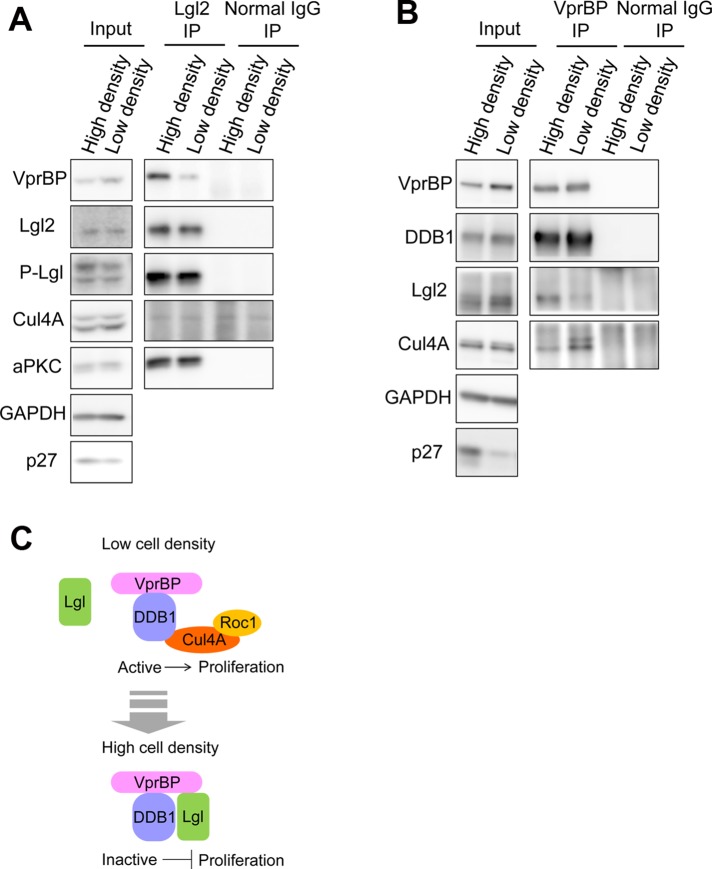
Lgl is a conserved tumor suppressor suggested to be involved in cell polarity regulation and suppression of cell proliferation. Lgl inhibits formation of the VprBP-DDB1-Cul4A-Roc1 ubiquitin E3 ligase complex, which is implicated in cell cycle progression, by promoting formation of the Lgl-VprBP-DDB1 complex to prevent overproliferation.
Abstract
Lethal giant larvae (Lgl) is an evolutionarily conserved tumor suppressor whose loss of function causes disrupted epithelial architecture with enhanced cell proliferation and defects in cell polarity. A role for Lgl in the establishment and maintenance of cell polarity via suppression of the PAR-aPKC polarity complex is established; however, the mechanism by which Lgl regulates cell proliferation is not fully understood. Here we show that depletion of Lgl1 and Lgl2 in MDCK epithelial cells results in overproliferation and overproduction of Lgl2 causes G1 arrest. We also show that Lgl associates with the VprBP-DDB1 complex independently of the PAR-aPKC complex and prevents the VprBP-DDB1 subunits from binding to Cul4A, a central component of the CRL4 [VprBP] ubiquitin E3 ligase complex implicated in G1- to S-phase progression. Consistently, depletion of VprBP or Cul4 rescues the overproliferation of Lgl-depleted cells. In addition, the affinity between Lgl2 and the VprBP-DDB1 complex increases at high cell density. Further, aPKC-mediated phosphorylation of Lgl2 negatively regulates the interaction between Lgl2 and VprBP-DDB1 complex. These results suggest a mechanism protecting overproliferation of epithelial cells in which Lgl plays a critical role by inhibiting formation of the CRL4 [VprBP] complex, resulting in G1 arrest.
INTRODUCTION
A defect in the organization of cell sheets is a hallmark of epithelial cancer. Mutation in the Drosophila tumor suppressor lethal giant larvae (lgl) leads to the “giant-larva” phenotype in which the imaginal epithelia and nervous system are aberrant; the proliferating cells fail to form flat epithelial sheets, whereas most nonproliferating larval tissues show normal structure. Of importance, mutant overproliferating cells also show defects in cell polarity; proteins that localize to the apical membrane or adherens junctions mislocalize (Gateff, 1978; Bilder, 2004). Further, mutant neuroblasts show mislocalization of basal determinants required for asymmetric cell division (Ohshiro et al., 2000; Peng et al., 2000). These observations suggested that the tumor suppressive function of Lgl is a consequence of the disruption of cell polarity. Vertebrates have two lgl orthologues, Lgl1/Llgl1/Hugl1 and Lgl2/Llgl2/Hugl2, which also function as tumor suppressors. In vertebrates, loss of Lgl1 in the retinal epithelia in zebrafish affects both apical domain size and cell proliferation (Clark et al., 2012). Epidermal cells in zebrafish lgl2 mutants hyperproliferate, and transplantation of lgl2 mutant cells results in epidermal tumors (Sonawane et al., 2005; Reischauer et al., 2009). The Lgl1-knockout mouse exhibits defects in neuroepithelial cells: disruption of apicobasal polarity, disorganization of the apical junctional complex, and disruption of asymmetric Numb localization, resulting in hyperproliferation and brain dysplasia (Klezovitch et al., 2004). Human Lgl1 is reduced in some cancers, and overexpression of Lgl1 decreases tumor size, whereas it increases cell adhesion and/or decreases cell motility (Grifoni et al., 2004; Schimanski et al., 2005; Kuphal et al., 2006; Tsuruga et al., 2007). These observations suggest that the tumor-suppressive function of Lgl, which prevents overgrowth and the formation of abnormal tissue structure with polarity defects, is evolutionarily conserved.
The mechanism by which Lgl regulates cell polarity involves direct inhibition of the PAR polarity complex. The PAR complex consists of PAR3, PAR6, and atypical protein kinase C (aPKC) (Ohno, 2001). Lgl competes with PAR3 to form an inactive complex with aPKC and PAR6, and the balance of Lgl and PAR3 is critically important during the establishment and maintenance of cell polarity (Chalmers et al., 2005; Yamanaka et al., 2006). Consistently, Drosophila aPKC mutants show reduced cell proliferation of both neuroblasts and epithelia, the opposite of the lgl tumor suppressor phenotype. These observations reinforce a close relationship between cell polarity and cell proliferation and are consistent with the notion that Lgl regulates proliferation and differentiation through regulation of cell polarity.
Mosaic analysis in Drosophila larval eye disks, however, revealed that lgl mutant clones maintaining apicobasal polarity show ectopic S phases and mitosis (Grzeschik et al., 2007). lgl was also identified as a dominant suppressor of a weak cyclin E mutant (Brumby et al., 2004). These results raised the possibility that Lgl directly regulates the cell cycle regulatory machinery in addition to regulating cell polarity (Humbert et al., 2008). Collectively, whether Lgl regulates cell proliferation through polarity defect or more direct regulation of cell cycle, and the mechanism by which Lgl regulates cell proliferation, remains unclear.
VprBP/DCAF1, originally identified as a binding partner of HIV1 protein Vpr (Zhang et al., 2001), forms a cullin-RING E3 ubiquitin ligase CRL4 [VprBP] complex with DDB1, Cul4A, and Roc1 (Angers et al., 2006; He et al., 2006) and is involved in cell cycle regulation (Hrecka et al., 2007; Li et al., 2010). A study revealed the conserved molecular interaction between Lgl and VprBP/Mahjong in Drosophila and mammals (Tamori et al., 2011). The nature of the Lgl-VprBP complex, however, and its role in cell cycle regulation remains unknown. In this study, we analyzed the role of Lgl in cell proliferation using a model system composed of cultured Madin–Darby canine kidney (MDCK) epithelial cells that recapitulates the formation of monolayer cell sheets with apicobasal polarity and contact-mediated inhibition of cell proliferation.
RESULTS
Lgl is involved in suppression of proliferation in confluent epithelial cells
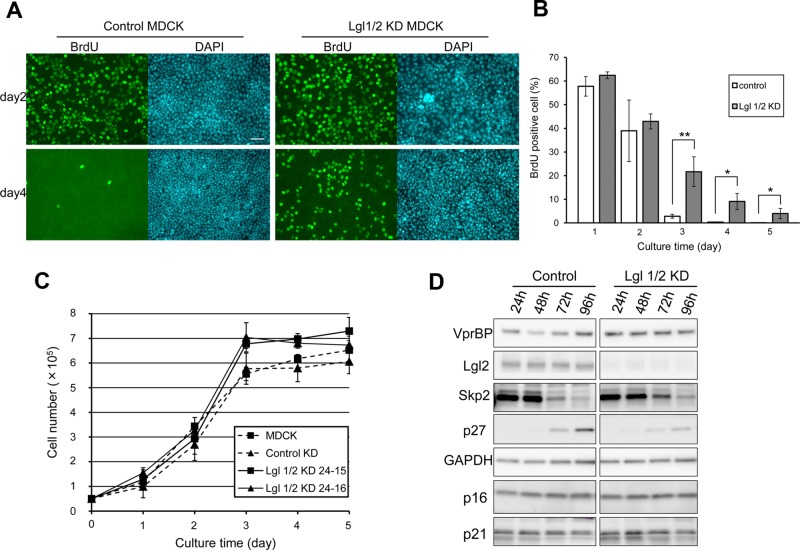
Previous studies revealed a defect in membrane polarity and failure of three-dimensional (3D) cyst formation in Lgl1 and Lgl2 double-knockdown (Lgl1/2 KD) MDCK cells (Yamanaka et al., 2003, 2006); however, the effect of Lgl1/2 KD on cell proliferation has not been analyzed. As shown in Figure 1, A and B, control cells cultured for 3 d reached confluency and rarely incorporated 5-bromo-2′-deoxyuridine (BrdU). In contrast, Lgl1/2 KD cells continued to enter S phase after reaching confluency and grew to higher saturation densities than control or parental cells, although they did not have a significantly higher proliferation rate under low-density conditions (Figure 1C). These results demonstrate the role of Lgl in suppression of cell proliferation in confluent culture conditions. At the molecular level, p27kip1 (p27), which binds to cyclin-CDK complexes and causes G1 cell cycle arrest, is up-regulated in G0/G1-arrested cells such as contact-inhibited or serum-starved cells (Polyak et al., 1994; Coats et al., 1996; Besson et al., 2008). In control cells, p27 but not other cell cycle inhibitors, p16 and p21, was up-regulated as cell density increased. However, up-regulation of p27 was attenuated in Lgl1/2 KD cells (Figure 1D). These results suggest that Lgl is involved in G1 cell cycle arrest at high cell density.
FIGURE 1:
Lgl is involved in suppression of proliferation in confluent epithelial cells. (A) Control MDCK and Lgl1/2 KD MDCK cells were seeded and cultured for the indicated times. BrdU was added 3 h before fixation, and BrdU-positive cells were visualized by immunofluorescence. Scale bar, 50 μm. (B) The ratio of BrdU-positive cells to total cells was determined, and averages of three independent experiments are plotted. Error bar indicates ±SD. Single and double asterisks denote significant differences p < 0.05 and p < 0.01, respectively, by Student's t test. (C) A total of 5 × 104 normal MDCK, control MDCK, and two Lgl1/2 KD MDCK cell clones was seeded in 12-well Transwell plates and counted using a hemocytometer. Error bars indicate ±SD of three independent experiments. Note that Lgl1/2 KD MDCK cells grew to a significantly higher saturation density than normal MDCK or control MDCK cells (p < 0.05, Student's t test between all four combinations at day 4). (D) Control MDCK and Lgl1/2 KD MDCK cells were seeded and cultured until the indicated times. Then levels of cell cycle inhibitors and Skp2 were monitored. Cell density–dependent induction of p27 and suppression of Skp2 were attenuated in Lgl1/2 KD MDCK cells.
Overexpression of Lgl2 arrests the cell cycle at G1 phase
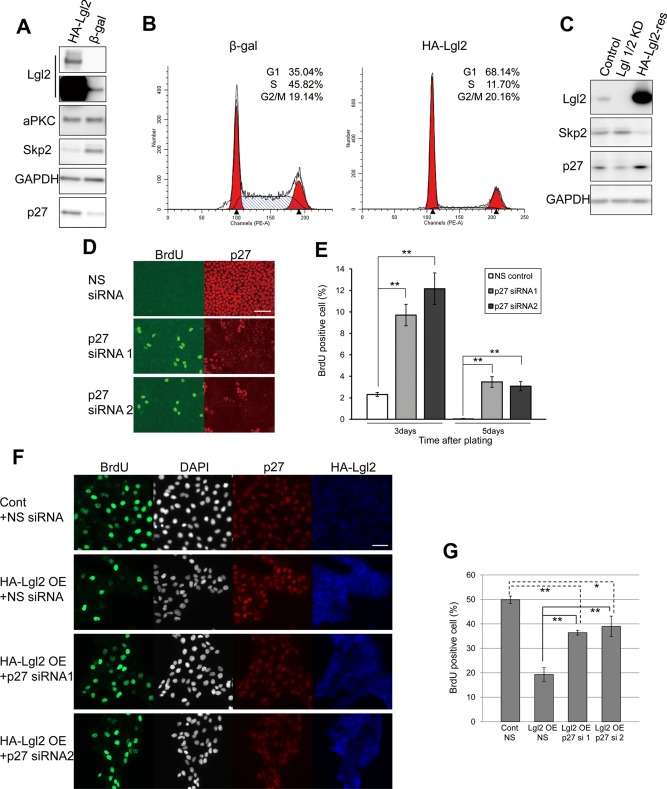
To evaluate further the role of Lgl on the cell cycle, we overexpressed hemagglutinin (HA)–tagged Lgl2 (HA-Lgl2) in sparsely seeded MDCK cells using an adenovirus vector (Figure 2A). HA-Lgl2-expressing cells proliferated more slowly than control cells expressing β-galactosidase (unpublished data). Flow cytometric analysis revealed that overexpression of HA-Lgl2 dramatically reduced the number of S-phase cells and increased the number of G1-phase cells, supporting that Lgl mediates G1 arrest (Figure 2B). Note that overexpression of HA-Lgl2 did not decrease the G2/M population in spite of G1 arrest, suggesting that Lgl may also have a weak effect on G2/M regulation. Because the levels of Lgl2 are not dependent on cell density (Figure 1D), these results imply that the antiproliferative activity of Lgl2 is weak at low cell density and strong at high cell density. Moreover, overexpression of HA-Lgl2 up-regulated p27 even at low cell density (Figure 2A). Skp2, which is down-regulated inversely to p27 at G1 phase (Carrano et al., 1999), was down-regulated by overexpression of HA-Lgl2 even at low cell density (Figure 2A). This effect was rescued in HA-Lgl2-res cells, in which endogenous Lgl1 and Lgl2 were knocked down and ectopic HA-Lgl2 was stably overexpressed (Figure 2C). The pattern of the amount of p27 and Skp2 also supports that Lgl mediates G1 arrest.
FIGURE 2:
Overexpression of Lgl2 arrests the cell cycle at G1 phase. (A, B) MDCK cells were infected with adenovirus expressing HA-Lgl2 or β-galactosidase. Western blot analysis confirmed expression of HA-Lgl2. Short and long exposures are shown for the immunoblotting of Lgl2 (A). Cells were stained with PI and subjected to flow cytometry (B). (C) Lgl1/2 KD MCDK was rescued by stable overexpression of HA-Lgl2 (lane 3), and levels of Skp2 and p27 were analyzed. (D) MDCK cells (1 × 105 cells/well) were plated on 12-well Transwell plates and transfected simultaneously with nonsilencing siRNA or siRNAs for p27, and the BrdU incorporation assay was performed after additional 3 or 5 d of culture. Representative images of cells cultured for 5 d. Scale bar, 50 μm. (E) The ratio of BrdU-positive cells to total cells in the experiment in D was determined, and averages of three independent experiments are plotted. Error bars indicate ±SD. Double asterisk denotes significant difference (p < 0.01) by Student's t test. (F) Lgl2-overexpressed MDCK cells were transiently introduced with siRNAs for p27 by electroporation. After culturing for 21 h, the BrdU incorporation assay was performed (summation of culturing time was 24 h). (G) The ratio of BrdU-positive cells to total cells in the experiment in F was determined, and averages of three independent experiments are plotted. Error bars indicate ±SD. Single and double asterisks denotes significant differences p < 0.05 and p < 0.01, respectively, by Student's t test.
Previous studies demonstrated the inhibitory role of p27 in cell cycle progression during contact inhibition (St Croix et al., 1998; Levenberg et al., 1999; Seluanov et al., 2009). Although p27-KO mice show multiple organ hyperplasia, however, contact inhibition remains intact in p27-deficient embryonic fibroblasts (Nakayama et al., 1996). Together, these observations suggest that the necessity of p27 for G1 cell cycle arrest is context dependent. Thus we next evaluated the growth-suppressive function of p27 in MDCK cells. MDCK cells were transfected with siRNA targeting p27 and cultured for an additional 3 or 5 d to induce contact inhibition, and BrdU incorporation was then evaluated. Knockdown of p27 significantly increased the number of BrdU-positive cells in both 3- and 5-d cultured cells (Figure 2, D and E). We also observed several mitotic cells distinguished by 4′,6-diamidino-2-phenylindole (DAPI) staining in p27-depleted cells (unpublished data). Next we tested the contribution of p27 for G1 arrest in Lgl2-overexpressing cells. Decreased BrdU incorporation of Lgl2-overexpressing cells was partially restored by transient introduction of siRNA for p27 (Figure 2, F and G). These observations suggest that p27 contributes to Lgl-mediated G1 arrest of MDCK cells at least to some extent. In addition, they also suggest the involvement of other pathways that regulate G1 arrest downstream of Lgl independently of p27.
Lgl physically interacts with VprBP and DDB1 independently of Cul4A and the PAR complex
To clarify the mechanism by which Lgl suppresses proliferation, we searched for its binding proteins. For this purpose, we established an MDCK cell line that stably expresses streptavidin-binding protein (SBP) tag–fused Lgl2. Tagged Lgl2–protein complex was purified from the confluently cultured cells and analyzed by mass spectrometry. We detected VprBP and DDB1, subunits of an E3 ubiquitin ligase in addition to aPKC and PAR6β (Supplemental Figure S1, A and B). VprBP is the substrate recognition subunit of the E3 ubiquitin ligase CRL4 [VprBP] complex, in which Roc1 mediates catalytic activity and Cul4A links Roc1 and DDB1, the adaptor protein that recruits VprBP to this complex (Angers et al., 2006; He et al., 2006). Although the interaction between Lgl and VprBP was reported (Tamori et al., 2011), the nature of the Lgl-interacting protein complex was unknown. Previous studies reported that VprBP localizes to nucleus and cytosol (Zhang et al., 2001). In MDCK cells, VprBP localizes to both the nuclear and cytosolic fractions, although the meaning of the cell density–dependent change in the localization is unclear (Supplemental Figure S2A). On the other hand, immunofluorescence staining of Lgl predominantly localizes as cortical (Betschinger et al., 2003; Yamanaka et al., 2003); however, Lgl2 is also fractionated to both cytosolic and nuclear fractions (Supplemental Figure S2A). Nuclear localization of Lgl2 was also confirmed by immunofluorescence after preextraction of membrane and cytosolic fraction, which enables relative enhancement of the nuclear Lgl2 signal by reduction of cortical Lgl2 staining (Supplemental Figure S2B). These results suggest that Lgl2 and VprBP could interact in each fraction.
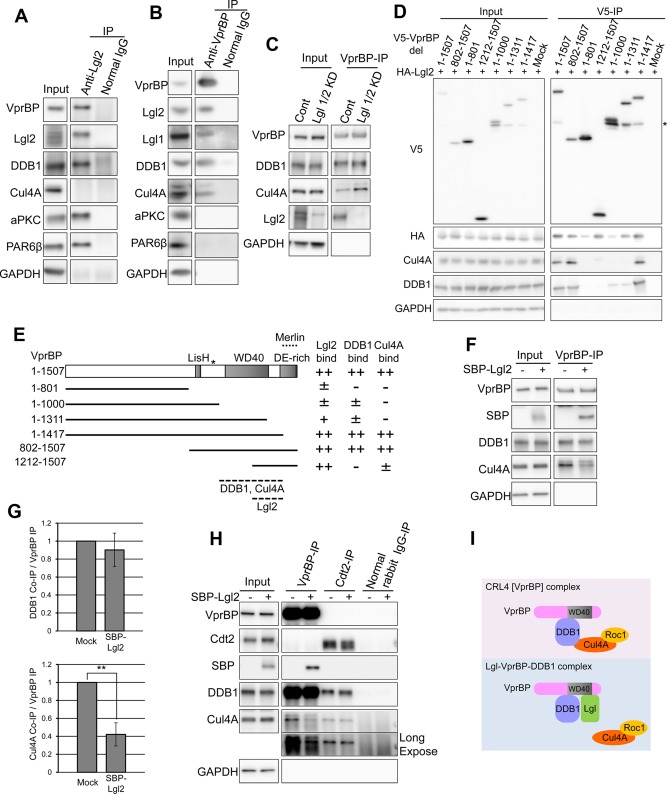
Immunoprecipitation analysis confirmed the interaction between Lgl2 and VprBP-DDB1. Of importance, we failed to detect Cul4A in the immunoprecipitates of Lgl2 (Figure 3A), whereas the immunoprecipitates of VprBP contained Cul4A in addition to DDB1 (Figure 3B). These results provide evidence for the presence of a novel protein complex, the Lgl2-VprBP-DDB1 complex, which may not function as an E3 ubiquitin ligase because it does not contain the core component Cul4A. Note that immunoprecipitates of VprBP did not contain components of the PAR complex, aPKC and PAR6 (Figure 3B), unlike Lgl2 immunoprecipitates (Figure 3A), suggesting that the Lgl2-VprBP-DDB1 complex is also independent of the PAR polarity complex. Consistently, VprBP-depleted cells did not show significant defects in the polarization and depolarization process (Supplemental Figure S3, A and B), indicating that VprBP is not critically involved in regulation of cell polarity.
FIGURE 3:
Lgl physically interacts with VprBP and DDB1 independently of Cul4A. (A) Lgl2 was immunoprecipitated from lysate prepared from confluently cultured MDCK cells. VprBP and DDB1 were coprecipitated with Lgl2, along with aPKC and PAR6β. (B) VprBP was immunoprecipitated from lysate prepared from confluently cultured MDCK cells. Lgl2, Lgl1, DDB1, and Cul4A were coprecipitated with VprBP, whereas aPKC and PAR6β were not. (C) VprBP was immunoprecipitated from control and Lgl1/2 KD MDCK cell lysate. Coimmunoprecipitation of Cul4A was up-regulated by depletion of Lgl1/2. (D) V5-VprBP deletion mutants and HA-Lgl2 were coexpressed in HEK293T cells. Immunoprecipitation was performed using anti-V5 antibody conjugated resin. Asterisk indicates the 110-kDa bands that were reproducibly detected in this experiment. VprBP may have an endo-cleavage site around amino acid 964. (E) Schematic representation of VprBP mutants and results of the experiment performed in D. Numbers in the left column represent amino acid number. Asterisk indicates position of amino acid 964. The binding site of Merlin, reported in Li et al. (2010), is also illustrated. (F) VprBP was immunoprecipitated from the lysates of HEK293T cells transfected with SBP-Lgl2 expression vector (lanes 2 and 4) or SBP expression vector (lanes 1 and 3). Note that the amount of Cul4A coimmunoprecipitated with VprBP was significantly decreased by expression of Lgl2. (G) Coimmunoprecipitated DDB1 and Cul4A were quantified by densitometry, and the average of three independent experiments is plotted. Error bars indicate ±SD. Double asterisks denotes significant difference (p < 0.01) by Student's t test. (H) VprBP or Cdt2 was immunoprecipitated from the common lysate of HEK293T cells transfected with SBP-Lgl2 expression vector (lanes 2, 4, 6, and 8) and also immunoprecipitated from the common lysate of cells transfected with SBP expression vector (lanes 1, 3, 5, and 7). Long-exposure image is also presented for Cul4A-immunoblot. (I) Hypothetical model for CRL4 [VprBP] and Lgl-VprBP-DDB1 complex. Lgl2 may sterically mask the Cul4A-binding domain of DDB1.
Because Lgl2-VprBP-DDB1 complex did not contain Cul4A, Lgl2 and Cul4A could each competitively bind to VprBP-DDB1. Consistent with this notion, the amount of Cul4A coimmunoprecipitated with VprBP was increased in Lgl1/2 KD cells (Figure 3C). Moreover, immunoprecipitation experiments using V5-tagged deletion mutants of VprBP coexpressed with HA-Lgl2 in HEK293T cells revealed that amino acids 1212–1417 of VprBP exhibited stronger affinity for Lgl2 compared with the other N-terminal fragments (Figure 3, D and E). This suggests that binding regions for Lgl2, DDB1, and Cul4A on VprBP overlap around the WD40 domain and implies that Lgl2 and DDB1/Cul4A share the same binding domain and can compete with each other for VprBP. To confirm this, an immunoprecipitation assay was performed; the interaction between VprBP and Cul4A was disrupted by overproduction of Lgl2, whereas the interaction between VprBP and DDB1 was not (Figure 3, F and G). Given that VprBP and Cul4A are connected by DDB1 (McCall et al., 2008), this suggests that the binding domains for Lgl2 and DDB1 in VprBP are very close but are not the same and that Lgl2 can sterically mask the Cul4A-binding domain of DDB1 (Figure 3I). Because DDB1 can interact with a number of substrate recognition subunits besides VprBP and recruit specific substrates to the corresponding CRL4 E3 complex, the effect of Lgl2 on CRL4 complexes should be specific to that containing VprBP. To confirm this, we assessed the effect of Lgl2 on one of the well-characterized CRL4 complexes, CRL4 [Cdt2] complex (Higa et al., 2006a; Jin et al., 2006). DDB1 and Cul4A were coimmunoprecipitated with Cdt2 irrespective of the overproduction of Lgl2, indicating that Lgl2 did not affect the interaction between Cul4A and Cdt2 (formation of the CRL4 [Cdt2] complex), whereas it disrupted the interaction between Cul4A and VprBP (formation of the CRL4 [VprBP] complex; Figure 3H). Note that the amount of DDB1-associated Cul4A was not largely affected by overexpression of Lgl2, consistent with the notion that VprBP is just one of the many binding partners of DDB1 (Supplemental Figure S4). This also suggests that Lgl is not the broad regulator of the DDB1-Cul4A E3 complexes but the specific regulator of the VprBP-DDB1-Cul4A complex. Taken together, the results indicate that the Lgl-VprBP-DDB1 complex and the VprBP-DDB1-Cul4A-Roc1 complex are mutually exclusive; Lgl can inhibit formation of the VprBP-DDB1-Cul4A-Roc1 E3 ligase complex by forming the Lgl-VprBP-DDB1 complex.
VprBP is involved in suppression of overproliferation mediated by Lgl
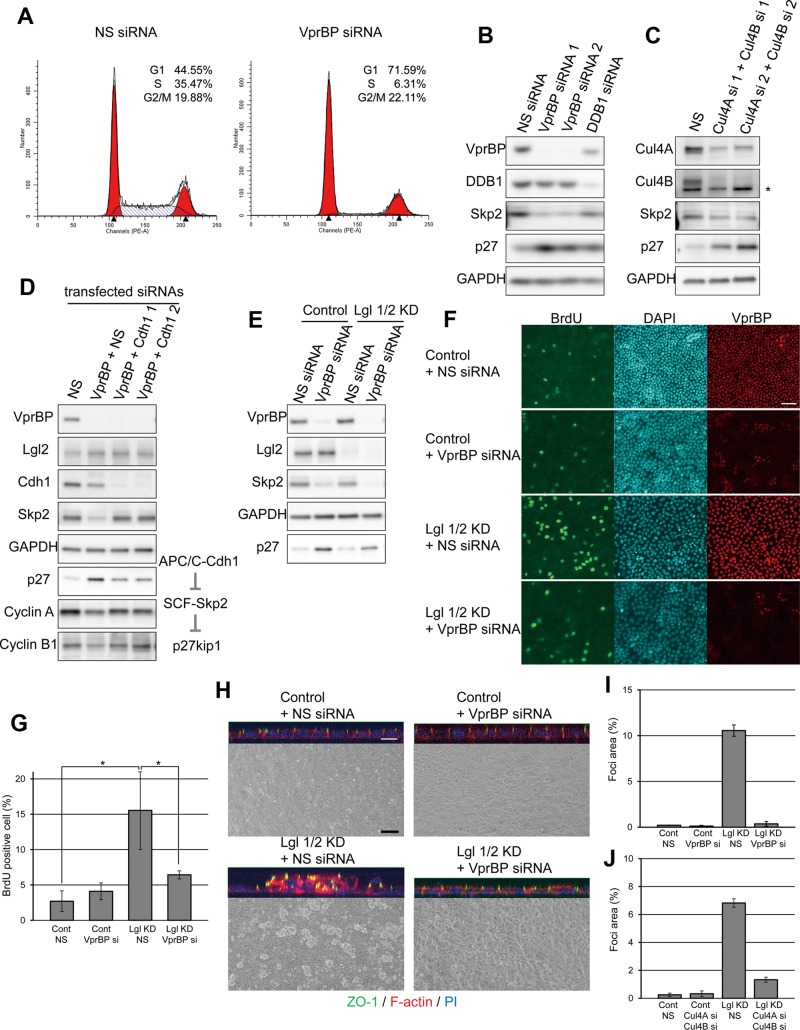
VprBP has been reported to be required for G1- to S-phase transition in other cell lines (Hrecka et al., 2007; Li et al., 2010). This raises the possibility that Lgl and VprBP may be functionally related with respect to the suppression of proliferation. We first tested whether VprBP-depleted MDCK cells were specifically arrested at G1. Similarly to overexpression of Lgl2, VprBP-knockdown cells proliferated slowly and were arrested specifically at G1 without changing the G2/M population (Figure 4, A and B; unpublished data). Previous studies showed that DDB1 and/or Cul4A regulate the level of p27 (Bondar et al., 2006; Higa et al., 2006b; Li et al., 2006; Miranda-Carboni et al., 2008), suggesting that VprBP may also take part in the regulation of p27 levels, possibly as a subunit of CRL4 [VprBP]. In support of this notion, knockdown of VprBP or DDB1 increased the amount of p27 and decreased Skp2 in sparsely cultured MDCK (Figure 4B) and HeLa cells (Supplemental Figure S5). In addition, depletion of both Cul4A and its orthologue, Cul4B, which is also suggested to interact with the VprBP-DDB1 complex (Jin et al., 2006), also results in up-regulation of p27 and down-regulation of Skp2 in MDCK cells, suggesting that CRL4 [VprBP] regulates levels of Skp2 and p27 (Figure 4C). Skp2 is a subunit of the SCF-Skp2 complex, which is the best-established E3 ubiquitin ligase for p27, and the level of Skp2 is also regulated by ubiquitination-dependent degradation mediated by the APC/C-Cdh1 complex (Carrano et al., 1999; Sutterluty et al., 1999; Bashir et al., 2004; Wei et al., 2004). We then evaluated the functional relationship between VprBP and Cdh1. Knockdown of VprBP down-regulated the level of Skp2 and cyclins A and B1, other substrates of APC/C-Cdh1, whereas double knockdown of VprBP and Cdh1 rescued Skp2, cyclin A, and cyclin B1 levels (Figure 4D), suggesting that VprBP may inactivate the APC/C-Cdh1 complex to sustain a Skp2 level that is appropriate for proliferation.
FIGURE 4:
VprBP is involved in suppression of overproliferation mediated by Lgl. (A) MDCK cells were transfected with the indicated siRNAs, stained with PI, and subjected to flow cytometry. (B) MDCK cells were transfected with siRNAs for VprBP or DDB1 and cultured at low density. Knockdown of VprBP or DDB1 up-regulated p27 and down-regulated Skp2. (C) MDCK cells were transfected with two siRNAs targeting for Cul4A and Cul4B and cultured at low density. Asterisk denotes nonspecific signal. (D) VprBP and Cdh1 were simultaneously knocked down in MDCK cells. Note that knockdown of Cdh1 alleviated the effects of VprBP knockdown. (E) Control or Lgl1/2 KD MDCK cells were transfected with siRNA for VprBP. After culturing for 2 d, the amounts of Skp2 and p27 were analyzed. (F) Control or Lgl1/2 KD MDCK cells were transfected with nonsilencing siRNA (NS) or siRNA for VprBP and seeded confluently to Transwells. After culturing for 2 d, the BrdU incorporation assay was performed. (G) The ratio of BrdU-positive cells to total cells in the experiment in F was determined, and averages of three independent experiments are plotted. Error bars indicate ±SD. Asterisk denotes significant difference (p < 0.05) by Student's t test. (H) Control or Lgl1/2 KD MDCK cells were transfected with nonsilencing siRNA (NS) or siRNA for VprBP and cultured until they reached confluency. Phase-contrast images and reconstituted confocal z-axis images of the epithelial sheets. Samples were stained with anti–ZO-1 (green), Alexa 647–phalloidin (red), and PI (blue). Scale bars, 10 μm (white), 30 μm (black). (I) The ratio of area of foci to total area was determined, and averages of three independent experiments are plotted. Error bars indicate ±SD. Note that knockdown of VprBP significantly suppressed formation of multilayered structures in Lgl1/2 KD MDCK cells. (J) Control or Lgl1/2 KD MDCK cells were transfected with nonsilencing siRNA (NS) or Cul4A siRNA 1 and Cul4B siRNA 1 and cultured until they reached confluency. Then the ratio of area of foci to total area was determined.
We next examined the functional relationship between Lgl and VprBP by knocking down VprBP in Lgl1/2 KD cells. Knockdown of VprBP up-regulated p27 in either sparsely seeded control or Lgl1/2 KD cells (Figure 4E) and inhibited BrdU uptake in confluent Lgl1/2 KD cells (Figure 4, F and G). Although control cells formed a monolayer, Lgl1/2 KD cells formed several multilayered structures (foci) throughout the epithelial sheet (Figure 4H). Depletion of VprBP in Lgl-depleted cells significantly reduced the multilayered structures of Lgl-depleted cells (Figure 4, H and I). We further confirmed the involvement of Cul4 in the overproliferation phenotype observed for Lgl-depleted cells; depletion of Cul4A and Cul4B inhibited multilayer formation of Lgl1/2 KD cells (Figure 4J and Supplemental Figure S6). These results support the notion that the CRL4 [VprBP] complex is involved in suppression of overproliferation mediated by Lgl.
Lgl inhibits formation of the VprBP-DDB1-Cul4A-Roc1 complex in confluent cells by forming the Lgl-VprBP-DDB1 complex
To evaluate the biological significance of the physical interaction between Lgl and VprBP, we monitored the interactions between Lgl2 and VprBP during the change in cell density. Immunoprecipitation assays using lysates from sparse or confluent cultures of MDCK cells revealed that the interaction between Lgl2 and VprBP was stronger in confluent cells than in sparse cells (Figure 5, A and B). On the contrary, the interaction between VprBP and Cul4A was weaker in confluent cells than in sparse cells (Figure 5B). These results are consistent with the notion that Lgl inhibits CRL4 [VprBP] complex formation by forming an Lgl-VprBP-DDB1 complex at high cell density (Figure 5C).
FIGURE 5:
Lgl2-VprBP-DDB1 complex was well formed with reduction of CRL4 [VprBP] complex in confluent cells. (A) Lgl2 was immunoprecipitated from the lysates of MDCK cells cultured in confluent or low-density conditions. The amount of coprecipitated VprBP was higher from confluent cells. (B) VprBP was immunoprecipitated from MDCK cell lysates cultured in confluent or low-density conditions. The amount of coprecipitated Cul4A was lower from confluent cells. (C) Hypothetical model of Lgl-mediated inhibition of the CRL4 [VprBP] complex and its downstream pathway. Lgl forms a complex with VprBP-DDB1 independently of Cul4A when cells reach confluency. Lgl-VprBP-DDB1 complex is catalytically inactive because it lacks Cul4A and Roc1, the active center that associates with E2 enzyme.
Phosphorylation of Lgl2 impairs the interaction between Lgl2 and VprBP, and phosphomimetic mutations attenuate the proliferation-suppressive function of Lgl2
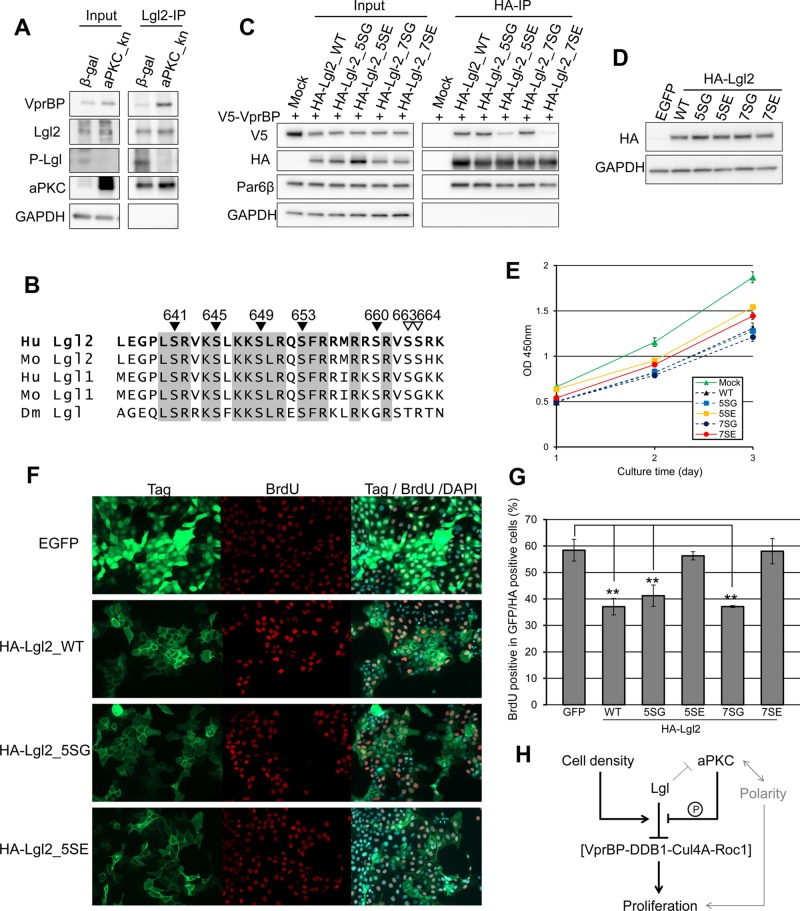
As described in the foregoing, Lgl binds to the aPKC-PAR6 complex and suppresses formation of the active PAR-aPKC ternary complex composed of PAR3, Par6, and aPKC. In addition, Lgl harbors conserved serine clusters phosphorylated by aPKC, and phosphorylation detaches Lgl from binding proteins, aPKC, and nonmuscle myosin II (Kalmes et al., 1996; Betschinger et al., 2003; Yamanaka et al., 2003). Therefore we tested the possibility that aPKC controls the affinity between Lgl2 and VprBP. Introduction of a kinase-negative form of aPKC, aPKC_kn, into MDCK cells decreased the phosphorylation level of Lgl2 and enhanced the interaction between Lgl2 and VprBP (Figure 6A). Serines 649, 653, and 660 of Lgl2 are effectively phosphorylated by aPKC in vitro. In addition to these sites, endogenous phosphorylation of Ser-655 of Lgl1, corresponding to Ser-645 of Lgl2, has been detected (PhosphoSite Plus; www.phosphosite.org). Thus we generated Lgl2 mutants on which sets of several serine residues were mutated (Figure 6B). We adopted the Ser-to-Gly mutant as a phosphoresistant form because Ala mutants were not expressed well in MDCK cells, for unknown reasons (Supplemental Figure S7). Phosphomimetic mutants of Lgl2 (5SE and 7SE) showed severely weakened interaction with VprBP (Figure 6C). Together, these results demonstrate that phosphorylation of Lgl2 inhibits the interaction between Lgl2 and VprBP. Next, we investigated whether these mutations affect the proliferation-inhibitory function of Lgl2. We used the pEB vector, which is episomally replicated in the cell, to permit sustainable expression of Lgl2 and its mutants in proliferating cells (Tanaka et al., 1999). This system successfully overexpressed Lgl2 and its mutants at similar levels, whereas not all cell populations were introduced (Figure 6, D and F). Lgl2 SE mutants showed weaker proliferation-inhibitory function than Lgl2 WT or SG mutants in the WST-8 cell proliferation assay (Figure 6E). Furthermore, uptake of BrdU was significantly inhibited in Lgl2 WT and SG mutant-expressing cells but not in SE mutant-expressing cells (Figure 6, F and G). These data suggest the underlying mechanism that aPKC-mediated phosphorylation of Lgl2 compromises binding between Lgl2 and VprBP, promoting cell proliferation. Thus, Lgl can regulate cell proliferation by at least two independent mechanisms: phosphorylation downstream of polarity signaling and an unknown mechanism downstream of the cell density (see also Discussion).
FIGURE 6:
Phosphorylation of Lgl2 impairs the interaction between Lgl2 and VprBP, and phosphomimetic mutations attenuate the proliferation-suppressive function of Lgl2. (A) MDCK cells were infected by adenovirus vector expressing LacZ or dominant-negative PKCλ (K273E). Then Lgl2 was immunoprecipitated from cell lysates. Note that the expression of the kinase-negative aPKC suppressed phosphorylation of Lgl2 and enhanced the interaction between Lgl2 and VprBP. (B) Alignment of amino acid sequences around aPKC-mediated phosphorylation sites of Lgl1 or 2 of human, mouse, and fly. The numbers show the amino acid sequence of human Lgl2. Closed inverted triangles show mutated residues in 5S mutants, and open inverted triangles show additional residues mutated in 7S mutants. (C) V5-VprBP and HA-Lgl2 or its mutants were overexpressed in HEK293T cells. Then HA-Lgl2 and its mutants were immunoprecipitated using anti-HA antibody. Note that phosphomimetic mutants of Lgl2 showed low affinity for VprBP. (D) Episomally stable transformants were obtained by selection with G418 after introduction of pEB vector encoding Lgl2 and its mutants, and equal expression was confirmed among these transformants. Immunoblot was performed using whole-cell lysates of 48 h–cultured cells after reseeding. (E) WST-8 cell proliferation assay using episomally stable MDCK transformants expressing Lgl2 and its mutants. Error bars indicate ±SD. (F) Episomally stable MDCK cells expressing Lgl2 and its mutants were reseeded sparsely and cultured for 2 d. Then the BrdU incorporation assay was performed. Note that not all cells expressed transgene. Cells with introduced pEB vector encoding enhanced GFP were used as a control. (G) The ratio of BrdU-positive cells to total cells in the experiment in F was determined, and averages of three independent experiments are plotted. Error bars indicate ±SD. Double asterisk denotes significant difference (p < 0.01) by Student's t test. (H) Schematic representation of Lgl-mediated proliferation inhibition. Lgl inhibits formation of the CRL4 [VprBP] complex, and this inhibition is controlled by aPKC-mediated phosphorylation, cell density, or yet-unknown mechanism. Cell polarity regulation may also affect regulation of proliferation.
DISCUSSION
A role for Lgl in the establishment and maintenance of cell polarity via suppression of PAR-aPKC polarity complex is established; however, the mechanism by which Lgl regulates cell proliferation is not fully understood. Here we use MDCK epithelial cells and show that depletion of Lgl1/2 causes overproliferation only at high cell density. On the contrary, overexpression of Lgl2 causes G1 cell cycle arrest. These results suggest a mechanism in normal epithelial cells by which Lgl mediates G1 arrest at high cell density. We identified VprBP and DDB1 as Lgl-binding partners and focused on VprBP, a conserved binding partner of Lgl (Tamori et al., 2011). VprBP is a component of a cullin-RING E3 ubiquitin ligase CRL4 [VprBP] complex (Angers et al., 2006; He et al., 2006) and is implicated in cell cycle regulation. We demonstrate that depletion of VprBP suppresses overproliferation of Lgl1/2-depleted cells, suggesting that Lgl mediates G1 cell cycle arrest at high cell density through a mechanism involving VprBP.
We also show that the interaction between Lgl2 and VprBP is enhanced when cell density is high and that Lgl2 overexpression can disrupt the interaction between VprBP-DDB1 and Cul4A by forming the Lgl2-VprBP-DDB1 complex. These results not only support the suppressive role of Lgl on cell proliferation, but they also suggest that cell density–dependent stimuli up-regulate the affinity between Lgl and VprBP and inhibit formation of the VprBP-DDB1-Cul4A-Roc1 ubiquitin E3 ligase complex to cause G1 cell cycle arrest. Of importance, the Lgl2-VprBP-DDB1 complex does not contain components of the PAR complex, aPKC and PAR6, and VprBP-depleted cells do not show significant defects in the polarization and depolarization process, suggesting that the Lgl2-VprBP-DDB1 complex is also independent of the PAR polarity complex. These results highlight the role of Lgl in suppressing cell proliferation by interacting with the VprBP-DDB1 complex in addition to its known role of regulating cell polarity by interacting with the aPKC-PAR6 complex.
The role of Lgl in control of cell polarity is regulated by phosphorylation by aPKC (Betschinger et al., 2003). We demonstrate that phosphorylated Lgl2 shows weak affinity for VprBP. Taking this together with the fact that the phosphomimetic form of Lgl2 showed a compromised proliferation-inhibitory effect, we suggest that phosphorylation of Lgl is involved in proliferation regulation. These results also suggest that Lgl can be regulated by the polarity signaling. Because phosphorylation levels of Lgl do not significantly differ between low-density and confluent cultures (Figure 5A), an additional mechanism may be required for cell density–dependent regulation of the affinity between Lgl and VprBP, such as alteration of posttranslational VprBP modifications. Thus interaction of Lgl and VprBP may be controlled by several factors, including polarity signaling, in a context-dependent manner (Figure 6H).
Although the direct target of CRL4 [VprBP] is unclear, this process was accompanied by decrease in the substrates of APC/C-Cdh1, such as Skp2, cyclin A, and cyclin B1, and an increase of p27. There are several possible factors that inactivate APC/C-Cdh1 during the G1 to S transition, including Emi1 and UBCH10 (Peters, 2006). CRL4 [VprBP] might destroy an upstream regulator of them or a component of the APC/C itself.
There are at least three possible roles of the physical interaction between Lgl and VprBP-DDB1. The first is that Lgl is a substrate of VprBP-containing E3 ligase. However, we failed to detect significant differences in the amount of Lgl between control and VprBP-depleted cells and between the presence or absence of MG132, a proteasome inhibitor, under sparse and confluent culture conditions (Supplemental Figure S8, A and B). The second is that Lgl is a component of the VprBP-containing E3 ligase complex, in which Lgl plays a role as a substrate receptor. The Lgl2-VprBP-DDB1 complex, which we have identified, does not contain Cul4A and is independent of the CRL4 [VprBP] E3 ligase complex. The involvement of VprBP-DDB1 in another E3 ligase complex, EDVP complex, is suggested (Maddika and Chen, 2009). However, we failed to detect EDD in immunoprecipitates of VprBP (unpublished data), suggesting that the EDVP complex is not involved in MDCK cells. These results do not support the second possibility, whereas the possibility of the presence of a yet-unidentified E3 ligase complex containing VprBP and DDB1 cannot be excluded. The third possibility is that the Lgl-VprBP-DDB1 complex inhibits the formation of VprBP-containing E3 ligase complexes such as CRL4 [VprBP]. This possibility is supported by the specific mode of molecular interaction between Lgl and VprBP and is confirmed by the observation that overexpression of Lgl2 decreases the affinity of the interaction between VprBP-DDB1 and Cul4A. The specific mode of molecular interaction between Lgl and VprBP also raises the intriguing possibility that the VprBP-DDB1 complex can compete with the aPKC-PAR6 complex for Lgl in certain situations, although we did not detect any significant effect on cell polarity by depletion of VprBP (Supplemental Figure S3, A and B).
Many cultured cells arrest the cell cycle at G0/G1 when they reach a certain density. This intrinsic inhibitory system, called contact inhibition of cell proliferation, can explain at least in part how normal tissue growth is regulated. Contact inhibition is usually disrupted in cancer cells, resulting in abnormal tissue growth and architecture (Fagotto and Gumbiner, 1996; Takai et al., 2008). This process involves cell surface receptors that are engaged by the physical interaction between cell surfaces and growth-regulatory signaling pathways, which are affected by those receptors in a contact-dependent manner. The former include E-cadherin, nectin, and nectin-like molecules, and the latter involves the recently identified Hippo pathway (Takai et al., 2008; McClatchey and Yap, 2012). In addition, contact inhibition of cell proliferation must involve the cell cycle regulatory machinery; the best-studied example is the cyclin-dependent kinase inhibitor p27kip1 (p27), which binds to cyclin-CDK complexes, causing cell cycle arrest at G1. However, the mechanism by which cell density affects the levels of p27 is unclear, although ubiquitin ligases that regulate p27 levels are known. In this study, we showed that p27 is necessary for complete inhibition of the proliferation of confluent MDCK cells and that depletion of Lgl caused attenuation of the up-regulation of p27 by affecting VprBP-DDB1-Cul4A complex formation in confluent MDCK cells. Further studies are needed to clarify how Lgl and VprBP sense cell density and which factor is the direct target of CRL4 [VprBP].
In this study, we showed that Lgl1/2 KD cells continued to proliferate even after reaching confluence and formed stratified structures. We analyzed these structures and found a moderate polarity defect, in that asymmetrically localized proteins diffusely localized in Lgl1/2 KD cells (Supplemental Figure S9). Together with the result that Lgl1/2 and VprBP-depleted cells form monolayers (Figure 4H), these results suggest that the polarity defect is not sufficient for formation of aberrant epithelial structure. Combination of both proliferation and the polarity defect may cause multilayered overgrowth.
Merlin/NF-2 is a hyperplastic tumor suppressor in Drosophila and is inactivated in neurofibromatosis type 2 and other sporadic human tumors (Okada et al., 2007). Merlin also interacts with VprBP to block substrate binding, resulting in inhibition of cell proliferation (Li et al., 2010). Although the functional relationship between Lgl and Merlin in inhibition of CRL4 [VprBP] is unclear, their effects on the E3 ligase are clearly different. Lgl disrupts complex formation of CRL4 [VprBP] by binding to the WD40 domain of VprBP, whereas Merlin binds to the extreme C-terminus of VprBP to inhibit substrate binding. Thus Lgl and Merlin can redundantly inhibit CRL4 [VprBP]. Considering that VprBP is the target of two different tumor suppressors, Lgl and Merlin, the C-terminal region of VprBP may be a promising target for anticancer drugs.
MATERIALS AND METHODS
Expression vectors and small interfering RNAs
To construct an expression vector for Flag-SBP-Lgl2, we generated a Flag-SBP tandem tag fragment by PCR using pNTAP (Stratagene, La Jolla, CA) as a template and subcloned it into the multiple cloning site (MCS) of pCAG-GS. An Lgl2 cDNA (Yamanaka et al., 2003) was subcloned into the MCS of pCAG-GS-Flag-SBP, and the neomycin-resistance cassette of pMC was subcloned into the SalI site of this vector. Information on pEB-CAG-HA-Lgl2 and how to generate transformants is given by Horikoshi et al. (2009). pEB vectors encoding phosphomimetic (SE) or nonphosphorylatable (SG) forms of Lgl2 were generated by PCR-based site-directed mutagenesis using pEB-CAG-HA-Lgl2. A VprBP/KIAA0800 cDNA was obtained from Flexi ORF clones (Nagase et al., 2008), and each deletion mutant was generated by PCR. These fragments were subcloned into pCAG-GS with a 5′ V5-tag sequence. A DDB1 cDNA was also obtained from Flexi ORF clones and subcloned into pEB vector with a 5′ Myc-tag sequence. Adenoviral vectors encoding HA-Lgl2, aPKC, aPKC_kn, and LacZ were described previously (Suzuki et al., 2001; Yamanaka et al., 2003). A hygromycin-resistance vector encoding HA-Lgl2 (pHyg-HA-Lgl2) was generated by exchanging the autonomous replication machinery of pEB-CAG-HA-Lgl2 with a hygromycin-resistance cassette. The PciI-SfiI fragment of pEB-CAG-HA-Lgl2 and the AhdI-SspI fragment of pTK-Hyg were blunted and ligated. The target sequences of small interfering RNAs (siRNAs) were as follows; VprBP #1 (GGAAGUGGCUUUACGGCAA), VprBP #2 (CCAUUGAUGUGAAACGGAA), DDB1 (ACACUUUGGUGCUCUCUUU), Cdh1 #1 (GGAUCAAUGAGAAUGAGAA), Cdh1 #2 (GCAACGAUGUGUCUCCCUA), p27kip1 #1 (CCAACAGAACAGAAGAAAA), p27kip1 #2 (CGACGAUUCCUCUCCUCAA), Cul4A #1 (GGAUAAUGAAGAUGAGAAA), Cul4A #2 (CCAUAUCAUUAGUGAUAAA), Cul4B #1 (GGAUAAAAUUAUGAUCAUA), Cul4B #2 (GCUGAAGGCCAAAAAUUAA), and nonsilencing scramble (1027281; Qiagen, Valencia, CA). Unless otherwise noted, VprBP #1 was used for VprBP knockdown.
Cell culture, transfection, and establishment of stable transformants
MDCK II, HEK293T, and HeLa cells were cultured in DMEM supplemented with 10% fetal bovine serum, 1 mM glutamine, and 100 U/ml penicillin/streptomycin at 37°C in 5% CO2. Lgl1/2 KD MDCK and control MDCK cells were described previously (Yamanaka et al., 2006). Clone 24-15 was used in this study unless otherwise indicated. To establish an MDCK cell line expressing Flag-SBP-Lgl2, pCAG-GS-Flag-SBP-Lgl2 was introduced into the previously described Lgl2-knockdown cell line (Yamanaka et al., 2006) by electroporation and selection using G418. The HA-Lgl2 rescue clone and overexpressing clone were established by introducing pHyg-HA-Lgl2 into Lgl1/2 KD MDCK cells and normal MDCK cells, respectively, and selecting with hygromycin. Nonsilenced control and VprBP-knockdown MDCK clones were established by introducing pSUPERIOR-neo vectors (OligoEngine, Seattle, WA) encoding a nonsilencing sequence (CAGUCGCGUUUGCGACUGG) and the sequences for VprBP, respectively. Transient plasmid transfection was performed using Lipofectamine LTX (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. To efficiently introduce siRNAs, MDCK cells were transfected twice using Lipofectamine RNAi MAX (Invitrogen); briefly, 2.5 × 105 cells/well were seeded with siRNA transfection complex in six-well plates and incubated for 24 h. For assaying the confluent state, 1 × 105 cells/well were reseeded with transfection complex in 12-well Transwell plates (Corning, Corning, NY) and cultured for an additional 48 h. For assaying low-density cultured cells, 5 × 104 cells/well were reseeded with transfection complex in 24-well plates and cultured for an additional 48 h. NEPA21 electroporator (Nepa Gene, Chiba, Japan) was also used to introduce siRNA; 0.1 nmol of siRNA and 5 × 105 cells were mixed in 50 μl of Opti-MEM, and parameters were set according to the manufacturer's instruction. WST-8 assay was performed using Cell Count Reagent SF (Nacalai Tesque, Kyoto, Japan) according to the manufacturer's instructions.
Antibodies
Anti-PAR6β (BC31AP), anti-Lgl2 (N13AP), and anti-Lgl2-S653P antibodies have been described previously (Yamanaka et al., 2003). Anti-GP135 (3F2/D8) was a kind gift from George K. Ojakian (State University of New York, Brooklyn, NY). Other antibodies were purchased as follows: Lgl2 (Abnova, Taipei, Taiwan), Lgl1 (Sigma-Aldrich, St. Louis, MO), VprBP (Proteintech Group, Chicago, IL), DDB1 (Bethyl, Montgomery, TX), Cul4A (Bethyl), Cul4B (Proteintech Group), Cdt2 (Novus Biologicals, Littleton, CO), PKC iota (BD, Franklin Lakes, NJ), zonula occludens-1 (ZO-1; Santa Cruz Biotechnology, Dallas, TX), p27kip1 (BD), p16INK4a (Cell Signaling, Danvers, MA), p21Waf1/Cip1 (Santa Cruz Biotechnology), Skp2 (Santa Cruz Biotechnology), Cdh1 (Abcam, Cambridge, United Kingdom), cyclin A (Santa Cruz Biotechnology), cyclin B1 (Santa Cruz Biotechnology), HSP70 (Enzo Life Sciences, Farmingdale NY), EDD1 (Bethyl), glyceraldehyde-3-phosphate dehydrogenase (Abcam), β-Actin (Sigma-Aldrich), E-cadherin (Sigma-Aldrich), V5 (Invitrogen), HA (Roche, Basel, Switzerland), SBP (Santa Cruz Biotechnology), Myc (Millipore, Billerica, MA; Cell Signaling), BrdU (BD; Abcam), and normal rabbit immunoglobulin G (Cell Signaling).
Protein identification
MDCK cells expressing Flag-SBP-Lgl2 were cultured and lysed with lysis buffer containing 25 mM Tris-HCl, pH 7.5, 140 mM NaCl, 0.5% Triton X-100, 2.5 mM MgCl2, 1 mM ethylene glycol tetraacetic acid, Complete (Roche), and PhosSTOP (Roche). After centrifugation, the soluble fraction was incubated with streptavidin-Sepharose (GE Healthcare, Waukesha, WI) for 2 h at 4°C with gentle rotation. After washing with lysis buffer, the affinity-purified protein complexes were eluted by incubation in lysis buffer containing 2 mM biotin at 4°C for 30 min. The eluted fractions were separated by SDS–PAGE and stained with SilverQuest (Invitrogen). Excised protein bands were digested and subjected to liquid chromatography–tandem mass spectrometry. Peptide sequences were analyzed using the Mascot search engine.
BrdU incorporation assays
Fifty thousand Lgl1/2 KD or control MDCK cells were seeded per well in 12-well Transwell plates and cultured for the indicated times. Before fixation, they were incubated in medium containing 100 μM BrdU. Then cells were fixed with 2% paraformaldehyde/phosphate-buffered saline (PBS) and permeabilized for 30 min in 2 N HCl and 0.1% Triton-X in water. BrdU-positive and -negative cells were visualized by immunofluorescence using anti-BrdU and DAPI counterstaining. More than 1000 cells across several locations were counted.
Immunoprecipitation
MDCK or 293T cells were lysed in lysis buffer. After centrifugation, the supernatants were subjected to immunoprecipitation with the indicated antibodies, followed by SDS–PAGE and Western blotting. To prepare lysates from confluent and low-density-cultured MDCK cells, 8 × 106 cells were seeded in one 10-cm dish and 3 × 106 cells were seeded in three 10-cm dishes, respectively. After culturing for 2 d, lysates were prepared and protein concentrations were adjusted, and they were subjected to immunoprecipitation.
Immunofluorescence
Cells were fixed with 2% paraformaldehyde in PBS and permeabilized with 0.5% Triton X-100 in PBS. Alexa Fluor–conjugated secondary antibodies (Invitrogen) were used for immunostaining. For staining of F-actin, rhodamine-phalloidin or Alexa 647–phalloidin was used, and for nuclei, DAPI or propidium iodide (PI) was used. For PI staining, samples were pretreated with RNase. Images were obtained using a conventional immunofluorescence microscope (AxioImager; Carl Zeiss, Oberkochen, Germany) or a laser confocal scanning microscope system (LSM 510, LSM700; Carl Zeiss).
Cell cycle analysis
MDCK cells (2.5 × 105) were seeded in 6-cm dishes and cultured for 18 h. Cells were then incubated for 6 h in low calcium medium (3 µM Ca2+, 5% FBS-containing DMEM) containing each adenovirus (1 × 108 pfu/ml). After incubating in normal medium for 24 h, cells were trypsinized and fixed with 70% ethanol overnight at 4°C. After washing in PBS, cells were incubated in 150 μl of PBS containing 50 μM PI and 0.6 mg/ml RNase at 37°C for 30 min. Data were collected with a BD FACSCanto flow cytometer, and the mathematical model MODFIT was used to calculate the percentage of cells in G1, S, and G2/M phases of the cell cycle. To analyze the effect of VprBP knockdown, 2.5 × 105 MDCK cells were seeded in 6-cm dishes and transfected with each siRNA simultaneously. After a 24-h incubation, a second transfection was performed in the same manner, and cells were then cultured for an additional 48 h. Cells were then harvested and analyzed by flow cytometry as described.
Supplementary Material
Acknowledgments
We thank A. Yamashita for technical advice concerning protein purification and H. Nakamura for mass spectrometry services. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (S.O. and H.H.), the Japan Society for the Promotion of Science (A.S. and S.O.), and the Yokohama Foundation for Advancement of Medical Science (K.Y.).
Abbreviations used:
- aPKC
atypical protein kinase C
- BrdU
5-bromo-2′-deoxyuridine
- DAPI
4′,6-diamidino-2-phenylindole
- MDCK
Madin–Darby canine kidney
- PI
propidium iodide
- SBP
streptavidin-binding protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E14-10-1462) on May 6, 2015.
REFERENCES
- Angers S, Li T, Yi X, MacCoss MJ, Moon RT, Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M. Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature. 2004;428:190–193. doi: 10.1038/nature02330. [DOI] [PubMed] [Google Scholar]
- Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–169. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bondar T, Kalinina A, Khair L, Kopanja D, Nag A, Bagchi S, Raychaudhuri P. Cul4A and DDB1 associate with Skp2 to target p27Kip1 for proteolysis involving the COP9 signalosome. Mol Cell Biol. 2006;26:2531–2539. doi: 10.1128/MCB.26.7.2531-2539.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumby A, Secombe J, Horsfield J, Coombe M, Amin N, Coates D, Saint R, Richardson H. A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S-phase entry in Drosophila. Genetics. 2004;168:227–251. doi: 10.1534/genetics.104.026617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- Chalmers AD, Pambos M, Mason J, Lang S, Wylie C, Papalopulu N. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development. 2005;132:977–986. doi: 10.1242/dev.01645. [DOI] [PubMed] [Google Scholar]
- Clark BS, Cui S, Miesfeld JB, Klezovitch O, Vasioukhin V, Link BA. Loss of Llgl1 in retinal neuroepithelia reveals links between apical domain size, Notch activity and neurogenesis. Development. 2012;139:1599–1610. doi: 10.1242/dev.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM. Cell contact-dependent signaling. Dev Biol. 1996;180:445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Schimanski CC, Schmitz G, Laurenti E, Galle PR, Pession A, Cavicchi S, Strand D. The human protein Hugl-1 substitutes for Drosophila lethal giant larvae tumour suppressor function in vivo. Oncogene. 2004;23:8688–8694. doi: 10.1038/sj.onc.1208023. [DOI] [PubMed] [Google Scholar]
- Grzeschik NA, Amin N, Secombe J, Brumby AM, Richardson HE. Abnormalities in cell proliferation and apico-basal cell polarity are separable in Drosophila lgl mutant clones in the developing eye. Dev Biol. 2007;311:106–123. doi: 10.1016/j.ydbio.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YJ, McCall CM, Hu J, Zeng Y, Xiong Y. DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev. 2006;20:2949–2954. doi: 10.1101/gad.1483206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol. 2006a;8:1277–1283. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- Higa LA, Yang X, Zheng J, Banks D, Wu M, Ghosh P, Sun H, Zhang H. Involvement of CUL4 ubiquitin E3 ligases in regulating CDK inhibitors Dacapo/p27Kip1 and cyclin E degradation. Cell Cycle. 2006b;5:71–77. doi: 10.4161/cc.5.1.2266. [DOI] [PubMed] [Google Scholar]
- Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J Cell Sci. 2009;122:1595–1606. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- Hrecka K, Gierszewska M, Srivastava S, Kozaczkiewicz L, Swanson SK, Florens L, Washburn MP, Skowronski J. Lentiviral Vpr usurps Cul4-DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc Natl Acad Sci USA. 2007;104:11778–11783. doi: 10.1073/pnas.0702102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, Richardson HE. Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene. 2008;27:6888–6907. doi: 10.1038/onc.2008.341. [DOI] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC. A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell. 2006;23:709–721. doi: 10.1016/j.molcel.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Kalmes A, Merdes G, Neumann B, Strand D, Mechler BM. A serine-kinase associated with the p127-l(2)gl tumour suppressor of Drosophila may regulate the binding of p127 to nonmuscle myosin II heavy chain and the attachment of p127 to the plasma membrane. J Cell Sci. 1996;109:1359–1368. doi: 10.1242/jcs.109.6.1359. [DOI] [PubMed] [Google Scholar]
- Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–571. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, Schimanski CC, Bataille F, Hofer P, Strand S, Strand D, Bosserhoff AK. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene. 2006;25:103–110. doi: 10.1038/sj.onc.1209008. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Yarden A, Kam Z, Geiger B. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 1999;18:869–876. doi: 10.1038/sj.onc.1202396. [DOI] [PubMed] [Google Scholar]
- Li B, Jia N, Kapur R, Chun KT. Cul4A targets p27 for degradation and regulates proliferation, cell cycle exit, and differentiation during erythropoiesis. Blood. 2006;107:4291–4299. doi: 10.1182/blood-2005-08-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, You L, Cooper J, Schiavon G, Pepe-Caprio A, Zhou L, Ishii R, Giovannini M, Hanemann CO, Long SB, et al. Merlin/NF2 suppresses tumorigenesis by inhibiting the E3 ubiquitin ligase CRL4(DCAF1) in the nucleus. Cell. 2010;140:477–490. doi: 10.1016/j.cell.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddika S, Chen J. Protein kinase DYRK2 is a scaffold that facilitates assembly of an E3 ligase. Nat Cell Biol. 2009;11:409–419. doi: 10.1038/ncb1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall CM, Miliani de Marval PL, Chastain PD, 2nd, Jackson SC, He YJ, Kotake Y, Cook JG, Xiong Y. Human immunodeficiency virus type 1 Vpr-binding protein VprBP, a WD40 protein associated with the DDB1-CUL4 E3 ubiquitin ligase, is essential for DNA replication and embryonic development. Mol Cell Biol. 2008;28:5621–5633. doi: 10.1128/MCB.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Yap AS. Contact inhibition (of proliferation) redux. Curr Opin Cell Biol. 2012;24:685–694. doi: 10.1016/j.ceb.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Miranda-Carboni GA, Krum SA, Yee K, Nava M, Deng QE, Pervin S, Collado-Hidalgo A, Galic Z, Zack JA, Nakayama K, et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121–3134. doi: 10.1101/gad.1692808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Yamakawa H, Tadokoro S, Nakajima D, Inoue S, Yamaguchi K, Itokawa Y, Kikuno RF, Koga H, Ohara O. Exploration of human ORFeome: high-throughput preparation of ORF clones and efficient characterization of their protein products. DNA Res. 2008;15:137–149. doi: 10.1093/dnares/dsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr Opin Cell Biol. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–596. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- Okada T, You L, Giancotti FG. Shedding light on Merlin's wizardry. Trends Cell Biol. 2007;17:222–229. doi: 10.1016/j.tcb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Peng CY, Manning L, Albertson R, Doe CQ. The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- Reischauer S, Levesque MP, Nusslein-Volhard C, Sonawane M. Lgl2 executes its function as a tumor suppressor by regulating ErbB signaling in the zebrafish epidermis. PLoS Genet. 2009;5:e1000720. doi: 10.1371/journal.pgen.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schafer SC, Lehr HA, Berger MR, et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24:3100–3109. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- Seluanov A, Hine C, Azpurua J, Feigenson M, Bozzella M, Mao Z, Catania KC, Gorbunova V. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci USA. 2009;106:19352–19357. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonawane M, Carpio Y, Geisler R, Schwarz H, Maischein HM, Nuesslein-Volhard C. Zebrafish penner/lethal giant larvae 2 functions in hemidesmosome formation, maintenance of cellular morphology and growth regulation in the developing basal epidermis. Development. 2005;132:3255–3265. doi: 10.1242/dev.01904. [DOI] [PubMed] [Google Scholar]
- St Croix B, Sheehan C, Rak JW, Florenes VA, Slingerland JM, Kerbel RS. E-Cadherin-dependent growth suppression is mediated by the cyclin-dependent kinase inhibitor p27(KIP1) J Cell Biol. 1998;142:557–571. doi: 10.1083/jcb.142.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- Tamori Y, Bialucha CU, Tian AG, Kajita M, Huang YC, Norman M, Harrison N, Poulton J, Ivanovitch K, Disch L, et al. Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 2011;8:e1000422. doi: 10.1371/journal.pbio.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J, Miwa Y, Miyoshi K, Ueno A, Inoue H. Construction of Epstein-Barr virus-based expression vector containing mini-oriP. Biochem Biophys Res Commun. 1999;264:938–943. doi: 10.1006/bbrc.1999.1617. [DOI] [PubMed] [Google Scholar]
- Tsuruga T, Nakagawa S, Watanabe M, Takizawa S, Matsumoto Y, Nagasaka K, Sone K, Hiraike H, Miyamoto Y, Hiraike O, et al. Loss of Hugl-1 expression associates with lymph node metastasis in endometrial cancer. Oncol Res. 2007;16:431–435. doi: 10.3727/000000007783980855. [DOI] [PubMed] [Google Scholar]
- Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG., Jr Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–198. doi: 10.1038/nature02381. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci. 2006;119:2107–2118. doi: 10.1242/jcs.02938. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Zhang S, Feng Y, Narayan O, Zhao LJ. Cytoplasmic retention of HIV-1 regulatory protein Vpr by protein-protein interaction with a novel human cytoplasmic protein VprBP. Gene. 2001;263:131–140. doi: 10.1016/s0378-1119(00)00583-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.