Abstract
During storage groundnut is attacked by number of stored grain pest and management of these insect pests particularly bruchid beetle, Caryedon serratus (Oliver) is of prime importance as they directly damage the pod and kernels. Hence, some essential oils were tested for their insecticidal and fungicidal properties. Highest total bruchid mortality was recorded with the application of neem oil and pongamia oil at 10% (v/w) concentration and lowest in eucalyptus oil at 5% (v/w). Number of eggs laid was recorded 2.3 in neem oil 10% (v/w) which was lowest and significantly superior over untreated control and was at par with castor oil 10% (v/w) which recorded 2.5 eggs per 100 g of groundnut pods. There was no adult emergence in the groundnut pods treated with castor oil, eucalyptus oil, neem oil and pongamia oil at 10% (v/w) concentration. Groundnut pods treated with castor oil, eucalyptus oil, neem oil and pongamia oil at 10% (v/w) and neem oil at 5% (v/w) concentrations recorded no damage to pods and kernels and also zero per cent weight loss. These oils effectively influenced groundnut bruchid establishment and reduce damage besides reduction in aflatoxin contamination.
Keywords: Arachis hypogaea, Bruchid beetle, Neem oil, Aflatoxin
Introduction
Groundnut, Arachis hypogaea L. is rich in nutrients, providing over 30 essential nutrients and phytonutrients. Groundnut seed contains about 44–56% oil and 22–30% protein on a dry seed basis and is a rich source of minerals (phosphorus, calcium, magnesium and potassium) and vitamins (E, K and B group) and also provides energy of 567 Kcal per 100 g (Savage and Keenan 1994). Groundnuts being an important source of nutrition in human diet and is often consumed either directly or as oil, are affected mainly by insects and pathogens. Bruchid beetle, Caryedon serratus (Oliver) is one of the major storage pests affecting the groundnut produce causing damage up to 70–80 % in stored groundnuts (Harish et al. 2012). It is speculated that in storage bruchids besides causing direct damage to groundnut, increases the contamination of aflatoxin in the stored groundnuts. Aflatoxin have assumed considerable significance due to its deleterious effects on human being, poultry and livestock (Abbas 2005; Chaytor et al. 2011; Diaz et al. 2010; Hifnawy et al. 2004; Iheshiulor et al. 2011; Taranu et al. 2010; Vijayasamundeeswari et al. 2009; Williams et al. 2010). It is a potent carcinogenic, mutagenic, and immunosuppressive agent (Diaz et al. 2010), produced as secondary metabolites by the fungi, Aspergillus flavus, A. parasiticus and A. nomius on a variety of food commodities (Essono et al. 2009; Kurtzman et al. 1987). Among these, A. flavus is the predominant species present in the Indian soil (Ahmad and Singh 1994). Infection of A. flavus and subsequent aflatoxin contamination in groundnut can occur at pre-harvest, harvest, post-harvest storage and processing. Aluminium phosphide a common grain preservative is extensively used for the management of bruchids in storage, all over the world particularly in the developing countries. However, recent research showed it was quite poisonous and could cause serious toxic effects to lungs, heart and blood vessels resulting in pulmonary oedema, shock and arrhythmias (Abder-Rahman 1999). Use of pesticides should be reduced as groundnut is often consumed directly hence, the biorational approaches are more appropriate. In order to retain nutritive value of groundnut eco-friendly approach was used to study the insecticidal properties of essential oils against bruchid beetle.
Material and methods
The laboratory study was conducted at Entomology section of Directorate of Groundnut Research (DGR), Junagadh, during the year 2011–2012. Four essential oils viz., castor oil, eucalyptus oil, neem oil and pongamia oil were used at two concentrations 5 and 10% (v/w), respectively for treating 100 g of pods along with these treatments, untreated and unreleased control were used as checks. Freshly emerged adults on the day of release were collected from the mother culture maintained in the lab and paired. Five pair of adults were released into plastic containers of 500 g capacity containing 100 g groundnut pods of variety GG 2. In untreated control there was no oil treatment but bruchid adults were released and in unreleased control groundnut pods were neither treated with oil nor bruchids were released. There were three replications for each treatment. The mouth of the containers were then covered with muslin cloth and held in place with the help of rubber bands and kept at 30°C in BOD incubator. During the emergence of first generation C. serratus, adults were removed and counted daily till no further adult emergence was observed for five consecutive days. Observations on number of eggs laid, adult emergence, number of damaged pod, weight of damaged pod, number of damaged kernel and weight of damaged kernel were recorded. Pod and kernel damage was expressed as a proportion of total pod and kernel, respectively. Pod and kernel weight loss was expressed as per centage of initial pod and kernel weight, respectively. Damage per centage was determined using the formula, 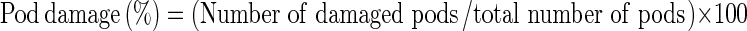 and similarly, kernel damage was also calculated, weight loss was calculated using count and weight method (Gwinner et al. 1996):
and similarly, kernel damage was also calculated, weight loss was calculated using count and weight method (Gwinner et al. 1996):
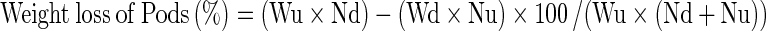 |
Where
- Wu
Weight of undamaged Pods
- Nu
Number of undamaged Pods
- Wd
weight of damaged Pods and
- Nd
Number of damaged Pods
Similarly, weight loss in kernel was also calculated.
A similar set was kept for recording mortality; here ten adults were released into plastic containers of 500 g capacity. Number of dead adults were recorded after 24, 48 and 72 h after treating pods with essential oils. Abbott’s formula was used to calculate actual mortality (Abbott 1925).
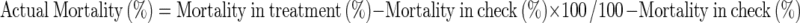 |
Contamination of aflatoxin in groundnuts was analyzed by indirect competitive ELISA (Ajitkumar et al. 2004). Indirect competitive ELISA uses aflatoxin B1-Bovine Serum Albumin conjugate coated on microtitre plate. The coated plate is incubated with the aflatoxin antibody labeled with enzyme and standard/sample in aflatoxin. The AFB1 in the standard/sample competes with aflatoxin on the solid phase for enzyme labeled antibody. The amount of antibody bound to the plate is ultimately determined by enzyme-substrate reaction.
Statistical analysis
There were three replications in each treatment. The experiment was repeated twice; the data were pooled and analyzed. One way analysis of variance in complete randomized design was employed and analyzed using DSAASTAT software (Onobri 2007) and analysis of variance was worked out. Differences between means were considered significant at p<0.05 by LSD test.
Results and discussion
Mortality is the number of insects dying per unit of time, during 24 h after treatment, highest mortality of 88.2% was recorded in neem oil and pongamia oil at 10% (v/w) concentration which was significantly superior over all other treatments where as eucalyptus oil at 5% (v/w) concentration recorded the lowest mortality of 20.6%. Similarly, at 48 h after treatment mortality was highest in pongamia oil at 5% (v/w) concentration followed by neem oil at 5% (v/w) concentration and at 72 h after treatment eucalyptus oil at 5% (v/w) concentration recorded highest mortality of 12.2%. However highest total mortality was recorded in neem oil and pongamia oil at 10% (v/w) concentration and lowest in eucalyptus oil at 5% (v/w) concentration (Table 1). Essential oils of plant origin are highly lipophilic and therefore have the ability to penetrate the cuticle of insects (Abdullahi et al. 2011). Neem oil was found to cause 100% mortality of Callasobruchus maculates after two days of treatment (Ram and Gopal 2000).
Table 1.
Mortality of Caryedon serratus on groundnut treated with different essential oils
| Treatments | Per cent mortality (h) | ||
|---|---|---|---|
| 24 | 48 | 72 | |
| Castor oil 5% (v/w) | 69.7 (56.9)a | 12.2 (17.8) | 3.8 (10.4) |
| Castor oil 10% (v/w) | 83.2 (66.9) | 2.2 (7.2) | 0.5 (4.1) |
| Eucalyptus oil 5% (v/w) | 20.6 (23.7) | 12.2 (19.8) | 12.2 (20.0) |
| Eucalyptus oil 10% (v/w) | 74.6 (60.9) | 5.5 (12.2) | 0.5 (4.1) |
| Neem oil 5% (v/w) | 37.7 (37.9) | 28.9 (32.4) | 5.5 (10.4) |
| Neem oil 10% (v/w) | 88.2 (73.8) | 0.5 (4.1) | 0.5 (4.1) |
| Pongamia oil 5% (v/w) | 37.4 (35.8) | 32.3 (34.4) | 0.5 (4.1) |
| Pongamia oil 10% (v/w) | 88.2 (73.8) | 0.5 (4.1) | 0.5 (4.1) |
| Unreleased | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Untreated | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| SEM ± | 7.2 | 3.5 | 2.5 |
| LSD (p<0.05) | 21.2 | 10.3 | 7.3 |
Each observation is based on three replicate, afigures in the parenthesis are arcsine (X+0.5) transformed values
Analysis of variance indicated that there was a significant difference among the various essential oils and different concentrations used for number of eggs laid, adult emergence, per cent damage in pods and kernels and per cent weight loss in pods and kernels (Table 2). Oils are commonly used in insect control because the oils are relatively effective against all stages of insects (Adedire 2002).
Table 2.
Number of eggs laid, adults emerged, per cent damage and per cent weight loss caused by bruchid beetles on groundnut
| Treatments | Eggs laid (Nos) | Adult emergence (Nos) | Damaged pods (%) | Damaged Kernels (%) | Weight loss Pods (%) | Weight loss Kernels (%) |
|---|---|---|---|---|---|---|
| Castor oil 5% (v/w) | 17.8 (4.1)a | 2.3 (1.4)a | 2.2 (8.0)b | 0.8 (6.6)b | 1.5 (5.0)b | 0.7 (4.9)b |
| Castor oil 10% (v/w) | 2.5 (1.6) | 0.5 (0.7) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Eucalyptus oil 5% (v/w) | 49.5 (7.0) | 15.5 (3.9) | 13.7 (21.6) | 1.9 (21.6) | 13.7 (7.8) | 2.0 (8.2) |
| Eucalyptus oil 10% (v/w) | 12.2 (3.2) | 0.5 (0.7) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Neem oil 5% (v/w) | 9.7 (3.1) | 1.0 (0.9) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Neem oil 10% (v/w) | 2.3 (1.5) | 0.5 (0.7) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Pongamia oil 5% (v/w) | 16.2 (3.9) | 1.0 (1.0) | 0.8 (5.1) | 0.6 (5.5) | 1.0 (4.5) | 0.6 (4.2) |
| Pongamia oil 10% (v/w) | 7.0 (2.6) | 0.5 (0.7) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Unreleased | 0.5 (0.7) | 0.5 (0.7) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) | 0.5 (4.1) |
| Untreated | 68.2 (8.0) | 63.3 (7.7) | 58.0 (50.2) | 19.0 (46.1) | 52.0 (23.3) | 9.1 (17.4) |
| SEM ± | 0.6 | 0.5 | 2.8 | 1.1 | 3.0 | 0.6 |
| LSD (p<0.05) | 1.9 | 1.4 | 8.1 | 3.3 | 8.9 | 1.9 |
Each observation is based on three replicate, afigures in the parenthesis are √X+0.5 transformed values, bfigures in the parenthesis are arcsine (X+0.5) transformed values.
The egg mortality and failure to hatch on the seed treated with oil has been attributed to the toxic component of the oil and also to the physical properties which cause changes in the surface tension and oxygen tension within the eggs (Singh 1978). Number of eggs laid was recorded 2.3 in neem oil 10% (v/w) which was lowest and significantly superior over untreated control and was at par with castor oil 10% (v/w) which recorded 2.5 eggs per 100 g of groundnut pods. Highest number of eggs was laid in untreated control followed by eucalyptus oil 5% (v/w) where the eggs laid were 49.9 per 100 g of groundnut pods. Oils of Azadirecta indica, Milletiaie ferruginea and Chrysanthemum cinerarefolium were most effective in partially or completely preventing egg laying and emergence of pulse beetle (Mulatu and Gebremedhin 2000).
There was no adult emergence recorded in the groundnut pods treated with castor oil, eucalyptus oil, neem oil and pongamia oil at 10% (v/w) concentration, however highest number of adults 15.5 emerged in eucalyptus oil 5% (v/w) treated groundnut after untreated control. Application of oils occlude seed funnel leading to the death of the developing embryo of the insect by asphyxia which ultimately reduced the emergence of the insect from the treated seed (Copping and Menn 2000).
Groundnut pods treated with castor oil, eucalyptus oil, neem oil and pongamia oil at 10% (v/w) and neem oil 5% (v/w) concentrations recorded no damage to pods and kernels and also zero per cent weight loss. However, eucalyptus oil at 5% (v/w) concentration recorded 13.7 and 1.9% damage in pods and kernels, respectively and also 13.7% and 2.0% weight loss in pods and kernels of groundnut, respectively which was significantly superior over other treatments except untreated control. The low grain damage in oil treatment might be due to the decrease in number of adult emergence that results in less weight loss and less kernel damage (Vijaya and Khader 1990).
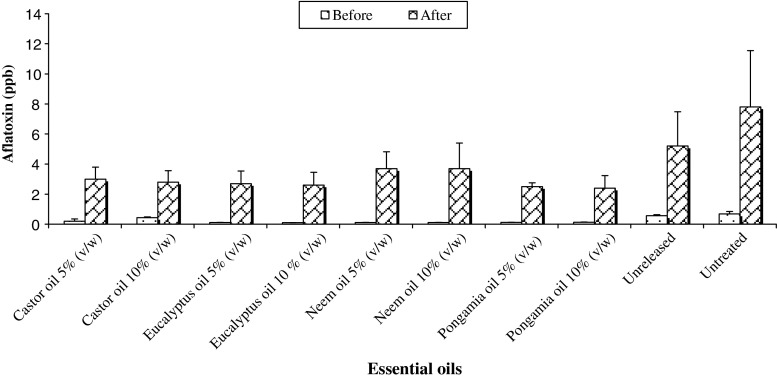
In addition to insect toxicity, some of the essential oils have the advantage of showing effectiveness against storage fungi also. Exposure of wheat to the essential oils of Caesulia axillaris Roxb. and Mentha arvensis resulted in complete control of insects, Sitophilus oryzae and Tribolium castaneum as well as the fungi including Aspergillus. flavus, A. niger and A. fumigatus, (Varma and Dubey 2001). Aflatoxin content is highest in untreated control (7.8 ppb) where groundnut is not treated with any essential oil but bruchids were released. All essential oils recorded less aflatoxin content compared to unreleased and untreated control, however pongamia oil 10% (v/w) recorded the lowest aflatoxin content of 2.4 ppb (Fig. 1). The effects of essential oil of clove and its principal components eugenol, on growth and mycotoxin production by some toxigenic fungal genera like Aspergillus spp. and Penicillium spp. (Cairns and Magan 2003). Antiaflatoxigenic actions of eugenol may be related to inhibition of the ternary steps of aflatoxin production, without any significant constraints on growth or events of primary metabolism (Jayashree and Subramanyam 1999).
Fig. 1.
Aflatoxin content (ppb) in groundnut treated with different essential oils. Bar represents Standard deviation (SD) of three replications
Conclusion
Use of essential oils for managing stored insect pests is one of the most commonly followed traditional practices in villages, of developing countries like India. Hence an attempt was made to know the insecticidal and fungicidal properties of some essential oils. Essential oils were found to be effective in managing the bruchid beetles and also showed some fungicidal properties at 10 % (v/w) concentration. However, eucalyptus oil at 5% concentration was not able to manage the bruchid as effectively as other essential oils. Bruchids infesting on stored groundnut may be managed using essential oil, which is an eco friendly method. Further research is required to ascertain insecticidal and fungicidal properties in all easily available essential oils (Tables 1 and 2).
References
- Abbas HK. Aflatoxin and food safety. USA: CRC Press; 2005. p. 587. [Google Scholar]
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Ent. 1925;18:265–267. [PubMed] [Google Scholar]
- Abder-Rahman H. Effect of aluminum phosphide on blood glucose level. Vet Hum Toxicol. 1999;41:31–32. [PubMed] [Google Scholar]
- Abdullahi N, Majeed Q, Oyeyi T. Studies on the efficacy of Vittallaria paradoxa seed oil on the Oviposition, hatchability of eggs and emergence of Callasobruchus maculates (F.) (Coleoptera: Bruchidae) on treated cowpea seed. J Ent. 2011;8:391–397. doi: 10.3923/je.2011.391.397. [DOI] [Google Scholar]
- Adedire CO. Use of nutmeg Myristica fragrans (Houtt.) Powder and oil for the control of cowpea storage bruchid, Callosobruchus maculates Fabricius. J Plant Dis Plant Prot. 2002;109:193–199. [Google Scholar]
- Ahmad SK, Singh PL. Distribution of Aspergillus flavus in soil and air of agricultural fields. Indian Phytopath. 1994;47:81–86. [Google Scholar]
- Ajitkumar A, Naik MK, Waliyar F, Reddy SV. Use of indirect competitive ELISA technique for detection of aflatoxins B1 contamination in chilli. In: Mayee CD, Manoharachary C, Tilak KVBR, Mukadam DS, Deshpande J, editors. Biotechnological Approaches for the Integrated Management of Crop Diseases. New Delhi: Daya Publishing House; 2004. pp. 8–14. [Google Scholar]
- Cairns V, Magan N. Impact of essential oils on growth and ochratoxin A production by Penicillium verrucosum and Aspergillus ochraceus on a wheat-based substrate. In: Credland PF, Armitage DM, Bell CH, Cogan PM, Highley E, editors. Proceedings of the eighth international working conference on stored product protection advances in stored product protection, 22–26 July 2002, York. Wallingford: CAB International; 2003. pp. 479–485. [Google Scholar]
- Chaytor AC, See MT, Hansen JA, de Souza ALP, Middleton TF, Kim SW. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J Anim Sci. 2011;89:124–135. doi: 10.2527/jas.2010-3005. [DOI] [PubMed] [Google Scholar]
- Copping LG, Menn JJ. Bio pesticides. A review of their action application and efficacy. Pest Manage Sci. 2000;56:651–676. doi: 10.1002/1526-4998(200008)56:8<651::AID-PS201>3.0.CO;2-U. [DOI] [Google Scholar]
- Diaz GJ, Murcia HW, Cepeda SM. Bioactivation of aflatoxin B1 by turkey liver microsomes: responsible cytochrome P450 enzymes. Br Poult Sci. 2010;51:828–837. doi: 10.1080/00071668.2010.528752. [DOI] [PubMed] [Google Scholar]
- Essono G, Ayodele M, Akoa A, Foko J, Filtenborg O, Olembo S. Aflatoxin-producing Aspergillus spp. and aflatoxin levels in stored cassava chips as effected by processing practices. Food Control. 2009;20:648–654. doi: 10.1016/j.foodcont.2008.09.018. [DOI] [Google Scholar]
- Gwinner J, Harnisch R, Muck O. Manual on the prevention of post harvest seed losses, post harvest project, GTZ, D-2000. FRG: Hamburg; 1996. p. 294. [Google Scholar]
- Harish G, Holajjer P, Savaliya SD, Gedia MV. Population density on damage of groundnut by Caryedon serratus. Ann Pl Protec Sci. 2012;20:217–219. [Google Scholar]
- Hifnawy MS, Mangoud AM, Eissa MH, Edin EN, Mostafa Y, Abouel-Magd Y, Sabee EI, Amin I, Ismail A, Morsy TA, Mahrous S, Afefy AF, El-Shorbagy E, El-Sadawy M, Ragab H, Hassan MI, El-Hady G, Saber M. The role of aflatoxin-contaminated food materials and HCV in developing hepatocellular carcinoma in Al-Sharkia Governorate. J Egypt Soc Parasitol. 2004;34:479–488. [PubMed] [Google Scholar]
- Iheshiulor OOM, Esonu BO, Chuwuka OK, Omede AA, Okoli IC, Ogbuewu IP. Effects of mycotoxins in animal nutrition: a review. Asian J Anim Sci. 2011;5:19–33. doi: 10.3923/ajas.2011.19.33. [DOI] [Google Scholar]
- Jayashree T, Subramanyam C. Antiaflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Lett Appl Microbiol. 1999;28:179–183. doi: 10.1046/j.1365-2672.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- Kurtzman CP, Horn BW, Hesseltine CW. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Van Leeuwenhoek. 1987;53:147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- Mulatu B, Gebremedhin T. Oviposition-Deterrent and Toxic Effects of Various Botanicals on the Adzuki Bean Beetle. Callosobruchus Chinensis L. Int J Trop Insect Sci. 2000;20:33–38. doi: 10.1017/S174275840001780X. [DOI] [Google Scholar]
- Onobri A (2007) Routine statistical analysis of field experiments by using an excel extension. Proceedings 6th national conference Italian Biometric Society: “La statistica nelle science della vita e dell anbi ante” 20–22 June, Pisa, pp 93–96
- Ram BP, Gopal PS. Use of botanicals for the management of pulse beetle (Callosobruchus maculates) in lentils. Nepal Agric Res J. 2000;4–5:27–30. [Google Scholar]
- Savage GP, Keenan JI. The composition and nutritive value of groundnut kernels. In: Smart J, editor. The groundnut crop: scientific basis for improvement. London: Chapman and Hall; 1994. pp. 173–213. [Google Scholar]
- Singh SR. Resistance to pestof cowpeas in Nigeria. In: Singh SR, Van Emden HF, Taylor, editors. Pests of grain legumes: ecology and control. London: Academic; 1978. pp. 267–269. [Google Scholar]
- Taranu IE, Marin DP, Burlacu R, Pinton P, Damian V, Oswald I. Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Arch Anim Nutr. 2010;64:383–393. doi: 10.1080/1745039X.2010.492140. [DOI] [PubMed] [Google Scholar]
- Varma J, Dubey NK. Efficacy of essential oils of Caesulia axillaris and Mentha arvensis against some storage pests causing biodeterioration of food commodities. Int J Food Microbiol. 2001;68:207–210. doi: 10.1016/S0168-1605(01)00506-2. [DOI] [PubMed] [Google Scholar]
- Vijaya, Khader Pachymerus chinensis, its biology and extent of damage to grains. Plant Prot Bull. 1990;25:23–28. [Google Scholar]
- Vijayasamundeeswari A, Mohankumar M, Karthikeyan M, Vijayanandraj S, Paranidharan V, Velazhahan R. Prevalence of aflatoxin B1 contamination in pre- and post-harvest maize kernels, food products, poultry and livestock feeds in Tamil Nadu, India. J Plant Protection Res. 2009;49:221–224. [Google Scholar]
- Williams JH, Grubb JA, Davis JW, Wang J, Jolly PE, Ankrah N, Ellis WO, Afriyie-gyawu E, Johnson NM, Robinson AG, Phillips TD. HIV and hepatocellular and esophageal carcinomas related to consumption of mycotoxin-prone foods in sub-Saharan Africa. Am J Clin Nutr. 2010;92:154–160. doi: 10.3945/ajcn.2009.28761. [DOI] [PubMed] [Google Scholar]