 0.01b | 0.12 ± 0.05d | 0.47 ± 0.18a | 0.86 ± 0.34a |
| 1 | 0.27 ± 0.00a | 0.04 ± 0.00b | 0.16 ± 0.00cd | 0.48 ± 0.00a | 0.91 ± 0.00a | |
| 2 | 0.23 ± 0.02a | 0.06 ± 0.03b | 0.33 ± 0.01b | 0.46 ± 0.01a | 0.88 ± 0.03a | |
| 3 | 0.21 ± 0.02a | 0.06 ± 0.01b | 0.26 ± 0.02bc | 0.49 ± 0.03a | 0.91 ± 0.01a | |
| 4 | 0.22 ± 0.01a | 0.06 ± 0.04b | 0.31 ± 0.01b | 0.51 ± 0.01a | 0.91 ± 0.10a | |
| 6 | 0.21 ± 0.01a | 0.06 ± 0.00b | 0.31 ± 0.04b | 0.48 ± 0.02a | 0.91 ± 0.03a | |
| 10 | 0.28 ± 0.00a | 0.19 ± 0.00a | 0.36 ± 0.01a | 0.45 ± 0.02a | 0.87 ± 0.02a | |
| 27 ± 1 | 0 | 0.18 ± 0.06ab | 0.03 ± 0.01d | 0.12 ± 0.05c | 0.47 ± 0.18a | 0.86 ± 0.34a |
| 1 | 0.14 ± 0.08abc | 0.19 ± 0.02d | 0.20 ± 0.13c | 0.34 ± 0.19a | 0.68 ± 0.00a | |
| 2 | 0.14 ± 0.08a | 0.26 ± 0.03d | 0.28 ± 0.13c | 0.46 ± 0.01a | 0.65 ± 0.02ab | |
| 3 | 0.16 ± 0.01ab | 0.53 ± 0.06cd | 0.67 ± 0.08b | 0.44 ± 0.01a | 0.44 ± 0.01ab | |
| 4 | 0.06 ± 0.00bc | 0.85 ± 0.09bc | 1.08 ± 0.01a | 0.47 ± 0.01a | 0.38 ± 0.01ab | |
| 6 | 0.00 ± 0.00c | 1.31 ± 0.03ab | 1.38 ± 0.02a | 0.47 ± 0.02a | 0.33 ± 0.01ab | |
| 10 | 0.00 ± 0.00c | 1.72 ± 0.11a | 1.21 ± 0.03a | 0.51 ± 0.01a | 0.10 ± 0.00b | |
| 40 ± 1 | 0 | 0.18 ± 0.06a | 0.02 ± 0.01e | 0.12 ± 0.05d | 0.47 ± 0.18a | 0.86 ± 0.34a |
| 1 | 0.17 ± 0.01a | 0.25 ± 0.04de | 0.34 ± 0.01cd | 0.43 ± 0.03a | 0.63 ± 0.04b | |
| 2 | 0.16 ± 0.00a | 0.49 ± 0.00cd | 0.68 ± 0.00bc | 0.51 ± 0.00a | 0.37 ± 0.00bc | |
| 3 | 0.00 ± 0.00b | 0.48 ± 0.09cd | 1.05 ± 0.21a | 0.59 ± 0.10a | 0.32 ± 0.01bc | |
| 4 | 0.00 ± 0.00b | 0.84 ± 0.20cb | 1.34 ± 0.17a | 0.53 ± 0.00a | 0.18 ± 0.01bc | |
| 6 | 0.00 ± 0.00b | 1.18 ± 0.13ab | 1.21 ± 0.16ab | 0.50 ± 0.05a | 0.10 ± 0.00c | |
| 10 | 0.00 ± 0.00b | 1.51 ± 0.02b | 0.96 ± 0.04ab | 0.49 ± 0.01a | 0.03 ± 0.00c | |
Each value in the table represents the mean ± standard deviation from three replications. Means within each column of each individual temperature with different superscript are significantly different (p<0.05)
Table 2 also shows that lactic acid content increased 59 fold and 52 fold (p<0.05) in durian pulp fermented at 27 and 40 °C, respectively, compared to the control (fresh durian). This major end product in fermented durian pulp was presumably produced from a number of substrates such as sucrose, glucose, fructose and malic acid. The metabolic conversion of these components into lactic acid is either through the phospho-ketolase or glycolytic pathway (Liu 2003; Kandler 1983). Besides that, the increase in acidity was also contributed by an increasing content of acetic acid at both temperatures. At 27 °C, acetic acid increased 11 fold on Day 6 from an initial value of 0.12 g/100 g before decreasing to 1.21 g/100 g at the end of the fermentation days. Fermentation at 40 °C showed a greater rate of increase where the content was 1.34 g/100 g on Day 4 fermentation. Thereafter, the content decreased, possibly due to the combine effect of decreased production and volatilization.
In the early stages in the production of lactic acid fermented vegetables such as sauerkraut and kimchi, heterofermentative LAB produce significant quantities of acetic acid in addition to lactic acid and are replaced later by more acid tolerant homofermentative species that produce lactic acid (Adams 1990). Higher amounts of acetic acid than lactic acid were produced in the first 4 days of fermentation at 40 °C and can be attributed to the activity of heterofermentative LAB groups such as Lb. brevis, Lb fermentum and Ln. mesenteroides (Leisner et al. 2001). The presence of substantial amounts of acetic acid in the durian pulp fermented at 40 °C and the fact that during fermentation, a lot of gas was entrapped inside the bottle, indicate that heterofermentative organisms at 40 °C were relatively more active than at 27 °C.
The production of lactic and acetic acids was higher at 27 °C compared to 40 °C. From the final concentrations of lactic and acetic acids (Table 2), ratios of lactic acid to acetic acid of 1.4:1 and 1.6:1 were obtained for durian fermented at 27 and 40 °C, respectively. The presence of higher quantity of lactic acid is probably due LAB being more active in the later stages of fermentation. Also, as can be seen in Table 2, the maximum production of lactic and acetic acids occurred in pulp fermented for 6 days at 27 °C and between 4–6 days at 40 °C indicating the development of a fermented product that is relatively acidic. Malic (1.46 g/100 g), lactic (0.34 g/100 g) and acetic acids (0.14 g/100 g) have been reported to be present in fermented durian pulp on Day 8 at 27 °C, but the presence of succinic and citric acid have not been reported (Yuliana and Garcia 2009). It was observed that citric acid content remained fairly stable throughout the fermentation at 27 and 40 °C with no significant difference (p<0.05). Thus, temperature and fermentation times did not have any effect on citric acid in durian pulp. Citric acid is reportedly involved in the production of acetic acid through the citric acid metabolic pathway and also in the formation of flavor compounds such as acetoin and 2, 3- butanediol (Caplice and Fitzgerald 1999). However, the utilization of citric acid could be retarded due to the presence of salt in anaerobic condition and partially inhibited in aerobic condition (Bobillo and Marshall 1991).
On average, the total sugar concentration in fresh durian was about 17 g/100 g (Table 3). The main sugar that is present in fresh durian pulp was sucrose (12.58 g/100 g), followed by glucose (2.21 g/100 g), fructose (1.60 g/100 g) and maltose (0.51 g/100 g). Lactose was not detected. Voon et al. (2007) reported that the sugar content of different durian cultivars ranged between 8.82–13.79 g/100 g and consisted of sucrose (5.57–10.47 g/100 g), glucose (1.86–2.77 g/100 g) and fructose (0.77–1.27 g/100 g). Voon et al. (2006) reported the presence of maltose content (0.5–0.6 g/100 g). They, too, did not detect lactose. At 15 °C for 10 days, fructose and glucose contents increased slightly by 0.72 % and 0.46 % (p<0.05), respectively. On the other hand, sucrose did not change significantly (p<0.05) during 6 days of fermentation but decreased significantly thereafter. The decrease in sucrose concentration could be due to hydrolysis via the activity of sucrose hydrolases and phosphorylases into fructose and glucose (Reid and Abratt 2005), thus accounting for the slight increase in both fructose and glucose concentrations. Generally, the concentration of maltose decreased significantly at the end of the fermentation period, indicating utilization.
Table 3.
Changes in sugar content during fermentation
| Fermentation temperature (°C) | Day | Sugar concentration (g/100 g) | |||
|---|---|---|---|---|---|
| Fructose | Glucose | Sucrose | Maltose | ||
| 15 ± 1 | 0 | 1.60 ± 0.04e | 2.21 ± 0.08b | 12.58 ± 0.33ab | 0.51 ± 0.01bc |
| 1 | 1.85 ± 0.04cde | 2.40 ± 0.20ab | 13.76 ± 1.14a | 0.69 ± 0.30ab | |
| 2 | 1.70 ± 0.03de | 2.26 ± 0.15b | 12.34 ± 0.54ab | 0.45 ± 0.00bc | |
| 3 | 1.93 ± 0.09bcd | 2.43 ± 0.09ab | 12.68 ± 0.84ab | 0.44 ± 0.02bc | |
| 4 | 1.99 ± 0.04bc | 2.55 ± 0.11ab | 12.37 ± 0.54ab | 0.91 ± 0.11a | |
| 6 | 2.16 ± 0.21ab | 2.49 ± 0.07ab | 12.35 ± 0.07ab | 0.32 ± 0.01c | |
| 10 | 2.32 ± 0.16a | 2.67 ± 0.12a | 11.38 ± 0.12b | 0.23 ± 0.05c | |
| 27 ± 1 | 0 | 1.60 ± 0.04c | 2.21 ± 0.08d | 12.58 ± 0.33a | 0.51 ± 0.01d |
| 1 | 2.18 ± 0.10c | 2.42 ± 0.06d | 11.44 ± 0.29a | 0.32 ± 0.03e | |
| 2 | 4.65 ± 0.04a | 3.07 ± 0.03d | 2.81 ± 0.07b | 0.80 ± 0.04c | |
| 3 | 5.20 ± 0.57a | 4.74 ± 0.46c | 1.18 ± 0.72c | 1.04 ± 0.12ab | |
| 4 | 5.04 ± 0.33a | 5.57 ± 0.35bc | 0.59 ± 0.08c | 1.09 ± 0.03a | |
| 6 | 4.43 ± 0.30a | 6.25 ± 0.50ab | 0.52 ± 0.13c | 0.84 ± 0.02c | |
| 10 | 3.47 ± 0.33b | 6.67 ± 0.51a | 0.54 ± 0.33c | 0.91 ± 0.07bc | |
| 40 ± 1 | 0 | 1.60 ± 0.04e | 2.21 ± 0.08e | 12.58 ± 0.33a | 0.51 ± 0.01a |
| 1 | 2.14 ± 0.17de | 2.80 ± 0.22e | 12.06 ± 1.03ab | 0.47 ± 0.04a | |
| 2 | 2.57 ± 0.07d | 3.71 ± 0.32d | 10.28 ± 0.29bc | 0.40 ± 0.01ab | |
| 3 | 3.16 ± 0.07c | 4.53 ± 0.28cd | 9.35 ± 0.76cd | 0.43 ± 0.25ab | |
| 4 | 3.43 ± 0.08c | 5.03 ± 0.16bc | 8.88 ± 0.07dde | 0.26 ± 0.02ab | |
| 6 | 4.05 ± 0.16b | 5.71 ± 0.12b | 7.82 ± 0.50de | 0.23 ± 0.03ab | |
| 10 | 5.08 ± 0.44a | 7.06 ± 0.62a | 6.73 ± 1.62e | 0.18 ± 0.06b | |
Each value in the table represents the mean ± standard deviation from three replications. Means within each column of each individual temperature with different superscripts are significantly different (p<0.05)
Fermentation of durian pulp at 27 °C showed apparent changes in sugar contents (Table 3) where fructose content increased from 1.60 to 5.20 g/100 g on Day 3, after which the concentration decreased to 3.47 g/100 g on Day 10. Also, glucose content gradually increased from 2.21 to 6.67 g/100 g until the end of the fermentation period. In comparison, the sucrose content decreased drastically from 12.58 to only 2.81 g/100 g on Day 2 of fermentation and by the end of the fermentation period, the loss in sucrose content was a significant 95 %. The decrease in sucrose concentration can, to a certain extent, account for the increase in the concentrations of fructose and glucose, due principally to enzymatic hydrolysis. Differences in the rates (obtained by plotting the sugar concentration against time of fermentation) of glucose (0.06 g/h) and fructose (0.10 g/h) utilization concentrations during the duration of fermentation were probably the result of different rates of utilization by microorganism involved in the fermentation of durian pulp. Tukey’s multiple range test revealed significant differences (p<0.05) in fructose and glucose concentrations after Day 2 and Day 3 of fermentation, respectively. For sucrose, it showed a significance difference (p<0.05) on the second day of fermentation, and thereafter showed no significant difference after the third day to the end of fermentation. Maltose content increased (p<0.05) to the value 1.09 g/100 g on Day 4 at 27 °C and then decreased slightly with further fermentation. The increase in maltose content could be due to hydrolysis of starch in durian pulp has been reported to contain ~11 % of starch (Brown 1997). It has been reported that α-amylase activities were lower at 16 °C than 21 °C in sugar apple fruit (Annona squamosa L.) (Wu et al. 1999) and the same may apply during durian fermentation.
Fermentation at 40 °C also caused apparent changes in the sugar content (Table 3). However, the trend in changes is different compared to the durian pulp fermented at 27 and 15 °C. Tukey’s multiple range test revealed significant differences (p<0.05) with regards to the concentrations of fructose and glucose from the onset of fermentation to the end of fermentation period. At this temperature, the levels of fructose and glucose continued to increase until the end of the fermentation period. Values obtained on Day 10 represented 3.2 folds increases over fresh sample values. Concomitant with the increases in fructose and glucose concentrations was the decrease in sucrose content which showed a significant difference (p<0.05) at each day of fermentation with only 53 % remaining after 10 days of fermentation. The trend in changes in fructose, glucose and sucrose concentrations supports the conclusion that there were greater levels of enzymatic and microbial activities at 27 °C compared to those at 40 °C. Contrary to durian pulp incubate at 27 °C, the maltose content decreased (p<0.05) to the value of 0.18 g/100 g in durian pulp incubate at 40 °C.
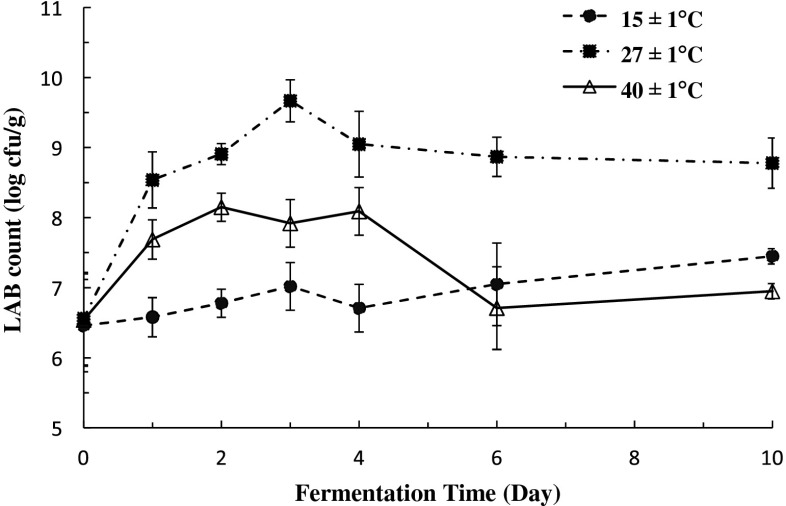
The initial LAB population of durian pulp after mashing and mixing with salt was 6.46 to 6.56 log cfu/g. As expected, fermentation temperature has a significantly effect on the growth of LAB as shown in Fig. 1. The LAB count of sample fermented at 15 °C remained fairly constant (p<0.05) throughout the fermentation. However, when fermented at 27 °C, the LAB count increased 1.5 times from 6.56 to 9.67 log cfu/g in the first 3 days of fermentation. Thereafter, the count decreased slightly. LAB count also increased at 40 °C, however, the rate of increase was lower than at 27 °C. After 3 days, the count was 7.92 log cfu/g which is 18 % less than the count at 27 °C. Unlike fermentation at 27 °C, a significant (p<0.05) rapid decline in LAB population was observed at 40 °C after 4 days of fermentation where at the end of the fermentation period, LAB count was similar to that at the start of fermentation.
Fig. 1.
Changes in lactic acid bacteria (LAB) count (log cfu/g) in durian pulp at different temperatures (n=3)
The increase in count of LAB during fermentation at 27 °C shows that the bacterial population was acid-tolerant since the pH of the pulp was already pH 4.70 at Day 2 of fermentation. The dominant microbial population in fermented durian pulp obtained from various markets in Malaysia has been reported to be LAB (ranging from 8.5 to 9.2 log cfu/g) including Lb. plantarum as the major group, Lb. brevis, Lb. fermentum, Lb. mali and Ln. mesenteroides (Leisner et al. 2001). In the present study, durian pulp subjected to 40 °C might be populated by different LAB population since these population bacteria are less able to tolerate pH below 4.30 and are less able to withstand the temperature of fermentation.
The aroma of durian pulp has been found to be due to the predominant presence of ester, sulfur and alcohol compounds (Voon et al. 2007; Chin et al. 2007; Zhang and Li 2007; Jiang et al. 1998). The strong durian aroma caused by sulfur compounds mixed the sweet and fruity notes in durian pulp is mainly correlated with ethyl propanoate, methyl butanoate, propyl 2-methyl propanoate and ethyl 3-butanoate (Voon et al. 2007; Chin et al. 2007). The ethyl ester compounds were reported to decrease but methyl, propyl and butyl ester compounds increased during storage of durian at 25 °C after 8 days Zhang and Li (2007). The creamy flavor of durian is contributed by the predominant presence of acetoin among the 108 compounds identified in the pulp (Jiang et al. 1998). Fermentation will, of course, change the character of the durian aroma. A zNoseTM (ultra-fast gas chromatograph) was used to profile the generation of aroma compounds during fermentation of durian pulp as earlier studies have successfully demonstrated the zNoseTM application in analysis of bacterial (Casalinuovo et al. 2006), honey (Lammertyn et al. 2004) and lilac flower Oh et al. (2008). In this study, the instrument was used in a qualitative manner to profile aroma changes that took place during fermentation of durian pulp.
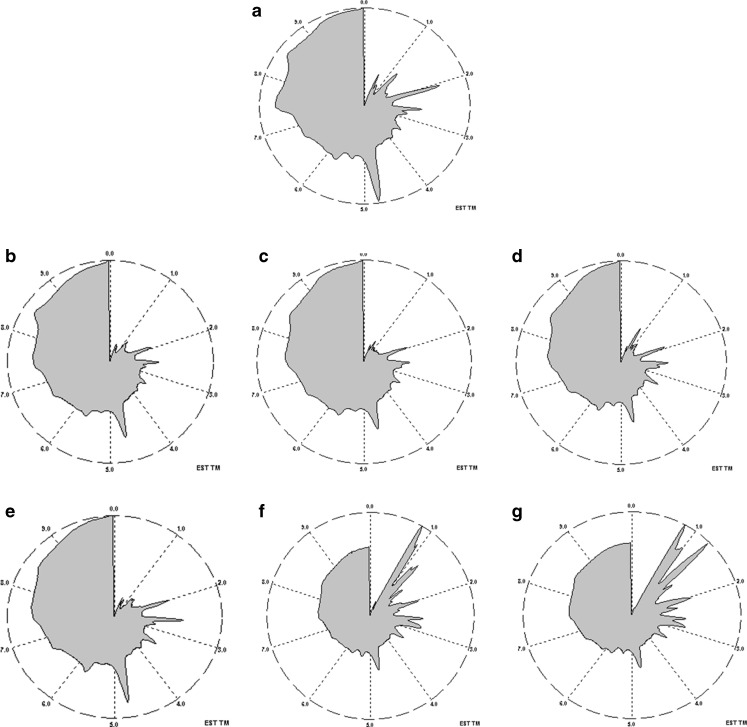
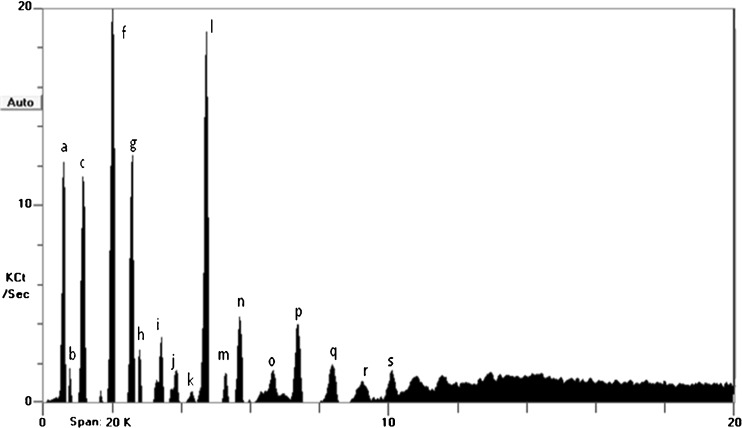
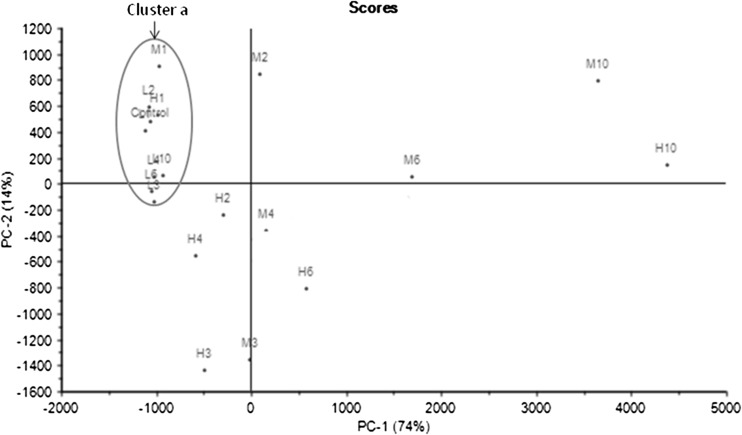
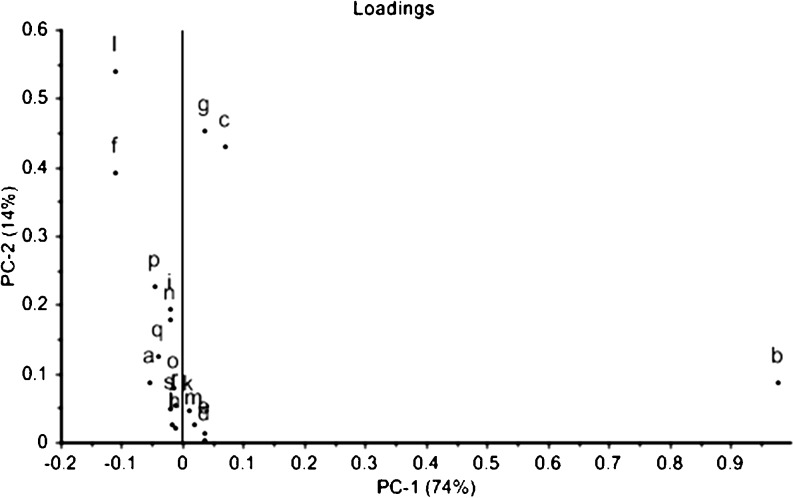
When using the zNoseTM, samples could be discriminated using two different analysis approaches: an olfactory image known as VaporPrintTM and chromatogram analysis using relative peaks area (Staples 2000). A zNoseTM chromatogram is a 2-dimensional olfactory image which corresponds to the instruments sensor frequency change as radial variables and the retention time as angular variables (Staples 2000), and separation of compounds using zNoseTM is based on their molecular weight. The lower molecular weight and more volatile organic compounds condense quickly from the SAW surface and elute first. Results obtained are shown in Fig. 2 (as VaporPrintTM) and chromatogram (Fig. 3). Each peak that appears during the running time corresponds to a specific volatile compound, although there could be compounds that co-elute. In this study, 19 individual compounds that were detected in 20 s of analysis time fresh durian pulp (Fig. 3). Depending on the fermentation temperature, changes in peak profile and peak heights occurred during fermentation. Data from the zNoseTM chromatograms (data not shown) were analyzed using the Principal Component Analysis (PCA) (Staples 2000). Analysis dataset of all peaks from the chromatographic approach using PCA shows a clear distinction between durian pulps fermented at different temperatures right from the onset (Day 0) until end of fermentation. The results showed that the first two principal components accounted for 88 % of the total variance (Fig. 4). This discrimination of volatiles using PCA demonstrated that the durian pulps samples (from Day 1 until Day 10) incubated at 15 °C tended to produce about similar volatiles concentration to the Control (Day 0) as displayed in ‘Cluster a’ in Fig. 4. As the temperature of fermentation increased to 27 and 40C, the durian pulp displayed changes in their volatiles concentrations fermentation progressed. A closer look at the loading plot displayed in Fig. 5 shows that there were five main volatile compounds i.e. b, c, f, g, and l, that contributed to changes in the aroma profile. Table 4 shows changes in the concentration of these volatile compounds. Most of volatile compounds increased in concentration during fermentation, and these compounds were also mostly the dominant compounds. Recent work by Neti et al. (2011) found that the spontaneous fermentation of durian pulp still containing sulfur volatile constituents and had lack of fruity aroma after 8 days storage at 30 °C. They reported the decrease of major sulfur compound (diethyl trisulfide, N-dimethylthioisophynil-3-amino and dipropyl trisulfide) and no substantially change of the major ester compounds (ethyl 2-methyl butanoate and ethyl propanoate) at the end of fermentation days. The formation of sulfur compound by LAB through transaminase and lyses activity on methionine and cystein has been reported in dairy, wine and cheese (Landaud et al. (2008). Zhang et al. (2007) reported the presence of 2-methyl butanoic acid responsible for the unpleasant taste during storage of durian pulp at 25 °C.
Fig. 2.
Vaporprint TM of durian pulp (a) on Day 0, during fermentation on Day 4 at (b) 15 °C, (c) 27 °C, (d) 40 °C, and during fermentation on Day 10 at (e) 15 °C, (f) 27 °C, (g) 40 °C
Fig. 3.
Aroma profile of fresh durian pulp (Day 0). ‘a-s’ indicate the different volatile compound peaks
Fig. 4.
PCA scores plot of durian pulp fermented at 15 °C (L), 27 °C (M), 40 °C (H). Fermentation days from Day 1 until Day 10 are indicated by the number 1–10. Cluster a comprises Control (Day 0), L1, L2, L3, L4, L6, L10, M1, and H1. PCA denotes Principal Component Analysis
Fig. 5.
PCA loading plot of the volatile compounds ‘a-s’ in durian pulp fermented at 15 °C 27 °C and 40 °C. PCA denotes Principal Component Analysis
Table 4.
Concentration of main volatile compounds that affected the aroma profile during fermentation
| Incubation Temperature (°C) | Day | Concentration of volatile compounds (counts) | ||||
|---|---|---|---|---|---|---|
| b | c | f | g | l | ||
| 15 ± 1 | 0 | 51.5 ± 36.88 | 1152.0 ± 223.80 | 1742.5 ± 148.20 | 948.8 ± 124.60 | 1909.5 ± 122.60 |
| 1 | ND | 931.5 ± 142.70 | 1756.5 ± 141.10 | 1027.8 ± 88.10 | 1854.8 ± 413.70 | |
| 2 | ND | 1250.3 ± 598.90 | 2013.5 ± 261.93 | 1270.5 ± 378.70 | 1488.3 ± 244.80 | |
| 3 | ND | 980.5 ± 795.40 | 1384.0 ± 540.00 | 889.0 ± 164.10 | 1260.0 ± 91.80 | |
| 4 | ND | 931.8 ± 361.70 | 1005.5 ± 88.40 | 1053.8 ± 115.50 | 1755.6 ± 46.70 | |
| 6 | ND | 663.3 ± 236.80 | 1223.0 ± 86.80 | 1207.8 ± 80.50 | 1458.8 ± 15.60 | |
| 10 | ND | 896.3 ± 626.10 | 715.3 ± 148.00 | 1532.8 ± 476.50 | 1632.5 ± 195.60 | |
| 27 ± 1 | 0 | 51.5 ± 36.88 | 1152.0 ± 223.80 | 1742.5 ± 148.20 | 948.8 ± 124.60 | 1909.5 ± 122.60 |
| 1 | 152.3 ± 31.91 | 801.5 ± 152.10 | 1737.8 ± 169.60 | 1719.0 ± 837.70 | 1719.0 ± 481.60 | |
| 2 | 1262.3 ± 522.90 | 431.8 ± 139.60 | 1964.3 ± 838.50 | 2106.8 ± 968.10 | 1738.5 ± 464.40 | |
| 3 | 877.5 ± 107.80 | 107.5 ±27.80 | 838.5 ± 113.30 | 545.0 ± 56.30 | 609.8 ± 40.50 | |
| 4 | 1159.0 ± 316.50 | 106.0 ± 104.90 | 1419.8 ± 340.10 | 1128.8 ± 198.30 | 1015.5 ± 80.60 | |
| 6 | 2659.5 ± 262.00 | 426.3 ± 194.60 | 1484.0 ± 157.10 | 1296.0 ± 225.80 | 1092.5 ± 172.10 | |
| 10 | 4502.3 ± 977.70 | 1248.0 ± 1291.20 | 905.8 ± 640.20 | 1492.0 ± 461.10 | 1438.0 ± 825.50 | |
| 40 ± 1 | 0 | 51.5 ± 36.88 | 1152.0 ± 223.80 | 1742.5 ± 148.20 | 948.8 ± 124.60 | 1909.5 ± 122.60 |
| 1 | 142.0 ± 28.31 | 524.0 ± 50.40 | 1641.8 ± 398.60 | 1478.3 ± 436.50 | 1795.0 ± 222.30 | |
| 2 | 747.5 ± 245.30 | 276.5 ± 59.70 | 1399.3 ± 447.50 | 1114.8 ± 464.00 | 1162.5 ± 503.30 | |
| 3 | 383.3 ± 50.60 | 83.5 ± 22.60 | 768.0 ± 103.00 | 576.5 ± 82.50 | 613.3 ± 181.40 | |
| 4 | 436.0 ± 140.30 | ND | 1188.0 ± 97.00 | 1062.5 ± 163.40 | 1148.0 ± 100.90 | |
| 6 | 1525.3 ± 240.60 | ND | 1255.8 ± 126.50 | 965.8 ± 98.40 | 640.0 ± 198.10 | |
| 10 | 5114.5 ± 121.60 | 1420.0 ± 494.10 | 740.8 ± 219.00 | 1255.0 ± 242.80 | 867.5 ± 252.00 | |
ND Not detected. Compounds b, c, f, g and l refer to those labeled in Fig. 5. Each value in the table represents the mean ± standard deviation from three replications
Other changes noted during durian pulp fermentation were a marked change of color from yellow to brownish, and thinning (liquefaction) of pulp stored at 40 °C. Storage at 15 and 27 °C had no or a slight effect on pulp color, while pulp at 27 °C became slightly softer. Pulp at 15 °C remained firm. Browning in durian pulp may due to a higher rate of the Maillard. The degree of viscosity in durian pulp has been correlated well with pectin degradation through the activity of the enzyme polygalacturonase (Imsabai et al. 2002). It was reported that low temperature (12 °C) inhibits the activity of the enzyme in durian pulp while the activity of polygalacturonase was high as fruit pulp held at 27 and 34 °C, and coincided with the rapid loss of firmness along with an increase in the content of water soluble pectin.
Conclusion
It can be concluded that fermented durian (tempoyak) production is affected by the fermentation time and temperature. To produce an acceptable product, durian pulp should be fermented at room temperature although low temperature has been successful in other fermented products such as sauerkraut. On the other hand, fermentation at 40 °C was not so favorable for the growth of LAB leaving high residual sucrose after Day 4. Also, long fermentation period at high temperature produced a product that had undesirable aroma and color making it unfit for consumption. Therefore, the best condition for the fermentation of durian pulp into tempoyak is 27 °C, and it is ready to be consumed between Days 4 and 6 when the acidity and sugar content had begun to stabilize.
Acknowledgment
Financial support in the form of a Graduate Research Fellowship from the Malaysian Government is fully appreciated.
References
- Adams MR. Topical aspects of fermented foods. Trends Food Sci Technol. 1990;4:140–144. doi: 10.1016/0924-2244(90)90111-B. [DOI] [Google Scholar]
- Amin AM, Jaafar Z, Khim LN. Effect of salt on tempoyak fermentation and sensory evaluation. J Biol Sci. 2004;4:650–653. doi: 10.3923/jbs.2004.650.653. [DOI] [Google Scholar]
- Amiza MA, Zakiah J, Ng LK, Lai KW. Fermentation of tempoyak using isolated tempoyak culture. Res J Microbiol. 2006;1:243–254. doi: 10.3923/jm.2006.243.254. [DOI] [Google Scholar]
- Battcock M, Ali SA. Fermented fruits and vegetables a global perspective. Italy: FAO Agricultural Service Bulletin; 1998. p. 134. [Google Scholar]
- Bobillo M, Marshall VM. Effect of salt and culture aeration on lactate and acetate production by Lactobacillus plantarum. Food Microbiol. 1991;8:153–160. doi: 10.1016/0740-0020(91)90008-P. [DOI] [Google Scholar]
- Bonestroo MH, Kusters BJM, De Wit JC, Rombouts FM. Glucose and sucrose fermenting capacity of homofermentative lactic acid bacteria used as starters in fermented salads. Int J Food Microbiol. 1992;15:365–376. doi: 10.1016/0168-1605(92)90070-J. [DOI] [PubMed] [Google Scholar]
- Brown JM. Durio-a bibliography review. India: International Plant genetic Resources Institutes; 1997. [Google Scholar]
- Caplice E, Fitzgerald GF. Food fermentation: role of microorganisms in food production and preservation. Int J Food Microbiol. 1999;50:131–149. doi: 10.1016/S0168-1605(99)00082-3. [DOI] [PubMed] [Google Scholar]
- Carr FJ, Chill D, Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- Casalinuovo IA, Di Pierro D, Bruno E, Di Francesco P, Coletta M. Experimental use of new surface acoustic wave sensor for the rapid identification of bacteria and yeast. Lett Appl Microbiol. 2006;42:24–29. doi: 10.1111/j.1472-765X.2005.01792.x. [DOI] [PubMed] [Google Scholar]
- Chin ST, Nazimah SAH, Quek SY, Che Man YB, Abdul Rahman R, Mat Hashim D. Analysis of volatile compounds from Malaysian durians (Durio zibethinus) using headspace SPME coupled to fast GC-MS. J Food Compos Anal. 2007;20:31–34. doi: 10.1016/j.jfca.2006.04.011. [DOI] [Google Scholar]
- Chin ST, Hamid NSA, Quek SY, Che Man YB, Rahman RA, Hashim DM. Changes of volatiles’ attribute in durian pulp during freeze- and spray-drying process. LWT-Food Sci Technol. 2008;41:1899–1905. doi: 10.1016/j.lwt.2008.01.014. [DOI] [Google Scholar]
- Doores S. Organic acid. In: Davidson PM, Branen AL, editors. Organic acid. New York: Marcel Dekker; 1993. pp. 95–136. [Google Scholar]
- Galdon BR, Rodriguez CT, Rodriguez ER, Romero CD. Organic acid contents in onion cultivars (Allium cepa L.) J Agr Food Chem. 2008;56:6512–6519. doi: 10.1021/jf800282h. [DOI] [PubMed] [Google Scholar]
- Holzapfel W, Schillinger U, Buckenhuskes H. Sauerkraut. In: Farnworth ER, editor. Handbooks of fermented functional foods. New York: CRC Press; 2008. pp. 343–359. [Google Scholar]
- Hugenholtz J, Kleerebezem M. Metabolic engineering of lactic acid bacteria: overview of the approaches and results of pathway rerouting involved in food fermentations. Curr Opin Biotech. 1999;10:492–497. doi: 10.1016/S0958-1669(99)00016-6. [DOI] [PubMed] [Google Scholar]
- Hunt DC, Jackson PA, Mortlock RE, Kirk RS. Quantitative determination of sugars in foodstuffs by high-performance liquid chromatography. Analyst. 1977;102:917–920. doi: 10.1039/an9770200917. [DOI] [Google Scholar]
- Imsabai W, Ketsa S, Van Doorn WG. Effect of temperature on softening and the activities of polygalacturonase and pectinesterase in durian fruit. Postharvest Biol Technol. 2002;26:347–351. doi: 10.1016/S0925-5214(02)00067-4. [DOI] [Google Scholar]
- Jamradloedluk J, Nathakaranakule A, Soponronnarit S, Prachayawarakorn S. Influences of drying medium and temperature on drying kinetics and quality attributes of durian chip. J Food Eng. 2007;78:198–205. doi: 10.1016/j.jfoodeng.2005.09.017. [DOI] [Google Scholar]
- Jaswir I, Che Man Y, Selamat J, Ahmad F, Sugisawa H. Retention of volatile components of durian fruit leather during processing and storage. J Food Process Preserve. 2008;32:740–750. doi: 10.1111/j.1745-4549.2008.00211.x. [DOI] [Google Scholar]
- Jiang J, Choo SY, Omar N, Ahamad N. GC-MS analysis of volatile compounds in durian (Durio zibethinus Murr.) Dev Food Sci. 1998;40:345–352. doi: 10.1016/S0167-4501(98)80058-7. [DOI] [Google Scholar]
- Johanningsmeier SD, Fleming HP, Breidt JF. Malolactic activity of lactic acid bacteria during sauerkraut fermentation. J Food Sci. 2004;69:222–227. doi: 10.1111/j.1365-2621.2004.tb09891.x. [DOI] [Google Scholar]
- Kandler O. Carbohydrate metabolism in lactic acid bacteria. Antonie Leeuwenhoek. 1983;49:209–224. doi: 10.1007/BF00399499. [DOI] [PubMed] [Google Scholar]
- Kang JH, Lee JH, Min S, Min DB. Changes of volatile compounds, lactic acid bacteria, and headspace gases in Kimchi, a traditional Korean fermented vegetable product. J Food Sci. 2003;68:849–854. doi: 10.1111/j.1365-2621.2003.tb08254.x. [DOI] [Google Scholar]
- Ketsa S, Pangkool S. The effect of humidity on ripening of durians. Postharvest Biol Technol. 1994;4:159–165. doi: 10.1016/0925-5214(94)90017-5. [DOI] [Google Scholar]
- Lammertyn J, Veraverbeke EA, Irudayaraj J. zNoseTM technology for the classification of honey based on rapid aroma profiling. Sensors Actuators B Chem. 2004;98:54–62. doi: 10.1016/j.snb.2003.09.012. [DOI] [Google Scholar]
- Landaud S, Helinck S, Bonnarme P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl Microbiol Biotechnol. 2008;77:1191–1205. doi: 10.1007/s00253-007-1288-y. [DOI] [PubMed] [Google Scholar]
- Lee PR, Saputra A, Yu B, Curran P, Liu SQ. Biotransformation of durian pulp by mono- and mixed-cultures of Saccharomyces cerevisiae and Williopsis saturnus. LWT-Food Sci Technol. 2012;46:84–90. doi: 10.1016/j.lwt.2011.10.022. [DOI] [Google Scholar]
- Leisner JJ, Vancanneyt M, Gulam R, Pot B, Lefebvre K, Fresi A, Tee LK. Identification of lactic acid bacteria constituting the predominating microflora in an acid-fermented condiment (tempoyak) popular in Malaysia. Int J Food Microbiol. 2001;63:149–157. doi: 10.1016/S0168-1605(00)00476-1. [DOI] [PubMed] [Google Scholar]
- Leisner JJ, Vancanneyt M, Lefebvre K, Vandemeulebroecke K, Hoste B, Euras Vivalta N, Rusul G, Swings J. Lactobacillus durianis sp. nov., isolated from an acid-fermented condiment (tempoyak) in Malaysia. Int J Syst Evol Micr. 2002;52:927–931. doi: 10.1099/ijs.0.02091-0. [DOI] [PubMed] [Google Scholar]
- Liu SQ. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int J Food Microbiol. 2003;83:115–131. doi: 10.1016/S0168-1605(02)00366-5. [DOI] [PubMed] [Google Scholar]
- McFeeters RF, Perez-Diaz I. Fermentation of cucumbers brined with calcium chloride instead of sodium chloride. J Food Sci. 2010;75:291–296. doi: 10.1111/j.1750-3841.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- McFeeters RF, Fleming HP, Daeschel MA. Malic acid degradation and brined cucumber bloating. J Food Sci. 1984;49:999–1002. doi: 10.1111/j.1365-2621.1984.tb10379.x. [DOI] [Google Scholar]
- Merican Z. Malaysian tempoyak. In: Steinkraus KH, editor. Handbook of indigenous fermented foods. New York and Basel: Marcel Dekker; 1977. p. 148. [Google Scholar]
- Neti Y, Erlinda ID, Virgilio VG. The effect of spontaneous fermentation on the volatile flavor constituents of durian. Int Food Res J. 2011;18:625–631. [Google Scholar]
- Oh SY, Shin HD, Kim SJ, Hong J. Rapid determination of floral aroma compounds of lilac blossom by fast gas chromatography combines with surface acoustic wave sensor. J Chromatogr A. 2008;1183:170–178. doi: 10.1016/j.chroma.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Paramithiotis S, Hondrodimou OL, Drosinos EH. Development of the microbial community during spontaneous cauliflower fermentation. Food Res Int. 2010;45:1098–1103. doi: 10.1016/j.foodres.2010.01.023. [DOI] [Google Scholar]
- Ranganna S. Manual of analysis of fruit and vegetable products. New Delhi: Tata Mc-Graw Hill; 1979. [Google Scholar]
- Reid SJ, Abratt VR. Sucrose utilisation in bacteria: genetic organisation and regulation. Appl Microbiol Biotechnol. 2005;67:312–321. doi: 10.1007/s00253-004-1885-y. [DOI] [PubMed] [Google Scholar]
- Roberts JR, Kidd DR. Lactic acid fermentation of onions. Lebensm-Wiss U Technol. 2005;3:185–190. doi: 10.1016/j.lwt.2004.05.007. [DOI] [Google Scholar]
- Staples EJ (2000) The zNoseTM, a new electronic nose using acoustic technology. http://www.estcal.com/tech_papers/papers/GeneralAnalysis/ASA2000.pdf. Accessed 22 September 2011
- Staples EJ (2003) Electronic Nose simulation of olfactory response containing 500 Orthogonal Sensors in 10 seconds. http://www.estcal.com/tech_papers/papers/GeneralAnalysis/IEEE99.pdf. Accessed 28 August 2012
- Steinkraus KH. Fermentation in world food processing. Compr Rev Food Sci. 2002;1:23–32. doi: 10.1111/j.1541-4337.2002.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Sturm K, Koron D, Stamfar F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003;83:417–422. doi: 10.1016/S0308-8146(03)00124-9. [DOI] [Google Scholar]
- Subhadrabandhu S, Ketsa S. Durian king of tropical fruit. New Zealand: CABI Publishing; 2001. [Google Scholar]
- Tassou CC, Panagou EZ, Katsaboxakis KZ. Microbiological and physicochemical changes of naturally black olives fermented at different temperatures and NaCl levels in the brines. Food Microbiol. 2002;19:605–615. doi: 10.1006/fmic.2002.0480. [DOI] [Google Scholar]
- Voon YY, Hamid NSA, Rusul G, Osman A, Quek SY. Physicochemical, microbial and sensory changes of minimally processed durian (Durio zibethinus cv. D24) during storage at 4 and 28 °C. Postharvest Biol Technol. 2006;42:168–175. doi: 10.1016/j.postharvbio.2006.06.006. [DOI] [Google Scholar]
- Voon YY, Hamid NSA, Rusul G, Osman A, Quek SY. Characterisation of Malaysian durian (Durio zibethinus Murr.) cultivars: relationship of physicochemical and flavor properties with sensory properties. Food Chem. 2007;103:1217–1227. doi: 10.1016/j.foodchem.2006.10.038. [DOI] [Google Scholar]
- Wills RBH, Balmer N, Greenfield H. Composition of Australian foods 2: methods of analysis. Food Tech Aust. 1980;32:198–204. [Google Scholar]
- Wu MC, Chen CH, Chen CS. Changes in sugars, starch and amylase activity during ripening of sugar apples at different storage temperatures. Food Preserv Sci. 1999;25:57–61. doi: 10.5891/jafps.25.57. [DOI] [Google Scholar]
- Yuliana N, Dizon EI. Phenotypic identification of lactic acid bacteria isolated from tempoyak (fermented durian) made in the Philippines. Int J Biol. 2011;3:145–152. doi: 10.5539/ijb.v3n2p145. [DOI] [Google Scholar]
- Yuliana N, Garcia VV. Influence of Pediococcus acidilactiti as a starter on the flavour of tempoyak (fermented durian) Indian J Biotechnol. 2009;8:304–310. [Google Scholar]
- Zhang ZM, Li GK. A preliminary study of plant aroma profile characteristic by a combination sampling method coupled with GC-MS. Microchem J. 2007;86:29–36. doi: 10.1016/j.microc.2006.09.003. [DOI] [Google Scholar]
- Zhang ZM, Zeng DD, Li GK. The study of the aroma profile characteristics of durian pulp during storage by the combination sampling method coupled with GC-MS. Flavour Fragr J. 2007;22:71–77. doi: 10.1002/ffj.1761. [DOI] [Google Scholar]