Abstract
Objective
To determine the relationships between Krüppel-like Factors (KLF) 2 and 4, immune-activation, and sub-clinical vascular disease in HIV-infected patients on antiretroviral therapy.
Design
Double blind, randomized, placebo-controlled trial
Methods
We studied 74 HIV-infected adults on antiretroviral therapy enrolled in a randomized clinical trial of statin therapy. KLF2 and KLF4 gene expression was measured by quantitative polymerase chain reaction from peripheral blood mononuclear cells (PBMCs) at baseline and after 24 weeks of 10mg daily rosuvastatin or placebo. At the same time points, T-cell and monocyte activation were assessed by flow cytometry and vascular health was assessed by cardiac computed tomography and carotid ultrasound.
Results
KLF4 expression was negatively correlated with duration of antiretroviral therapy (r=−0.351, p=0.004) and positively correlated with measures of immune activation: pro-inflammatory monocytes [CD14+CD16+ (r=0.343, p=0.003)], patrolling monocytes [CD14dimCD16+ (r=0.276, p=0.017] and activated CD8+ T-lymphocytes [CD8+DR+CD38+ (r=0.264, p=0.023)]. KLF2 expression was negatively correlated with subclinical atherosclerosis: mean-mean common carotid artery intima media thickness (CIMT) (r= −0.231, p=0.048), mean-max CIMT (r= −0.271, p=0.020) and coronary artery calcium score (r=−0.254, p=0.029). There were no statistically significant changes in KLF2/4 expression in PBMCs after 24 weeks of rosuvastatin.
Conclusions
Expression of KLF4 in PBMCs positively correlates with cellular markers of immune activation, while KLF2 expression negatively correlates with markers of subclinical atherosclerosis in this HIV-infected population on antiretroviral therapy. Additional studies are needed to determine if targeted interventions might alter KLF2/4 expression to reduce inflammation and vascular risk in humans.
Keywords: HIV, Cardiovascular Disease, Krüppel-like Factor, Inflammation, Immune Activation
Introduction
There is increasing evidence that immune activation and inflammation contribute to cardiovascular disease (CVD) in the general population[1, 2]. Patients infected with human immunodeficiency virus (HIV) have an increased risk for CVD compared to HIV-uninfected controls despite effective antiretroviral therapy, possibly due to residual immune dysregulation[3, 4]. The mechanisms of immune-mediated vascular disease in controlled HIV infection are complex and include alterations in both innate and adaptive immune cell function in the myeloid[5–7] and lymphocyte[8–10] compartments, respectively. In addition, markers of coagulation, immune activation and inflammation are associated with HIV disease progression and mortality[11–13]. Yet, the processes that regulate inflammation and CVD in HIV are incompletely understood.
Activated monocytes are critical to the initiation and progression of atherosclerotic CVD[14]. There exist three distinct subsets of monocytes based on CD14 and CD16 expression and each have distinct immunophenotypes[15–17]. Canonical monocytes, CD14+CD16−, are phagocytic and antigen presenting; patrolling monocytes, CD14dimCD16+, express pathogen-recognition receptors and home to the endothelium; pro-inflammatory monocytes, CD14+CD16+, produce inflammatory cytokines in response to antigen stimulation. HIV-infected patients without known coronary artery disease and HIV-uninfected patients that have had an acute coronary syndrome have similarly high levels of inflammatory and patrolling monocytes suggesting a shared pro-atherothrombotic phenotype[18].
The role of T-cells in the progression of atherosclerosis is well described[1]. HIV infection causes heightened T-cell activation—often defined as co-expression of CD38 and/or HLA-DR—that is only partially reversed by antiretroviral therapy[19] and is an important predictor of mortality[20]. Further, cross-sectional correlations between T-cell activation and subclinical vascular disease have been described[8–10], but the mechanisms linking T-cell activation and vascular disease in HIV+ patients remain elusive.
Coronary artery calcification (CAC), as detected by ECG-gated non-contrast computed tomography (CT), is a marker of subclinical coronary atherosclerosis that predicts coronary events better than other surrogate measures of vascular risk[21]. Compared to HIV-uninfected controls, HIV-infected patients on stable antiretroviral therapy (ART) have a higher CAC burden[22, 23]. Similarly, high-resolution ultrasound measures of carotid intima media thickness (CIMT) also predict CVD risk in the general population, particularly stroke[24, 25]. Traditional and non-traditional risk factors are associated with elevated CAC and CIMT in patients with HIV[8, 9, 22, 23, 26–29]. To date, transcriptional modulators that may explain these observations have not been identified.
The Krüppel-like Factor (KLF) family of transcription factors has regulatory roles in cellular differentiation, metabolism, homeostasis and cellular differentiation. Hemovascular expression of two members of the KLF family, KLF2 and KLF4, has been shown to modulate thrombosis, atherosclerosis, inflammation, barrier function, and vasomotor tone in cell and animal models of disease[30–38]. In addition, statins (HMG-CoA reductase inhibitors) significantly induce levels of KLF2 in mouse and human T-cells[39] and KLF2 and KLF4 in human umbilical vein endothelial cell cultures[36]. The in vivo effect of statins on KLF expression in humans is, however, as yet unknown. Given the important roles of KLF2 and KLF4 in modulating CVD progression and inflammation in cell and animal models of CVD, we hypothesized that KLF2 and KLF4 may play a role in mediating the inflammatory CVD phenotype in HIV+ patients.
To address this question, we designed the current study to evaluate the associations between immune activation, subclinical vascular disease, and KLF2/4 expression in PBMCs of a population of HIV infected subjects treated with antiretroviral therapy. We further aimed to compare changes in KLF2/4 expression in PBMCs after 24 weeks of daily rosuvastatin versus matching placebo.
Methods
Study population
This is a sub-study of the Stopping Atherosclerosis and Treating Unhealthy Bone with Rosuvastatin in HIV (SATURN-HIV) trial. SATURN-HIV is a randomized, double-blind, placebo-controlled trial aimed at elucidating the physiological effects of 10mg/daily rosuvastatin on markers of subclinical cardiovascular disease, inflammation and skeletal health in subjects with HIV on stable antiretroviral therapy (clinicaltrials.gov identifier: NCT01218802). The institutional review board at University Hospitals Case Medical Center approved the study and written informed consent was obtained from each participant.
Participants in SATURN-HIV were HIV-infected adults ≥18 years of age, with HIV-1 RNA < 1,000 copies/mL, on stable antiretroviral therapy (ART) for at least 3 months and cumulative ART for at least 6 months, fasting LDL cholesterol (LDL-C) of ≤130mg/dL and fasting triglyceride level of ≤500mg/dL. Additional inclusion criteria included evidence of heighted T-cell activation (CD8+ T cells expressing CD38+HLA-DR+ ≥19%) and/or screening high-sensitivity C-reactive protein (hs-CRP) ≥2mg/L. Exclusion criteria included known history of coronary artery disease or diabetes, pregnant or lactating, or uncontrolled inflammatory condition.
A medical history, demographic characteristics and physical exam including height, weight, waist and hip measurements were obtained at the initial screening visit. Metabolic syndrome was defined according to Adult Treatment Panel III guidelines[40] as having 3 of the following 5 criteria: (1) waist circumference >102 cm (men), >88 cm (women); (2) triglycerides ≥150 mg/dL; (3) high density lipoprotein <40 mg/dL (men), <50 mg/dL (women); (4) blood pressure ≥130/≥85 mmHg or on anti-hypertensive treatment; and (5) fasting glucose ≥110 mg/dL. Twelve-hour fasting venous blood draw was obtained at study entry and at 24 weeks. Participants’ HIV-1 RNA levels and CD4+ counts were obtained as part of routine clinical care.
Blood sample preparation
PBMCs were separated from whole blood by centrifugation through a Ficoll-Hypaque gradient. All SATURN-HIV participants had PBMCs collected for T-cell and monocyte analysis. PBMCs not used for these analyses were stored for future studies. This KLF sub-study was performed using only those participants (n=74) who had sufficient quantities of PBMCs available for further analyses at both baseline and at week 24.
Quantitative polymerase chain reaction
RNA was isolated from PBMCs using Trizol. Reverse transcription was then performed using a BioRad iScript kit and quantitative polymerase chain reaction (qPCR) was performed using the probe-based Lightcycler 480 system. KLF2, KLF4, HPRT and GAPDH expression levels were measured and the data herein represents an average copy number of KLF2 and KLF4 expression normalized to both housekeeping genes- HPRT and GAPDH. To determine absolute quantification of gene transcripts, we created a standard curve (copy number vs. Cp) using purified amplicon from each gene above, as described previously[41]. We subsequently derived copy number from a line of best fit for the standard curve using the Cp of each gene. The number of copies of KLF2/4 mRNA reported is that present in 20 picograms of total cellular mRNA. Each subject’s PBMC sample was run at least three times in triplicate.
Monocyte subsets
Whole blood was incubated for 15 minutes on ice in FACS lysis buffer (BD Biosciences) and washed in phosphate-buffered saline containing 1% bovine serum albumin and 0.1% sodium azide. The cells were then stained for 30 minutes in the dark on ice, washed and fixed in 1% paraformaldehyde. Monocyte subsets were defined by expression of CD14, CD16, size and granularity[18]. In order to quantify expression of surface markers, we utilized isotype gating. The following fluorescent antibodies were used to identify monocytes: anti-CD14 (Pacific Blue, BD Pharmingen) and anti-CD16 (PE-conjugated, BD Pharmingen). Monocytes were analyzed for differential expression of CD14 and CD16 using a Miltenyi MACS quant flow cytometer and MACS Quantify software. Monocyte subsets were defined using relative expression of CD14 and CD16.
T-cell activation
T cells were quantified by size, granularity and positive expression of CD3 and either CD4 or CD8. T-cell activation was determined using PE-conjugated anti-CD38, FITC-conjugated anti-HLA-DR, peridinin-chlorophyll-protein-complex-conjugated anti-CD3, allophycocyanin-cy7-conjugated anti-CD8 and allophycocyanin-conjugated anti-CD4 antibodies all from BD Biosciences. T-cell activation was defined as expression of co-expression of CD38 and HLA-DR. Measurements were performed using a LSR II flow cytometer and analyzed using FACSDiva software.
Coronary artery calcium score
Coronary artery calcium (CAC) score was performed as previously described[42]. Briefly, all patients underwent a ECG-gated non-contrast computed tomography (CT) scan of the chest. Calcified lesions were quantified as areas of ≥6 pixels with density > 130 Hounsfield units. The Agatston method was used to quantify CAC. Subjects were categorized dichotomously as a score of 0 or >0.
Carotid intima media thickness
Vascular ultrasound to assess carotid intima media thickness (CIMT) was performed as described previously[42]. CIMT was measured over the distal one-centimeter segment of the common carotid artery at three angles bilaterally (i.e. 6 segments). Each of these 6 segments has a max value and a mean value over that 1 cm segment. Two measures were used for analysis: average of the 6 mean values (mean-mean IMT) and the average of the 6 max values (mean-max IMT).
Statistical Analysis
Characteristics of study participants were described as median (inter-quartile range) for continuous variables and frequency (%) for categorical variables. Characteristics of those participants included in the KLF analysis were compared to the rest of the study population using an unpaired t-test or Wilcoxon Rank Sum test as distributionally appropriate for continuous variables and Fisher’s exact test for categorical variables. Spearman correlation was used to examine the relationships between KLF expression and markers of immune activation, subclinical vascular disease, and HIV-specific factors. Changes in KLF2/4 copy number from 0 to 24 weeks were expressed as percentage change from baseline and were compared between statin and placebo groups using paired t-tests. All statistical tests were two-sided and considered significant at a level of p<0.05. Analyses were performed using SAS v. 9.2 (The SAS Institute, Carey, North Carolina, USA).
Results
Of 147 total participants in SATURN-HIV, 74 had sufficient quantities of PBMCs for inclusion in this KLF sub-study. Baseline characteristics of the 74 study participants, including CVD risk factors and HIV disease status, are displayed in Table 1. Median age was 47 and the majority were male. Participants had well-controlled HIV-infection with high median CD4+ T-cell count and 78% with undetectable HIV-1 RNA. Although median 10-year Framingham risk score was low (5% risk of cardiovascular disease), two-thirds were smokers and nearly one-quarter of participants had metabolic syndrome. Participants in the KLF analysis were similar to those who were not included (n=73), except that included participants were more likely to have undetectable (<48c/ml) HIV-1 RNA levels (78% vs. 63%, p=0.04, included vs. excluded; p>0.05 for all other characteristics shown in Table 1). Total white blood cell count was higher among those who were included in the study compared to those who were not included (6.7 vs. 5.7 ×109 cells/L); however, total lymphocytes and total monocytes were not different (both p>0.2). All participants in this sub-study completed a full 24 weeks of study therapy.
Table 1.
Baseline characteristics of study participants
| Demographics | n=74 |
|---|---|
| Age (years) | 47 (42–52) |
| Male | 60 (81%) |
| African-American | 49 (66%) |
| HIV Parameters | |
| Current CD4+ (cells/mm3)† | 628 (473–807) |
| Nadir CD4+ (cells/mm3) | 231 (114–312) |
| HIV Duration (years) | 14 (7.4–19) |
| ART Duration (years) | 5.2 (2.8–7.1) |
| Undetectable HIV-1 RNA (<48c/ml) | 58 (78%) |
| CVD Risk Factors | |
| Systolic Blood Pressure (mmHg) | 120 (110–130) |
| HDL Cholesterol (mg/dL) | 45 (35–57) |
| LDL Cholesterol (mg/dL) | 102 (77–114) |
| Body-Mass Index (kg/m2) | 27 (24–31) |
| Metabolic Syndrome | 16 (22%) |
| Current Smoker | 49 (66%) |
| Family History of MI | 25 (34%) |
| 10-year Framingham Risk Score (%) | 5 (1–9) |
| Current Medication Use | |
| Anti-hypertensive Medication | 15 (20%) |
| Protease Inhibitor | 33 (45%) |
| AZT or D4T | 2 (3%) |
Data presented as median (inter-quartile range) or frequency (%).
ART, antiretroviral therapy; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction; AZT, zidovudine; D4T, stavudine
Normal CD4+ T-cell count for HIV-uninfected individuals is 350–2740 cells/
KLF4 positively correlates with monocyte and T-cell activation
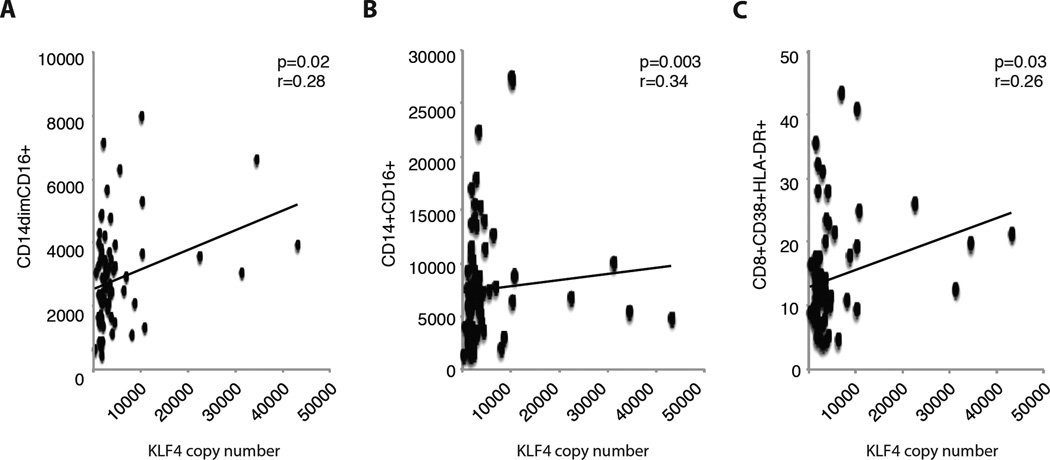
We hypothesized that expression levels of KLF2/4 in PBMCs would be related to a study participant’s level of immune activation at baseline. Copy number of KLF mRNA was determined using standard qPCR techniques. In a cross-sectional analysis of baseline data, KLF4 was positively correlated with two activated monocyte subsets: CD14+CD16+ monocytes (r=0.34, p<0.01) and CD14dimCD16+ monocytes (r=0.28, p=0.02) (Fig. 1A). Similarly, we observed that KLF4 was positively correlated with activated CD8+CD38+HLA-DR+ T-cells (r=0.26, p=0.03) (Fig. 1B). Despite a borderline statistically significant positive correlation with total lymphocyte count (r=0.220, p=0.06) and a borderline negative correlation with total monocyte count (r=−0.213, p=0.07), we observed no significant correlations between KLF2 and markers of activated monocytes or T-cells (all p>0.1).
Figure 1.
Baseline expression of KLF4 in peripheral blood mononuclear cells was correlated with the percentage of (A) CD14dimCD16+ monocytes, (B) CD14+CD16+ monocytes, and (C) CD8+ T-cell activation.
KLF2 negatively correlates with coronary calcium score and carotid intima media thickness
Secondly, we hypothesized that KLF2 and KLF4 would also be related to measures of subclinical vascular disease. Indeed, KLF2 was negatively correlated with mean-mean IMT (Table 2; r= −0.231, p=0.048), mean-max IMT (r= −0.271, p=0.020), and with the presence of coronary calcium (r= −0.254, p=0.029). We found no significant correlation between KLF4 and CAC or IMT (Table 2; all p >0.05).
Table 2.
Relationship between KLF2 and KLF4 expression and measures of subclinical atherosclerosis
| KLF2 | KLF4 | |||
|---|---|---|---|---|
| r | p-value | r | p-value | |
| CAC >0 | −0.254 | 0.029 | 0.215 | 0.066 |
| Mean-mean CIMT | −0.231 | 0.048 | 0.177 | 0.131 |
| Mean-max CIMT | −0.271 | 0.020 | 0.151 | 0.199 |
KLF, Krüppel-like Factor; CAC, coronary artery calcium; CIMT, carotid intima-media thickness
KLF4 positively correlates with measures of HIV disease duration and ART use
We further explored whether KLF2 and KLF4 would be related to measures of HIV disease severity and duration. KLF4 was negatively correlated with duration of HIV infection (r=−0.297, p=0.01) and duration of ART (r=−0.351, p=0.004), including PI duration (r=−0.322, p=0.02) and nucleoside reverse transcriptase inhibitor (NRTI) duration (r=−0.307, p=0.009), but not non-nucleoside reverse transcriptase inhibitor (NNRTI) duration (r=0.104, p=0.433). We did not observe any relationship between these measures and KLF2. Neither KLF2 nor KLF4 was associated with HIV-1 RNA level, current CD4+ T-cell count, or nadir CD4+ T-cell count (all p>0.6).
Rosuvastatin therapy for 24 weeks does not alter the expression of KLF2 or KLF4 in PBMCs
After 24 weeks of therapy, there were no significant changes in KLF2 or KLF4 expression in PBMCs of those treated with 10mg of daily rosuvastatin compared to those who received placebo [mean (standard deviation) change of +0.4(1.8)% vs. +0.1(0.7)%, p=0.638 for KLF2 and +2.7(6.2)% vs. +3.4(8.8)%, p=0.799 for KLF4, statin vs. placebo, respectively].
Discussion
Studies to elucidate the mechanisms that link immune activation and premature vascular disease in the HIV-infected population are urgently needed in order to develop targeted therapies that improve outcomes. In this clinical study of HIV infected patients on antiretroviral therapy, we have uncovered important relationships between two transcriptional regulators of atherothrombotic vascular disease—KLF2 and KLF4—and levels of cellular immune activation, subclinical atherosclerosis, duration of HIV infection, and ART use.
KLF2 and KLF4 represent two of the 18 members of the family of transcription factors called Krüppel-like factors. While the KLFs have diverse roles in fundamental physiological processes including cell differentiation and homeostatic balance, immune cell expression of KLF2 and KLF4 in particular have been implicated in regulation of essential aspects of hemovascular biology[43, 44]. In animal models of disease, KLF2 and/or KLF4 expression in immune cells have been shown to protect from development of atherosclerosis[35], insulin resistance and obesity[37], and regulate the immune response in inflammatory arthritis[45], myocarditis[39], and polymicrobial infection[46]. The precise transcriptional and signaling pathways by which KLF2/4 exert their immune effects is a subject of intense investigation. Several studies have shown roles for KLF2 or KLF4 in the regulation of T-cell and monocyte/macrophage activation in vitro and in vivo using cell culture and animal models[39, 47–50]. Early in vivo mouse studies suggested that the anti-inflammatory transcription factor KLF2 maintains T-cells in a quiescent state; however subsequent studies indicate that KLF2 regulates a composite of T cell functions, trafficking in particular, that can give the impression that it has a direct effect on quiescence[47, 48]. As reviewed by Hart et al.[43], constitutive expression of KLF2 in naïve T-cells is extinguished after T-cell stimulation, and cytokines involved in effector differentiation repress re-expression of KLF2. Loss of KLF2 leads, in turn, to transcriptional down-regulation of sphingosine 1-phosphate receptor 1 and thus activated T-cells are retained in lymphoid sites[51]. Furthermore, once CD8+ T-cells are activated, elevated levels of KLF2 are required to maintain the memory cell phenotype[52]. Of note, KLF2 also regulates expression of CCR5 in response to activation, thus making it a potential target to modify susceptibility to R5-tropic HIV-1 infection[53]. Susceptibility of CD4+ T-cells to HIV-1 infection may be further increased by KLF2 regulated expression of CD62L (L-selectin)[54]. Finally, more recent data suggests that in addition to its important role in T-cell biology, KLF2 is also a negative regulator of monocyte activation[49]. Data regarding immune expression of KLF2 in humans is limited. Expression was shown to be decreased in circulating monocytes from patients with coronary artery disease[49] or by RNA analysis of whole blood in patients with sepsis[46]. In our study, we found no correlation between KLF2 copy number in PBMCs and levels of inflammatory monocyte subsets or CD38+HLA-DR+ T-cells. Relationships between KLF2 and markers of T-cell turnover and senescence—which are altered by HIV infection—should be explored in future studies.
The important role of the transcription factor KLF4 in monocyte and tissue macrophage activation has been previously described[50, 55]. Its role in T-cell activation is less clear, though it does appear to be required for T helper 17 differentiation[56, 57] and is expressed in CD8+ T cells where it contributes to T-cell quiescence downstream of Ets transcription factor ELF[58]. Inflammatory cytokines such as interferon and lipopolysaccharide induce tissue macrophages to produce KLF4 and overexpression of KLF4 induces macrophage activation[50]. As for KLF2, the expression in and role of KLF4 in immune tissue from human samples is limited. One study demonstrated a 50% reduction KLF4 expression in adipose tissue from obese (versus lean) patients[36]. Decreased KLF4 signal was attributed to loss of expression in adipose tissue macrophages and hypothesized to play a role in the metabolic dysregulation seen in obese patients[36]. In this study, we report modest positive correlations between KLF4 copy number in PBMCs and activated CD8+CD38+HLA-DR+ T-cells and pro-inflammatory CD14+CD16+ monocytes. KLF4 was also positively correlated with CD14dimCD16+ monocytes, so-called “patrolling monocytes.” These patrolling monocytes play a homeostatic role in maintaining a healthy endothelium, but can become activated in response to certain viral infections[16]. Higher levels of these monocytes have been associated with CAC progression[59] and carotid stiffness[60] in HIV-infected subjects. Initiation of ART reduces T-cell activation[19] and starting ART earlier in the course of HIV infection is associated with a lower T-cell activation setpoint[61]. The negative correlation between KLF4 levels and duration of ART in our study is, therefore, intriguing; however, whether KLF4 mediates the relationship between ART and immune activation requires further investigation in longitudinal clinical trials of ART.
Because our study population was extensively characterized by non-invasive vascular imaging tests, we were able to explore the relationship between KLF expression in PBMCs and the extent of subclinical atherosclerosis. We observed that HIV-infected subjects with detectable coronary calcium and higher carotid IMT had lower levels of KLF2. It has long been recognized that KLF2 expression in endothelial cells is induced by shear stress[62] and that carotid IMT is thicker in areas of low shear stress such as the carotid bifurcation[63]; however, to our knowledge, this inverse relationship between peripheral blood KLF2 expression and carotid IMT has not previously been described in humans. It is important to acknowledge that we did not directly measure endothelial KLF expression in this study. Despite correlations with cellular immune activation markers, KLF4 was not associated with any measure of subclinical atherosclerosis in our cross-sectional analysis.
Statins profoundly reduce the risk of cardiovascular events in multiple populations, and part of this risk reduction appears to be related to reductions in inflammation that are independent of LDL-lowering[64, 65]. Prior studies have suggested that KLF2 and KLF4 may be important transcriptional regulators of this anti-inflammatory effect of statins[36, 39, 66, 67]. Surprisingly in the present study, there was no effect of 24 weeks of statin therapy on KLF2/4 levels in PBMCs. This is an important observation that must be confirmed in larger human studies. A potential explanation may be that the concentrations used in prior cell culture and small animal experiments (0.1–10 µM) are much higher than the blood concentration of statins typically achieved in humans (0.001–0.015 µM)[68] or that timing of blood draws and medication administration was mismatched to detect statin-mediated KLF expression. We measured KLF RNA expression in our study as a surrogate marker of protein levels in PBMCs. Many studies have shown strong correlation between RNA and protein[37, 38, 46, 55]; however, there are no data on whether this relationship is altered by HIV infection. In this study, KLF RNA expression was measured in PBMCs. Future studies should assess the effect of statins on KLF expression in vascular endothelial cells. Finally, whether this lack of statin effect is unique to HIV-infected individuals is unknown and should be explored in HIV-uninfected populations.
This study describes a novel examination of factors that transcriptionally regulate inflammation and vascular health in chronic HIV-infection. These transcription factors may also be altered by duration of ART use. The novel findings of this study are strengthened by the extensive immunophenotyping and subclinical vascular disease characterization of study subjects. Limitations of this study include a relatively small sample size due in part to the time and expense required to measure KLF with qPCR; however it is larger than any previous study using human PBMCs. We cannot exclude the possibility that the observed relationships are explained by unmeasured confounding. Additionally, cross-sectional associations cannot determine causality.
In conclusion, KLF2 and KLF4 may play a role in mediating the inflammatory CVD phenotype in HIV-infected patients. Future studies should examine longitudinal relationships between KLF2/4, immune activation, and subclinical vascular disease. Regulation of KLF2/4 expression may represent a novel therapeutic target to prevent vascular disease in HIV infection and other chronic inflammatory diseases.
Acknowledgements
A.T.H and C.T.L performed data collection, study design, data analysis and drafted the manuscript. Y.J. and S.M.D performed data analysis. D.E.L and N.S. performed data collection and provided administrative support. A.H. and G.A.M performed data collection, study design, data analysis and drafted the manuscript.
The authors would like to thank the study participants. We also acknowledge Dr. Mukesh K. Jain for his support and encouragement.
Funding: This work was supported by the National Institutes of Health (grant numbers R01NR012642 to GAM, K23HL123341 to CTL, and R01HL113570 to AH) and by a Wolf Family Foundation Scholars Grant to CTL. Technical assistance was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219). The study is registered with clinicaltrials.gov, number NCT01218802.
CTL has received grants from Bristol-Myers Squibb and the Medtronic Foundation. GAM has served as a scientific advisor or speaker for Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Gilead Sciences, has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences, and has served as the DSMB Chair for a Pfizer-sponsored study.
Footnotes
Conflicts of Interest:
ATH, YJ, SD, DEL, NS, and AH have no disclosures.
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. The Journal of infectious diseases. 2012;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longenecker CT, Triant VA. Initiation of antiretroviral therapy at high CD4 cell counts: does it reduce the risk of cardiovascular disease? Current opinion in HIV and AIDS. 2013 doi: 10.1097/COH.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. The Journal of infectious diseases. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA : the journal of the American Medical Association. 2012;308:379–386. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burdo TH, Lo J, Abbara S, Wei J, Delelys ME, Preffer F, et al. Soluble CD163, a Novel Marker of Activated Macrophages, Is Elevated and Associated With Noncalcified Coronary Plaque in HIV-Infected Patients. The Journal of infectious diseases. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. The Journal of infectious diseases. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. The Journal of infectious diseases. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ancuta P, Wang J, Gabuzda D. CD16+ monocytes produce IL-6, CCL2, and matrix metalloproteinase-9 upon interaction with CX3CL1-expressing endothelial cells. J Leukoc Biol. 2006;80:1156–1164. doi: 10.1189/jlb.0206125. [DOI] [PubMed] [Google Scholar]
- 16.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 18.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt PW. HIV and inflammation: mechanisms and consequences. Current HIV/AIDS reports. 2012;9:139–147. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 20.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 21.Yeboah J, McClelland RL, Polonsky TS, Burke GL, Sibley CT, O'Leary D, et al. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA : the journal of the American Medical Association. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Post WS, Budoff M, Kingsley L, Palella FJ, Jr, Witt MD, Li X, et al. Associations Between HIV Infection and Subclinical Coronary Atherosclerosis. Ann Intern Med. 2014;160:458–467. doi: 10.7326/M13-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsue PY, Ordovas K, Lee T, Reddy G, Gotway M, Schnell A, et al. Carotid intima-media thickness among human immunodeficiency virus-infected patients without coronary calcium. The American journal of cardiology. 2012;109:742–747. doi: 10.1016/j.amjcard.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262–269. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
- 25.Craven TE, Ryu JE, Espeland MA, Kahl FR, McKinney WM, Toole JF, et al. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation. 1990;82:1230–1242. doi: 10.1161/01.cir.82.4.1230. [DOI] [PubMed] [Google Scholar]
- 26.Longenecker C, Jiang Y, Orringer C, Gilkeson R, Debanne S, Funderburg N, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014 doi: 10.1097/QAD.0000000000000158. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23:1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Currier JS, Kendall MA, Zackin R, Henry WK, Alston-Smith B, Torriani FJ, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure. AIDS. 2005;19:927–933. doi: 10.1097/01.aids.0000171406.53737.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein JH, Brown TT, Ribaudo HJ, Chen Y, Yan M, Lauer-Brodell E, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. AIDS. 2012 doi: 10.1097/QAD.0b013e32835ce27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nayak L, Goduni L, Takami Y, Sharma N, Kapil P, Jain MK, et al. Kruppel-like factor 2 is a transcriptional regulator of chronic and acute inflammation. Am J Pathol. 2013;182:1696–1704. doi: 10.1016/j.ajpath.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shatat MA, Tian H, Zhang R, Tandon G, Hale A, Fritz JS, et al. Endothelial Kruppel-like factor 4 modulates pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2014;50:647–653. doi: 10.1165/rcmb.2013-0135OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nayak L, Shi H, Atkins GB, Lin Z, Schmaier AH, Jain MK. The thromboprotective effect of bortezomib is dependent on the transcription factor Kruppel-like factor 2 (KLF2) Blood. 2014;123:3828–3831. doi: 10.1182/blood-2014-01-547448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, et al. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest. 2012;122:4727–4731. doi: 10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, et al. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30:1952–1959. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, et al. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.SenBanerjee S, Lin Z, Atkins GB, Greif DM, Rao RM, Kumar A, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, et al. Kruppel-like factor 4 regulates macrophage polarization. J Clin Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 39.Bu DX, Tarrio M, Grabie N, Zhang Y, Yamazaki H, Stavrakis G, et al. Statin-induced Kruppel-like factor 2 expression in human and mouse T cells reduces inflammatory and pathogenic responses. J Clin Invest. 2010;120:1961–1970. doi: 10.1172/JCI41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 41.Hale AT, Tian H, Anih E, Recio FO, 3rd, Shatat MA, Johnson T, et al. Endothelial Kruppel-like factor 4 regulates angiogenesis and the Notch signaling pathway. J Biol Chem. 2014;289:12016–12028. doi: 10.1074/jbc.M113.530956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Longenecker CT, Jiang Y, Orringer CE, Gilkeson RC, Debanne S, Funderburg NT, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS. 2014;28:969–977. doi: 10.1097/QAD.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hart GT, Hogquist KA, Jameson SC. Kruppel-like factors in lymphocyte biology. J Immunol. 2012;188:521–526. doi: 10.4049/jimmunol.1101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Z, Sun X, Icli B, Wara AK, Feinberg MW. Role of Kruppel-like factors in leukocyte development, function, and disease. Blood. 2010;116:4404–4414. doi: 10.1182/blood-2010-05-285353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das M, Lu J, Joseph M, Aggarwal R, Kanji S, McMichael BK, et al. Kruppel-like factor 2 (KLF2) regulates monocyte differentiation and functions in mBSA and IL-1beta-induced arthritis. Curr Mol Med. 2012;12:113–125. doi: 10.2174/156652412798889090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahabeleshwar GH, Kawanami D, Sharma N, Takami Y, Zhou G, Shi H, et al. The myeloid transcription factor KLF2 regulates the host response to polymicrobial infection and endotoxic shock. Immunity. 2011;34:715–728. doi: 10.1016/j.immuni.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo CT, Veselits ML, Leiden JM. LKLF: A transcriptional regulator of single-positive T cell quiescence and survival. Science. 1997;277:1986–1990. doi: 10.1126/science.277.5334.1986. [DOI] [PubMed] [Google Scholar]
- 48.Buckley AF, Kuo CT, Leiden JM. Transcription factor LKLF is sufficient to program T cell quiescence via a c-Myc-dependent pathway. Nat Immunol. 2001;2:698–704. doi: 10.1038/90633. [DOI] [PubMed] [Google Scholar]
- 49.Das H, Kumar A, Lin Z, Patino WD, Hwang PM, Feinberg MW, et al. Kruppel-like factor 2 (KLF2) regulates proinflammatory activation of monocytes. Proc Natl Acad Sci U S A. 2006;103:6653–6658. doi: 10.1073/pnas.0508235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feinberg MW, Cao Z, Wara AK, Lebedeva MA, Senbanerjee S, Jain MK. Kruppel-like factor 4 is a mediator of proinflammatory signaling in macrophages. J Biol Chem. 2005;280:38247–38258. doi: 10.1074/jbc.M509378200. [DOI] [PubMed] [Google Scholar]
- 51.Mudd JC, Murphy P, Manion M, Debernardo R, Hardacre J, Ammori J, et al. Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood. 2013;121:2914–2922. doi: 10.1182/blood-2012-07-445783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schober SL, Kuo CT, Schluns KS, Lefrancois L, Leiden JM, Jameson SC. Expression of the transcription factor lung Kruppel-like factor is regulated by cytokines and correlates with survival of memory T cells in vitro and in vivo. J Immunol. 1999;163:3662–3667. [PubMed] [Google Scholar]
- 53.Richardson MW, Jadlowsky J, Didigu CA, Doms RW, Riley JL. Kruppel-like factor 2 modulates CCR5 expression and susceptibility to HIV-1 infection. J Immunol. 2012;189:3815–3821. doi: 10.4049/jimmunol.1201431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trinite B, Chan CN, Lee CS, Mahajan S, Luo Y, Muesing MA, et al. Suppression of Foxo1 activity and down-modulation of CD62L (L-selectin) in HIV-1 infected resting CD4 T cells. PLoS One. 20149:e110719. doi: 10.1371/journal.pone.0110719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alder JK, Georgantas RW, 3rd, Hildreth RL, Kaplan IM, Morisot S, Yu X, et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180:5645–5652. doi: 10.4049/jimmunol.180.8.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lebson L, Gocke A, Rosenzweig J, Alder J, Civin C, Calabresi PA, et al. Cutting edge: The transcription factor Kruppel-like factor 4 regulates the differentiation of Th17 cells independently of RORgammat. J Immunol. 2010;185:7161–7164. doi: 10.4049/jimmunol.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.An J, Golech S, Klaewsongkram J, Zhang Y, Subedi K, Huston GE, et al. Kruppel-like factor 4 (KLF4) directly regulates proliferation in thymocyte development and IL-17 expression during Th17 differentiation. FASEB J. 2011;25:3634–3645. doi: 10.1096/fj.11-186924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamada T, Park CS, Mamonkin M, Lacorazza HD. Transcription factor ELF4 controls the proliferation and homing of CD8+ T cells via the Kruppel-like factors KLF4 and KLF2. Nat Immunol. 2009;10:618–626. doi: 10.1038/ni.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker JV, Hullsiek KH, Singh A, Wilson E, Henry K, Lichtenstein K, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS. 2014;28:831–840. doi: 10.1097/QAD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Longenecker C, Jiang Y, Debanne S, McComsey G. Carotid Distensibility and Immune Activation in HIV-infected Patients without Coronary Calcium; 21st Conference on Retroviruses and Opportunistic Infections; Boston, MA USA. 2014. [Google Scholar]
- 61.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 63.Gnasso A, Carallo C, Irace C, Spagnuolo V, De Novara G, Mattioli PL, et al. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996;94:3257–3262. doi: 10.1161/01.cir.94.12.3257. [DOI] [PubMed] [Google Scholar]
- 64.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: a prospective study of the JUPITER trial. Lancet. 2009;373:1175–1182. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]
- 65.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4:977–987. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 66.Maejima T, Inoue T, Kanki Y, Kohro T, Li G, Ohta Y, et al. Direct evidence for pitavastatin induced chromatin structure change in the KLF4 gene in endothelial cells. PLoS One. 2014;9:e96005. doi: 10.1371/journal.pone.0096005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villarreal G, Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, Garcia-Cardena G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun. 2010;391:984–989. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bjorkhem-Bergman L, Lindh JD, Bergman P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br J Clin Pharmacol. 2011;72:164–165. doi: 10.1111/j.1365-2125.2011.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]