Abstract
This study evaluated the hypolipidemic effect of crude polyphenol fraction from Clerodenrdon colebrookianum (CPCC) leaves in cholesterol fed rats. Crude polyphenol fraction was obtained from the ethyl acetate extract of Clerodenrdon colebrookianum (CC). Investigation was conducted by administering graded oral doses (0.25 g, 0.5 g and 1 g/kg b. w. /day) of the CPCC for a period of 28 days. Significant (p < 0.01) rise in plasma total cholesterol (TC), triglycerides (TG), phospholipids (PL), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL-C) and decrease in high-density lipoprotein cholesterol (HDL-C) were observed in cholesterol fed rats. Increased lipid profile has been depleted and high-density lipoprotein cholesterol (HDL-C) has been increased after chronic feeding of CPCC. In addition, CPCC extract enhanced the excretion of fecal cholesterol (FC) but could not arrest the 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase activities. Histopathological observations showed loss of normal liver architecture in cholesterol fed rats which were retained in CPCC treated groups. Moreover, the analysis of CC extract demonstrated the presence of substantial amount of total polyphenols, flavonoids and tannins content, further HPLC analysis led to the identification and quantification of two most important biologically active secondary metabolites i.e. (+) Catechin (432 ppm) and Quarcetin (105 ppm). The findings of this study suggested that CPCC had a strong hypolipidemic function and could be used as a supplement in healthcare foods and drugs.
Keyword: Clerodendron colebrookianum, Crude polyphenols, Hyperlipidemia
Introduction
Atherosclerosis is a complex disease characterized by an excessive inflammatory, fibro-fatty, proliferative response to damage of the artery wall involving several cell types, particularly smooth muscle cells, monocyte-derived macrophages, T-lymphocyte and platelets (Schwartz et al. 1993). Hyperlipidemia constitutes a major etiopathological factor for atherosclerosis. Recent studies have suggested several mechanisms by which hyperlipidemia may participate directly in atherogenesis. The markedly enhanced retention of oxidized LDL-C plays an important role in the progression of atherosclerosis (Mary et al. 2001). Therefore, increased in TG, TC, LDL-C and VLDL-C, and depletion in HDL-C were chosen as indices to determine the risk for atherosclerosis. Natural polyphenols range from simple molecules (phenolic acids) to highly polymerized compounds (tannins) being the current therapeutic interest, contribute against various degenerative diseases including cardiovascular risk. Lipid lowering activity of polyphenols obtained from Ecklonia cava, Ichnocarpus frutescens, Morus alba, Hibiscus sabdariffa, Red grape have already been well documented. (Hyeon et al. 2011; Chidambaram et.al. 2007; Mon et al. 2011, 2010; Alberto et al. 2006). Reducing lipid profile in circulating blood and inhibiting LDL-C oxidation by natural compounds is one of the best approaches to prevent atherosclerosis (Chi et al. 2006).
Clerodendron colebrookianum (CC) Walp (East Indian Glory Bower) is a perennial shrub belonging to the family Verbenaceae and most well known among different species of Clerodendron. The species is found in tropical regions of Asia including India, Myanmar, Bangladesh, Malayasia, Indonesia, Thailand, Bhutan and Nepal and also in temperate China (Wu and Raven 1994). In India it is confined to the Northeast (NE) region including West Bengal and Sikkim. Most of the tribal people of the Northeast India are constantly using various parts of CC against various treatments like cough, dysentery, blood pressure etc. (Singh et al. 1996; Singh and Singh 2006). More notably the Mizo people uses the leaves as vegetable (local name-phuinum) and there are very few hypertensive patients reported among their community members (Sharma et al. 2001). It is now widely used as a popular household remedy for hypertension throughout NE region of India (Nath and Bordoloi 1991; Bordoloi and Borthakur 1997).
Earlier studies on CC leaf extract showed the antioxidant and hypolipidemic activity in rat model (Devi et al. 2003; Devi and Sharma 2004). Hypolipidemic effect of glycosides from CC leaf has already been reported in C3H mice (Sharma and Bhuyan 2006). Lipid lowering activity of polyphenols has now been widely accepted, yet little is known about this positive effect of CC polyphenols despite of its hypolipidemic potentiality was documented earlier. Therefore, the present study was designed to address two questions: (i) to determine whether chronic ingestion of crude polyphenols from CC leaves (CPCC) has beneficial effect on hyperlipidemia and (ii) to elucidate the quantity of polyphenols present in CC leaves. Statin, a hypolipidemic drug was used as a positive control for its hypolipidemic influence in rats.
Materials and methods
Plant material
Tender leaves of the plant CC was collected from different parts of Guwahati, Kamrup district of Assam (situated in between 25° 43′-26° 53′ North latitude and 90° 39′–92° 11′ East longitude) in the month of July. The plant was authenticated and reconfirmed in the Department of Botany, Gauhati University, Assam. A voucher specimen (IASST/LSD/PM-10) was deposited at the medicinal and aromatic plant section, Life Sciences Department of IASST, Assam. The collected leaves were sorted, cleaned and shade dried.
Preparation of crude polyphenolic fraction of CC (CPCC)
Shade-dried CC leaves (500 g) was extracted with water and the water soluble fraction was dissolved in hot water and washed with chloroform. Aqueous layer was extracted with ethyl acetate and the ethyl acetate layer containing polyphenols was separated out and evaporated to dryness (Hara 1994). It was resuspended in water and used for the experiments. The percentage yield of crude polyphenols was 9 % w/w.
Experimental design and treatment schedule
Experiment was conducted using laboratory bred male Wistar albino rats, in accordance with the internationally accepted guideline for experimental animals use and care and the study was approved by the Institute Animal Ethics Committee (IAEC)(902/AC/05/CPCSEA).
The basal diet consisted of (g): Whole wheat 62.5, yellow corn 37.5, barley 37.5, milk 37.5, bone meal 2.5, calcium chloride 2.5, salt 2.5, oil 37.5, vitamin B12 one tablet. Each individual animal was given 12 g of diet per day (Farris and Ed 1950).
A total number of 36 adult male Wistar rats weighing between 150 and 200 g were obtained from the animal house of the Institute of Advanced Study in Science and Technology (IASST) after the approval of IAEC.
Following groups were considered for the study: (Khanna et al. 2002).
-
Group A:
normal diet and water (Control) upto 28 days.
-
Group B:
normal diet + cholesterol (Sigma Aldrich) (25 mg/kg body weight (b. w.) /day) upto 28 days.
-
Group C:
normal diet + cholesterol (25 mg/kg b. w. /day) + statin (5 mg/kg b.w. /day) upto 28 days.
-
Group D:
normal diet + cholesterol (25 mg/kg b.w. /day) + CPCC (0.25 g/kg b.w. /day) upto 28 days.
-
Group E:
normal diet + cholesterol (25 mg/kg b.w. /day) + CPCC (0.5 g/kg b.w. /day) upto 28 days.
-
Group F:
normal diet + cholesterol (25 mg/kg b.w. /day) + CPCC (1 g/kg b.w./day) upto 28 days.
The cholesterol solution was prepared under the requirement of 25 mg/kg b.w. of rat by dissolving the cholesterol in refined groundnut oil (0.5 % w/v) (Khanna et al. 2002).
At the end of the experimental duration overnight- fasted animals were sacrificed under light ether anesthesia. Blood (2–3 mL) was collected by heart puncture and serum was separated by centrifugation. Fecal samples were collected on 28th day of the experiment and kept at −20°C until analyzed.
Acute toxicity study
Acute toxicity test was conducted in C3H mice (n = 6) following the OECD protocol (OECD 2002). The animals were kept fasting overnight except water ad libitum and administered with a single dose of 5 mg/kg b.w. CPCC and kept under observation for a period of 14 days. As per the protocol mandate, (i) if mortality was observed in two out of three animals, then the dose administered was assigned as toxic dose, (ii) If mortality took place for single out of three, the dose was repeated to confirm the toxicity, (iii) If there was no mortality at all, the dose specificity had been raised to the maximum of 2,000 mg/kg b.w..
Biochemical parameters
The body weights (b.w.) of rats were measured on the 1st and 28th day of the study. Plasma TC was determined by the method of Zlaktkins et al. 1953. Plasma TG, PL and HDL-C were determined using test kits from Randox. LDL-C and VLDL-C were calculated using the Friedwald’s formula (Friedwald’s et al. 1972).
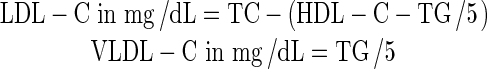 |
HMG CoA reductase [EC 1.1.1.34] activity was determined by the method of Rao (Rao and Ramakrishnan 1975) in the liver tissue of rats. Fecal cholesterol (FC), liver cholesterol (LC) and heart cholesterol (HC) were extracted by the method of Floch et al. 1957 and estimated using the method mentioned above.
Histopathology
For histopathological analysis liver samples were collected from all groups and fixed in 10 % buffered formalin. After routine processing, the tissue was embedded in paraffin, sectioned at 5 μm, stained with routine hematoxylin-eosin (H-E) and examined under light microscope.
Phytochemical analysis
Determination of total polyphenols content of CC
One gram shade dried powdered CC leaves was extracted with 50 mL of 70 % aqueous acetone under 20 min sonication in an ultrasonic bath at 30 °C. The extract was rapidly vacuum filtered and kept refrigerated before use. Total polyphenols were determined by Folin-Ciocalteu procedure (Hagerman et al. 2000). Aqueous acetone extracts were diluted 200 times and to the aliquots of 0.5 mL Folin-Ciocalteu reagent (0.25 mL) and 1.25 mL 20 % aqueous sodium carbonate solution were added. Samples were vortexed and the absorbance of blue colored mixtures recorded after 40 min at 725 nm against a blank containing 0.5 mL of 70 % aqueous acetone, 0.25 mL of Folin-Ciocalteu reagent and 1.25 mL 20 % aqueous sodium carbonate solution. The amount of total polyphenols was calculated as a catechin equivalent from the calibration curve of catechin standards solutions and expressed as mg catechin /g dry plant material. All measurement was done in triplicate.
Determination of flavonoids content
For the determination of flavonoids, powdered CC leaves (1 g) was homogenized with the extracting solvent (140:50:10 MeOH-H2O-CH3COOH, 20 mL) and filtered into volumetric flasks. The final volume was adjusted to 100 mL by addition of additional extracting solvent. To prepare the solutions for analysis the aliquots of 2.5 mL were transferred into 50 mL volumetric flasks and adjusted with water. Now to the 10 mL of analysis solutions, water (2 mL) and AlCl3 reagent (133 mg crystalline aluminium chloride and 400 mg crystalline sodium acetate dissolved in 100 mL of extracting solvent, 5 mL) were added and the absorbances were recorded at 430 nm against a blank (10 mL of analyzed solution plus 5 mL of water). The amount of flavonoids was calculated as a Quercetin equivalent from the calibration curve of Quercetin standard solutions and expressed as Quercetin/g dry plant material.
Determination of tannins
Total tannin contents were determined by Folin-Cioclateu procedure as described above, after removal of tannins by their adsorption to Polyvinylpolypyrrolidone (PVPP) (Liao and Shi 2005). In brief, 20 mL of aqueous acetone CC leaves extract were homogenized with 200 mg of PVPP and mixture was stirred for 1 h. After filtration, no adsorbed phenolics determined by Folin-Ciocalteu procedure as described. Calculated values were substracted from total polyphenols contents and the amount of total tannins expressed as mg catechin/g dry plant material. All measurement was done in triplicate.
HPLC analysis
The HPLC analysis of crude extract of CC leaves was performed using a Shimazdu HPLC system (Kyoto, Japan) equipped with an SCL-10Avp controller, two LC-10ADvp pumps, a DGU-14 A degasser, and an SPD-M 10vp diode array detector. The column used was a Luna C18 (250 × 4.6 mm i.d., 5 μm) purchased from Phenomenex (Torrance, CA). A mixture of water, acetonitrile, isopropanol, and formic acid (78:12:10:0.1, v/v) was used as a mobile phase in isocratic mode. The mobile phase flow rate was 0.8 mL/min. The sample volume injected was 10μL of crude CC extract, centrifuged (3,000 rpm) and filtered through 0.45 μm membrane filters before analysis. Data were acquired using a photodiode array detector in the range of 190–370 nm, and the chromatograms have been extracted at 283 nm. Time constant was 0.64 s and frequency 1.5625 Hz. Data acquisitions was performed by Shimadzu LC solution software.
Pure standards (+)-Catechin and Quercetin were purchased from Sigma-Aldrich and the Catechin and Quercetin in the sample were identified by comparison of retention times and spectra for each peak with the corresponding standard. All measurement was done in triplicate.
Statistical analysis
Results for b.w. were presented as mean ± standard deviation (S.D.) while those for TC, TG, PL, HDL-C, LDL-C and VLDL-C were presented as mean ± S.E. (n = 3 for in vitro experiment and n = 6 for in vivo experiment). Analysis to determine differences in experimental and control group was done by unpaired student’s t-test using statistical software program, SYSTAT 10.2 and p < 0.05 was set as significant.
Results and discussion
Acute toxicity studies revealed the non-toxic nature of the CPCC. After the administration of CPCC, mice were immediately observed for 2 h for behavioral, neurological and autonomic profiles for any changes or lethality for the next 48 h. There was no lethality or any toxic reactions recorded at any of the doses selected until the end of the study period. However, at the above mentioned doses, the CPCC as such could not produce any untoward effects on behavioral response, normal reflexes and so on. According to OECD guidelines for acute oral toxicity, an LD50 dose of 2,000 mg/kg and above is characterized as unclassified and hence the test material is found to be safe.
Oral administration of CPCC extract for 28 days did not alter the feeding pattern of the treated rats. As such, the pattern of weight gain in the treated groups (from 177 ± 30 g to 185 ± 30 g in group B, 175 ± 16 g to 182 ± 13 g in group C, 170 ± 15 g to 188 ± 27 g in group D, 175 ± 15 g to 187 ± 21 g in group E and 183 ± 21 g to 195 ± 29 g in group F on days 1st & 28th , respectively) was not significantly different from that of control ( from 173 ± 10 g to 180 ± 20 g on days 1st and 28th , respectively). Thus the CPCC has no significant effect on the pattern of weight gain in the treated rats.
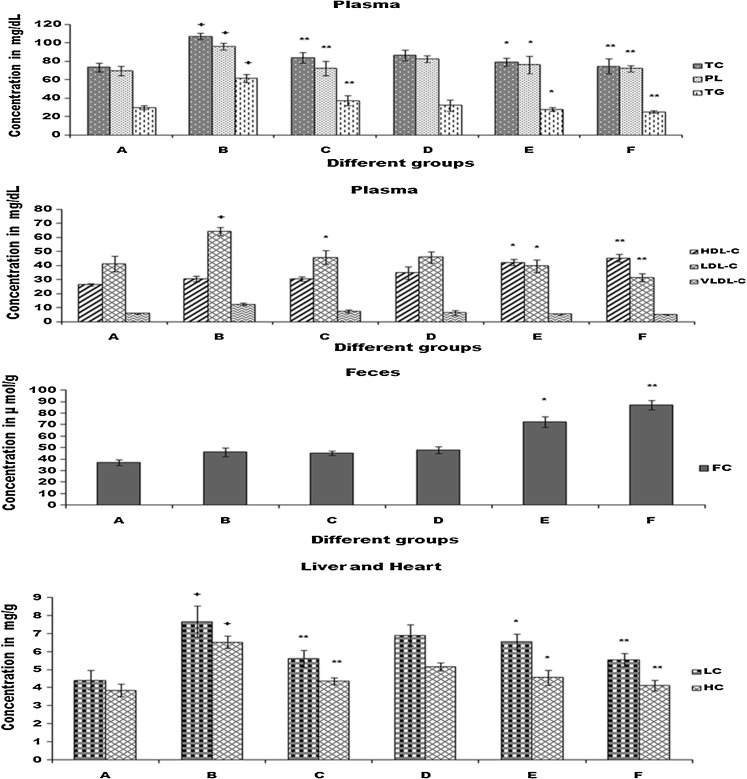
Serum lipid profiles As shown in Fig. 1, the plasma concentration of total cholesterol (TC), triglycerides (TG) and phospholipids(PL) increased significantly (p < 0.001) in group B after feeding cholesterol for 28 days. The administration of CPCC significantly attenuated the increase of TC, TG and PL in a dose dependent manner in Group D, E and F which is quite comparable with standard drug Statin (sigma-Aldrich) administered group C. After the oral administration of CPCC gradual increase of HDL-C in group D, E and F was notable. In addition, the administration of CPCC also attenuated the increase of LDL-C and VLDL-C in a dose dependent manner in Group D, E and F.
Fig. 1.
Biochemical changes in plasma, feces, liver and heart in rats of 6 different groups (A–F). Group A = control, B = cholesterol fed, C = cholesterol + statin fed, D = cholesterol + CPCC (0.25 g/kg) fed, E = cholesterol + CPCC (0.5 g/kg) fed, F = cholesterol + CPCC (1 g/kg) fed. Values are mean ± SE. N = 6. * P < 0.05 vs B, **P < 0.01 vs B, +P < 0.001 vs A
Fecal cholesterol (FC) were significantly (P < 0.01) increase in group F (1 g/kg b.w. /day) against cholesterol fed group B and presented a dose dependent elevation.
Liver and heart cholesterol levels were significantly (p < 0.01) elevated in cholesterol fed group B. Expectedly the oral administration of CPCC in group D, E and F dose dependently decreased TC content in liver and heart.
Table 1 shows the effects of CPCC extract on the HMG CoA reductase activity in liver tissue of different groups of rats. The ratio between the HMG CoA and mevalonate is inversely proportional to HMG CoA reductase activity, i.e. an increase in ratio indicates decreased activity. No significant change was observed after graded oral dose administration of CPCC.
Table 1.
HMG-CoA reductase activity in liver tissue of rats of different groups. Refer to Fig. 1 for rat groups A–F
| Different groups | Ratio of HMG CoA/Mevalonate |
|---|---|
| Group A | 1.23 ± 0.18 |
| Group B | 1.17 ± 0.23 |
| Group C | 1.42 ± 0.27 |
| Group D | 1.00 ± 0.14 |
| Group E | 1.39 ± 0.12 |
| Group F | 1.36 ± 18 |
Polyphenols of natural origin attribute enough in the reduction of the nasty cholesterol, responsible for heart diseases. Thus the effect of polyphenol present in CC leaves could be considered as highly positive. Yang et al. (2001) investigated the effects of polyphenol rich green, oolong, and black tea extracts on serum lipids of a hyperlipidemic animal model induced by a high-sucrose diet and observed hypolipidemic effect. In addition, only oolong and black tea extracts decreased weight gain in the 25 day experimental period. However, in our experiment, no effect on weight gain in normal and experimental animals was observed. These findings are consistent with our preliminary study where we have tested only single dose of crude extract of CC leaves and found that it could lower the baseline level of serum lipid profile significantly (Devi and Sharma 2004).
The plasma cholesterol level is regulated by the rate of biosynthesis and uptake through the liver low density lipoprotein receptors (LDLR) by way of elevation of hepatic clearance. It was observed that CPCC could not promote the HMG CoA reductase activity, key regulatory enzyme of cholesterol biosynthesis, but it enhanced the removal of fecal cholesterol. It has been reported that polyphenols from Red Grape Juice alter cholesterol homeostasis and increase LDLR activity in human cells (Alberto et al. 2006). Regarding the mechanism of action, CPCC might have down regulated HMG CoA reductase mRNA and up regulated the expression of LDLR mRNA. In parallel to the result, there existed more LDLR on the liver cell surface to accomplish more LDL uptake and hepatic clearance, thus reducing the plasma cholesterol concentration.
The decrease of serum TG level is an important finding of this experiment. Recent studies also showed that triglycerides are independently related to coronary heart diseases (Bainton et al. 1992) and most of the anti-hyperlipidemic drugs don’t decrease TG levels, but CPCC lowered it significantly and this effect might be co-related to increase the endothelium bound lipoprotein lipase which hydrolysis the TG into fatty acids. Lipoprotein lipase played a major role in the transport and metabolism of TG of exogenous origin (Taskimen 1987). It is the key enzyme, which regulates the disposal of lipid fuels in the body.
Enhancement of cardioprotective lipid HDL-C in a dose dependent manner after oral administration of CPCC extract was another interesting finding of the present study. Our study suggests that CPCC leaf could be a potent cardioprotective agent, in view of the inverse correlation between HDL-C levels and the risk of coronary heart disease (Tall 1998). This effect was not observed in our earlier study where we tested a single dose of CC leaves extract (Devi and Sharma 2004). Present study also showed that CPCC extract lowered TC and LDL-C and enhanced the HDL-C significantly. Therefore, it is expected that crude polyphenols of this plant might have a great advantage in the care and prevention of hyperlipidemia especially among Indians where HDL-C is the prevalent lipoprotein abnormality (Gupta et al. 1994, 1995).
Administration of CPCC significantly decreased the elevation of cholesterol in liver and heart, probably activated AMP-Activated Protein Kinase (AMPK), which results in hepatic and heart cholesterol lowering activity. AMPK is a key metabolic regulator in liver, skeletal muscle, and heart that responds to increased cellular AMP-to-ATP ratio and upstream signaling pathways stimulated by cellular stress (Hardie 2003). In turn, AMPK regulates fatty acid oxidation and lipid synthesis, two important determinants of tissue lipids and hyperlipidemia (Fryer and Carling 2005). In diabetic LDLR deficient mice, polyphenols stimulate AMP-Activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis (Mengwei et al. 2006).
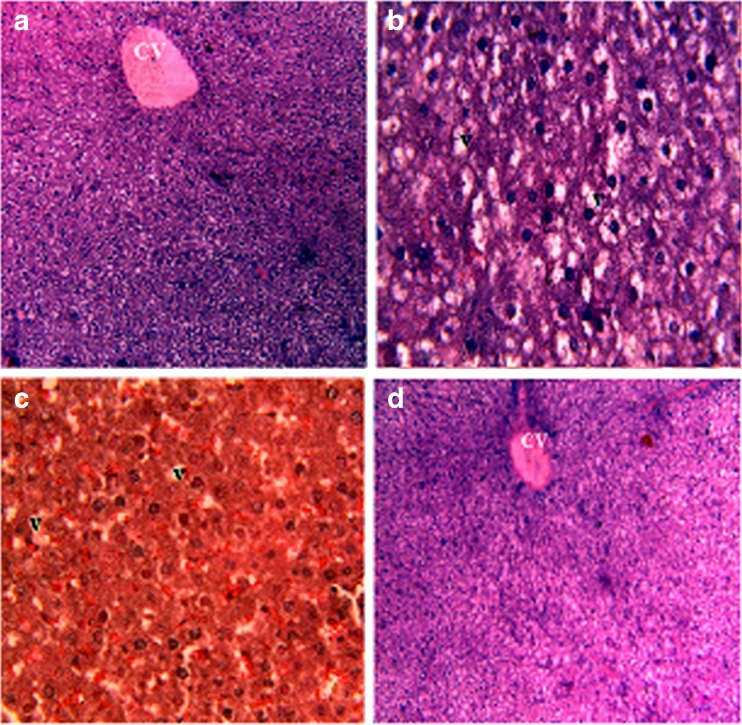
The visual estimation of tissue entities under the microscope is the most direct and still the most enquired method to determining the cellularity of a given tissue (Greenstein 1954). The technique is free from subjective error with sufficient numbers of records, is capable of attaining a high degree of accuracy in the most heterogeneous tissue. Thus it is attempted to observe the cellular structure of hepatocytes in rats fed with a high cholesterol diet and treatment with CPCC. Degenerative changes and lipid droplets were observed in the hepatocytes of hyperlipidemic rats (Fig. 2). The increase in lipid accumulation in hyperlipidemic group can be explained due to increased amount of triglycerides depot in liver. As the hepatocytes in the treatment group showed the sufficient reduction of lipid droplets and simultaneously degenerative changes, so the histopathological analysis of the liver supported the biochemical data and indicated a protective effect of CPCC on the development of liver damage due to hyperlipidemic diet.
Fig. 2.
Histopathological effects of CPCC in liver of cholesterol fed rat. a Control rat (magnification ×10) (b) Cholesterol fed rat (magnification ×40) (c) Cholesterol fed rat given 1 g/kg b.w. dose of CPCC (magnification ×40) (d) Cholesterol fed rat given standard drug statin (magnification ×40). V-Vacuole, CV-Centrilobular Vein
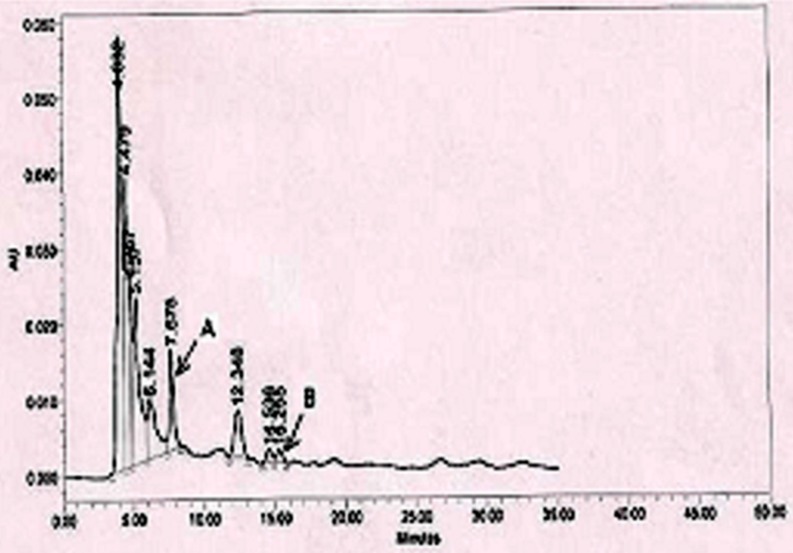
Phytochemical assessment of CC leaves extract showed the presence of total polyphenols, flavonoids and tannin as 103.04 ± 7.52 mg/g dry leaves, 63.41 ± 2.18 mg/g dry leaves and 28.16 ± 1.42 mg/g dry leaves respectively. Analysis reveals that the CC leaves contain high concentrations of polyphenols, which can be used as potential source of polyphenols. Further HPLC analysis led to the identification and quantification of polyphenolic compound Catechin and Quercetin in the extract (Fig. 3). HPLC was able to identified and quantified Catechin and Quercetin in the sample by comparison of retention times and spectra for each peak with the corresponding standard. HPLC analysis has the advantage over total phenolics content determined by the Folin-Ciocalteu method, as it provides more precise information of individual compounds, however, further studies are underway to identify other polyphenolic compound present in the CC leaves.
Fig. 3.
HPLC chromatogram of Clerodendron colebrookianum leaf extract at 283 nm. Peaks: a, catechin (432 ppm); b, quercetin (105 ppm)
Conclusions
The present study showed that the CPCC possesses hypolipidemic property and it needs some special attention to conduct further research to explain its molecular mechanism of its hypolipidemic effect. Data generated in the present study clearly indicated that CC leaf is a rich source of polyphenols which could be used as supplement in healthcare foods and drugs.
Acknowledgments
The authors acknowledge the financial support from the Indian Council of Medical Research (ICMR), Government of India, New Delhi, India, (No 59/30/2005/BMS/TRM) for this work.
Conflict of interest
The authors have declared that there is no conflict of interest.
References
- Alberto D, Carlos FH, Francisca C, Javier MB, Diego GC, Carmen GC, Miguel A. Red grape juice polyphenols alter cholesterol homeostasis and increase LDL receptor activity in human cells in vitro. J Nutr. 2006;136:1766–1773. doi: 10.1093/jn/136.7.1766. [DOI] [PubMed] [Google Scholar]
- Bainton D, Miller NE, Bolton CH, Yarnell JWG, Sweetnam PM, Baker IA, Lewis B, Elwood PC. Plasma triglyceride and high density lipoprotein cholesterol a predictors of ischemic heart disease in Britain man. Br Heart J. 1992;68:60–66. doi: 10.1136/hrt.68.7.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoloi B, Borthakur SK. Botanical identity of ‘Phuinum’ a folk remedy for hypertension. BMEBR. 1997;18(1):18–29. [Google Scholar]
- Chi F, Mao T, Simon J. Analysis of antioxidant as a therapeutic agent for atherosclerosis. Curr Pharm Anal. 2006;2:369–384. doi: 10.2174/157341206778699546. [DOI] [Google Scholar]
- Chidambaram T, Kumarappan T, Rao N, Mandal SC. Polyphenolic extract of Ichnocarpus frutescens modifies hyperlipidemia status in diabetic rats. J Cell Mol Biol. 2007;6(2):175–187. [Google Scholar]
- Devi R, Sharma DK. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J Ethnopharmacol. 2004;90:63–68. doi: 10.1016/j.jep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Devi R, Banerjee SK, Sood S, Maulik SK. In-vitro and in-vivo antioxidant activity of different extracts of the leaves of Clerodendron colebrookianum Walp in rat. J Pharma Pharmacol. 2003;55:1680–1688. doi: 10.1211/0022357022296. [DOI] [PubMed] [Google Scholar]
- Farris E, Ed J. The care and breeding of laboratory animals. John Wiley Songs. 1950;XVI:66–67. [Google Scholar]
- Floch J, Lees M, Stanley A simple method for the isolation and purification of total lipids from animal’s tissues. J Biochem. 1957;226:497–509. [PubMed] [Google Scholar]
- Frieedwalds WT, Levy IR, Frederickson SD. Estimation of concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Fryer GD, Carling D. AMP-activated protein kinase and the metabolic syndrome. Biochem Soc Trans. 2005;33:362–366. doi: 10.1042/BST0330362. [DOI] [PubMed] [Google Scholar]
- Greenstein JP (1954) Biochemistry of Cancer. New York: Academic Press, pp. 323
- Gupta R, Gupta HP, Kumar N, Joshi AK, Gupta VP. Lipoprotein lipids and prevalence of hyperlipidaemia in rural India. J Cardiovasc Risk. 1994;1:179–183. doi: 10.1177/174182679400100213. [DOI] [PubMed] [Google Scholar]
- Gupta R, Kaul V, Prakash H. Profiles of cholesterol and other lipids in Indian men. Indian Heart J. 1995;47:264–636. [Google Scholar]
- Hagerman A, Harvey-Mueller I, Makkar PS (2000) Quantification of tannins in tree foliage-A laboratory manual FAO/IAEA, Vienna b:4–7
- Hara Y. Prophylatic functions of tea polyphenols. Food phytochemicals for cancer prevention. Washington: American Chemical Society; 1994. pp. 34–35. [Google Scholar]
- Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179–5183. doi: 10.1210/en.2003-0982. [DOI] [PubMed] [Google Scholar]
- Hyeon CS, Seong HK, Yongju P, Bong HL, Hye JH (2011) Effects of 12 week oral supplementation of Ecklonia cava polyphenols on anthropometric and blood lipid parameters in overweight Korean individuals: a double–blind randomized clinical trial. Phytother Res. doi:10.1002/ptr.3559 [DOI] [PubMed]
- Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol. 2002;82:19–22. doi: 10.1016/S0378-8741(02)00136-8. [DOI] [PubMed] [Google Scholar]
- Liao XO, Shi B. Selective removal of tannins from medicinal plant extracts using a collagen fibre adsorbent. J Sci Food Agric. 2005;85:1285–1291. doi: 10.1002/jsfa.2114. [DOI] [Google Scholar]
- Mary YC, Susan PP, Thomas NW, Alan C. Oxidised LDL bind to nonproteoglycan components of smooth muscle extracellular matrices. J Res. 2001;42:824–833. [PubMed] [Google Scholar]
- Mengwei Z, Shanqin X, Karlene A, Maitland T, Adriana Z, Xiuyun H, Bingbing J, Michel W, Tony JV. Polyphenols stimulate AMP activated protein kinase lower lipids and inhibit accelerated atherosclerosis in diabetic LDL Receptor deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- Mon YY, Chiung HP, Kuei CC, Yi SY, Chien NH, Chau JW. The hypolipidemic effect of Hibiscus sabdariffa polyphenols via inhibiting lipogenesis and promoting hepatic lipid clearance. J Agric Food Chem. 2010;58:850–859. doi: 10.1021/jf903209w. [DOI] [PubMed] [Google Scholar]
- Mon YY, Chien NH, Kuei CC, Yi SY, Chiung HP, Chau JW. Mulberry leaf polyphenols possess antiatherogenesis effect via inhibiting LDL oxidation and foam cell formation. J Agric Food Chem. 2011;59:1985–1995. doi: 10.1021/jf103661v. [DOI] [PubMed] [Google Scholar]
- Nath SC, Bordoloi DN. Clerodendrum colebrookianum, a folk remedy for the treatment of hypertension in northeastern India. J Pharmacog. 1991;29(2):127–129. [Google Scholar]
- OECD (Organization for Economic Co-operation and Development). OECD Guidelines for the Testing of Chemicals/Section 4. (2002) Health effects test No. 423: acute oral toxicity-acute toxic class method. OECD, Paris
- Rao AV, Ramakrishnan S. Indirect assessment of hydroxyl methyl glutaryl-CoA reductase (NADPH) activity in liver tissue. Clin Chem. 1975;1(10):1523–1525. [PubMed] [Google Scholar]
- Schwartz CJ, Valente AJ, Sprague EA. A modern view of atherogenesis. Am J Cardiol. 1993;71:9b–14b. doi: 10.1016/0002-9149(93)90139-4. [DOI] [PubMed] [Google Scholar]
- Sharma DK, Bhuyan SK. Hypolipidemic effect of Clerodendron colebrookianum Walp glycosides in C3H mice. Planta Medica. 2006;72:1041. [Google Scholar]
- Sharma HK, Chhangte L, Dolui AK. Traditional medicinal plants in Mizoram, India. Fitoterapia. 2001;72(2):146–161. doi: 10.1016/S0367-326X(00)00278-1. [DOI] [PubMed] [Google Scholar]
- Singh NR, Singh MS. Wild medicinal plants of Manipur included in the Red List. Asian Agric Hist. 2006;13(3):221–225. [Google Scholar]
- Singh J, Bhuyan TC, Ahmed A. Ethnobotanical studies on the Mishing tribes of Assam with special reference to food and medicinal plants-1. J Econ Taxon Bot. 1996;12(1):350–356. [Google Scholar]
- Tall A. An overview of reverse cholesterol transport. Eur Heart J. 1998;19(suppl):A31–A35. [PubMed] [Google Scholar]
- Taskimen MR. Lipiprotin lipase in diabetes. Diabetes Metab Rev. 1987;3:551–570. doi: 10.1002/dmr.5610030208. [DOI] [PubMed] [Google Scholar]
- Wu ZY, Raven PH. Clerodendrum colebrookianum Walpers. Flora of China. 1994;17:40. [Google Scholar]
- Yang MH, Wang CH, Chen HL. Green, oolong and black tea extracts module lipid metabolism in hyperlipidemia rats fed high sucrose diet. J Nutr Biochem. 2001;12:14–20. doi: 10.1016/S0955-2863(00)00140-6. [DOI] [PubMed] [Google Scholar]
- Zlaktkins A, Zaak B, Boyle AJ. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953;41:486–491. [PubMed] [Google Scholar]