Abstract
Objective
HIV-infected individuals are susceptible to development of chronic lung diseases but little is known regarding prevalence and risk factors associated with different spirometric abnormalities in this population. We sought to determine the prevalence, risk factors and performance characteristics of risk factors for spirometric abnormalities among HIV-infected individuals.
Design
Cross-sectional cohort study
Methods
We analyzed cross-sectional US data from the NHLBI-funded Lung-HIV consortium, a multi-center observational study of heterogeneous groups of HIV-infected participants in diverse geographic sites. Logistic regression analysis was performed to determine factors statistically significantly associated with spirometry patterns.
Results
A total of 908 HIV-infected individuals were included. The median age of the cohort was 50 years, 78% were male and 68% current smokers. An abnormal spirometry pattern was present in 37% of the cohort: 27% had obstructed and 10% had restricted spirometry patterns. Overall, age, smoking status and intensity, history of Pneumocystis infection, asthma diagnosis and presence of respiratory symptoms were independently associated with an abnormal spirometry pattern. Regardless of presence of respiratory symptoms, five HIV-infected participants would need to be screened with spirometry to diagnose two individuals with any abnormal spirometry pattern.
Conclusions
Nearly 40% of a diverse US cohort of HIV-infected individuals had an abnormal spirometry pattern. Specific characteristics including age, smoking status, respiratory infection history and respiratory symptoms can identify those at risk for abnormal spirometry. The high prevalence of abnormal spirometry and the poor predictive capability of respiratory symptoms to identify abnormal spirometry should prompt clinicians to consider screening spirometry in HIV-infected populations.
Keywords: HIV; lung disease, obstructive; respiratory tract disease; lung function; spirometry
INTRODUCTION
Human immunodeficiency virus (HIV) infection has become a controllable chronic disease in the current era of effective anti-retroviral therapy (ART).[1–3] As morbidity and mortality from opportunistic infections has decreased, it has become evident that HIV-infected individuals are susceptible to chronic diseases at greater rates than those observed in HIV-uninfected persons.[4–7] A large body of literature has described an association between HIV infection and chronic lung diseases such as asthma and chronic obstructive pulmonary disease (COPD).[8–15] While risk factors such as smoking, opportunistic infections and injection drug use contribute to the increased risk for chronic lung disease, HIV infection and markers of HIV disease severity also have been associated with increased risk.[16]
Chronic lung diseases can be categorized by the patterns of abnormalities detected with spirometry testing. An obstructive airflow pattern, as can be seen in asthma, bronchiectasis and COPD, is characterized by a disproportional reduction in the forced expiratory volume in 1 second (FEV1) compared to the forced vital capacity (FVC), generally with a reduction in the FEV1/FVC<0.70 used to define disease.[17]. Restrictive patterns of airflow, seen in interstitial lung disease and respiratory muscle insufficiency, are defined by a normal FEV1/FVC (≥0.70), but an FVC<80% of predicted value. Both obstructive and restrictive spirometry patterns are associated with increased mortality in the general population.[18–20] The prevalence and clinical risk factors for these spirometric patterns are under-explored in HIV-infected populations despite the increased frequency of chronic lung disease in this population.[13, 21]
The multi-center Lung-HIV consortium has collected spirometric and clinical data from heterogeneous groups of HIV-infected participants in diverse geographic sites,[22] providing a unique opportunity to define patterns of lung function abnormalities among HIV-infected individuals. Using cross-sectional spirometric and clinical data from United States sites, we determine the prevalence and risk factors for any abnormal pattern as well as for obstructed and restricted spirometry patterns. To help inform clinical evaluation, we also characterize the performance characteristics [i.e., sensitivity, specificity and area under the curve (AUC)] of different risk factors for abnormal spirometry, and determine the number of HIV-infected individuals needed to screen with spirometry to detect one abnormal result. Some of these results have been previously reported in abstract form.[23]
METHODS
Study Cohort
The Lung-HIV Study was a NHLBI-sponsored consortium that collected data on HIV-associated lung diseases from 2008 to 2013. The consortium included eight clinical centers and a data coordinating center. Clinical centers enrolled different risk groups of HIV-infected and HIV-uninfected individuals. While each site had unique research initiatives, site investigators collaborated to create common data collection forms and standardized pulmonary testing procedures, permitting harmonized clinical and spirometric datasets. Five US clinical centers collected spirometry measurements along with demographic and clinical data, at clinical enrollment sites that included Atlanta GA, Baltimore MD, Columbus OH, Houston TX, Los Angeles CA, New York (including Manhattan and the Bronx) NY, Pittsburgh PA, and San Francisco CA (see Crothers[22] for details of individual site enrollment criteria and consortium integration). For this analysis, all HIV-infected Lung-HIV participants from the United States sites who contributed clinical and demographic data, spirometric measures, and HIV-related laboratory testing were included (see Supplemental Digital Content 1 for contributing centers and participant selection). While individual sites have previously included data on lung function in subsets of participants included in this analytical cohort,[10, 21, 24–27] prior analyses included spirometry measurements obtained at different time points during follow-up or in the context of different research objectives. Prior publications have not published on restrictive physiology patterns or compared risk factors for obstruction and restriction versus normal spirometry pattern among HIV-infected individuals. The Lung-HIV study protocol was approved by the Institutional Review Boards (IRB) at each clinical site and the data coordinating center. All participants provided written, informed consent to participate in both their local site’s research study as well as in Lung-HIV group studies.
Data Collection
Demographic and clinical data was collected using standardized Lung-HIV forms. History of cigarette smoking, injection drug use, and ART use were obtained through self-report. History of asthma, COPD, and respiratory infections (bacterial, Pneumocystis, tuberculosis) were determined through self-report of physician diagnosis rather than medical records abstraction because self-reported data could be standardized and collected across the diverse Lung-HIV clinical centers. Respiratory symptoms were assessed using a modified version of the American Thoracic Society respiratory questionnaire,[28] which included the following questions: “Do you usually have a cough?”, “Do you usually bring up phlegm from your chest?” and “Have you ever had an attack of wheezing or whistling in your chest that made you feel short of breath?”. Dyspnea was assessed using the modified Medical Research Council (mMRC) questionnaire with a validated 0–4 scale, with a higher score indicating worse dyspnea.[29, 30] CD4 cell counts and HIV viral load were obtained from participant records obtained in the context of research or clinical visits closest to the date of pulmonary function testing based on individual site protocols.
Categories of Spirometric Patterns
Pre-bronchodilator spirometry FEV1 and FVC measurement was performed in accordance with American Thoracic Society guidelines.[31] Percent predicted values were calculated using standard formulas.[32] Separate categories of spirometric patterns were defined using prior published definitions[33, 34] as follows: obstructed (FEV1/FVC<0.70), restricted (FEV1/FVC≥0.70 and FVC<80% predicted and any FEV1 value), undefined (FEV1/FVC≥0.70 and FVC≥80% predicted and FEV1<80% predicted) and normal (FEV1/FVC≥0.70 and FVC≥80% predicted and FEV1≥80% predicted). In separate analyses, lower limit of normal (LLN) criterion to define an obstructive ventilatory defect were calculated using reference equations.[35] All spirometry testing was done when patients were in stable condition free of recent respiratory infections.
Statistical Analysis
For comparisons of demographic and clinical characteristics across different spirometry patterns, Kruskall-Wallis tests and chi-square tests were used. Logistic regression analysis was performed to determine factors associated with three separate spirometry groups: any abnormal spirometry versus normal pattern, obstructed versus normal spirometry pattern, and restricted versus normal spirometry pattern. Respiratory symptoms were examined individually and also with a combined variable “any respiratory symptoms” defined as present if participant answered yes to any of the questions regarding cough, phlegm, wheeze or had an mMRC score>2. Predictors identified as significant in univariable analyses (i.e., p<0.20) were included in multivariable models. Parsimonious models were generated to determine characteristics independently associated with abnormal spirometric patterns. The AUC and performance characteristics (sensitivity, specificity) of covariates included in multivariable models were calculated. A p-value of <0.05 was used to infer statistical significance. Stata 13.0 (College Station, Texas) software was used for statistical analyses.
RESULTS
Study Participants
After excluding 22 participants with an undefined spirometry pattern, 908 HIV-infected individuals contributed clinical and spirometric data to analysis (Table 1). The median age of the cohort was 50 years, 78% were male and 64% self-identified as black race. Cigarette smoking was common, with 68% reporting current smoking and 19% reporting former tobacco use. The median smoking exposure was 18 pack-years (IQR 8–33). Overall, 382 (44%) participants reported a history of injection drug use. The median CD4 cell count for the cohort was 425 cells/μl (IQR 262–627) with 16% having a CD4 cell count less than 200 cell/μl. The median log HIV viral load was 3.87 copies/ml (IQR 3.69–7.58). A total of 69% had an undetectable HIV viral load and 73% reported current ART use. Of the cohort, 21% self-reported a physician-diagnosis of asthma while 14% self-reported a physician-diagnosis of COPD. There was a weak, but statistically significant correlation between self-report of asthma and COPD diagnoses (r=0.234; p<0.001). Prior respiratory infections were frequent in the cohort, with 26% reporting bacterial pneumonia, 11% Pneumocystis pneumonia and 4% pulmonary tuberculosis.
Table 1.
Clinical characteristics of participants (N=908)
| Age, years | 50 (44–55) |
|
| |
| African-American race | 581 (64) |
|
| |
| Male | 709 (78) |
|
| |
| Smoking status | |
| Current | 614 (68) |
| Former | 169 (19) |
| Never | 125 (14) |
|
| |
| Pack-years smoking | 17.8 (7.8–33.0) |
|
| |
| History of injection drug use | 382 (44) |
|
| |
| CD4 count, cells/mm3 | 425 (262–627) |
|
| |
| CD4 category, cells/μl | |
| <100 | 53 (6) |
| 100–199 | 96 (11) |
| 200–349 | 202 (23) |
| 350+ | 554 (61) |
|
| |
| Viral load, log10(copies/ml) | 3.87 (3.69–7.58) |
|
| |
| Viral load undetectable | 555 (69) |
|
| |
| Current ART use | 571 (73) |
|
| |
| History of asthma | 185 (21) |
|
| |
| History of COPD | 128 (14) |
|
| |
| History of pneumonia | 229 (26) |
|
| |
| History of Pneumocystis | 101 (11) |
|
| |
| History of tuberculosis | 39 (4) |
|
| |
| Cough present | 289 (32) |
|
| |
| Phlegm present | 312 (35) |
|
| |
| Wheeze present | 322 (36) |
|
| |
| MRC score | 0 (0–1) |
|
| |
| FEV1, Absolute (L) | 2.96 (2.37–3.56) |
| FEV1, % predicted | 92.0 (80.6–103.5) |
|
| |
| FVC, Absolute (L) | 3.98 (3.31–4.71) |
| FVC, % predicted | 97.8 (87.9–107.5) |
|
| |
| FEV1/FVC ratio | 0.76 (0.69–0.80) |
|
| |
| FEV1 severity category | |
| >70% predicted | 788 (87) |
| 50–69% predicted | 95 (10) |
| <50% predicted | 25 (3) |
|
| |
| Category of impairment | |
| Restricted | 92 (10) |
| Obstructed | 244 (27) |
| Normal | 572 (63) |
All values median (IQR) or n (%). ART= antiretroviral therapy; COPD= chronic obstructive pulmonary disease
Spirometric Pattern
Overall, the median FEV1 and FVC measures were near normal, with median FEV1 and FVC 92.0 and 97.8 percent predicted, respectively. The median FEV1/FVC was 0.76 (IQR 0.69–0.80). Despite the overall preserved mean values of spirometry measures, 37% of the cohort had an abnormal spirometry pattern, with 27% having an obstructed and 10% having a restricted spirometry pattern (Figure 1).
Figure 1.
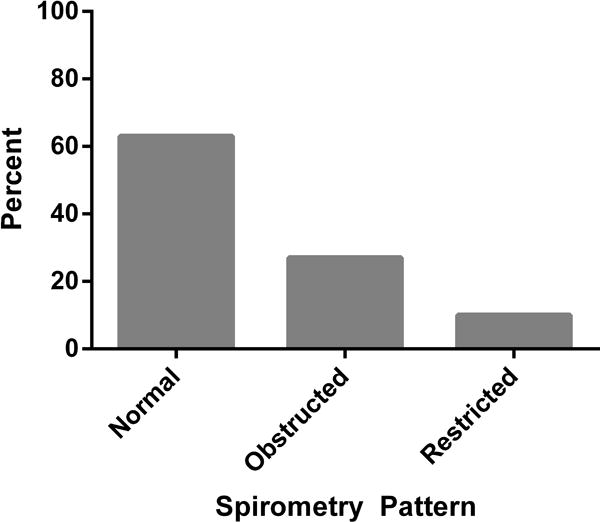
Prevalence of abnormal, obstructed and restricted spirometry patterns among 908 Lung-HIV cohort participants. See methods section for definitions of spirometric categories.
Compared to participants with a normal spirometry pattern, those with an obstructed pattern were older, more likely to be current smokers and had higher pack-years smoked (Table 2). Participants with airflow obstruction were also more likely to report a diagnosis of asthma, COPD, respiratory symptoms and have a history of more bacterial pneumonia and Pneumocystis infection. Notably, among participants with spirometry-confirmed airflow obstruction, only 21% reported a diagnosis of COPD, 29% asthma and 10% both asthma and COPD. A total of 60% of participants with airflow obstruction had no prior diagnosis of either asthma or COPD. Of those reporting a diagnosis of asthma, 43% met spirometric criteria for airflow obstruction while 46% of participants reporting a diagnosis of COPD also met spirometric criteria for airflow obstruction. When defining obstructive ventilatory pattern using LLN rather than fixed ratio criterion, 95% of participants had similar designation of diseased or normal airflow patterns.
Table 2.
Clinical characteristics of HIV-infected participants by Spirometry Pattern
| Obstructed Spirometry | Restricted Spirometry | Normal Spirometry | P –Obstructed vs. Normal | P –Restricted vs. Normal | |
|---|---|---|---|---|---|
| N | 244 | 92 | 572 | ||
|
| |||||
| Age, years | 52 (47–57) | 51 (44–56) | 48 (43–53) | <0.001 | 0.011 |
|
| |||||
| African-American race | 142 (58) | 70 (76) | 369 (65) | 0.23 | 0.027 |
|
| |||||
| Male | 196 (80) | 70 (76) | 443 (77) | 0.36 | 0.77 |
|
| |||||
| Smoking status | |||||
| Current | 186 (76) | 57 (62) | 371 (65) | ||
| Former | 36 (15) | 23 (25) | 110 (19) | ||
| Never | 22 (9) | 12 (13) | 91 (16) | 0.004 | 0.40 |
|
| |||||
| Pack-years smoking | 37 (13–40) | 18 (8–32) | 17 (8–37) | <0.001 | 0.18 |
|
| |||||
| Prior injection drug use | 104 (46) | 44 (49) | 234 (43) | 0.52 | 0.30 |
|
| |||||
| CD4 count, cells/mm3 | 408 (255–611) |
419 (242–600) |
429 (269–636) |
0.47 | 0.45 |
|
| |||||
| CD4 category, cells/μl | |||||
| <100 | 16 (7) | 4 (4) | 33 (6) | ||
| 100–199 | 24 (10) | 9 (8) | 63 (11) | ||
| 200–349 | 56 (23) | 26 (28) | 123 (22) | ||
| 350+ | 148 (61) | 53 (58) | 353 (62) | 0.90 | 0.53 |
|
| |||||
| Viral load, log10(copies/ml) | 3.87 (3.69–6.11) |
3.87 (3.69–7.93) |
3.87 (3.69–7.90) |
0.082 | 0.88 |
|
| |||||
| Viral load undetectable | 159 (74) | 58 (67) | 338 (68) | 0.091 | 0.98 |
|
| |||||
| Current ART use | 159 (74) | 58 (72) | 354 (72) | 0.70 | 0.91 |
|
| |||||
| History of asthma | 71 (29) | 21 (23) | 93 (16) | <0.001 | 0.13 |
|
| |||||
| History of COPD | 51 (21) | 17 (19) | 60 (11) | <0.001 | 0.021 |
|
| |||||
| History of pneumonia | 75 (32) | 21 (23) | 133 (24) | 0.020 | 0.89 |
|
| |||||
| History of Pneumocystis | 41 (17) | 10 (11) | 50 (9) | 0.001 | 0.50 |
|
| |||||
| History of tuberculosis | 9 (4) | 8 (9) | 22 (4) | 0.94 | 0.04 |
|
| |||||
| Cough present | 106 (43) | 33 (36) | 150 (26) | <0.001 | 0.06 |
|
| |||||
| Phlegm present | 121 (50) | 28 (31) | 163 (29) | <0.001 | 0.69 |
|
| |||||
| Wheeze present | 118 (48) | 37 (41) | 167 (29) | <0.001 | 0.03 |
|
| |||||
| MRC score | 1 (0–3) | 0 (0–3) | 0 (0–1) | <0.001 | 0.01 |
|
| |||||
| FEV1, Absolute (L) | 2.39 (2.01–2.89) |
2.31 (1.92–2.59) |
3.28 (2.80–3.82) |
<0.001 | <0.001 |
|
| |||||
| FEV1, % predicted | 77.6 (64.5–87.3) |
71.8 (70.0–77.6) |
99.4 (90.9–108) |
<0.001 | <0.001 |
|
| |||||
| FVC, Absolute (L) | 3.93 (3.32–4.64) |
2.85 (2.40–3.23) |
4.21 (3.59–4.86) |
<0.001 | <0.001 |
|
| |||||
| FVC, % predicted | 96.4 (86.6–107) |
72.9 (67.0–77.0) |
101 (92.9–109) |
<0.001 | <0.001 |
|
| |||||
| FEV1/FVC ratio | 0.64 (0.58–0.68) |
0.79 (0.74–0.83) |
0.80 (0.75–0.83) |
<0.001 | 0.05 |
All values median (IQR) or n (%). ART= antiretroviral therapy; COPD= chronic obstructive pulmonary disease; P-value via Kruskall-Wallis or χ2 test
Compared to participants with a normal spirometry pattern, those with a restricted pattern of airflow were older, more likely to be African-American and report a diagnosis of COPD (Table 2). Wheeze, dyspnea and history of tuberculosis were more frequent in restricted compared with normal spirometry patterns. Across the different spirometry patterns, there were no differences in CD4 count, viral load or ART use.
Univariable and Multivariable Predictors of Specific Spirometric Patterns
Univariable logistic regression determined demographic and clinical characteristics associated with any abnormal spirometry pattern, an obstructed pattern, or a restricted spirometry pattern (Supplemental Digital Content 2). Several covariates were associated with any abnormal spirometry pattern. These included increasing age, current smoking status, history of asthma, history of COPD, history of Pneumocystis and presence of any respiratory symptoms. Greater smoking intensity, measured by pack-years smoked, was linearly associated with abnormal spirometry using thresholds of 10, 20, 30 and 40 pack-years, with the highest odds ratio for abnormal spirometry comparing >40 to ≤40 pack-years. A similar cluster of covariates were associated with an obstructed ventilatory pattern. These included increasing age, current smoking, history of asthma, history of COPD, history of Pneumocystis infection and presence of any respiratory symptoms. Also similar to the analysis of any abnormal spirometry pattern, greater smoking intensity was associated with an increased odds of an obstructed pattern, with the greatest association in those who smoked more than 20 pack-years. Unlike any abnormal pattern, a history of bacterial pneumonia was also associated with an obstructed ventilatory pattern. Slightly different characteristics were associated with a restrictive ventilatory pattern. These included increasing age, African-American race, >40 pack-year smoking history, history of COPD, history of tuberculosis and presence of any respiratory symptoms.
Multivariable modeling incorporating factors identified from the univariable analyses are summarized in Table 3. Several factors remained statistically associated with the presence of any abnormal spirometry: age (per 10 years, OR 1.75; 95% CI 1.45–2.13), current smoking status (OR 1.42 compared to non-smokers; 95% CI 1.02–1.98), history of Pneumocystis (OR 1.72; 95% CI 1.10–2.67) and presence of any respiratory symptoms (OR 1.95; 95% CI 1.40–2.73). While both self-report of asthma and COPD were associated with abnormal spirometry pattern in univariable analysis, inclusion of both asthma and COPD in multivariable analysis resulted in statistical significance only for self-report of asthma diagnosis (OR 1.69; 95% CI 1.17–2.44). Incorporation of different thresholds for pack-years smoked into multivariable models demonstrated that a history of smoking >30 pack-years was the lowest level associated with abnormal spirometry (OR 1.52; 95% CI 1.12–2.08). The overall AUC for this multivariable model was 0.690.
Table 3.
Multivariable models of factors associated with spirometry patterns
| Odds Ratio (95% CI) | P-value | |
|---|---|---|
| Any Abnormal Spirometry | ||
| Age (per 10 years) | 1.75 (1.45–2.13) | <0.001 |
| Current smoking | 1.42 (1.02–1.98) | 0.037 |
| >30 pack-years smoking | 1.52 (1.12–2.08) | 0.008 |
| History of asthma | 1.69 (1.17–2.44) | 0.005 |
| History of Pneumocystis | 1.72 (1.10–2.67) | 0.017 |
| Respiratory symptoms* | 1.95 (1.40–2.73) | <0.001 |
| Obstructed Spirometry Pattern | ||
| Age (per 10 years) | 1.92 (1.54–2.39) | <0.001 |
| Current smoking | 1.77 (1.20–2.62) | 0.004 |
| >20 pack-years smoking | 1.68 (1.20–2.36) | 0.003 |
| History of asthma | 1.81 (1.21–2.71) | 0.004 |
| History of Pneumocystis | 1.97 (1.22–3.18) | 0.005 |
| Respiratory symptoms* | 1.88 (1.28–2.76) | 0.001 |
| Restricted Spirometry Pattern | ||
| Age (per 10 years) | 1.46 (1.11–1.93) | 0.007 |
| African-American race | 1.75 (1.05–2.94) | 0.032 |
| Respiratory symptoms* | 2.23 (1.35–3.71) | 0.002 |
Answers yes to any of the following: cough, phlegm, wheeze, mMRC≥2; Only variables from univariable models with p<0.2 included in multivariable model (see supplementary table 2 for univariate model results)
When examining characteristics independently associated with an obstructed spirometry pattern, a similar list of factors was identified. These included increasing age (per 10 years, OR 1.92; 95% CI 1.54–2.39), current smoking status (OR 1.77 compared to non-smokers; 95% CI 1.20–2.62), history of Pneumocystis (OR 1.97; 95% CI 1.22–3.18) and presence of any respiratory symptoms (OR 1.88; 95% CI 1.28–2.76). Compared to analysis of any abnormal spirometry pattern, a lower pack-years threshold (>20 pack-years) was associated with the presence of an obstructed pattern (OR 1.68; 95% CI 1.20–2.36). As was observed with the analysis of any abnormal spirometry pattern, self-report of asthma, but not COPD, was statistically associated with an obstructed airflow pattern (OR 1.81; 95% CI 1.21–2.71). The overall AUC for this multivariable model was 0.717. Multivariable models using LLN criteria to define obstructed spirometry pattern demonstrated similar associations (Supplemental Digital Content 3).
Only three factors remained associated with a restricted spirometry pattern in multivariable analysis: increasing age (per 10 years, OR 1.46; 95% CI 1.11–1.93), African-American race (OR 1.75; 95% CI 1.05–2.94) and presence of any respiratory symptoms (OR 2.23; 95% CI 1.35–3.71). The overall AUC for this multivariable model was 0.648.
Performance Characteristics of Covariates
The sensitivity and specificity for individual characteristics incorporated into multivariable models of any abnormal spirometry are provided in Table 4. Age greater than 40 years had the highest sensitivity for predicting the presence of any abnormal spirometry, with a sensitivity of 93%. Current smoking status and smoking history exceeding 10 pack-years demonstrated lower sensitivities of 72% and 80%, respectively. While self-report of asthma diagnosis or prior Pneumocystis infection had good specificity for predicting any abnormal spirometry (84% and 91%, respectively), the sensitivity was relatively poor (28% and 16%, respectively). The presence of any respiratory symptom demonstrated 78% sensitivity and 41% specificity for predicting any abnormal spirometry patterns. Other performance characteristics are summarized in Supplemental Digital Content 4 and 5.
Table 4.
Performance characteristics of factors associated with presence of any abnormal spirometry pattern
| Variable | Sensitivity | Specificity | AUC |
|---|---|---|---|
| Age | |||
| >40 years | 93 | 15 | 0.543 |
| >50 years | 63 | 56 | 0.594 |
| > 60 years | 14 | 92 | 0.527 |
|
| |||
| Current smoker | 72 | 35 | 0.537 |
|
| |||
| Pack-years smoked | |||
| >10 | 80 | 31 | 0.554 |
| >20 | 57 | 58 | 0.578 |
| >30 | 42 | 73 | 0.575 |
| >40 | 27 | 85 | 0.561 |
|
| |||
| History of asthma | 28 | 84 | 0.556 |
|
| |||
| History of Pneumocystis | 16 | 91 | 0.534 |
|
| |||
| Respiratory symptoms* | 78 | 41 | 0.592 |
Answers yes to any of the following: cough, phlegm, wheeze, mMRC≥2
Given the clinical relevance, analysis restricted to participants with any respiratory symptoms was performed. Using the presence of respiratory symptoms to predict abnormal spirometry would result in 66% of the cohort being diagnosed with a chronic lung disease. However, of those with respiratory symptoms, only 261 (44%) had any abnormal spirometry pattern, resulting in a false positive rate of 59% when using respiratory symptoms alone to define chronic lung disease. Of the 44% of participants with respiratory symptoms and abnormal spirometry, 32% demonstrated an obstructive pattern and 11% a restrictive pattern. Compared to the 44% prevalence of abnormal spirometry pattern among those with respiratory symptoms, the prevalence of abnormal spirometry among those without symptoms was 24% (p<0.001). The performance characteristics of factors identified in the multivariable analysis of any abnormal spirometry pattern among those with respiratory symptoms were comparable to the entire cohort (Supplemental Digital Content 6).
DISCUSSION
In this analysis of 908 HIV-infected participants recruited from multiple US cities, we found that abnormal spirometry-defined airflow patterns are common, with nearly 40% of participants demonstrating abnormalities. An obstructed spirometry pattern was three times as common as a restrictive spirometry pattern. In this cohort, HIV-specific indices such as CD4 cell count and HIV viral load were not associated with specific patterns. The presence of specific risk factors among HIV-infected individuals should prompt an evaluation for undiagnosed lung impairment during the clinical care of HIV-infected patients. Regardless of respiratory symptoms, five HIV-infected participants would need to be screened with spirometry to diagnose two individuals with abnormal spirometry.
To our knowledge, this report describes the largest analysis of US HIV-infected individuals in the post-ART era using spirometric testing to define airflow abnormalities. We found that abnormal spirometry results were common in this population, with 27% and 10% having obstructed and restricted airflow patterns, respectively. These rates are higher than reported in studies of general US populations of HIV-uninfected individuals, with obstructed and restricted spirometry prevalence of 7–14% and 7%, respectively.[36–38] A portion of the increased prevalence of spirometric abnormalities in our cohort likely reflects increased risk factors for airflow obstruction such as smoking and respiratory infections. However, among the general population, only 13% of current smokers and 9% of former smokers have obstructive airflow patterns on spirometry.[36] Prior publications from individual Lung-HIV sites focusing on other research questions have demonstrated prevalence of airflow obstruction ranging from 16–18%.[10, 26, 27] The analysis presented here includes a larger, more diverse cohort of HIV-infected individuals, reinforcing the importance of recognizing the risk for lung disease among HIV-infected individuals.
Our results suggest that the use of screening spirometry among at-risk HIV infected individuals will likely increase estimates of disease prevalence in this population. However, compared to Centers for Disease Control data on US HIV-infected individuals,[39] current tobacco and ever drug use are over-represented in Lung-HIV. Thus, we may have observed a higher prevalence of spirometry abnormalities than a cohort with lower rates of smoking and drug use. The benefits of screening spirometry may differ in HIV-infected groups with different risk profiles and studies are needed.
In this analysis, several characteristics were identified which were independently associated with an abnormal spirometry pattern. These characteristics included older age, current smoking, greater smoking history, prior diagnosis of asthma, history of Pneumocystis infection and presence of any respiratory symptoms. Many of these characteristics are similar to established risk factors for abnormal spirometry in HIV-uninfected individuals.[33, 36, 38] Respiratory infections are known to contribute to the development of chronic lung diseases in general populations[40] as well as HIV-infected populations.[41] In our analysis, we observed that Pneumocystis infection, but not bacterial respiratory infections were associated with obstructed physiology. Pneumocystis has been implicated as a causative agent for the development of COPD in both HIV-infected and uninfected individuals.[42–44] The detection of prior pneumonia may represent a proxy for severe immunodeficiency, which can be associated with late access to health care as well as end-organ harms of uncontrolled HIV. Nadir CD4 and duration of HIV illness were not available from all Lung-HIV sites, thus this analysis is not able to determine the independent effects of respiratory infections from severe immunodeficiency and access to care. Studies in the pre-ART era have shown pneumonia to be a primary risk factor for lung abnormalities among HIV-infected individuals independent of CD4 count[41] as well as in persons without underlying HIV [40, 45], but these data may not be applicable to patients with less severe immunodeficiency. Prior studies have described an association between tuberculosis infection and lung function impairment,[45–47] an association not observed in this analysis, likely reflecting the low occurrence of tuberculosis in this cohort (4%).
Our analysis did not reveal an association between markers of HIV infection and presence of abnormal spirometry. Several studies have observed that increased HIV viral load or reduced CD4 cell count are associated with an increased prevalence of obstructed spirometry pattern,[10] diagnosis of COPD[48] and more rapid lung function decline over time.[16] These studies included a substantial number of individuals with poorly controlled disease. Nearly 69% of this cohort had an undetectable HIV viral load, attenuating the ability to detect impact of uncontrolled HIV on lung manifestations. However, our findings highlight that even in the era of effective ART, spirometry-defined lung function abnormalities remain a substantial burden.
Studies have highlighted the burden of respiratory symptoms among HIV-infected individuals.[49–52] In this analysis, the presence of any respiratory symptom approximately doubles the likelihood of abnormal spirometry testing. Therefore, clinicians caring for HIV-infected individuals should inquire about the presence of non-specific respiratory symptoms, and when present should consider spirometric testing. However, it is important to note that the presence of respiratory symptoms alone was a relatively poor predictor of abnormal airflow patterns, and should not substitute for spirometry when diagnosing lung disease among HIV-infected individuals. While current guidelines do not specifically address the role of screening spirometry in all HIV-infected individuals, the high prevalence of abnormal spirometry and the poor predictive capability of respiratory symptoms alone suggest this may be warranted.
This study has limitations. The selection of subsets of individuals from larger study cohorts introduces the potential for selection bias. The cross-sectional nature of the analysis limits the determination of causal relationships between participant characteristics, spirometric abnormalities and long-term clinical outcomes. Historical data obtained via self-report are susceptible to recall bias. Post-bronchodilator testing was not performed at all Lung-HIV sites; thus, a subset of individuals may have partially or fully reversible airflow obstruction. Our analysis was limited to spirometry data only. Performance of lung volume testing is required to confirm restrictive lung disease. Because not all sites within the Lung-HIV consortium enrolled HIV-uninfected individuals, this analysis is not able to provide estimates of background prevalence of abnormal spirometry patterns. The prevalence of obstructed spirometry pattern among HIV-uninfected individuals from published cohorts of smoking-injection drug users and MACS/WIHS ranged from 16–20%. [10, 26] These estimates were lower than the 27% observed in this analysis of HIV-infected individuals.
In conclusion, using data from multiple US cohorts of HIV-infected individuals, we have found that abnormal spirometry patterns are common, with nearly 40% of HIV-infected individuals having physiologic lung impairment. Specific risk factors are associated with obstructive lung disease, including older age, tobacco use, history of asthma and Pneumocystis infection. Restrictive lung impairments were less common, and associated with older age and black race. The presence of respiratory symptoms was strongly associated with abnormal airflow patterns, and should prompt formal spirometry testing when encountered in the clinical care of HIV-infected individuals. However, the use of respiratory symptoms in the absence of spirometry testing is inadequate to ascertain the presence of airflow abnormalities. Screening five HIV-infected participants would lead to diagnose of abnormal spirometry in two individuals. These findings highlight the importance of recognizing and diagnosing chronic lung diseases in HIV-infected populations.
Supplementary Material
Acknowledgments
A subset of participant data analyzed in this manuscript were collected in part by the Multicenter AIDS Cohort Study (MACS) with centers (Principal Investigators) at: Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels), U01-AI35040; University of Pittsburgh (Charles Rinaldo), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson), UM1-AI35043. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR000424 (JHU CTSA). Data in this manuscript were also collected by the Women’s Interagency HIV Study (WIHS). WIHS (Principal Investigators): U01-AI-103408; Connie Wofsy Women’s HIV Study, Northern California (Ruth Greenblatt, Bradley Aouizerat, and Phyllis Tien); Southern California WIHS (Alexandra Levine and Marek Nowicki), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). WIHS data collection was also supported by UL1-TR000004 (UCSF CTSA). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Funding:This work was supported by the following NIH awards: K23 HL103192 (MBD); R01 HL090335 and K24 HL087713 (LH); R01HL090313 (PTD); R01HL090483 (GDK); UCLA CTSI UL1TR000124 (EK); R01 HL090339 (AM) and the University of Pittsburgh CTSI (UL1 RR024153), NIAID and NCI UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041 (Multicenter AIDS Cohort); NIAID and NICHHD UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, UO1-AI-42590, and UO1-HD-32632 (WIHS); R01 HL090316 (WR); R01HL090331 (BT); R01 HL090342 (KC)
Footnotes
AUTHOR CONTRIBUTIONS
MB DRUMMOND: Lung HIV site co-investigator; Primary statistical analysis; Substantial contribution to study conception and design; Analysis and interpretation of data; Drafting work; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for accuracy and integrity of work
L HUANG: Lung HIV Site PI; Substantial contribution to study conception and design; Acquisition, analysis and interpretation of data; Drafting work; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
PT DIAZ: Lung HIV Site PI; Substantial contribution to study conception and design; Acquisition and interpretation of data; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
GD KIRK: Lung HIV Site PI;Substantial contribution to study conception and design; Acquisition and interpretation of data; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
EC KLEERUP: Lung HIV Site co-investigator; Substantial contribution to study conception and design; Acquisition and interpretation of data; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
A MORRIS: Lung HIV Site PI; Substantial contribution to study conception and design; Acquisition and interpretation of data; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
W ROM: Lung HIV Site PI; Substantial contribution to study conception and design; Acquisition and interpretation of data; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
MD WEIDEN: Lung HIV Site co-investigator; Substantial contribution to study conception and design; Interpretation of data; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
E ZHAO: Lung HIV Data Coordinating Center Data Management Statistician; Analysis and interpretation of data; Drafting work; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
B THOMPSON: Lung HIV Data Coordinating Center Data Management PI; Substantial contribution to study conception and design; Analysis and interpretation of data; Drafting work; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
K CROTHERS: Lung HIV Site PI; Substantial contribution to study conception and design; Acquisition, analysis and interpretation of data; Drafting work; Critical revisions for intellectual content; Final approval of published version; Agreement to be accountable for parts of work personally completed
All authors meet the ICMJE criteria for authorship as defined as substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
All authors report no relevant conflicts of interest related to this manuscript.
References
- 1.Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, Sax PE, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 2.Braithwaite RS, Justice AC, Chang CC, Fusco JS, Raffanti SR, Wong JB, et al. Estimating the proportion of patients infected with HIV who will die of comorbid diseases. Am J Med. 2005;118:890–898. doi: 10.1016/j.amjmed.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 3.Wood E, Hogg RS, Lima VD, Kerr T, Yip B, Marshall BD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300:550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 4.Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salter ML, Lau B, Go VF, Mehta SH, Kirk GD. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011;53:1256–1264. doi: 10.1093/cid/cir673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett CF, Hsue PY, Machado RF. Pulmonary hypertension: an increasingly recognized complication of hereditary hemolytic anemias and HIV infection. JAMA. 2008;299:324–331. doi: 10.1001/jama.299.3.324. [DOI] [PubMed] [Google Scholar]
- 8.Gingo MR, Wenzel SE, Steele C, Kessinger CJ, Lucht L, Lawther T, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J Allergy Clin Immunol. 2012;129:708–714. doi: 10.1016/j.jaci.2011.11.015. e708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med. 2011;183:388–395. doi: 10.1164/rccm.201006-0836OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummond MB, Kirk GD, Astemborski J, Marshall MM, Mehta SH, McDyer JF, et al. Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax. 2012;67:309–314. doi: 10.1136/thoraxjnl-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris A, George MP, Crothers K, Huang L, Lucht L, Kessinger C, et al. HIV and chronic obstructive pulmonary disease: is it worse and why? Proc Am Thorac Soc. 2011;8:320–325. doi: 10.1513/pats.201006-045WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond MB, Kirk GD. HIV-associated obstructive lung diseases: insights and implications for the clinician. Lancet Respir Med. 2014;2:583–592. doi: 10.1016/S2213-2600(14)70017-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingo MR, George MP, Kessinger CJ, Lucht L, Rissler B, Weinman R, et al. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am J Respir Crit Care Med. 2010;182:790–796. doi: 10.1164/rccm.200912-1858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunisaki KM. Will expanded ART use reduce the burden of HIV-associated chronic lung disease? Curr Opin HIV AIDS. 2014;9:27–33. doi: 10.1097/COH.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen MJ, Lou Y, Kvale PA, Rao AV, Jordan MC, Miller A, et al. Pulmonary function tests in HIV-infected patients without AIDS. Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 1995;152:738–745. doi: 10.1164/ajrccm.152.2.7633736. [DOI] [PubMed] [Google Scholar]
- 16.Drummond MB, Merlo CA, Astemborski J, Marshall MM, Kisalu A, McDyer JF, et al. The effect of HIV infection on longitudinal lung function decline among injection drug users: a prospective cohort. AIDS. 2013;27:1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44:207–215. doi: 10.1016/j.amepre.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65:499–504. doi: 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speizer FE, Fay ME, Dockery DW, Ferris BG., Jr Chronic obstructive pulmonary disease mortality in six U.S. cities. Am Rev Respir Dis. 1989;140:S49–55. doi: 10.1164/ajrccm/140.3_Pt_2.S49. [DOI] [PubMed] [Google Scholar]
- 20.Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313:711–715. doi: 10.1136/bmj.313.7059.711. discussion 715–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond MB, Merlo CA, Astemborski J, Kalmin MM, Kisalu A, McDyer JF, et al. The effect of HIV infection on longitudinal lung function decline among IDUs: a prospective cohort. AIDS. 2013;27:1303–1311. doi: 10.1097/QAD.0b013e32835e395d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crothers K, Thompson BW, Burkhardt K, Morris A, Flores SC, Diaz PT, et al. HIV-associated lung infections and complications in the era of combination antiretroviral therapy. Proc Am Thorac Soc. 2011;8:275–281. doi: 10.1513/pats.201009-059WR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond MB, Zhao E, Wong M, Kirk GD, Rom WN, Diaz PT, et al. Prevalence of Spirometric Abnormalities Among HIV-Infected Individuals. Am J Respir Crit Care Med. 2014;189:A1196. [Google Scholar]
- 24.Drummond MB, Kirk GD, Astemborski J, McCormack MC, Marshall MM, Mehta SH, et al. Prevalence and risk factors for unrecognized obstructive lung disease among urban drug users. Int J Chron Obstruct Pulmon Dis. 2011;6:89–95. doi: 10.2147/COPD.S15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fitzpatrick ME, Gingo MR, Kessinger C, Lucht L, Kleerup E, Greenblatt RM, et al. HIV infection is associated with diffusing capacity impairment in women. J Acquir Immune Defic Syndr. 2013;64:284–288. doi: 10.1097/QAI.0b013e3182a9213a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crothers K, McGinnis K, Kleerup E, Wongtrakool C, Hoo GS, Kim J, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr. 2013;64:271–278. doi: 10.1097/QAI.0b013e3182a9215a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campo M, Oursler KK, Huang L, Goetz MB, Rimland D, Hoo GS, et al. Association of chronic cough and pulmonary function with 6-minute walk test performance in HIV infection. J Acquir Immune Defic Syndr. 2014;65:557–563. doi: 10.1097/QAI.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 29.Fletcher CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45:577–584. [PubMed] [Google Scholar]
- 30.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 32.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 33.Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9:613–621. [PubMed] [Google Scholar]
- 34.Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2015. Available from: http://www.goldcopd.org/. Accessioned March 1, 2015. [Google Scholar]
- 35.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 36.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: data from the National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2000;160:1683–1689. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 37.Hankinson JL, Bang KM. Acceptability and reproducibility criteria of the American Thoracic Society as observed in a sample of the general population. Am Rev Respir Dis. 1991;143:516–521. doi: 10.1164/ajrccm/143.3.516. [DOI] [PubMed] [Google Scholar]
- 38.Mannino DM, Ford ES, Redd SC. Obstructive and restrictive lung disease and markers of inflammation: data from the Third National Health and Nutrition Examination. Am J Med. 2003;114:758–762. doi: 10.1016/s0002-9343(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 39.Blair JM, Fagan JL, Frazier EL, Do A, Bradley H, Valverde EE, et al. Behavioral and clinical characteristics of persons receiving medical care for HIV infection – Medical Monitoring Project, United States, 2009. MMWR Surveill Summ. 2014;63(Suppl 5):1–22. [PubMed] [Google Scholar]
- 40.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 41.Morris AM, Huang L, Bacchetti P, Turner J, Hopewell PC, Wallace JM, et al. Permanent declines in pulmonary function following pneumonia in human immunodeficiency virus-infected persons. The Pulmonary Complications of HIV Infection Study Group. Am J Respir Crit Care Med. 2000;162:612–616. doi: 10.1164/ajrccm.162.2.9912058. [DOI] [PubMed] [Google Scholar]
- 42.Morris A, Sciurba FC, Norris KA. Pneumocystis: a novel pathogen in chronic obstructive pulmonary disease? COPD. 2008;5:43–51. doi: 10.1080/1541255070181756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L, Morris A, Limper AH, Beck JM, Participants ATSPW An Official ATS Workshop Summary: Recent advances and future directions in pneumocystis pneumonia (PCP) Proc Am Thorac Soc. 2006;3:655–664. doi: 10.1513/pats.200602-015MS. [DOI] [PubMed] [Google Scholar]
- 44.Christensen PJ, Preston AM, Ling T, Du M, Fields WB, Curtis JL, et al. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect Immun. 2008;76:3481–3490. doi: 10.1128/IAI.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–38. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, et al. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998;12:351–356. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]
- 47.Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest. 1998;113:933–943. doi: 10.1378/chest.113.4.933. [DOI] [PubMed] [Google Scholar]
- 48.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–1333. doi: 10.1378/chest.130.5.1326. [DOI] [PubMed] [Google Scholar]
- 49.Drummond MB, Kirk GD, Ricketts EP, McCormack MC, Hague JC, McDyer JF, et al. Cross sectional analysis of respiratory symptoms in an injection drug user cohort: the impact of obstructive lung disease and HIV. BMC Pulm Med. 2010;10:27. doi: 10.1186/1471-2466-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui Q, Carruthers S, McIvor A, Smaill F, Thabane L, Smieja M. Effect of smoking on lung function, respiratory symptoms and respiratory diseases amongst HIV-positive subjects: a cross-sectional study. AIDS Res Ther. 2010;7:6. doi: 10.1186/1742-6405-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Madeddu G, Fois AG, Calia GM, Babudieri S, Soddu V, Becciu F, et al. Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection. 2013;41:347–353. doi: 10.1007/s15010-012-0330-x. [DOI] [PubMed] [Google Scholar]
- 52.Diaz PT, Wewers MD, Pacht E, Drake J, Nagaraja HN, Clanton TL. Respiratory symptoms among HIV-seropositive individuals. Chest. 2003;123:1977–1982. doi: 10.1378/chest.123.6.1977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


