Abstract
Utilization of waste from fish processing industry for production of value added products has attracted substantial attention. Blackspotted croaker (Protonibea diacanthus) is a marine fish having the potential of abundant supply of raw skins for production of gelatin. This study was aimed to optimize the extraction conditions for gelatin production from skin of Blackspotted croaker. Response surface methodology (RSM) was adopted by following central composite design to determine the optimal conditions of four independent variables namely concentration of NaOH (X1), soaking time (X2), extraction temperature (X3) and extraction time (X4) for three response variables namely yield, gel strength and melting point. The models obtained by RSM produced a satisfactory fit to the data with respect to gelatin extraction (for gelatin yield, R2 = 0.867, P = 0.0003; for gel strength, R2 = 0.837, P = 0.007; for melting point, R2 = 0.765, P = 0.01). Based on these models, the optimum conditions to achieve the predicted maximum values were: yield of 17.21 % at X1 = 0.23 %, X2 = 46.19 min, X3 = 55.29 °C and X4 = 17.29 h; gel strength of 422.69 g at X1 = 0.22 %, X2 = 44.56 min, X3 = 59.02 °C and X4 = 15.35 h and melting point of 23.48 °C at X1 = 0.20 %, X2 = 46.68 min, X3 = 56.23 °C and X4 = 15.21 h. It can be concluded from the present study that Blackspotted croaker skin is a prospective source to produce gelatin in good yield with desirable quality attributes comparable to commercially available mammalian gelatins.
Keywords: Blackspotted croaker, Gelatin, Gel strength, Response surface methodology
Introduction
Gelatin, a denatured protein derived from animal collagen by thermo-hydrolysis, is one of the most important biopolymers with widespread applications in various industries. In the food industry, gelatin is used extensively in the manufacturing of desserts, candies, bakery products, jellied meats, ice cream, and dairy products. In the pharmaceutical industry, the manufacture of pharmaceutical capsules, ointments, cosmetics, tablet coatings, and emulsions often involve gelatin as one of the ingredients. Gelatin also finds application in photography and some specialized industries (Djagny et al. 2001).
The major sources of collagen for the production of gelatin are porcine skin, cattle hides and bones. Available reports indicate that the annual world output of gelatin is nearly 326,000 tons with pig skin-derived gelatin accounting for the highest (46 %) output (GME 2005). The demand for gelatin has been increasing at a steady rate of approximately 2 % per annum thus resulting in high prices for gelatin. Due to the outbreak of mad cow disease or Bovine Spongiform Encephalopathy (BSE) as well as the unacceptability of gelatin produced from bovine and/or porcine sources for Muslims, Jews and Hindus, the search for alternative sources has been initiated and accelerated. Fish processing waste is abundant and could be a valuable source of gelatin. It also offers an alternative for those applications where ethical or religious reasons exclude the use of mammalian gelatin products (Badii and Howell 2006).
Gelatin extraction from fish processing waste including skin, bones and/or scales, has received increasing attention (Jongjareonrak et al. 2006a). Processing of fish leads to enormous amounts of waste. It is estimated that fish processing waste after filleting accounts for approximately 75 % of the total fish weight (Shahidi 1994) and 30 % of the waste is in the form of bone and skin (Gómez-Guillén et al. 2002). This fish skin and bone can be processed into gelatin, thus contributing to solve the problem of waste disposal and in addition creating a value-added product. Therefore research on fish gelatin assumes significance for the development of methods for the efficient utilization of by-product of fish as replacement for mammalian sources (Montero and Gómez-Guillén 2000). Accordingly, many fish species have been investigated as raw materials for gelatin extraction like lumpfish (Osborne et al. 1990), tilapia (Grossman and Bergman 1992; Jamilah and Harvinder 2002), conger eel and squid (Kim and Cho 1996), cod (Gudmundsson and Hafsteinsson 1997), shark (Yoshimura et al. 2000), megrim (Montero and Gómez-Guillén 2000), Nile perch (Muyonga et al. 2004), Alaska pollock (Zhou and Regenstein 2004), sin croaker and shortfin scad (Cheow et al. 2007), grass carp (Kasankala et al. 2007), shark cartilage (Cho et al. 2004), yellowfin tuna (Cho et al. 2005), bigeye snapper and brownstripe snapper (Jongjareonrak et al. 2006b) and channel catfish (Yang et al. 2007; Liu et al. 2008).
Blackspotted croaker (Protonibea diacanthus) is a popular warm water marine fish which is well-accepted by consumers. This species is harvested in sufficient quantity which may have commercial potential for gelatin production. As Blackspotted croaker is usually processed or sold as skinless fillet, there is an abundant quantity of raw skin available. So far, no studies have been conducted on extraction and evaluation of Blackspotted croaker fish gelatin. Considering all these facts, Blackspotted croaker fish was chosen as the raw material for this study.
Gelatin quality is typically evaluated on the basis of several functional properties like gel strength, gelling and melting points and viscosity. Functional properties of gelatin differ with the extraction time, extraction temperature, soaking time and concentration of NaOH used for treating the skin. To maximize the yield and functionalities of gelatin obtained, the extraction process needs to be optimized.
Response surface methodology (RSM) is a useful statistical technique for the investigation and optimization of complex processes. It uses quantitative data from an appropriate experimental design to determine and simultaneously solve multivariate equation (Rastogi et al. 2010). Besides, this experimental methodology can generate a mathematical model and optimize the process conditions (Bas and Boyaci 2007; Yang et al. 2008; Ganesan et al. 2009; Garg and Singh 2010; Singh et al. 2012). Central composite design (CCD) is a widely used response surface design when the experimental region is defined by the upper and lower limits of each factor and not extended beyond them (Neter et al. 1996). A combination of factors generating a certain optimal response can be identified and also significant interactions between variables can be identified and quantified by this technique.
The aim of the present study was to model and evaluate the combined effects of concentration of NaOH, soaking time, extraction temperature and extraction time on gelatin yield, gel strength and melting point by applying the response surface methodology.
Methods and materials
Raw material
Blackspotted croaker (Protonibea diacanthus) fish skin with average size of 70–100 cm was purchased fresh from Shivaji fish market (Mumbai, India) and transported in ice in the ratio 1:1 to the laboratory. The skin was washed and cut into small pieces (1 × 1 cm2). The prepared skin was stored at −20 °C until used.
Chemicals
Sodium hydroxide, sulphuric acid and citric acid were purchased from Qualigens, Mumbai (India) and all are of analytical grade.
Experimental design
Software Unscrambler (version 9.5, CAMO, Norway) was employed to establish the optimum extraction conditions for gelatin from Blackspotted croaker fish skin using central composite design (CCD) with quadratic model to study the individual, interactive and square effects of the four independent variables (sodium hydroxide concentration (%), soaking time (min.), extraction temperature (°C) and extraction time (h)). These four independent variables are coded as X1, X2, X3 and X4 respectively. Five levels were chosen for each independent variable in the form −2, −1, 0, +1, and +2, where −2 and +2 were the lowest and highest levels, respectively, with 0 level as the center point. A total of 27 combinations including three replicates of the centre point were carried out in random order according to CCD configuration for the four chosen variables. The gelatin yield, gel strength and melting point were chosen as the dependent variables or responses. The design matrix and variable composition levels are given in the Table 2.
Table 2.
Design matrix for the optimization of gelatin extraction procedure
| Design points | Independent variable levels | |||
|---|---|---|---|---|
| X1 | X2 | X3 | X4 | |
| 1 | −2 | 0 | 0 | 0 |
| 2 | +2 | 0 | 0 | 0 |
| 3 | 0 | +2 | 0 | 0 |
| 4 | 0 | −2 | 0 | 0 |
| 5 | 0 | 0 | +2 | 0 |
| 6 | 0 | 0 | +2 | 0 |
| 7 | 0 | 0 | 0 | +2 |
| 8 | 0 | 0 | 0 | +2 |
| 9 | −1 | −1 | −1 | 0 |
| 10 | +1 | −1 | −1 | 0 |
| 11 | −1 | +1 | −1 | 0 |
| 12 | +1 | +1 | −1 | 0 |
| 13 | −1 | −1 | +1 | 0 |
| 14 | +1 | −1 | +1 | 0 |
| 15 | −1 | +1 | +1 | 0 |
| 16 | +1 | +1 | +1 | 0 |
| 17 | −1 | −1 | −1 | +1 |
| 18 | +1 | −1 | −1 | +1 |
| 19 | −1 | +1 | −1 | +1 |
| 20 | +2 | +1 | −1 | +1 |
| 21 | −1 | −1 | +1 | +1 |
| 22 | +1 | −1 | +1 | +1 |
| 23 | −1 | +1 | +1 | +1 |
| 24 | +1 | +1 | +1 | +1 |
| 25 | 0 | 0 | 0 | 0 |
| 26 | 0 | 0 | 0 | 0 |
| 27 | 0 | 0 | 0 | 0 |
X1 = Concentration of NaOH, X2 = Soaking time (min.), X3 = Extraction temp., X4 = Extraction time (h).
Gelatin extraction
Gelatin was extracted following the procedure described by Gudmundsson and Hafsteinsson (1997) with some modification. Thawed Blackspotted croaker fish skin was thoroughly cleaned and rinsed with excess water to remove superfluous material and then treated with alkali (NaOH) solution at varying concentrations and soaking time according to the experimental design (Tables 1 and 2). Then, it was soaked with 0.2 % sulphuric acid for 40 min. and followed by soaking with 0.7 % citric acid for 40 min. After each soaking treatment, the skin was washed under running tap water until they had a pH of about 7.0. Each soaking and washing treatment was repeated three times with a total time of 2 h for each treatment. The ratio of skin to washing liquid used was 1 kg skin (wet weight) to 7 L of acid or alkali solution for each treatment. The skins were then subjected to a final wash with distilled water to remove any residual matter. The final extraction was carried out in distilled water at varying temperature and time according to the experimental design (Tables 1 and 2). The ratio of skin: water used was 1:3 (w/v). The clear extract obtained was filtered with Whatman filter paper (No.1), using a Buchner funnel. The filtrate was then kept in a tray and dried in oven at 60 °C for 16 h. The thin film of dried matter was powdered, weighed and packed in Zip pack bags, stored at ambient temperature for further study. The yield of gelatin was calculated on wet weight basis of raw material and expressed as percentage yield. Percentage yield of extracted gelatin was calculated by the following formula.
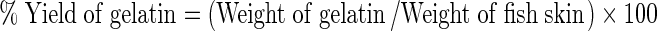 |
Table 1.
Experimental values and coded levels of the independent variables used for production of Blackspotted croaker skin gelatin
| Independent variables | Symbol | Coded variable level | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | ||
| Sodium hydroxide concentration (%) | X1 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
| Soaking time (min.) | X2 | 15 | 30 | 45 | 60 | 75 |
| Extraction temperature (°C) | X3 | 35 | 45 | 55 | 65 | 75 |
| Extraction time (h) | X4 | 8 | 12 | 16 | 20 | 24 |
Determination of gel strength
The gel strength of gelatin gel was determined according to the method described by Wainewright (1977). The gel was formed by dissolving a 6.67 % (w/v) dry gelatin powder in distilled water at 60 °C. The glass jar (46 mm diameter and 74 mm height, flat bottom) was covered and allowed to cool for 15 min at room temperature. The jars with solution were kept in a refrigerator at 7 °C (maturation temperature) for 18 h. Gel strength was determined on TA.XT2i Texture Analyzer (Stable micro systems, Godalming, Surrey, U.K) with a load cell of 5 kg, crosshead speed 1 mm/s and equipped with a 0.5 inch diameter, flat bottomed plunger. The jar was placed centrally under the plunger and the penetration test was then performed. The maximum force (in g) was determined when the probe proceeded to penetrate into the gel to a depth of 4 mm. The reading was the average of three determinations.
Determination of melting point of gel
The melting point measurement was done by a method modified from Wainewright (1977). Gelatin solutions, 6.67 % (w/w), were prepared and a 5 ml aliquot of each sample was transferred to a small glass tube (borosilicate tube, 12 mm × 75 mm2). The samples were degassed in vacuum desiccators for 5 min. The tubes were then covered with Para film and heated in a water bath at 60 °C for 15 min. The tubes were immediately cooled in ice-chilled water and matured at 10 °C for 18 h. Five drops of a mixture of 75 % chloroform and 25 % reddish brown dye (food colour) was placed on the surface of the gel. The gels were kept on a water bath at 10 °C and the bath was heated at the rate of 0.2 to 0.4 °C/min. The temperature of the bath was read using an electronic digital thermometer (Fisher Scientific, Germany). The temperature at which the dye drops began to move freely down the gel was taken as the melting point.
Statistical analysis
The effect of independent (design) variables on the dependent variables (gel strength, gelatin yield, and melting point) was studied using response surface methodology (Unscrambler, CAMO, Norway). The dependent variables were expressed individually as a function of the independent variables known as response function. The variance for each factor assessed was partitioned into linear, quadratic and interactive components and were represented using the second order polynomial equation.
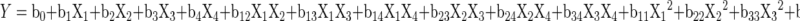 |
In this equation, Y represents the dependent variable and Xi (i = 1 to 4) represent the independent variables. Coefficients of the polynomial were represented by b0 (constant term), b1, b2, b3 and b4 (linear coefficients), b11, b22, b33, and b44 (quadratic coefficients) and b12, b13, b14, b23, b24 and b34 (interactive coefficients). From the ANOVA table, less significant terms were ignored to improve the model adequacy. The response surface plots for each response were generated as a function of two variables, while keeping other two variables at their centre points. ANOVA of each response was studied for finding the significance of variables and its interaction and square effects. The significance of all terms in the polynomial functions were assessed statistically using P value of 0.001, 0.01 and 0.05. R2 values were used to judge the adequacy of the models.
Results and discussion
Optimization of the gelatin extraction procedure
The response variables namely yield, gel strength and melting point were measured on gelatin extracted following different combinations of extraction conditions (independent variables) obtained by central composite design (Table 2). The data obtained for all the responses were analyzed by response surface methodology. Regression model was developed for each response by ignoring insignificant terms and the resulting models were found statistically significant with the observed P values of gelatin yield, gel strength and melting point as < 0.0003, <0.007 and <0.01 respectively. The coefficients of linear, quadratic and interaction terms for each model and their R2 values are shown in the Table 3. The mathematical equations obtained for the maximum predicted values for different response variables are provided in Table 4, where the independent variables are in terms of original values. The measured and predicted response values for individual design points are given in Table 5. The high values of correlation (r) for gelatin yield (0.9311), gel strength (0.915) and melting point (0.8746) suggest that there is a good agreement between the measured values obtained from the experiment and predicted values derived from the model.
Table 3.
Coefficient of variables in the model and their corresponding R 2 values
| Variables | Gelatin yield | Gel strength | Melting point |
|---|---|---|---|
| B0 | 17.130 | 408.5 | 23.433 |
| X1 | 2.508 | 642.067* | 6.0 |
| X2 | −0.007 | 0.954 | 0.0094 |
| X3 | 0.049* | 0.615 | 0.2667 |
| X4 | 0.113* | 4.282 | −0.0678 |
| X12 | 0.378 | −18.375 | −1.062** |
| X13 | −0.586* | −15.744 | −0.538 |
| X14 | ----- | 22.587 | 0.588* |
| X23 | ----- | 19.079 | ------- |
| X24 | 0.549* | 17.948 | 0.277 |
| X34 | −0.694** | 17.602 | -------- |
| X1 2 | 0.174 | −26.146* | −0.262 |
| X2 2 | −0.903*** | −44.288** | −0.354 |
| X3 2 | −0.430* | −55.896*** | −1.0** |
| X4 2 | 0.378* | −50.692*** | −0.573* |
| R2 | 0.867 | 0.837 | 0.765 |
| P Value | 0.0003*** | 0.007** | 0.01* |
*Significant at P < 0.05, **Significant at P < 0.01, ***Significant at P < 0.001, b0 = model constant, X1 = Concentration of NaOH (%), X2 = Soaking time (min.), X3 = Extraction temperature (°C), X4 = Extraction time (h)
Table 4.
Response surface models for processing conditions of gelatin from Blackspotted croaker (Protonibea diacanthus) skin
| Responses | Quadratic polynomial model |
|---|---|
| Yield |
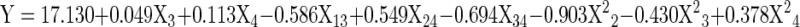
|
| Gel strength |
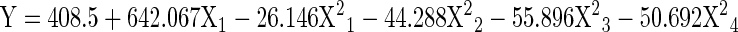
|
| Melting point |
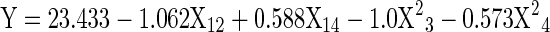
|
X1 = concentration of NaoH (%), X2 = soaking time (min), X3 = extraction temperature (°C) and X4 = extraction time (h), Y = predicted value
Table 5.
Measured and predicted response values for individual design points
| Design points | Dependent variables | |||||
|---|---|---|---|---|---|---|
| Gelatin yield (%) | Bloom value (g) | Melting point (°C) | ||||
| Measured | Predicted | Measured | Predicted | Measured | Predicted | |
| 1 | 17.010 | 17.631 | 210.320 | 230.993 | 21.500 | 21.700 |
| 2 | 18.530 | 18.133 | 330.010 | 359.407 | 22.000 | 22.900 |
| 3 | 13.910 | 13.448 | 122.400 | 187.977 | 20.900 | 21.617 |
| 4 | 12.300 | 12.986 | 260.700 | 245.193 | 21.800 | 22.183 |
| 5 | 13.290 | 14.283 | 110.200 | 153.977 | 17.500 | 18.567 |
| 6 | 17.020 | 16.251 | 172.300 | 178.593 | 19.600 | 19.633 |
| 7 | 18.120 | 17.861 | 110.600 | 154.577 | 21.200 | 21.500 |
| 8 | 19.190 | 19.673 | 217.000 | 223.093 | 19.600 | 20.400 |
| 9 | 15.550 | 14.946 | 214.800 | 193.723 | 19.100 | 20.029 |
| 10 | 14.650 | 15.647 | 270.500 | 282.917 | 23.100 | 22.429 |
| 11 | 13.130 | 12.708 | 189.700 | 181.919 | 22.700 | 22.013 |
| 12 | 15.750 | 15.046 | 262.800 | 191.488 | 21.200 | 19.813 |
| 13 | 18.140 | 18.702 | 190.000 | 160.669 | 22.200 | 21.337 |
| 14 | 17.100 | 16.866 | 253.200 | 181.638 | 22.600 | 22.188 |
| 15 | 16.050 | 16.464 | 238.000 | 231.539 | 23.200 | 23.321 |
| 16 | 15.980 | 16.265 | 115.800 | 172.883 | 18.300 | 19.571 |
| 17 | 16.230 | 16.167 | 142.800 | 102.019 | 19.600 | 17.604 |
| 18 | 17.890 | 16.868 | 349.000 | 289.088 | 23.200 | 22.554 |
| 19 | 16.830 | 16.306 | 162.800 | 167.989 | 20.300 | 20.788 |
| 20 | 18.510 | 18.644 | 229.800 | 275.434 | 20.400 | 21.137 |
| 21 | 16.970 | 16.916 | 140.300 | 145.240 | 17.900 | 18.912 |
| 22 | 13.960 | 15.079 | 240.000 | 264.084 | 22.200 | 22.313 |
| 23 | 17.830 | 17.055 | 290.000 | 293.885 | 22.600 | 22.096 |
| 24 | 16.860 | 16.856 | 378.400 | 333.104 | 22.800 | 20.896 |
| 25 | 17.530 | 17.130 | 366.400 | 408.500 | 23.100 | 23.433 |
| 26 | 15.840 | 17.130 | 420.100 | 408.500 | 23.500 | 23.433 |
| 27 | 18.020 | 17.130 | 439.000 | 408.500 | 23.700 | 23.433 |
Gelatin yield
Gelatin yield is considered one of the most important parameters by the gelatin industry because of its potential economic importance. The regression model gave R2 value 0.867 (Table 3) indicating that the model sufficiently explained the system. The maximum predicted value of 17.21 % was obtained from the mathematical equation at optimal extraction conditions of NaOH concentration (X1) = 0.23 %, soaking time (X2) = 46.19 min, extraction temperature (X3) = 55.29 °C and extraction time (X4) = 17.29 h. The ANOVA (Table 3) for gelatin yield shows that the significant linear variables affecting yield were X3 and X4. Among the square terms, X22 showed the highest significant (99.9 %) influence on gelatin yield (Table 3).
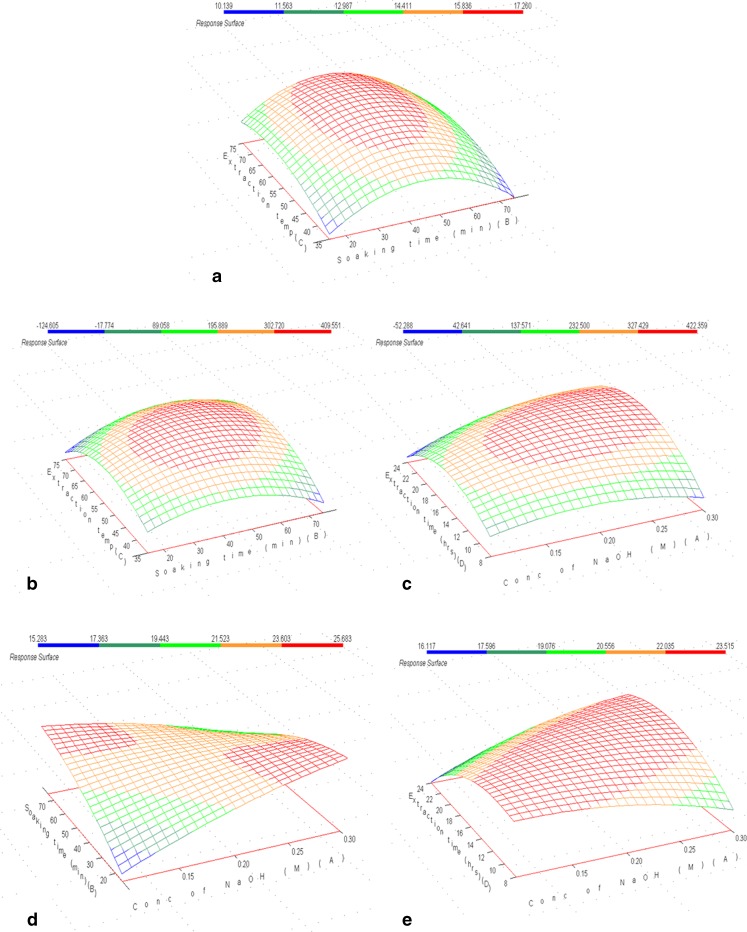
The three-dimensional response surface plot (Fig. 1a) showed the effect of extraction temperature and soaking time on gelatin yield. An increase in soaking time from 15 to 50 min. and extraction temperature 35 to 60 °C caused an increase in yield and thereafter decreased the yield. The increase could be because of the breakdown of collagen molecules into chains like α, β and high molecular weight compounds. When extraction temperature and time further increase, there will be further degradation of α and β chains into low molecular weight molecules. These low molecular weight molecules will be leached away during different processing steps which cause decrease in gelatin yield (Jamilah and Harvinder 2002).
Fig. 1.
Response surface plots showing the effect of extraction temperature, soaking time and/or NaOH concentration on (a) gelatin yield, (b) & (c) gel strength, (d) & (e) melting point of gel
In the present study the gelatin yield varied between 12.3 and 19.19 % depending on the extraction conditions (Table 4). Though the highest gelatin yield obtained was 19.19 % for sample 8 which is higher than the predicted maximum gelatin yield (17.21 %) extracted at optimum conditions, it showed low gel strength (217.5 g) and low melting point (19.6 °C) (Table 4). This may be due to the more extraction time (24 h), which must have resulted in small peptides. This yield was higher than those reported by Grossman and Bergman (1992) for tilapia skin (2.6–7.81 %), Osborne et al. (1990) for lumpfish skin (14.3 %) and Gudmundsson and Hafsteinsson (1997) for cod skin (11–14 %).
Gel strength
Gel strength is the second most important attribute of gelatin which determines the quality of produced gelatin. Fish gelatin typically shows less gel strength compared to gelatin obtained from mammalian sources (Gilsenan and Ross-Murphy 2000). The gelling strength of commercial gelatin ranges from 100 to 300. The model developed in the present study for the gel strength accounted for 83.7 % of observed variations (R2 value 0.837, Table 3). It was found that the square effects of extraction temperature and extraction time were found the most significant (P < 0.001) influencing factors. The other variable which had significant effect on gel strength was individual effect of NaOH concentration. The square effect of all the variables showed significant influence and all these effects were negative as illustrated by the response surface plots with curvature effects (Fig. 1b and c).
The gel strength varied between 110.2 and 439 g (Table 5) and by prediction with computing program, the maximum gel strength obtained was 422.69 g at the optimum levels of extraction conditions of concentration of NaOH = 0.22 %, soaking time = 44.56 min, extraction temperature = 59.02 °C and extraction time = 15.35 h (Table 6). The gel strength of gelatin extracted from Blackspotted croaker skin showed higher values compared to those reported by Jamilah and Harvinder (2002) & Grossman and Bergman (1992) for Tilapia fish (180.76 blooms and 263 g respectively), Muyonga et al. (2004) for Nile perch fish (229 g), Yang et al. (2007) for channel catfish (276 g) and similar to the values reported by Cho et al. (2005) for Yellow fin tuna skin gelatin (426 g) and Zhou and Regenstein (2004) for Pollock skin gelatin (460 g). The differences in the value of gel strength in all studies could be explained by differences in the manufacturing process used and the intrinsic properties of collagen which vary among fish species. Johnston-Banks (1990) reported that the average molecular weight of gelatin is largely responsible for its gelling behaviour. Nevertheless, the results obtained suggest that Blackspotted croaker skin can be used for extraction of good quality gelatin under optimum conditions.
Table 6.
Optimum values of independent variables for extraction of gelatin
| Variable | Gelatin yield (17.21 %) | Bloom value (422.69 g) | Melting point of gel (23.48 °C) |
|---|---|---|---|
| NaOH conc. (%) | 0.23 | 0.22 | 0.20 |
| Soaking time (min) | 46.19 | 44.56 | 46.68 |
| Extraction temp. (°C) | 55.29 | 59.02 | 56.23 |
| Extraction time (h) | 17.29 | 15.35 | 15.21 |
Melting point
The model developed for predicting Blackspotted croaker skin gelatin melting point could explain 76.5 % of the variation observed in respect of this functional property (Table 3). The interactive effect of conc. of NaOH and soaking time was observed to be the most significant (P < 0.05) influencing factor which showed negative effect on melting point (Fig. 1d). Other variables which had significant effect on melting point were interactive effect of conc. of NaOH and extraction time, and square effects of extraction temperature and extraction time. It was found that both the square effects of extraction temperature and extraction time showed negative effect on melting point but interactive effect of conc. of NaOH and extraction time showed synergistic effect on melting point (Fig. 1e).
Fish gelatin especially those extracted from cold-water species have low melting point compared to mammalian gelatin. This is due to the lower imino acid content of fish gelatin, which in turn reduces the propensity for intermolecular helix formation (Choi and Regenstein 2000). In this study, the melting point of gelatin varied between 17.9 and 23.7 °C and the maximum predicted value was 23.48 °C at optimum extraction conditions of X1 = 0.20 %, X2 = 46.68 min, X3 = 56.23 °C and X4 = 15.21 h. The values obtained in this study were far higher than those reported values for cod skin (8–10 °C) (Gudmundsson and Hafsteinsson 1997), hake (14 °C), sole (19.4 °C), megrim (18.8 °C) (Gómez-Guillén et al. 2002) and grass carp (19.5 °C) (Kasankala et al. 2007). Further, the melting point of Blackspotted croaker skin gelatin was very close to the bovine (23.8 °C) and porcine (25.6 °C) values reported by Cho et al. (2005). High gelling and melting points expand the range of gelatin applications.
Conclusions
This study demonstrated that Blackspotted croaker skin can be a potential source for production of gelatin with high yield. Compared to several fish species studied earlier for gelatin production, the Blackspotted croaker skin was found to yield gelatin with desirable functional properties such as high bloom value and melting point. Thus, Blackspotted croaker fish skin gelatin can be used in food applications to extend the gelatin market to some of the religious groups which do not accept the porcine and bovine gelatin.
Acknowledgments
Authors are grateful to Dr W.S. Lakra, Director and Vice-Chancellor, Central Institute of Fisheries Education, Mumbai, for providing the valuable support in conducting this research work. Authors wish to express their thanks to Avinash Sable and Bhanudas T. Phande for the technical support.
References
- Badii F, Howell NK. Fish gelatin: structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll. 2006;20:630–640. doi: 10.1016/j.foodhyd.2005.06.006. [DOI] [Google Scholar]
- Bas D, Boyaci IH. Modeling and optimization I: usability of response surface methodology. J Food Engg. 2007;78:836–845. doi: 10.1016/j.jfoodeng.2005.11.024. [DOI] [Google Scholar]
- Cheow CS, Norizah MS, Kyaw ZY, Howell NK. Preperation and characterisation of gelatins from the skins of sin croaker (Johnius dussumieri) and shortfin scad (Decapterus macrosoma) Food Chem. 2007;101:386–391. doi: 10.1016/j.foodchem.2006.01.046. [DOI] [Google Scholar]
- Cho SM, Kwak KS, Park DC, Gu YS, Ji CI, Jang DH. Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocoll. 2004;18:573–579. doi: 10.1016/j.foodhyd.2003.10.001. [DOI] [Google Scholar]
- Cho SM, Gu S, Kim SB. Extraction optimization and physical properties of yellowfin tuna (Thunnus albacares) skin gelatin compared to mammalian gelatins. Food Hydrocoll. 2005;19:221–229. doi: 10.1016/j.foodhyd.2004.05.005. [DOI] [Google Scholar]
- Choi SS, Regenstein JM. Physicochemical and sensory characteristics of fish gelatin. J Food Sci. 2000;65:194–199. doi: 10.1111/j.1365-2621.2000.tb15978.x. [DOI] [Google Scholar]
- Djagny KB, Wang Z, Xu S. Gelatin a valuable protein for food and pharmaceutical industries-review. Crit Rev Food Sci Nutr. 2001;4:481–492. doi: 10.1080/20014091091904. [DOI] [PubMed] [Google Scholar]
- Ganesan P, Pradeep MG, Sakhare PZ, Suresh PV, Bhaskar N. Optimization of conditions for natural fermentation of freshwater fish processing waste using sugarcane molasses. J Food Sci Technol. 2009;46(4):312–315. [Google Scholar]
- Garg SK, Singh DS. Optimization of extrusion conditions for defatted soy-rice blend extrudates. J Food Sci Technol. 2010;47(6):606–612. doi: 10.1007/s13197-010-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsenan PM, Ross-Murphy SB. Viscoelasticity of thermo reversible gelatin gels from mammalian and piscine collagen. J Rheology. 2000;44:871–882. doi: 10.1122/1.551118. [DOI] [Google Scholar]
- GME (2005) Standard methods for the testing of edible gelatin, gelatin monograph, Gelatin Manufacturers of Europe
- Gómez-Guillén MC, Turnay J, Fernández-Díaz MD, Olmo N, Lizarbe MA, Montero P. Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocoll. 2002;16:25–34. doi: 10.1016/S0268-005X(01)00035-2. [DOI] [Google Scholar]
- Grossman S, Bergman M. Process for the production of gelatin from fish skins. US Patent. 1992;5:093–474. [Google Scholar]
- Gudmundsson M, Hafsteinsson H. Gelatin from cod skin as affected by chemical treatments. J Food Sci. 1997;62(1):37–39. doi: 10.1111/j.1365-2621.1997.tb04363.x. [DOI] [Google Scholar]
- Jamilah B, Harvinder KG. Properties of gelatins from skins of fish-black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica) Food Chem. 2002;77:81–84. doi: 10.1016/S0308-8146(01)00328-4. [DOI] [Google Scholar]
- Johnston-Banks FA (1990) Gelatin. In: Harris P (ed) Food gels. Elsevier Applied Food Science Series, New York, pp 233–289
- Jongjareonrak A, Benjakul S, Visessanguan W, Tanaka M. Effects of plasticizers on the properties of edible films from skin gelatin of bigeye snapper and brownstripe red snapper. Eur Food Res Technol. 2006;222:229–230. doi: 10.1007/s00217-005-0004-3. [DOI] [Google Scholar]
- Jongjareonrak A, Benjakul S, Visessanguan W, Prodpran T, Tanaka M. Characterization of edible films from skin gelatin of brownstripe red snapper and bigeye snapper. Food Hydrocoll. 2006;20:492–501. doi: 10.1016/j.foodhyd.2005.04.007. [DOI] [Google Scholar]
- Kasankala LM, Xue Y, Weilong Y, Hong SD, He Q. Optimization of gelatin extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Biores Technol. 2007;98:3338–3343. doi: 10.1016/j.biortech.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Kim JS, Cho SY. Screening for raw material of modified gelatin in marine animal skins caught in coastal offshore water in Korea. Han’guk Nonghwa Hakhoechi. 1996;39:134–139. [Google Scholar]
- Liu H, Li D, Guo SD. Rheological properties of channel catfish (Ictalurus punctaus) gelatin from fish skins preserved by different methods. LWT. 2008;41:414–419. doi: 10.1016/j.lwt.2007.03.027. [DOI] [Google Scholar]
- Montero P, Gómez-Guillén MC. Extracting conditions for Megrim (Lepidorhombus boscii) skin collagen affect functional properties of the resulting collagen. J Food Sci. 2000;65(2):1–5. [Google Scholar]
- Muyonga JH, Colec CGB, Duodub KG. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004;8:581–592. doi: 10.1016/j.foodhyd.2003.08.009. [DOI] [Google Scholar]
- Neter J, Kutner MH, Nachtsheim CJ, Wasserman W. Applied linear statistical models. 4. Chicago: McGraw-Hill; 1996. [Google Scholar]
- Osborne K, Voight MN, Hall DE. Utilization of lumpfish carcasses for production of gelatin. In: Voight, Botta, editors. Advances in fisheries technology and biotechnology for increased profitability. Lancaster: Technomic Publishing Co; 1990. pp. 143–153. [Google Scholar]
- Rastogi NK, Nguyen LT, Jiang B, Balasubramaniam VM. Improvement in texture of pressure-assisted thermally processed carrots by combined pretreatment using response surface methodology. Food Bioproc Technol. 2010;3(5):762–771. doi: 10.1007/s11947-008-0130-6. [DOI] [Google Scholar]
- Shahidi F. Seafood processing by-products. In: Shahidi F, Botta JR, editors. Seafoods chemistry processing technology and quality. Glasgow: Blackie; 1994. pp. 320–334. [Google Scholar]
- Singh RKR, Majumdar RK, Venkateshwarlu G (2012) Optimum extrusion-cooking conditions for improving physical properties of fish-cereal based snacks by response surface methodology. J Food Sci Technol. doi:10.1007/s13197-012-0725-9 [DOI] [PMC free article] [PubMed]
- Wainewright FW. Physical tests for gelatin and gelatin products. In: Ward AG, Courts A, editors. The science and technology of gelatins. London: Academic; 1977. pp. 508–531. [Google Scholar]
- Yang H, Wang Y, Jiang M, Oh JH, Herring J, Zhou P. 2-Step optimization of the extraction and subsequent physical properties of channel catfish (Ictalurus punctatus) skin gelatin. J Food Sci. 2007;72(4):C188–C195. doi: 10.1111/j.1750-3841.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhao MM, Shi J, Yang N, Jiang YM. Effect of ultrasonic treatment on the recovery and DPPH radical scavenging activity of polysaccharides from longan fruit pericarp. Food Chem. 2008;106:685–690. doi: 10.1016/j.foodchem.2007.06.031. [DOI] [Google Scholar]
- Yoshimura K, Terashima M, Hozan D, Ebato T, Nomura Y, Ishii Y. Physical properties of shark gelatin compared with pig gelatin. J Agric Food Chem. 2000;48:2023–2027. doi: 10.1021/jf990887m. [DOI] [PubMed] [Google Scholar]
- Zhou P, Regenstein JM. Optimization of extraction conditions for Pollock skin gelatin. J Food Sci. 2004;69:393–398. [Google Scholar]