Abstract
Objective
We evaluated the comparative effectiveness of Mindfulness-based cognitive therapy (MBCT) versus an active control condition (ACC) for depression relapse prevention, depressive symptom reduction, and improvement in life satisfaction.
Method
Ninety-two participants in remission from Major Depressive Disorder with residual depressive symptoms were randomized to either an 8-week MBCT or a validated ACC that is structurally equivalent to MBCT and controls for non-specific effects (e.g., interaction with a facilitator, perceived social support, treatment outcome expectations). Both interventions were delivered according to their published manuals.
Results
Intention-to-treat analyses indicated no differences between MBCT and ACC in depression relapse rates or time to relapse over a 60-week follow-up. Both groups experienced significant and equal reductions in depressive symptoms and improvements in life satisfaction. A significant quadratic interaction (group x time) indicated that the pattern of depressive symptom reduction differed between groups. The ACC experienced immediate symptom reduction post-intervention and then a gradual increase over the 60-week follow-up. The MBCT group experienced a gradual linear symptom reduction. The pattern for life satisfaction was identical but only marginally significant.
Conclusions
MBCT did not differ from an ACC on rates of depression relapse, symptom reduction, or life satisfaction, suggesting that MBCT is no more effective for preventing depression relapse and reducing depressive symptoms than the active components of the ACC. Differences in trajectory of depressive symptom improvement suggest that the intervention-specific skills acquired may be associated with differential rates of therapeutic benefit. This study demonstrates the importance of comparing psychotherapeutic interventions to active control conditions.
Keywords: mindfulness-based cognitive therapy, major depression, relapse prevention, depressive symptoms, active control condition
Major depressive disorder (MDD) is a leading cause of disability worldwide (Ferrari et al., 2013). The 50–80% rate of depression relapse (Judd, 1997) has prompted a focus on treatments aimed at preventing relapse/recurrence. Maintenance antidepressant medication (mADM) is the most common strategy to prevent relapse and recurrence but is associated with poor adherence (ten Doesschate, Bockting, & Schene, 2009), side effects (Kelly, Posternak, & Alpert, 2008), and modest clinical benefits after discontinued use (Dobson et al., 2008; Hollon, Stewart, & Strunk, 2006). These limitations have increased interest in psychological therapies that target depression relapse/recurrence.
Mindfulness-based cognitive therapy (MBCT) is one such therapy that reduces depression relapse in patients with recurrent MDD (≥ 3 previous episodes) (Ma & Teasdale, 2004; Teasdale et al., 2000) and depressive symptoms in individuals with current MDD (Munshi, Eisendrath, & Delucchi, 2013; Strauss, Cavanagh, Oliver, & Pettman, 2014) and in remission with residual symptoms (Kingston, Dooley, Bates, Lawlor, & Malone, 2007). MBCT is as effective as mADM for reducing depression relapse/recurrence (Kuyken et al., 2008; Segal et al., 2010). While this evidence is compelling, nearly exclusively, studies have compared MBCT to treatment as usual (TAU), a heterogeneous combination of ADM and psychotherapy or waitlist controls. A critical next step in evaluating MBCT is to test whether reductions in depression relapse rates and depressive symptoms are specific to MBCT or whether other psycho-educational interventions may produce similar benefits. This is important because MBCT is not yet widely available or accessible, and a generalized psycho-education treatment may have cost, accessibility, and dissemination advantages (Parikh et al., 2012). Further, to begin to understand the active ingredients of MBCT, it is necessary to compare it to a structurally equivalent and therapeutically credible active control condition (ACC) that is matched to MBCT on non-specific factors (e.g., social support, treatment-related activity outside of class, interaction with a facilitator, expected positive outcomes, etc.), but lacks mindfulness and cognitive therapy components (Kirsch, 2005).
To our knowledge only two randomized controlled trials (RCTs) have compared MBCT to an ACC for depression relapse prevention (Meadows et al., 2014; Williams et al., 2014). Meadows et al. (2014) compared the effects of MBCT + depression relapse active monitoring (DRAM) to DRAM alone. DRAM was comprised of training in self-management of depression that was promoted, in part, through monthly assessments of depressive symptoms. This study found that fewer MBCT participants relapsed compared to controls and that time to first depressive episode was significantly reduced in the MBCT, but only among the per-protocol sample (those who attended 4 or more sessions of MBCT) and who were either receiving usual care in a specialist setting or taking antidepressants or mood stabilizers. Although therapeutically equally credible, the ACC was not matched to MBCT on other important and potentially therapeutic ingredients (e.g., social support, alliance with an instructor) and was thus unable to address questions about the active components of MBCT. Williams et al., 2014 conducted a 3-arm study designed to dismantle the mindfulness component of MBCT by comparing MBCT to cognitive psycho-education and TAU. Results indicated no group differences in time to relapse, except for individuals with a history of childhood trauma, who benefited most from MBCT and least from TAU. Conclusions about the specificity of MBCT’s effects (e.g., whether mindfulness is the ‘active’ ingredient) are difficult to draw from this study because the ACC did not require equivalent treatment-related activity outside of class.
The present study compared MBCT to a structurally equivalent and validated ACC and evaluated the comparative effectiveness and specificity of MBCT’s effects for preventing depression relapse. In secondary analyses, we examined the effects of MBCT vs. ACC on depressive symptoms and life satisfaction. We predicted that MBCT, compared to the ACC, would be more effective for preventing depression relapse, reducing depressive symptoms, and improving life satisfaction. This prediction is based on the fact that although extant literature indicates that the components of the ACC used in this study (e.g., physical activity, nutrition, and music therapy), can reduce depressive symptoms (Craft & Perna, 2004; Maratos, Crawford, & Procter, 2011; Opie, O’Neil, Itsiopoulos, & Jacka, 2014), stronger empirical evidence (e.g., more rigorous RCTs) exists for the effects of MBCT for depression outcomes, particularly depression relapse prevention (Ma & Teasdale, 2004; Segal et al., 2010; Teasdale et al., 2000; Williams et al., 2014). In exploratory analyses we examined several plausible moderators of intervention effects (e.g., severity of baseline depressive symptoms, age of onset of depression).
Method
Study Design and Participants
The study design was a randomized (1:1), controlled, 8-week parallel comparison of MBCT and a validated ACC. Consistent with the initial landmark MBCT studies (Ma & Teasdale, 2004; Teasdale et al., 2000) as well as subsequent MBCT trials (Bondolfi et al., 2010; Crane et al., 2014; Godfrin & van Heeringen, 2010; Kuyken et al., 2008; Williams et al., 2014), participants were followed over the course of 60-weeks. The study protocol was approved by the institutional review board at the sponsoring institution. Written consent was obtained from all participants. Participants were recruited from an urban area in the Rocky Mountain West through referrals from community mental health centers and local advertisements. Eligibility was assessed via a combination of an online screening assessment and an in-person administration of The Structured Clinical Interview for the DSM-IV (SCID I/II), which confirmed MDD-related and co-morbid Axis I/II diagnostic eligibility for the study (First, Gibbon, Spitzer, Williams, & Benjamin, 1997; First, Spitzer, Gibbon, & Williams, 1994). The SCID occurred approximately one week after the online screening. Inclusion criteria were: a) English speaking; b) age between 18 and 65 years; c) minimum of 1 prior episode of MDD; d) at least 1 prior episode of MDD within 2 years of interview assessment; e) current remission from MDD for at least 1 month prior to interview assessment; f) no change in type or dose of ADM for at least 3 weeks prior to online screening; g) residual depressive symptoms indicated by a Beck Depression Inventory-II (BDI-II) (Beck, Steer, & Brown, 1996) score between 4 and 30 at online screening. Exclusion criteria were: a) substance dependence within 12 months prior to online screening; b) current suicidal ideation or suicide attempt within 3 months prior to online screening; c) current diagnosis of bipolar disorder, schizophrenia, borderline or antisocial personality disorder, obsessive compulsive disorder, or eating disorder. Participants were permitted to seek treatment outside of the study interventions.
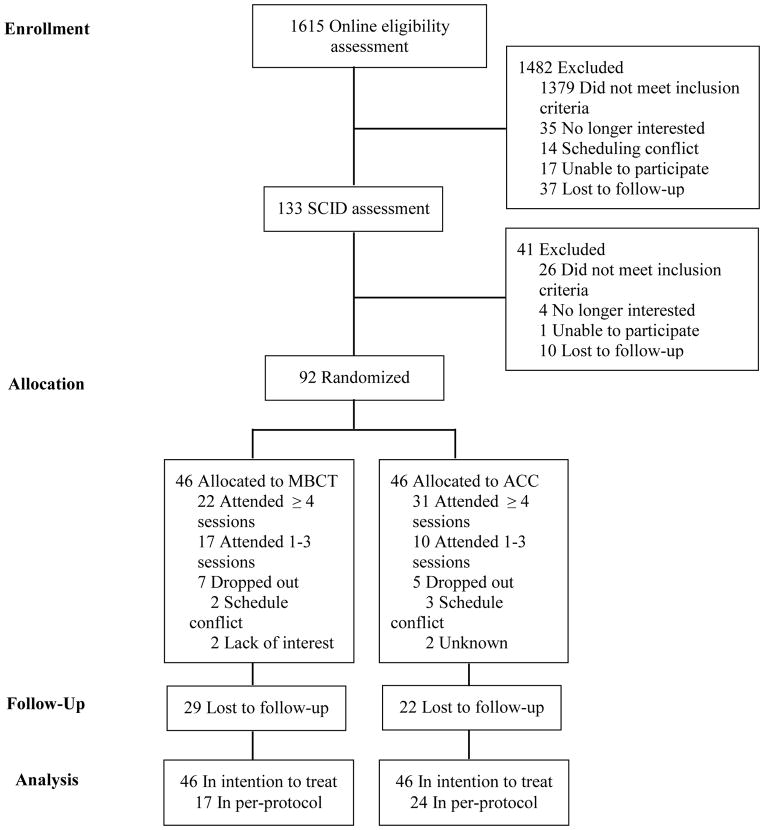
One thousand, six hundred and fifteen participants were assessed in the online screening questionnaire. Of these, 1379 did not meet inclusion criteria (23.3% due to meeting diagnostic criteria consistent with current MDD). An additional 103 were excluded due to miscellaneous reasons (e.g., scheduling conflicts), leaving 133 participants eligible for a SCID interview. Of these, 26 participants did not meet inclusion criteria (due primarily to a diagnosis of current MDD) and an additional 15 participants were excluded due to miscellaneous reasons (same as above). The final sample included 92 participants randomized to MBCT (n=46) or ACC (n=46) (Figure 1).
Figure 1.
CONSORT flow diagram. MBCT=mindfulness based cognitive therapy; ACC=active control condition; SCID=Structured Clinical Interview for DSM-IV.
Interventions
Mindfulness-based cognitive therapy (MBCT)
MBCT is a manualized 8-week group intervention that integrates components of cognitive behavioral therapy (Beck, 1979) and mindfulness-based stress reduction (MBSR) (Kabat-Zinn, 1990). MBCT is designed to prevent relapse of major depression by improving individuals’ ability to recognize and disengage from ruminative thinking and to process depressogenic information in ways that is hypothesized to decrease risk for depression relapse (Segal, Williams, & Teasdale, 2013). Each MBCT group was led by one of two Ph.D. level clinical psychologists and co-facilitated with one of two fourth-year doctoral trainees in clinical psychology. Both study therapists attended a 7-day residential training with one of the developers of the MBCT protocol. The two co-facilitators received organized training in MBCT facilitated by one of the study therapists before the start of the study.
Active control condition
The ACC was based on the validated and manualized Health Enhancement Program (HEP) (MacCoon et al., 2011), which was designed as an active control group for mindfulness-based interventions. It included classes in four therapeutic components including physical activity, functional movement, music therapy, and nutrition, each of which lacked a mindfulness element. In accordance with the HEP guidelines, ACC groups were led by facilitators with expertise and credentials in each of the focus areas. Facilitators included a board-certified music therapist, a certified personal trainer and group fitness instructor, and a Master Nutrition Therapist.
Although the therapeutic content differed between groups, several aspects of the HEP mapped onto key features of MBCT. For example, the physical activity component included moderate intensity walking as well as static and dynamic stretching. Functional movement involved exercises focused on improving balance, core stability, agility, and mobility. These body-focused components of HEP, which were designed to improve cardiovascular fitness, posture and strength, paralleled movement-based activities in MBCT (e.g., walking meditation) designed to foster non-judgmental awareness of physical sensations. The music therapy sessions focused on active music making, drumming, song writing, and supportive music and imagery (which involved listening to a piece of classical music while simultaneously drawing images that arise). These exercises were designed to match the body scan in MBCT, the primary difference being the emphasis on music as the sensory experience and therapeutic ingredient rather than MBCT’s emphasis on awareness of one’s own internal states. The nutrition component imparted knowledge about recommended dietary intake (specific to age, sex, and activity level) as well as strengths and weaknesses of participants’ current diet and how to make modifications consistent with the revised food guide pyramid. The didactic presentations on nutrition paralleled the instructive components of MBCT.
The ACC was matched to the MBCT group on course structure including: in-class time (weekly classes lasted 2.5 hours for 8 weeks), group size (10–12 participants in each group), and time outside of class for homework (approximately 50 minutes per day). The ACC controls for non-specific effects including: amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance, therapeutic alliance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes.
Measures
Therapist training and fidelity
All MBCT and HEP group sessions were audiotaped. Therapists’ degree of adherence to the MBCT protocol was monitored using the 17-item MBCT Adherence Scale (Segal, Teasdale, Williams, & Gemar, 2002). Scores were calculated based on a rating of ‘0’=no evidence for item to ‘2’=definite evidence for 17 different items delivered across the entire protocol. An independent, Master’s-level psychologist with training in, and familiarity with, the MBCT protocol and treatment adherence, rated a subset of MBCT sessions (one entire 8-week protocol for each therapist). Each therapist’s rating indicated acceptable adherence (Therapist 1, mean=1.68, SD = .5, range =1–2; Therapist 2, mean=1.76, SD=.5, range=1–2).
Treatment adherence for the HEP was assessed with the 17-item HEP Adherence Scale (HEP-AS) (Eisendrath et al., 2014), a newly developed but not yet validated measure modeled after the MBCT Adherence Scale. The HEP-AS assesses features of the HEP that are delivered across the entire protocol. Items are scored from ‘1’=no evidence to ‘3’ definite evidence. A Bachelor’s-level trainee with understanding of and familiarity with the HEP manual rated a subset of HEP sessions (one entire 8-week protocol). Unlike the MBCT-AS, the design of the HEP-AS does not allow for accurately parsing individual therapists’ adherence ratings. Thus, the rater’s score of 2.6 (SD= .5, range=2–3), which indicated quality adherence, reflects the degree to which all therapists collectively adhered to the HEP intervention manual.
Treatment credibility
Treatment credibility was assessed with the Credibility and Expectancy questionnaire (Devilly & Borkovec, 2000) immediately following the first class. The measure includes two subscales (credibility and expectancy) and asks participants to rate how logical they consider the treatment, the degree to which they believe the treatment will be successful in treating their symptoms, and their confidence in recommending the intervention to a friend with the same problem (‘1’=not at all to ‘9’=completely). Two additional questions asked how much improvement in their depression they believe will occur as a result of the interventions, (‘0%’=no improvement to ‘100%’=complete improvement). Percentage ratings were subjected to a linear transformation with a minimum of 1 and a maximum of 9 (Smeets et al., 2008). A summed score was calculated for each subscale ranging from 3 to 27. Internal consistency for the credibility and expectancy subscales was α = .89 and α = .85, respectively.
Social support
We assessed social support using the standardized Interpersonal Support Evaluation List (ISEL-12) (Cohen, Mermelstein, Kamarck, & Hoberman, 1985) immediately following the intervention. Response options range from ‘0’=de nitely false to ‘3’=de nitely true and index perceived availability of appraisal, belonging, and tangible support. Internal consistency for the ISEL was α = .85.
Primary and secondary outcomes
The primary outcome was incidence of depression relapse and time to relapse over 60 weeks using the depression module of the SCID (First et al., 1994). Clinical interviews were conducted by two fifth-year doctoral trainees in psychology. Interviewers were blind to group assignment and were supervised by an experienced research clinician, also blind to group assignment. All interviews were audio recorded and agreement between the two raters for the diagnosis of a major depressive episode in a subset of taped interviews (n=33) yielded a κ coefficient (Fleiss, Levin, & Paik, 2003) of .84. The secondary outcomes were depressive symptoms and life satisfaction assessed at baseline (T1), immediately following the 8-week intervention phase (T2), and at 6 and 12 months (T3 and T4, respectively). Depressive symptoms were measured with the BDI-II. Internal consistency for all BDI-II assessments was α >.85. Life satisfaction was measured with the Satisfaction with Life Scale (SWL) (Diener, Emmons, Larsen, & Griffin, 1985). Internal consistency for all SWL assessments was α >.85.
Randomization
A research assistant assigned participants to MBCT or ACC based on computer-generated random number sequencing. A stratified, block randomization (block size=4) procedure was implemented based on BDI-II scores at baseline (4–12 or 13–30), sex, and number of previous episodes of depression (≤2 prior episodes or ≥3 prior episodes). Blinding and equipoise were strictly maintained by emphasizing to intervention staff and to participants the prospective clinical validity of each group.
Data Analyses
Primary outcomes: relapse and time to relapse
Baseline characteristics of randomized participants were compared using Fisher’s exact and Mann–Whitney U-tests. Fisher’s exact test was used to compare the proportions of depression relapse between groups. Cox proportional hazards (Cox & Oakes, 1984) was used to estimate survival curves. Participants with missing data and those who did not relapse were treated as censored observations. Results were analyzed separately for intention-to-treat (ITT) and per-protocol samples (PP). The PP sample was comprised of all randomized participants who received the suggested minimum effective dose of MBCT (≥ 4 sessions). (Ma & Teasdale, 2004; Teasdale et al., 2000) The minimum effective treatment dose for the HEP intervention is unknown and was thus set to be identical to MBCT.
Secondary outcomes: depressive symptoms and life satisfaction
Full information maximum likelihood (FIML) (Enders, 2001) mixed effects regression models were used to analyze change in BDI-II and SWL scores across time between groups. Time was coded in weeks and treated as a continuous variable in order to examine differential change in depressive symptoms and SWL between groups over time and to aid interpretability of our results given our unequally spaced assessment intervals. This approach is consistent with other MBCT trials with similar assessment points. In our analyses, time was mean-centered and both linear and quadratic (partialed time-squared) changes were assessed. The group x linear time and group x quadratic time interactions were tested by specifying time as a random factor and using an unstructured covariance matrix. In the group x linear time interaction models, main effects of linear time and group were included as covariates. In the group x quadratic time interactions models, main effects of group, linear and quadratic time, as well as the group x linear interaction were included as covariates.
Results
Preliminary Analyses
The study took place from July 30, 2010 to June 15, 2013. Figure 1 shows the results for screening, randomization, and attrition. Ninety-two participants were randomized to MBCT (n=46) or ACC (n=46). Prior to the start of the intervention, 7 participants (15%) in the MBCT group and 5 (11%) in the ACC group dropped out of the study (OR=1.47; 95% CI, .387–5.93; p=.76). Attrition rates did not differ between groups at any of the follow up time points (ORs < 1.63; 95% CIs, .516–4.29, ps>.40). Per-protocol completion (attending ≥ 4 sessions) did not vary between groups (MBCT=47.8%, ACC=67.4%; OR=2.25; 95% CI, .892–5.75; p=.09).
Table 1 shows the sociodemographic and clinical characteristics of the ITT sample. Groups were comparable on all baseline variables, except the proportion of White relative to non-White participants was higher in the ACC group. Randomization procedures were carefully followed, thus this difference appears strictly due to chance (Assmann, Pocock, Enos, & Kasten, 2000). Results remained unchanged when including this variable as a covariate; therefore, unadjusted results are reported.
Table 1.
Socio-demographic and Clinical Characteristics
| Variable | Group | P value | |
|---|---|---|---|
| MBCT (n = 46) | ACC (n = 46) | pa | |
| Socio-demographic characteristics (baseline)
| |||
| Female | 36 (76) | 35(76) | 1 |
| White | 29 (69) | 40 (87) | .015* |
| Age (in years) M (SD) | 36.7 (12.8) | 33 (9.6) | .218 |
| Marital Status | .373 | ||
| Married/Partnered/cohabiting | 20 (43.4) | 20 (43.4) | |
| Single | 18 (39.1) | 20 (43.5) | |
| Divorced/separated/widowed | 8 (17.4) | 6 (13) | |
| Years of education | .127 | ||
| Partial high school | 1 (2.2) | 0 (0) | |
| Completed high school | 1 (2.2) | 2 (4.3) | |
| Partial college | 12 (26.1) | 20 (43.5) | |
| Completed college | 22 (47.8) | 17 (37) | |
| Professional or graduate school | 10 (21.7) | 7 (15.2) | |
| Family income per year | .091 | ||
| $10,000 | 3 (6.5) | 1 (2.2) | |
| $10,000–$30,000 | 15 (32.6) | 9 (19.6) | |
| $30,000–$50,000 | 9 (19.6) | 10 (21.7) | |
| $50,000–$100,000 | 12 (26) | 13 (28.2) | |
| $100,000 + | 3 (6.5) | 3 (6.5) | |
|
| |||
| Clinical characteristics (baseline) | |||
|
| |||
| BDI-II M (SD) | 12.1 (7.5) | 11.9 (6.6) | .879 |
| SWL M (SD) | 3.3 (1.5) | 3.3 (1.4) | .879 |
| Age (in years) of first onset of depression M (SD) | 15.7 (8.2) | 16.5 (5.7) | .252 |
| Number of previous episodes of depression (≥3) | 44 (95.7) | 43 (93.5) | 1 |
| Current antidepressant medication use | 14 (30.4) | 12 (26.1) | .817 |
|
| |||
| Clinical Characteristics during and post-intervention | |||
|
| |||
| BDI-II (T2) M (SD) | 11.9 (7.2) | 7.1 (6.49) | <.01 |
| BDI-II (T3) M (SD) | 8.2 (6.9) | 6.2 (5.7) | .295 |
| BDI-II (T4) M (SD) | 7.0 (6.1) | 7.2 (6.0) | .914 |
| SWL (T2) M (SD) | 3.5 (1.5) | 4.0 (1.8) | .198 |
| SWL (T3) M (SD) | 3.9 (1.7) | 4.3 (1.7) | .451 |
| SWL (T4) M (SD) | 4.3 (1.4) | 4.1 (1.6) | .603 |
| Treatment outside of interventions | |||
| Antidepressant use | 9 (28.1) | 8 (22.2) | .590 |
| One or more depression-related visits to GP | 14 (45.2) | 13 (39.4) | .801 |
| Counseling or psychotherapy | 8 (25.8) | 8 (24.2) | .885 |
| Psychiatric treatment | |||
| Outpatient | 2 (6.5) | 5 (15.2) | .428 |
| Day patient | 0 | 0 | |
| Inpatient | 0 | 0 | |
| Per-protocol completion (attended ≥4 sessions) | 22 (47.8) | 31 (67.4) | .091 |
| Number of intervention sessions completed M (SD) | 3.87 (2.93) | 4.72 (2.60) | .213 |
| Credibility of intervention M (SD) | 19.04 (5.21) | 19.91 (4.53) | .617 |
| Expectation of intervention effectiveness | 16.11 (5.39) | 16.51 (4.64) | .994 |
| Perceived social support M (SD) | 2.86 (.118) | 3.13 (.089) | .148 |
Note. Data are presented as number (percentage) unless otherwise indicated. Sxs=Symptoms; BDI-II = Beck Depression Inventory II; SWL=Satisfaction with Life; T2=Time 2 (immediately following treatment); T3=Time 3 (6-month follow up); T4=Time 4 (12-month follow up), GP=General Practitioner.
Fisher’s exact test for proportions and Mann–Whitney U-test for continuous and ordinal variables.
p < .05
To assess if data were missing at random, we ran Fisher’s exact or Mann-Whitney tests on relapse incidence, time to relapse, and depressive symptoms and life satisfaction at all four time points, comparing PP completers to non-completers. We additionally conducted these tests on outcomes comparing PP completers with present versus missing or incomplete data. For PP completers versus non-completers, no significant differences emerged on any of the outcome variables, p>.15. For PP completers with present versus missing or incomplete follow up data, no significant differences were found for any of the outcome variables, ps>.26. These non-significant comparisons are consistent with the conclusion that data were missing completely at random (Little, 1988).
Primary outcomes: depression relapse and time to relapse
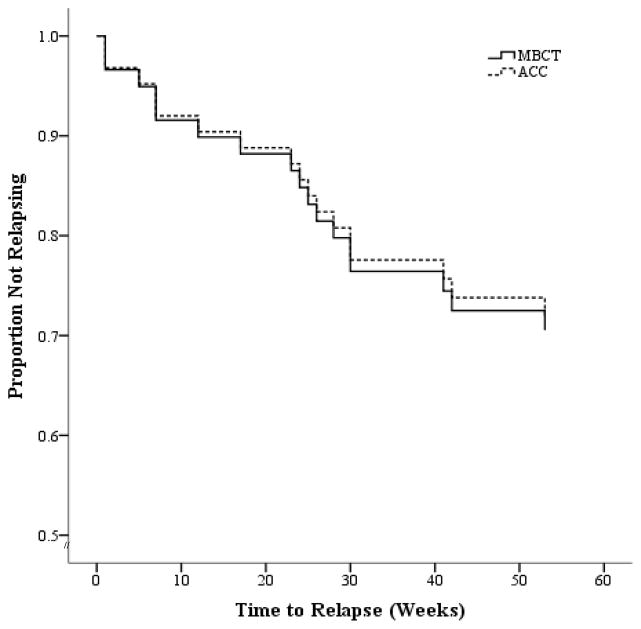
For ITT, sensitivity analyses were conducted whereby missing values in the MBCT and ACC conditions were imputed based on the observed relapse rates in the opposite arm (Proschan et al., 2001). Over the 60-week study period, in the ITT sample, 32.6% (15/46) of the MBCT participants relapsed compared to 30.4% (14/46) of the ACC participants (OR=1.10; 95% CI, .419–2.92; p=1). In the PP sample, 23.5% (4/17) of the MBCT participants relapsed, compared to 29.2% (7/24) of the ACC participants (OR=1.34; 95% CI, .264–7.02; p=.736). Figure 2 shows ITT survival curves over 60 weeks for both groups. Cox regression indicates no difference in probability of relapse between the two groups; ITT: Wald (1, n=92)=.014; hazard ratio (HR)=.945 (95% CI, .364–2.45); p=.91; and PP: Wald (1, n=53)=.063; HR=1.17 (95% CI, .342–4.00); p=.80.
Figure 2.
Survival (non-relapse/recurrence) curves comparing relapse/recurrence of major depression for Mindfulness Based Cognitive Therapy (MBCT) and Active Comparison Condition (ACC) over a 60-week follow up period (intention-to-treat sample). Zero weeks=baseline.
To examine potential subgroups for which treatment effects differ, we tested several potential moderators of treatment effects for incidence of and time to relapse. Each of the following moderators were examined in separate ITT regression analyses by entering interaction terms between group and each moderator into the model: number of prior episodes of depression; age of onset of depression; severity of residual depressive symptoms; sex; per-protocol completion; and total practice time outside of class. None of these variables interacted with treatment condition to predict incidence of or time to relapse (HRs/ORs ≤5.3; 95% CIs, .029–44.2, ps≥.12). We were unable to examine the number of prior episodes (≤2 vs. ≥ 3) as a moderator due to low power and a lack of variance (i.e., none of the participants in the MBCT group (n= 2) or ACC group (n =3) with ≤2 prior depressive episodes experienced a relapse). Importantly, the pattern and significance of results remain unchanged when excluding individuals with ≤2 prior episodes (n=5) 1.
Secondary outcome: depressive symptoms and life satisfaction
Table 2 summarizes parameters from the mixed effects regression analyses for depressive symptoms and life satisfaction. Analyses indicate a significant overall linear decrease in depressive symptoms and increase in life satisfaction over the study course. The non-significant group x linear time interaction indicates that this change did not differ between groups for either outcome. A significant overall quadratic effect across treatment was also observed for both depressive symptoms and life satisfaction. As depicted in Figure 3, Panel A, the quadratic effect differed between groups for depressive symptoms. Specifically, the MBCT group exhibited only linear reductions in depressive symptoms with no quadratic effect. In contrast, the ACC group exhibited a quadratic effect indicating that initial improvement was followed by a leveling off and gradual increase in depressive symptoms over time. For life satisfaction, the group x quadratic time interaction was marginally significant and, as depicted in Figure 3, Panel B, the pattern of results paralleled that for depressive symptoms such that the MBCT group exhibited linear increases in life satisfaction but no quadratic effect, while the ACC group exhibited a quadratic effect whereby life satisfaction increased initially but then leveled off and gradually decreased over time.
Table 2.
Summary of the Mixed Effects Regression Tests of Linear and Quadratic Change and Differential Change Between Groups for depressive symptoms (BDI) and life satisfaction (SWL).
| Effect (BDI) | B | SEB | Error df | p | 95% CI |
|---|---|---|---|---|---|
| Linear Time | −.0773 | .0129 | 63.1 | <.001 | −.1031 to −.0515 |
| Quadratic Time | .0021 | .0006 | 125.0 | <.001 | .0010 to .0033 |
| Group x Linear Time | −.0228 | .0257 | 61.2 | .337 | −.0741 to .0285 |
| Group x Quadratic Time | −.0023 | .0001 | 124.6 | .049 | .0011 to .0141 |
|
| |||||
| Effect (SWL) | B | SEB | Error df | p | 95% CI |
|
| |||||
| Linear Time | 0.0125 | 0.0021 | 60.6 | <.001 | .0083 to .0168 |
|
| |||||
| Quadratic Time | −0.0003 | 0.0001 | 131.9 | 0.011 | −.0004 to −.0001 |
|
| |||||
| Group x Linear Time | 0.0004 | 0.0043 | 60.6 | 0.919 | −.0081 to 0090 |
|
| |||||
| Group x Quadratic Time | 0.0003 | 0.0002 | 132.5 | 0.097 | −.0001 to .0007 |
Note. Interaction models controlled for all main effects (group, linear time, quadratic time). Parameter estimates can be interpreted as change in depressive symptoms and life satisfaction per 1 unit change in time (i.e., one week). B = Unstandardized regression weight; SEB = Standard error of B; df = degrees of freedom.
Figure 3.
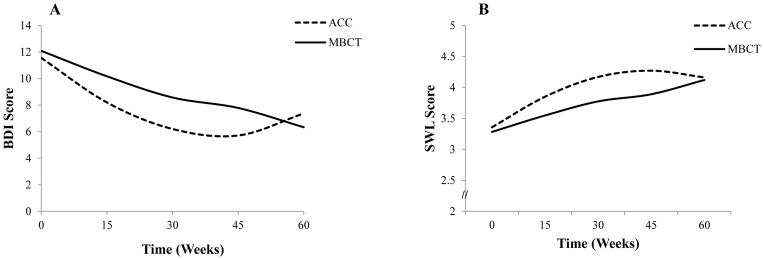
Differential pattern of quadratic change in depressive symptoms (BDI-Panel A) and life satisfaction (SWL-Panel B) between groups. Zero weeks=baseline. BDI-II scale range: 0–63; SWL scale range: 1–7.
Discussion
To our knowledge, this is the first RCT to examine the effects of MBCT, as compared to a structurally equivalent ACC, for preventing depression relapse, reducing depressive symptoms, and improving life satisfaction. Results indicated no group differences in relapse rates, time to relapse, depressive symptoms, or life satisfaction over a 60-week follow-up. Analyses for depressive symptoms indicated a significant quadratic group x time interaction such that the ACC experienced immediate symptom reduction post-intervention and then a gradual increase over the 60-week follow-up. In contrast, the MBCT group experienced a gradual linear symptom reduction. Analyses for life satisfaction indicated a marginal quadratic group x time interaction, with a pattern similar to depressive symptoms, whereby the ACC group experienced an immediate increase in life satisfaction followed by a gradual decline over the 60-week follow-up. The MBCT group experienced a gradual linear improvement in life satisfaction.
Lack of Group Differences between MBCT and ACC
The lack of differences between MBCT and ACC may be surprising provided previous evidence for MBCT’s effects compared to treatment as usual (Ma & Teasdale, 2004; Teasdale et al., 2000). However, four considerations support that the equal effectiveness of MBCT and ACC found here is reliable. First, the present results converge with findings demonstrating no significant differences between MBCT and other therapeutically credible active control conditions for depression relapse (Meadows et al., 2014; Williams et al., 2014) and depressive symptoms (Manicavasgar, Parker, & Perich, 2011; Oken et al., 2010; Philippot, Nef, Clauw, de Romree, & Segal, 2012). Moreover, studies comparing the same ACC used here to mindfulness-based stress reduction, the intervention upon which MBCT is based, have reported a similar lack of significant group differences on self-reported anxiety, psychological distress, physical health symptoms, and inflammation (MacCoon et al., 2012; Rosenkranz et al., 2013).
Second, the pattern and significance of results remain unchanged when examining several plausible moderators (number of prior episodes of depression; age of onset of depression; severity of residual depressive symptoms; sex; per-protocol completion; and total practice time outside of class). Importantly, the pattern and significance of our results did not change when excluding individuals with ≤2 prior episodes of depression (n=5) from our analyses. This suggests that the lack of group differences cannot be explained by including individuals in our primary analyses who have not benefited from MBCT in some studies (those with ≤2 prior episodes) (Ma & Teasdale, 2004; Teasdale et al., 2000). This finding is consistent with research suggesting that MBCT’s effectiveness may not be contingent on number of prior depression episodes (Geschwind, Peeters, Huibers, van Os, & Wichers, 2012). One moderator that we were unable to test was childhood trauma. Williams et al. (2014) found that MBCT was only more effective than a stringent comparison condition for individuals with childhood trauma. The hypothesized explanation for these results is that MBCT is most effective for individuals who are at highest risk of relapse. Although we did not assess childhood trauma, we did measure age of onset of depression, which has been found to fully mediate the relationship between childhood neglect and increased risk of relapse (Bifulco, Brown, Moran, Ball, & Campbell, 1998). This makes age of onset of depression a reasonable proxy for childhood trauma., Age of onset of depression did not moderate intervention effects in the present study (HR=.952; 95% CI, .802–1.13; p=.58), and the mean age of depression onset in our sample (16.1 years) was approximately 5 years younger than in the Williams 2014 study. While we cannot definitely rule out the possibility that MBCT might have been more effective than the ACC for individuals with a history of childhood trauma, these considerations are not consistent with childhood trauma as a moderator of the present effects. Overall, the present data are inconsistent with the hypothesis that MBCT might have been more effective for a subgroup of participants.
Third, one might question whether a lack of group effect might have been due to inferior delivery of the MBCT intervention, rendering MBCT ineffective. However, this concern is mitigated by three considerations. First, results indicated high therapist adherence and treatment expectancy and credibility ratings, which were similar to other MBCT studies (Kuyken et al., 2008; Meadows et al., 2014; Segal et al., 2010). Second, the relapse rates in the MBCT (32.6%) and ACC (30.4%) groups were comparable to the 32% relapse rate across several MBCT studies [for review see (Chiesa & Serretti, 2011)], which suggests that both treatments were equally effective rather than equally ineffective. The low relapse rates in both groups are unlikely to be due to a low base-rate likelihood of relapse, because participants were at very high risk for relapse as evidenced by an early age of onset of depression and a large proportion of participants (95%, n=87) who had ≥ 3 prior episodes of MDD. Finally, the 5-point reduction in depressive symptoms over the 60-week follow up, from mild depression to scores within the normative range, represents a clinically significant improvement (Brouwer, Meijer, & Zevalkink, 2013). Relatedly, depressive symptoms did not increase over the 60-week follow-up period, an important finding in support of both interventions’ effectiveness given that our sample was at high risk for experiencing elevated depressive symptoms.
Finally, the two interventions failed to differ across four outcomes (depression relapse rates, time to relapse, depressive symptoms, and life satisfaction), thus corroborating the robustness of the present findings. All effect sizes were small, suggesting that lack of statistical significance was not due to lack of power. Further, it should be noted that although baseline depressive symptom scores were relatively low (a feature common to other relapse prevention studies that required that participants did not meet criteria for current MDD), the significant reduction in BDI and SWL scores across time for both groups suggests that there was no floor effect that might have prevented group differences from emerging.
Overall, the convergence of our findings with other studies, the absence of effect moderation, high adherence, expectancy, and credibility ratings for the interventions, overall effectiveness of the MBCT and the ACC group, and consistency of small effect sizes across three indicators of depression (relapse rates, time to relapse, and depressive symptoms) as well as life satisfaction substantiate the lack of differences between MBCT and ACC.
Differences in Course of Symptomatic Improvement between MBCT and ACC
While there were no main effects of MBCT versus ACC, there was a significant quadratic group x time interaction predicting depressive symptoms and a marginally significant effect for life satisfaction. Results for both outcomes indicated that the ACC conferred immediate benefits, which were gradually reduced over time, whereas the MBCT experienced gradual benefits over the 60-week follow-up. Thus, while overall outcomes were not different between groups, the type of intervention did affect the trajectory of symptomatic improvement.
This difference in trajectory of improvement could be explained in two ways. First, it is possible that the skills associated with each intervention are learned at different rates. For example, participants were likely more familiar with physical activity and healthy eating than they were with mindfulness meditation. Thus, intervention-specific knowledge and skills may have been more easily and immediately acquired and implemented in the ACC compared to the MBCT group. A second, and perhaps more parsimonious explanation, is that the intervention-specific skills acquired are associated with differential rates of therapeutic benefit. Changes associated with instruction in physical activity, nutrition, and music therapy may lead to more immediate reductions in depressive symptoms through distraction, improved self-efficacy, or neuro-hormonal mechanisms (e.g., increases in serotonin, dopamine) (Craft & Perna, 2004; Maratos et al., 2011; Opie et al., 2014). These effects, however, might not be sustained over the longer-term. On the other hand, the neurocognitive improvements in attention regulation and self-referential processing that are associated with mindfulness meditation (Chiesa, Calati, & Serretti, 2011; Goldin, Ziv, Jazaieri, & Gross, 2012) might lead to more gradual (Grossman, Niemann, Schmidt, & Walach, 2004) but cumulative and sustained reductions in depressive symptoms (Mathew, Whitford, Kenny, & Denson, 2010).
Either explanation leads to the prediction that MBCT would show significant advantage over ACC at follow-up assessments past 12 months. Some initial evidence is consistent with this idea. For example, the study conducted by Meadows et al. (2014) that compared MBCT plus depression relapse active monitoring (DRAM) to DRAM alone demonstrated that, for individuals on anti-depressant medication, fewer individuals in the MBCT group experienced a relapse/recurrence between 12 and 26 months compared to the DRAM group. In contrast, the present study and the two other RCTs that compared MBCT to rigorous control conditions (Segal et al., 2010; Williams et al., 2014) using an 18-month and a 12-month follow-up yielded no significant differences between groups for relapse prevention. Future studies comparing MBCT to rigorous control conditions should include follow up assessments well beyond 12 months to test whether MBCT may have relatively slow but cumulative effects on depression relapse.
Implications for Understanding the Effects of MBCT
One strength of this study is that we compared MBCT to a rigorous and structurally equivalent ACC. This credible active control condition was comparable to MBCT on several key variables, including amount of group contact, treatment-related activity outside of class, interaction with a facilitator, therapeutic allegiance, emphasis on self-monitoring and behavior change, perceived social support, and expected positive outcomes. This feature allows us to begin to address some questions about the specificity of effects of MBCT beyond other studies comparing MBCT to psycho-educational comparison conditions that did not control for all of these factors (Williams et al., 2014; Meadows et al., 2014).
However, while the ACC was designed to be comparable to MBCT on as many variables as possible, some variables still differed. For example, the number of areas of concentration varied between groups with the ACC focusing on four areas (physical activity, functional movement, and music therapy) and MBCT focusing on only two (mindfulness and cognitive therapy). An ideal control condition would ensure equal variety in topics, as this could affect participants’ attentiveness and interest. Additionally, the therapeutic approach differs between groups with the ACC having a stronger behavioral activation component (e.g., engaging in adaptive externally-focused activities like exercise) and the MBCT having a stronger focus on self-referential processing (e.g., increased awareness of internal experiences—e.g., thoughts and emotions). Finally, the ACC focused most squarely on physical well-being while MBCT focused primarily on psychological health as well as mental and emotional processes. This could have implications for the therapeutic relationship. For example, therapeutic engagement might have been stronger in the MBCT condition given its focus on addressing emotionally salient experiences with the facilitator and the group. Of note, this difference would lead one to expect an advantage of MBCT.
In sum, although not all possible sources of therapeutic change could be held constant in this study, the ACC was matched to MBCT on several key variables. This means that one possible interpretation of our findings is that the previously found effects of MBCT, compared to TAU, are due to non-specific factors (e.g., social support, expectations of positive outcomes) rather than to mindfulness and/or cognitive therapy. However, it could also be that effects of MBCT are due to mindfulness and/or cognitive therapy components while effects of ACC are due to the active components of the Health Enhancement Program. This explanation is supported by the fact that each of the components of our ACC is associated with reductions in depressive symptoms (Craft & Perna, 2004; Maratos et al., 2011; Opie et al., 2014). A more definitive test of these hypotheses requires a pre-post assessment of the proposed mechanisms of change (e.g., increased mindfulness, decreased rumination, increased physical activity), and a demonstration that the mechanisms underlying the effects of MBCT differ from those underlying the ACC.
The most judicious conclusion that can be drawn from the present results is that mindfulness and cognitive therapy are not more effective than the active components of the ACC. This result raises an important question about whether MBCT, which requires a skilled clinician for delivery and is by extension not widely available or accessible, is the ideal treatment approach given the nearly epidemic proportions of depression-related morbidities and mortalities (Whiteford et al., 2013) and the scarcity of resources for mental health. Further exploration is needed to fully address this question and to determine whether the active components of the ACC are more scalable as a treatment for depression at the population level.
Limitations and Directions for Future Research
Despite its novel contributions, this study had three notable limitations. First, although the sample size was comparable to recent investigations comparing MBCT to other stringent conditions (e.g., CBT and mADM) (Manicavasgar et al., 2011; Segal et al., 2010) and results indicated small effect sizes for all analyses, null results may be due to modest statistical power. A priori power calculations were based on less stringent RCTs comparing MBCT to heterogeneous TAU controls and thus may have resulted in low power for the current investigation. Studies based on power analyses from emerging investigations comparing MBCT to ACCs are needed.
Second, although every effort was made to ensure participant retention, attrition rates were higher than anticipated and the proportion of participants who received an adequate dose of MBCT (≥4 sessions) was lower than previous studies (Segal et al., 2010; Williams et al., 2014). Data were established to be missing completely at random and attrition did not vary between groups, thus FIML estimation procedures are justifiable. Low retention may be explained by greater racial/ethnic diversity in our sample compared to other studies (25% non-Caucasian in our sample and 1% in three other MBCT trials with higher retention) (Ma & Teasdale, 2004; Teasdale et al., 2000; Williams et al., 2014). As has been reported in previous studies (Gonzalez, Weersing, Warnick, Scahill, & Woolston, 2011; Kearney, Draper, & Baron, 2005), Caucasian compared to non-Caucasian participants attended a greater number of sessions (r=.242, p=.02). Lower retention may reflect the need to culturally tailor the MBCT and HEP protocols.
Finally, because only one rater was used in the MBCT adherence ratings, no assessment of inter-rater reliability was possible. Although therapist adherence guidelines and quantitative scores have been established by the previously validated MBCT adherence rating scale (MBCT-AS) (Segal et al., 2002), and the use of only one rater has been a standard approach (Kuyken et al., 2008; Meadows et al., 2014; Segal et al., 2010), stronger confirmation of therapists’ adherence to the MBCT protocol would have been provided by at least two raters.
Concluding Comment
Our findings indicate that MBCT and HEP are equally effective for preventing depression relapse, reducing depressive symptoms, and improving life satisfaction at a 60-week follow up. The trajectory of depressive symptom improvement varies between groups. This investigation underscores the importance of comparing psychotherapeutic interventions to active control conditions in order to help isolate specific vs. non-specific therapeutic components and to test the comparative effectiveness of treatment approaches that may have differential cost and dissemination advantages.
Public Health Significance.
This is the first randomized controlled trial that compares MBCT to a structurally equivalent active comparison condition that may have cost and dissemination advantages. Our investigation is additionally designed to help isolate specific vs. non-specific therapeutic components of MBCT.
Acknowledgments
This study was supported by NIH-NCCIH, National Center for Complementary and Integrative Health (1 F32 AT004879).
Footnotes
Results when including only individuals with ≥ 3 prior episodes of depression (n=87): Over the 60-week study period, in the ITT sample, 31.8% (14/44) of the MBCT participants relapsed compared to 30.2% (13/43) of the ACC participants (OR=.929; 95% CI, .374–2.30; p=1). In the PP sample, 25% (4/16) of the MBCT participants relapsed, compared to 30.4% (7/23) of the ACC participants (OR=1.31; 95% CI, .311–5.53; p=.1). Cox regression indicates no difference in probability of relapse between the two groups; ITT: Wald (1, n=87)=.011; hazard ratio (HR)=.951 (95% CI, .367–2.47); p=.92; and PP: Wald (1, n=50)=.046; HR=1.15 (95% CI, .335–3.91); p=.83.
Contributor Information
Amanda J. Shallcross, New York University School of Medicine
James J. Gross, Stanford University
Pallavi D. Visvanathan, Manhattan Center for Cognitive Behavioral Therapy
Niketa Kumar, St. John’s University.
Amy Palfrey, St. John’s University.
Brett Q. Ford, University of California, Berkeley
Sona Dimidjian, University of Colorado, Boulder.
Stephen Shirk, University of Denver.
Jill Holm-Denoma, University of Denver.
Kari M. Goode, University of Denver
Erica Cox, University of Denver.
William Chaplin, St. John’s University.
Iris B. Mauss, University of California, Berkeley
References
- Assmann SF, Pocock SJ, Enos LE, Kasten LE. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet. 2000;355(9209):1064–1069. doi: 10.1016/S0140-6736(00)02039-0. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy of depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bifulco A, Brown GW, Moran P, Ball C, Campbell C. Predicting depression in women: the role of past and present vulnerability. Psychol Med. 1998;28(1):39–50. doi: 10.1017/s0033291797005953. [DOI] [PubMed] [Google Scholar]
- Bondolfi G, Jermann F, der Linden MV, Gex-Fabry M, Bizzini L, Rouget BW, Bertschy G. Depression relapse prophylaxis with Mindfulness-Based Cognitive Therapy: replication and extension in the Swiss health care system. Journal of affective disorders. 2010;122(3):224–231. doi: 10.1016/j.jad.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer D, Meijer RR, Zevalkink J. Measuring individual significant change on the Beck Depression Inventory-II through IRT-based statistics. Psychother Res. 2013;23(5):489–501. doi: 10.1080/10503307.2013.794400. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. Mindfulness based cognitive therapy for psychiatric disorders: a systematic review and meta-analysis. Psychiatry Res. 2011;187(3):441–453. doi: 10.1016/j.psychres.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman H. Social Support: Theory, Research and Applications. In: Sarason IG, Sarason B, editors. Measuring the functional components of social support. The Hague: Martinus Nijhoff; 1985. [Google Scholar]
- Cox DR, Oakes D. Analysis of survival data. London, UK: Chapman and Hall; 1984. [Google Scholar]
- Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry. 2004;6(3):104–111. doi: 10.4088/pcc.v06n0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane C, Crane RS, Eames C, Fennell MJ, Silverton S, Williams JM, Barnhofer T. The effects of amount of home meditation practice in Mindfulness Based Cognitive Therapy on hazard of relapse to depression in the Staying Well after Depression Trial. Behav Res Ther. 2014;63:17–24. doi: 10.1016/j.brat.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–75. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Dobson KS, Hollon SD, Dimidjian S, Schmaling KB, Kohlenberg RJ, Gallop RJ, Jacobson NS. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. Journal of Consulting and Clinical Psychology. 2008;76(3):468–477. doi: 10.1037/0022-006X.76.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendrath SJ, Gillung EP, Delucchi KL, Chartier M, Mathalon DH, Sullivan JC, Feldman MD. Mindfulness-based cognitive therapy (MBCT) versus the health-enhancement program (HEP) for adults with treatment-resistant depression: a randomized control trial study protocol. BMC Complement Altern Med. 2014;14:95. doi: 10.1186/1472-6882-14-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educational and Psychological Measurement. 2001;61(5):713–740. [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for Axis I DSM-IV Disorders—Patient Edition. Washington, D.C: American Psychiatric Press; 1994. [Google Scholar]
- Fleiss JL, Levin B, Paik MC. Statistical methods for rates and proportions. 3. Hoboken, N.J: J. Wiley; 2003. [Google Scholar]
- Geschwind N, Peeters F, Huibers M, van Os J, Wichers M. Efficacy of mindfulness-based cognitive therapy in relation to prior history of depression: randomised controlled trial. British Journal of Psychiatry. 2012;201(4):320–325. doi: 10.1192/bjp.bp.111.104851. [DOI] [PubMed] [Google Scholar]
- Godfrin KA, van Heeringen C. The effects of mindfulness-based cognitive therapy on recurrence of depressive episodes, mental health and quality of life: A randomized controlled study. Behavior Research and Therapy. 2010;48(8):738–746. doi: 10.1016/j.brat.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Goldin P, Ziv M, Jazaieri H, Gross JJ. Randomized controlled trial of mindfulness-based stress reduction versus aerobic exercise: effects on the self-referential brain network in social anxiety disorder. Front Hum Neurosci. 2012;6:295. doi: 10.3389/fnhum.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Weersing VR, Warnick EM, Scahill LD, Woolston JL. Predictors of treatment attrition among an outpatient clinic sample of youths with clinically significant anxiety. Administration and Policy in Mental Health. 2011;38(5):356–367. doi: 10.1007/s10488-010-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res. 2004;57(1):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Stewart MO, Strunk D. Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annual Review of Psychology. 2006;57:285–315. doi: 10.1146/annurev.psych.57.102904.190044. [DOI] [PubMed] [Google Scholar]
- Judd LL. The clinical course of unipolar major depressive disorders. Archives of General Psychiatry. 1997;54(11):989–991. doi: 10.1001/archpsyc.1997.01830230015002. [DOI] [PubMed] [Google Scholar]
- Kabat-Zinn J. Full catastrophe living : Using the wisdom of your body and mind to face stress, pain, and illness. New York, N.Y: Delacorte Press; 1990. [Google Scholar]
- Kearney LK, Draper M, Baron A. Counseling utilization by ethnic minority college students. Cultural Diversity and Ethnic Minority Psychology. 2005;11(3):272–285. doi: 10.1037/1099-9809.11.3.272. [DOI] [PubMed] [Google Scholar]
- Kelly K, Posternak M, Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues in Clinical Neuroscience. 2008;10(4):409–418. doi: 10.31887/DCNS.2008.10.4/kkelly. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston T, Dooley B, Bates A, Lawlor E, Malone K. Mindfulness-based cognitive therapy for residual depressive symptoms. Psychology and Psychotherapy. 2007;80(Pt 2):193–203. doi: 10.1348/147608306X116016. [DOI] [PubMed] [Google Scholar]
- Kirsch I. Placebo psychotherapy: Synonym or oxymoron? Journal of Clinical Psychology. 2005;61(7):791–803. doi: 10.1002/jclp.20126. [DOI] [PubMed] [Google Scholar]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Teasdale JD. Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. Journal of Consulting and Clinical Psychology. 2008;76(6):966–978. doi: 10.1037/a0013786. [DOI] [PubMed] [Google Scholar]
- Little RJ. A test of missing completeley at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–1202. [Google Scholar]
- Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. Journal of Consulting and Clinical Psychology. 2004;72(1):31–40. doi: 10.1037/0022-006X.72.1.31. [DOI] [PubMed] [Google Scholar]
- MacCoon DG, Imel ZE, Rosenkranz MA, Sheftel JG, Weng HY, Sullivan JC, Lutz A. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR) Behavior Research and Therapy. 2012;(50):3–12. doi: 10.1016/j.brat.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicavasgar V, Parker G, Perich T. Mindfulness-based cognitive therapy vs cognitive behaviour therapy as a treatment for non-melancholic depression. Journal of Affective Disorders. 2011;130(1–2):138–144. doi: 10.1016/j.jad.2010.09.027. [DOI] [PubMed] [Google Scholar]
- Maratos A, Crawford MJ, Procter S. Music therapy for depression: it seems to work, but how? Br J Psychiatry. 2011;199(2):92–93. doi: 10.1192/bjp.bp.110.087494. [DOI] [PubMed] [Google Scholar]
- Mathew KL, Whitford HS, Kenny MA, Denson LA. The long-term effects of mindfulness-based cognitive therapy as a relapse prevention treatment for major depressive disorder. Behav Cogn Psychother. 2010;38(5):561–576. doi: 10.1017/S135246581000010X. [DOI] [PubMed] [Google Scholar]
- Meadows GN, Shawyer F, Enticott JC, Graham AL, Judd F, Martin PR, Segal Z. Mindfulness-based cognitive therapy for recurrent depression: A translational research study with 2-year follow-up. Aust N Z J Psychiatry. 2014;48(8):743–755. doi: 10.1177/0004867414525841. [DOI] [PubMed] [Google Scholar]
- Munshi K, Eisendrath S, Delucchi K. Preliminary long-term follow-up of Mindfulness-based cognitive therapy-induced remission of depression. Mindfulness (N Y) 2013;4(4):354–361. doi: 10.1007/s12671-012-0135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Fonareva I, Haas M, Wahbeh H, Lane JB, Zajdel D, Amen A. Pilot controlled trial of mindfulness meditation and education for dementia caregivers. Journal of Alternative and Complementary Medicine. 2010;16(10):1031–1038. doi: 10.1089/acm.2009.0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opie RS, O’Neil A, Itsiopoulos C, Jacka FN. The impact of whole-of-diet interventions on depression and anxiety: a systematic review of randomised controlled trials. Public Health Nutr. 2014:1–20. doi: 10.1017/S1368980014002614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SV, Zaretsky A, Beaulieu S, Yatham LN, Young LT, Patelis-Siotis I, Streiner DL. A randomized controlled trial of psychoeducation or cognitive-behavioral therapy in bipolar disorder: a Canadian Network for Mood and Anxiety treatments (CANMAT) study [CME] The Journal of Clinical Psychiatry. 2012;73(6):803–810. doi: 10.4088/JCP.11m07343. [DOI] [PubMed] [Google Scholar]
- Philippot P, Nef F, Clauw L, de Romree M, Segal Z. A randomized controlled trial of mindfulness-based cognitive therapy for treating tinnitus. Clinical Psychology and Psychotherapy. 2012;19(5):411–419. doi: 10.1002/cpp.756. [DOI] [PubMed] [Google Scholar]
- Proschan MA, McMahon RP, Shih JH, Hunsberger SA, Geller NL, Knatterud G, Wittes J. Sensitivity analysis using an imputation method for missing binary data in clinical trials. Journal of Statistical Planning and Inference. 2001;96:155–165. [Google Scholar]
- Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behavior and Immunity. 2013;27(1):174–184. doi: 10.1016/j.bbi.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Levitan RD. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Archives of General Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal ZV, Teasdale JD, Williams JMG, Gemar MC. The mindfulness-based cognitive therapy adherence scale: inter-rater reliability, adherence to protocol and treatment distinctiveness. Clinical Psychology & Psychotherapy. 2002;9(2):131–138. doi: 10.1002/cpp.320. [DOI] [Google Scholar]
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression. 2. New York, NY: Guilford Press; 2013. [Google Scholar]
- Smeets RJ, Beelen S, Goossens ME, Schouten EG, Knottnerus JA, Vlaeyen JW. Treatment expectancy and credibility are associated with the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. Clin J Pain. 2008;24(4):305–315. doi: 10.1097/AJP.0b013e318164aa75. [DOI] [PubMed] [Google Scholar]
- Strauss C, Cavanagh K, Oliver A, Pettman D. Mindfulness-based interventions for people diagnosed with a current episode of an anxiety or depressive disorder: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e96110. doi: 10.1371/journal.pone.0096110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. Journal of Consulting and Clinical Psychology. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- ten Doesschate MC, Bockting CLH, Schene AH. Adherence to continuation and maintenance antidepressant use in recurrent depression. Journal of Affective Disorders. 2009;115(1–2):167–170. doi: 10.1016/j.jad.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, Russell IT. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: A randomized dismantling trial. Journal of Consulting and Clinical Psychology. 2014;82(2):275–286. doi: 10.1037/a0035036. [DOI] [PMC free article] [PubMed] [Google Scholar]