Peptide release from single dense-core vesicles (DCVs) was detected at a native nerve terminal for the first time. Synaptic release is slow, dynamin dependent, and mediated exclusively by kiss-and-run exocytosis. Presynaptic peptide release is independent of DCV arrival time, bouton location, and transport direction into the synapse.
Abstract
Neurons release neuropeptides, enzymes, and neurotrophins by exocytosis of dense-core vesicles (DCVs). Peptide release from individual DCVs has been imaged in vitro with endocrine cells and at the neuron soma, growth cones, neurites, axons, and dendrites but not at nerve terminals, where peptidergic neurotransmission occurs. Single presynaptic DCVs have, however, been tracked in native terminals with simultaneous photobleaching and imaging (SPAIM) to show that DCVs undergo anterograde and retrograde capture as they circulate through en passant boutons. Here dynamin (encoded by the shibire gene) is shown to enhance activity-evoked peptide release at the Drosophila neuromuscular junction. SPAIM demonstrates that activity depletes only a portion of a single presynaptic DCV's content. Activity initiates exocytosis within seconds, but subsequent release occurs slowly. Synaptic neuropeptide release is further sustained by DCVs undergoing multiple rounds of exocytosis. Synaptic neuropeptide release is surprisingly similar regardless of anterograde or retrograde DCV transport into boutons, bouton location, and time of arrival in the terminal. Thus vesicle circulation and bidirectional capture supply synapses with functionally competent DCVs. These results show that activity-evoked synaptic neuropeptide release is independent of a DCV's past traffic and occurs by slow, dynamin-dependent partial emptying of DCVs, suggestive of kiss-and-run exocytosis.
INTRODUCTION
Neuropeptides, neurotrophins, and enzymes are packaged in dense-core vesicles (DCVs) for synaptic release by Ca2+-induced exocytosis. In contrast to rapid neurotransmission by small synaptic vesicles (SSVs) at active zones, peptidergic transmission by DCVs typically requires bouts of activity, occurs away from active zones, and is slow, suggesting that presynaptic SSVs and DCVs differ in their release properties. Based on green fluorescent protein (GFP) imaging in cultured cells, peptide release from single DCVs has been detected at the neuron soma, growth cones, neurites, axons, and dendrites (Han et al. 1999; Ng et al., 2003; Lochner et al., 2006; de Wit et al., 2009; Matsuda et al., 2009; Xia et al., 2009). These in vitro studies have shown variable properties for release from single DCVs, ranging from complete release, indicative of full-collapse fusion, to partial release, suggestive of kiss-and-run exocytosis, which is accepted for DCVs but controversial for SSVs (Wu et al., 2014). However, activity-induced release from single neuronal DCVs has not been imaged in vivo from nerve terminals. Thus the kinetics and exocytic mechanism underlying peptidergic neurotransmission remain unknown.
Dynamin, in addition to its role in recycling SSVs by pinching off endocytic vesicles after exocytosis at active zones, participates in endocrine DCV exocytosis. For example, dynamin promotes kiss-and-run exocytic peptide release by pancreatic β-cells (Min et al., 2007). Furthermore, many studies suggest that dynamin facilitates dilation of the fusion pore to promote kiss-and-run release from adrenal chromaffin cells (Fulop et al., 2008; Anantharam et al., 2011; Gonzalez-Jamett et al., 2013; Trouillon and Ewing, 2013). However, dynamin has also been proposed to limit release of catecholamines (Graham et al., 2002) and tissue plasminogen activator (Tsuboi et al., 2004), the latter of which is slow because of its interaction with the fusion pore from inside the DCV (Weiss et al., 2014). Given these diverse effects in endocrine cells, it is interesting that dynamin's role in synaptic neuropeptide release is unknown.
The lack of mechanistic understanding of presynaptic DCV exocytosis during peptidergic neurotransmission led us to image the intact Drosophila larval neuromuscular junction (NMJ). Type Ib motoneuron terminals in this preparation possess glutamatergic SSVs and DCVs containing neuropeptides and a bone morphogenic protein (Anderson et al., 1988; James et al., 2014). Furthermore, a temperature-sensitive dynamin mutant (shibirets1 [shits1]) is effective at this synapse (e.g., Ramaswami et al., 1994). Finally, simultaneous photobleaching and imaging (SPAIM) can track individual presynaptic DCVs for minutes in the intact Drosophila NMJ (Wong et al., 2012). Although SPAIM was used to understand the supply of DCVs to en passant boutons by vesicle circulation and capture, we reasoned that this method could be used to detect release from individual presynaptic DCVs. Here experiments with mutant dynamin animals and SPAIM are performed to study neuropeptide release at the intact NMJ. Activity is found to induce dynamin-dependent partial emptying of DCVs. Kinetic and traffic studies are integrated to address how activity induces slow sustained synaptic peptide release.
RESULTS
Dynamin enhances synaptic peptide release
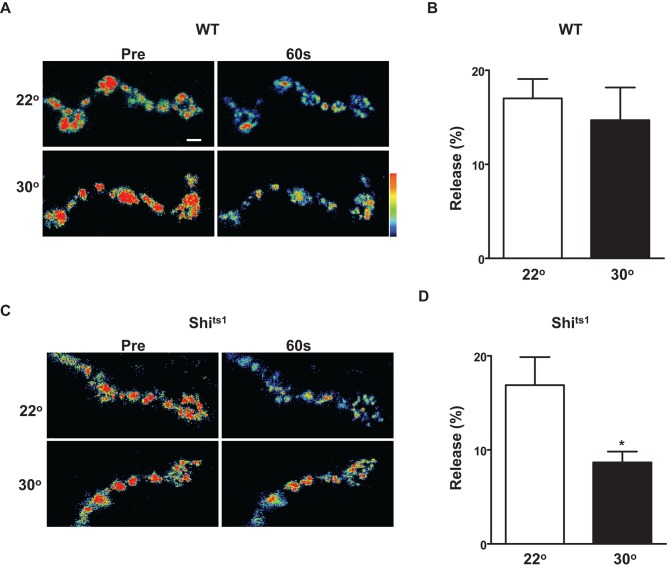
To test whether dynamin affects activity-dependent neuropeptide release at the Drosophila NMJ, we expressed GFP-tagged prepro–Drosophila insulin-like peptide 2 (Dilp2) in wild-type (WT) animals and the temperature-sensitive dynamin mutant shits1. Release at the larval neuromuscular junction was evoked by tetanic nerve stimulation (70 Hz, 1 min) at 22°C (i.e., a permissive temperature) or after heating to 30°C to acutely inactivate shits1. Imaging showed that the temperature change did not affect synaptic neuropeptide release in WT animals (Figure 1, A and B). However, temperature-dependent dynamin inhibition halved activity-evoked peptide release (Figure 1, C and D). Temperature-dependent dynamin inhibition does not affect presynaptic Ca2+ responses to activity (Macleod et al., 2004). Therefore, at a native synapse, dynamin enhances activity-evoked peptide release.
FIGURE 1:
Dynamin enhances activity-dependent synaptic neuropeptide release. (A) Pseudocolor images of Dilp2-GFP in WT motoneuron synaptic boutons before and after 60 s of activity at 22°C (top) or 30°C (bottom). Bar, 2 μm. (B) Quantification of activity-induced release by WT boutons at 22°C (N = 7) and 30°C (N = 5). Note absence of temperature effect. (C) Pseudocolor images of Dilp2-GFP in shits1 motoneuron synaptic boutons before and after 60 s of activity at 22°C (top) or 30°C (bottom). Bar, 2 μm. (D) Quantification of the effect of temperature on synaptic peptide release at shits1 NMJs (N = 10 for 22oC; N = 11 for 30oC). *p < 0.05.
Fractional release from synaptic DCVs
Dynamin dependence stimulated us to consider the hypothesis that neuropeptide release occurs by kiss-and-run exocytosis. Therefore we set out to determine whether release incompletely empties single presynaptic DCVs. However, because total internal reflection microscopy and oil immersion objectives are not amenable to imaging the intact NMJ in filleted larva, simultaneous photobleaching and imaging (SPAIM) on a confocal microscope (Wong et al., 2012) was used to image nerve terminal DCVs. Of importance, a single punctum in these experiments likely corresponds to a single DCV. First, previous analysis suggested that a single fluorescent punctum represents a single DCV detected by electron microscopy (Shakiryanova et al., 2006). Second, in SPAIM experiments, DCVs are tracked as they enter a large photobleached area, are transported rapidly into a bouton, and eventually become immobilized (i.e., docked) before being stimulated to undergo exocytosis. Throughout this extended tracking, puncta never split or merged, suggesting that they comprise one DCV. Indeed, to our knowledge, there is no evidence from electron microscopy or subresolution light microscopy (Scalettar et al., 2014) that demonstrates that neuronal DCVs travel or localize in clusters. Therefore SPAIM was used to analyze release from single DCVs.
In unstimulated preparations, Dilp2-GFP DCV fluorescence did not change (Figure 2Ai). Furthermore, although nerve stimulation (70 Hz, 60 s) did not affect all DCVs (Figure 2Aii), most DCVs released in response to activity (Figure 2Aiii). Indeed, for DCVs located near the bouton edge in the plane of focus, 20 of 22 DCVs responded. Therefore synaptic neuropeptide release is initiated reliably by activity, especially for membrane proximal DCVs. However, quantification of release in 33 DCVs showed that depletion was always incomplete (i.e., <50%; Figure 2B).
FIGURE 2:
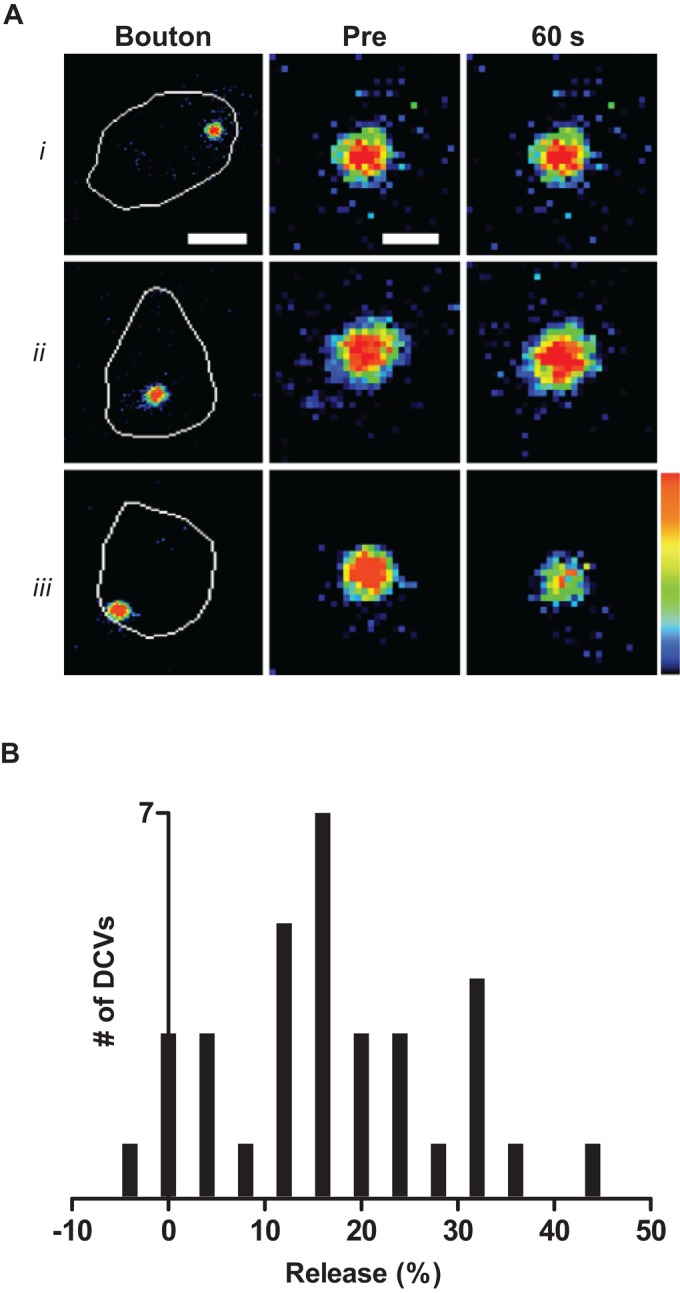
Activity evokes partial emptying of DCVs in the nerve terminal. (A) Pseudocolor images of single Dilp2-GFP DCVs in type Ib boutons, which are outlined in white in left images, before (Pre) and after 60 s. (i) DCV in an unstimulated terminal; (ii) unresponsive DCV localized in a bouton that was stimulated for 60 s; (iii) responsive DCV in a bouton that was stimulated for 60 s. Note the drop in signal for iii only. Bars, 1.75 μm (bouton images on the left), 0.5 μm (magnified DCV images). (B) Histogram quantifying the range of responses to stimulation in 33 DCVs. No DCV released more than half of its neuropeptide content.
Variable kinetics of synaptic peptide release by individual DCVs
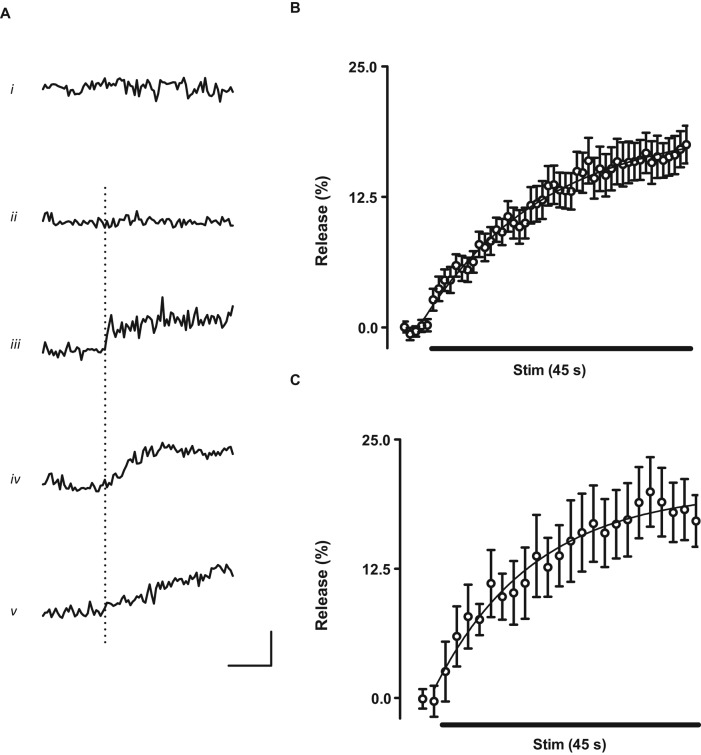
Further insights came from examining the kinetics of release from individual Dilp2-GFP DCVs. In unstimulated preparations, time courses were noisy but showed no significant change (Figure 3Ai). Time courses of some DCVs looked similar to unstimulated controls (Figure 3Aii) implying that they did not respond. However, most DCVs displayed release responses soon after initiation of activity (Figure 3, iii–v, dotted line shows start of tetani). However, responses had varying kinetics: in some cases, there were stepwise changes in content, implying rapid release (Figure 3Aiii), whereas others were slower stepwise (Figure 3Aiv) or gradual (Figure 3v) events. The latter events were irreversible, monotonic, and not correlated with movement, precluding an artifact due to increased DCV diffusion as occurs with mobilization (Shakiryanova et al., 2005). Furthermore, because Drosophila SSVs and DCVs are not very acidic (Sturman et al., 2006) and the emerald GFP variant used here is not sensitive to pH, these responses cannot be attributed to proton flux through the fusion pore. Rather, slow release is likely limited by permeation through the fusion pore or dispersion in the synaptic cleft. Of greatest importance, these data show that the kinetics of activity-evoked partial peptide release from single DCVs at a native synapse is surprisingly variable.
FIGURE 3:
Kinetics of neuropeptide release from single DCVs. (A) Time courses from single Dilp2-GFP DCVs. Horizontal bar, 20 s; vertical bar, 25%. Dotted line indicates start of 70-Hz stimulation. Release is plotted as a positive (upward) change. (i) Data from an unstimulated synapse; (ii–v) Data from stimulated synapses. (B) Ensemble-average time course from 33 DCVs. Curve is a single-exponential fit of data (τ = 21.5 s). (C) Average response from seven boutons. Curve is a single-exponential fit of data (τ = 17. 6 s). Horizontal bars in B and C indicate 45 s of 70-Hz stimulation.
Postfusion steps limit the rate of synaptic peptide release
To further analyze release kinetics, ensemble averages from single Dilp2-GFP DCV records were calculated. Of interest, the extent of release deduced from single DCVs (Figure 3B) was comparable (∼20%) to that obtained from macroscopic whole-bouton measurements (Figure 3C). Similarly, release kinetics was similar for ensemble-averaged single-DCV data (Figure 3B) and data from whole boutons (Figure 3C). Thus single DCVs revealed by SPAIM, which arrived within minutes, are representative of the typical behavior of the hundreds of DCVs present in type Ib boutons that have a half-life of many hours (Shakiryanova et al., 2006). This finding is surprising, given the less efficient release of older DCVs in endocrine cells (e.g., Duncan et al., 2003).
DCVs in cultured hippocampal neurons have a long latency for the onset of individual release events (τ = 16 s; Xia et al., 2009). If the onset of exocytosis was rate limiting for activity-dependent peptide release at the NMJ, then latencies in single Dilp2-GFP DCV records should have a mean similar to the time constant derived from an exponential fit of ensemble averaged records (21.5 s in Figure 3B). However, the latency of onset of unitary synaptic Dilp2-GFP events at the NMJ was much shorter (2.4 ± 0.5 s, N = 25). The dramatic difference in values derived from the latency in single DCV records and the time constant from ensemble averages and the individual DCV time courses (Figure 3A) shows that initiation of exocytosis is rapid compared with subsequent slow release.
Multiple rounds of release from single presynaptic DCVs
Resident DCVs in the Drosophila NMJ turn over slowly (t1/2 ∼ 6 h; Shakiryanova et al., 2006). Therefore maintaining peptidergic transmission would be problematic if each DCV could undergo exocytosis only once. In fact, no such limitation was detected at the intact NMJ. First, the effect of stimulating motor nerves twice with 10-s tetani was examined. Specifically, an ensemble average was generated from Dilp2-GFP DCVs that produced a stepwise response to the first bout of stimulation. Examination of the resultant time course showed that, when stimulated again 1 min later, a second response was elicited (Figure 4A). Indeed, two separate responses could be detected from a single DCV (Figure 4B). Second, single Dilp2-GFP DCVs subjected to prolonged tetanic stimulation (e.g., for 60 s) sometimes (5 of 33 DCVs) produced two responses (Figure 4C, i and ii). Such double responses are unlikely to have been produced by two independent, coincidentally colocalized DCVs because, as described earlier, a single punctum likely corresponds to a single DCV. Furthermore, the latency to first exocytosis is short (see earlier discussion) and never exceeded 9 s, but longer latencies were sometimes evident for the second event evoked by a prolonged tetanus (Figure 4C). This would not be expected for two independent DCVs but is consistent with repeated kiss-and-run release from a single DCV with loss of VAMP (synaptobrevin) and synaptotagmin (Tsuboi et al., 2004). In addition, the size of responses varied dramatically between DCVs but not among two responses in a single experiment (i.e., large events in Figure 4Ci, small events in Figure 4Cii), which is not expected for independent DCVs. Finally, there was no correlation between fluorescence intensity and the presence of two release events even though a pair of DCVs should have double the fluorescence of a single DCV. Therefore the simplest interpretation of the experimental data is that single DCVs can undergo two rounds of activity-evoked partial release.
FIGURE 4:
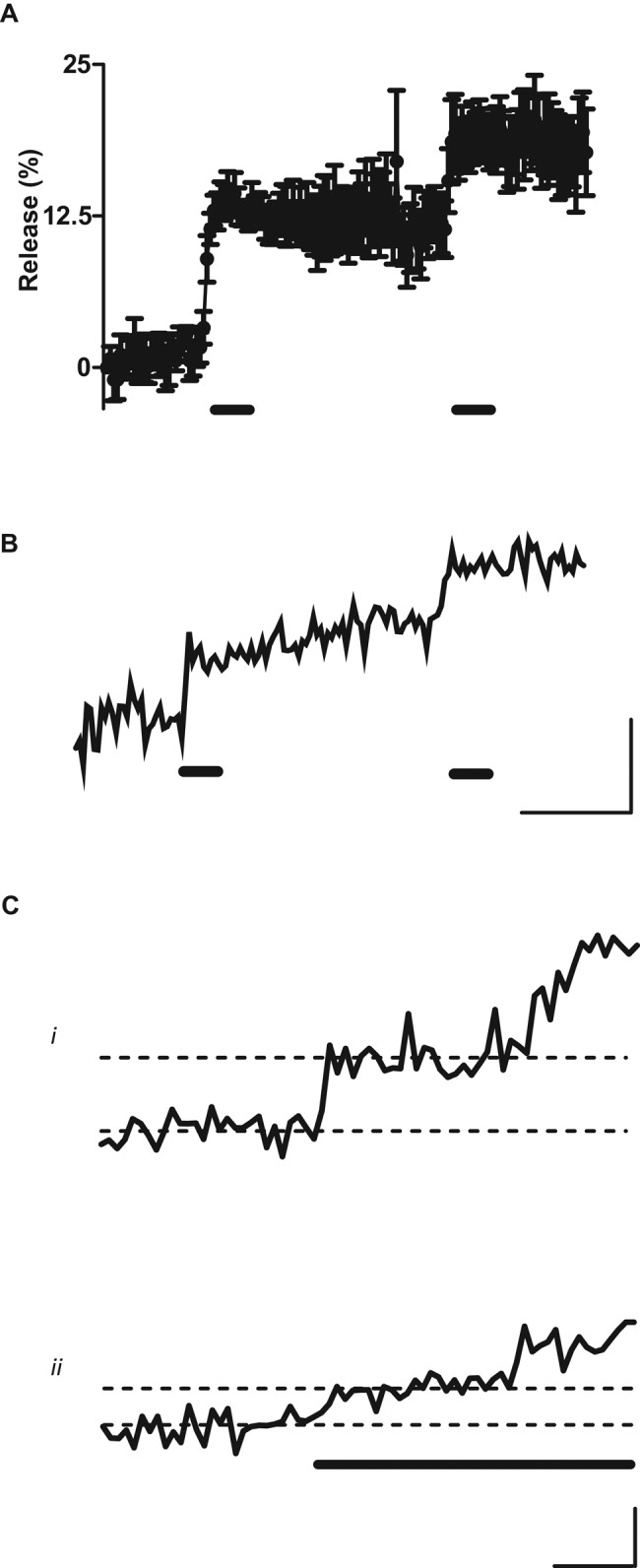
A single DCV can release twice. (A) Ensemble average from Dilp2-GFP DCVs stimulated twice for 10 s at 70 Hz (indicated by thick horizontal bars). Data are from six DCVs that displayed stepwise responses to the first tetanus. (B) Single-DCV time course with two stepwise responses to the double stimulation protocol in A. (C) Time courses of single Dilp2-GFP DCVs responding twice during a single prolonged tetanus (indicated by thick horizontal bar). Both responses were relatively large in i and small in ii. Horizontal dashed lines distinguish the baseline and signal after the first release event. Thin horizontal bars, 10 s; thin vertical bars, 10% release.
The capacity for multiple responses (Figure 4) and the range of single responses (Figure 3A) suggest that the three peaks in the histogram (Figure 2B) can be interpreted as representing unresponsive DCVs, DCVs that responded to activity once with ∼16% release, and DCVs that responded twice with ∼32% release (i.e., 2 × 16). In terms of synaptic function, multiple bouts of partial release by individual DCVs are significant because they further sustain activity-evoked release and provide a means for DCVs to function throughout their long lives in the nerve terminal. Finally, multiple release events are notable because they exclude a model in which DCVs completely empty by full-collapse fusion but peptide is retained at the membrane due to failure to disperse (i.e., binding); in such a model, a second response could not be initiated because peptide is already outside of the bouton after the first round of exocytosis. Therefore, although there may be some effect of slow or incomplete dispersion after release, multiple unitary responses demonstrate that release is partial and can occur repeatedly from a single presynaptic DCV to sustain peptidergic transmission.
Newly arrived DCVs are competent for activity-evoked synaptic release
On entering synaptic boutons, some DCVs are captured, whereas others visit the bouton transiently (i.e., they enter the bouton and then leave within 2 min; Wong et al., 2012). However, release from each of these subpopulations has not been measured. Therefore, to distinguish whether captured DCVs are optimized for release, atrial natriuretic factor (ANF)–GFP DCV records were sorted based on whether DCVs at the onset of stimulation had been in a bouton for at least 2 min (i.e., the threshold for capture) or <1 min. To simplify kinetic analysis and improve signal-to-noise ratio for these dimmer vesicles, we calculated ensemble time courses using data combined in 5-s periods (i.e., time binned) for the top 70% of responses (i.e., for ANF-GFP DCVs, >18% release identified responders). Furthermore, to take into account that not all DCVs fell into this class, we also quantified the fraction of responders (i.e., release probability). This quantification showed that release probability, kinetics, and extent were similar in visiting and captured DCVs (Figure 5, A and B). Therefore DCVs are competent for synaptic release soon after their arrival in synaptic boutons.
FIGURE 5:
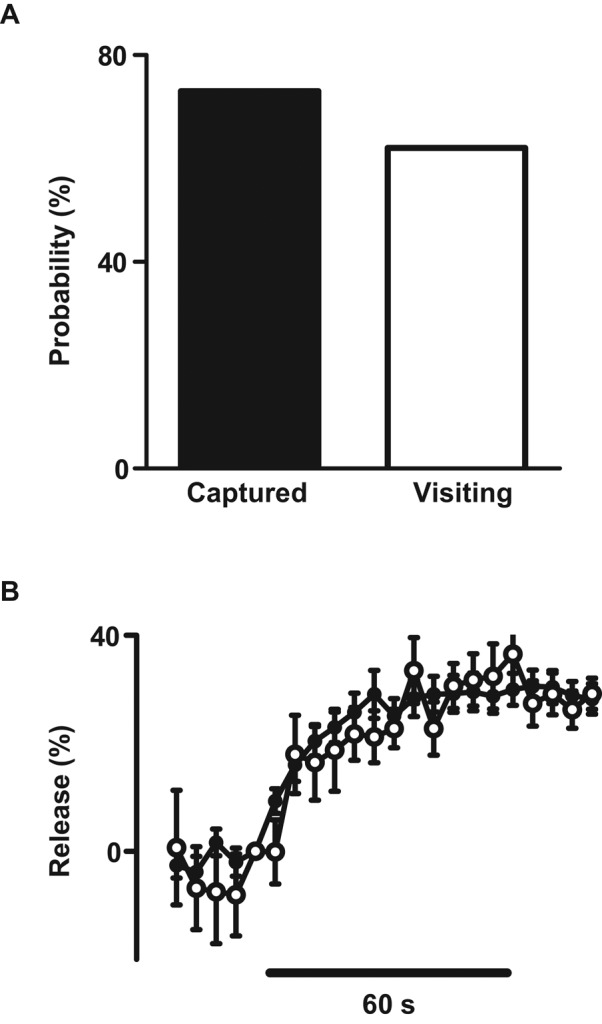
Comparable release from visiting and captured DCVs. At the onset of tetanic stimulation, visiting DCVs had been present in the bouton for <1 min, whereas captured DCVs had been present >2 min. (A) Release probability for 26 captured and 16 visiting ANF-GFP DCVs. (B) Time courses of release from 19 captured (filled circles) and 8 visiting (open circles) ANF-GFP DCVs.
Peptide release is consistent throughout the terminal
Synaptic release of glutamate packaged in SSVs is greatest at the most distal bouton, which is characterized by higher Ca2+ responses to single action potentials and tetani (Guerroro et al., 2005; Lnenicka et al., 2006). To determine whether location in the terminal affects synaptic neuropeptide release similarly, we sorted single anterograde ANF-GFP DCV records according to the bouton location at which capture occurred; the most distal bouton was assigned number 1, and proximal boutons were numbered in order (numbers 2–4). This analysis revealed no obvious difference in release probability among the most distal and proximal boutons (Figure 6A). Similarly, the time course and average release by responders were indistinguishable between proximal and most distal boutons (Figure 6B). Therefore, in contrast to glutamatergic transmission by SSVs at active zones, there is no detectable difference in activity-induced synaptic neuropeptide release based on location in the terminal.
FIGURE 6:
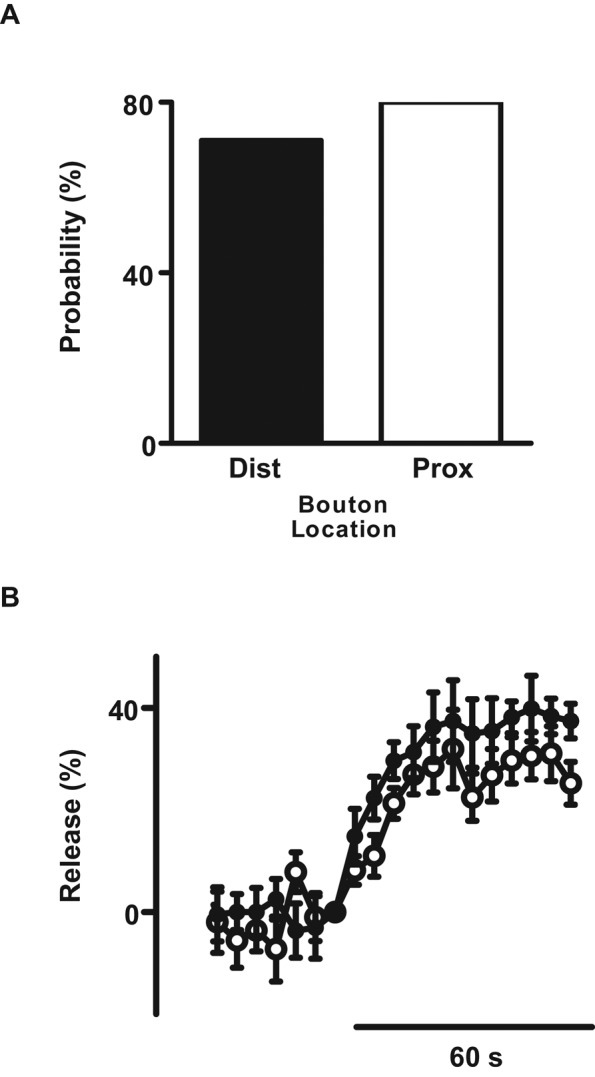
Single-DCV release is similar in proximal and the most distal boutons. (A) Single ANF-GFP DCV release probability in the most distal bouton (Dist, N = 7) and proximal boutons 2–4 (Prox, N = 10). (B) Ensemble-average time course from five distally (closed circles) and eight proximally (open circles) captured ANF-GFP DCVs. Data were time binned in 5-s intervals. All DCVs here arrived by anterograde transport.
DCVs supplied by anterograde and retrograde transport are competent for release
DCVs circulate bidirectionally through the motoneuron terminal (Shakiryanova et al., 2006; Wong et al., 2012). However, the release properties of anterograde and retrograde DCVs are unknown. Therefore SPAIM experiments were set up to examine whether the direction of entry into the bouton affects release. Ensemble averages from single ANF-GFP DCVs showed that release probability (Figure 7A) and time course and extent of release (Figure 7B) were unaffected by whether the DCVs entered the bouton by anterograde or retrograde transport. This functional equivalence implies that bidirectional capture of circulating DCVs effectively supplies en passant boutons with functionally competent DCVs for activity-induced synaptic neuropeptide release.
FIGURE 7:
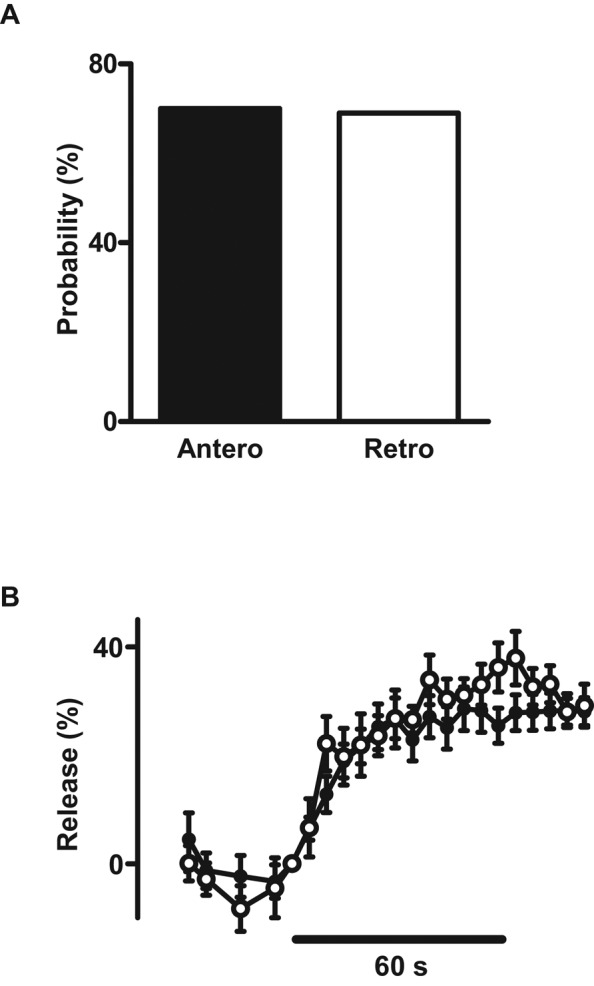
DCVs delivered by anterograde and retrograde transport are functionally equivalent. (A) Single ANF-GFP release probability for DCVs that entered boutons by anterograde (Antero, N = 23) or retrograde (retrograde, N = 16) transport. (B) Ensemble-average time course from 16 anterograde (filled circles) and 11 retrograde (open circles) ANF-GFP DCVs. Data were time binned in 5-s intervals.
DISCUSSION
Previous studies with cultured cells revealed great variation in single-DCV-mediated peptide release between experimental preparations. Even with the same peptide (ANF-GFP), extent and latency to initiation of release varied greatly according to cell type and neuronal compartment (Han et al., 1999; Barg et al., 2002; Duncan et al., 2003; Ng et al., 2003; Xia et al., 2009). However, past in vitro studies did not examine nerve terminals, the location of peptidergic synaptic transmission. Furthermore, results in culture may have been affected by the absence of the normal surroundings found in vivo that could influence release and subsequent dispersal of neuropeptides in the synaptic cleft. Finally, many prior studies used unphysiological sustained stimuli, such as bath-applied K+, nicotine, and long-lasting voltage clamp steps. Therefore, here, peptide release from single DCVs was induced by activity at the intact Drosophila NMJ. Imaging showed that synaptic neuropeptide release from single DCVs is dependent on dynamin and always incomplete, which are traits associated with kiss-and-run exocytosis (Wu et al., 2014). The limited depletion of DCVs is reminiscent of depolarization-evoked release from the hippocampal neuron soma (Xia et al., 2009). However, the latency to initiation of release was closer to that found with robust release from endocrine DCVs (Barg et al., 2002) than from cultured hippocampal neurons (Xia et al., 2009). Indeed, initiation of exocytosis is not rate limiting, as electrical activity reliably initiates synaptic peptide release and can induce by multiple rounds of partial emptying of DCVs. Thus synaptic release can be sustained without completely emptying DCVs, which bypasses the need to replace every used DCV by axonal transport from the distant soma. In this way, the apparent exclusive use of kiss-and-run exocytosis (and the corollary absence of full-collapse fusion) is well suited for peptidergic transmission.
Of interest, variable release kinetics from single DCVs had not been reported previously based on expression of GFP-tagged neuropeptides (e.g., neuropeptide Y and ANF). However, slow neuropeptide release can be produced by other peptides packaged in the same DCV (de Wit et al., 2009). Such an effect could reflect action from the DCV lumen to regulate fusion pore dilation (Weiss et al., 2014) or prevention of peptide dispersion from the matrix after release (de Wit et al., 2009). Alternatively, variability in release could occur with a single cargo if fusion pore dilation and peptide dispersion through the fusion pore or away from the presynaptic bouton are affected by the highly structured synaptic cleft, which features many synapse-spanning proteins. Regardless of its cause, the relatively slow release from presynaptic DCVs is, along with partial emptying and repeated exocytosis, well suited for peptidergic neurotransmission and release of neurotrophic factors and enzymes that influence synaptic development, maintenance, and remodeling.
Imaging single presynaptic DCVs was also used to determine the dependence of release on location in the nerve terminal. In contrast to SSV-mediated transmission in the same preparation (Guerrero et al., 2005), peptide release was not polarized (i.e., greater at the most distal bouton). This was surprising because the location dependence of glutamate release was attributed to differences in presynaptic Ca2+ (Guerroro et al., 2005; Lnenicka et al., 2006), which also triggers DCV exocytosis. However, a toxin that blocks Cav2 channels and SSV exocytosis does not affect neuropeptide release at the larval NMJ (James et al., 2014), suggesting that another Ca2+ channel mediates DCV exocytosis. Hence, total Ca2+ may not reflect the activity of channels that selectively mediate synaptic neuropeptide release, which occurs away from active zones (Zupanc, 2006). Of interest, lack of polarized release fits with the fact that released peptides can diffuse over large distances to produce neuromodulation.
This study explored whether single DCV release is affected by routing to or residence time in synaptic boutons. Traditionally, DCVs were believed to be delivered to the nerve terminal by anterograde axonal transport, with the time for transitioning between axonal transport and capability for release being unknown. However, SPAIM experiments recently revealed that DCVs are distributed among en passant boutons by being captured as they circulate back and forth between the proximal axon and the most distal bouton in the terminal (Wong et al., 2012). In the type Ib boutons studied here, capture is inefficient, so that DCVs often visit boutons only transiently. Indeed, it is the combination of routing by vesicle circulation and inefficient capture that ensures that DCVs are distributed evenly to en passant boutons (Wong et al., 2012; Kuznetsov and Kuznetsov, 2013; Bulgari et al., 2014). The effectiveness of this mechanism in supporting peptidergic transmission depends, however, on the maintenance of DCV functionality while circulating over long distances. However, the effect of vesicle circulation on DCV competence for release was unknown. Here this issue was addressed by showing that anterograde and retrograde DCVs have indistinguishable release properties. Thus vesicle circulation and bidirectional capture effectively provide functionally competent DCVs for activity-dependent synaptic peptide release.
Single-DCV experiments also suggest that newly arrived DCVs are as functionally competent as resident DCVs. First, the time course and extent of release deduced from ensemble averages deduced from single DCVs that had arrived within minutes after photobleaching were comparable to release measured by imaging whole boutons, which are dominated by DCVs that are hours old (Shakiryanova et al., 2006). Second, sorting of single-DCV records showed that release is similar for newly arrived DCVs (<1 min) and DCVs that were in boutons for >2 min, the threshold for capture (Wong et al., 2012). Therefore the transition from axonal transport to capability for exocytosis occurs rapidly upon entering the bouton. This finding supports the proposal that activity-dependent capture supplies DCVs to immediately support release (Shakiryanova et al., 2006; Frischknecht et al., 2008). In addition to maintaining release of constitutively expressed neuropeptides, the secretory competence of newly arrived DCVs also reduces the delay between synthesis of newly induced neuropeptides and their release. Such efficiency is desirable because many neuropeptide genes are subject to dynamic transcriptional control. Therefore the competence of recently arrived DCVs may enable peptidergic transmission to rapidly reflect plasticity in neuropeptide gene expression.
Despite this utility, the functional equivalence of recently arrived and resident DCVs was not expected, as newly synthesized DCVs dock and release more efficiently in endocrine cells (Duncan et al., 2003). This difference could reflect the increase in cytoplasmic DCV mobility induced by activity in nerve terminals (Shakiryanova et al., 2005, 2007), which may allow resident and newly arrived DCVs to dock to the membrane equivalently. In other words, when activity is intense, nerve terminal DCVs are not held in reserve by immobilization in the cytoplasm (Burke et al., 1997). In addition, it should be noted that because of vesicle circulation (Wong et al., 2012), newly arrived DCVs may have not been newly synthesized. Apparently, to allow axonal transport to terminals, which can take days in humans, the effect of time on neuronal DCVs is effectively suspended to prevent the age discrimination found in endocrine cells.
The shits1 dynamin mutant completely halts endocytosis and fast transmission by SSVs at active zones but was shown here to attenuate but not abolish synaptic neuropeptide release. This is of practical interest because selective expression of shits1 is used to identify neurons that affect behavior in Drosophila (Kitamoto, 2001). Our results show, however, that this mutant cannot distinguish whether a neuron influences behavior by virtue of its SSVs or DCVs. They also suggest that dynamin is not absolutely required for synaptic peptide release but instead plays a regulatory role for DCVs. In agreement with this proposal, preliminary single-DCV experiments failed to reveal a qualitative change in peptide release kinetics with the reduced release produced by inhibiting dynamin. Therefore more investigation will be required to fully discern the function of dynamin on synaptic DCVs (e.g., to test for effects on fusion pore dilation and docking). Nevertheless, the studies here establish shits1 as a tool for studying peptidergic transmission and demonstrate that dynamin facilitates synaptic DCV-mediated peptide release, which is mediated exclusively by kiss-and-run exocytosis.
MATERIALS AND METHODS
Stimulation of peptide release
Prior in vitro studies detected robust release of ANF-GFP from single DCVs in chromaffin cells, undifferentiated PC12 cells, and differentiated PC12 cell growth cones and more limited ANF-GFP release from cultured hippocampal neurons (Han et al., 1999; Barg et al., 2002; Duncan et al., 2003; Ng et al., 2003; Xia et al., 2009). However, the stimuli used in these studies are not amenable to imaging transgenically expressed ANF-GFP at the native Drosophila NMJ: bath-applied K+ directly evokes muscle contraction that interferes with presynaptic imaging, nicotine is not effective at this glutamatergic synapse, and type Ib synaptic boutons, which are surrounded by the subsynaptic reticulum of the postsynaptic muscle, have not been voltage clamped. Therefore, as described previously (Levitan et al., 2007), activity-evoked release at Drosophila third-instar larval type Ib boutons on muscle 6/7 was induced by 70-Hz electrical nerve stimulation in the presence of extracellular glutamate to desensitize postsynaptic receptors and inhibit muscle contraction.
Imaging single DCVs
Fluorescent puncta likely representing single DCVs (Shakiryanova et al., 2006) entering type Ib boutons were imaged at 1 Hz by SPAIM on an upright Olympus (Center Valley, PA) FluoView 1000 microscope with a dipping water immersion objective (60×, numerical aperture 1.1) as described previously (Wong et al., 2012). Because DCVs had to stay in view for >1 min in these experiments, studied DCVs tended to display little mobility. The axial resolution of these optics (i.e., the vertical thickness of an optical section) is comparable to the size of a bouton. Therefore it was not possible to ascertain whether a DCV was close to the bouton plasma membrane above or below the plane of focus. However, resolution in the plane of focus is much greater, making it possible to detect when DCVs were relatively close to the bouton edge that was evident before bleaching. Limitations in spatial resolution, however, do not affect measuring changes of fluorescence intensity, which are instead limited by the signal-to-noise ratio. Single DCVs in elav-GAL4 UAS-ANF-GFP (elav>ANF-GFP) larva (Rao et al., 2001) are dim. Therefore 1-Hz data from those animals were averaged in 5-s periods to improve signal-to-noise ratio. Furthermore, time courses included only DCVs that stayed within a bouton throughout the whole period shown.
To produce brighter DCVs with greater signal-to-noise ratio, a new chromosome 2 recombinant line was generated featuring a stronger motoneuron driver (OK6-GAL4) and a construct based on inserting GFP into Drosophila proinsulin-like peptide 2 (Wong et al., 2012), which is released natively by neurons. In the latter line (i.e., OK6>UAS-Dilp2-GFP/CyO), fluorescence from single DCVs in type Ib boutons is approximately double that seen in elav>ANF-GFP animals.
Because the emerald GFP variant used in both constructs is insensitive to pH, release was measured as the percentage loss in fluorescence (100 ΔF/F0, where ΔF = F0 − Ft). Accordingly, the loss of GFP fluorescence is quantified as an increase in release (i.e., a positive number). Peptide release was comparable in the two lines.
Dynamin mutant experiments
Drosophila expresses only one gene for dynamin, shibire (shi). Because the temperature-sensitive shits1 mutation is recessive and located on the X chromosome, the X chromosome recombinant elav>ANF-GFP line was not convenient for examining the effect of dynamin on peptide release. Therefore single crosses were performed with OK6>UAS-Dilp2-GFP/CyO males and virgin shits1 females at 18°C. GFP-expressing male larva (i.e., shits1/y; Ok6-GAL4 UAS-Dilp2-GFP/+) were used for confocal imaging of boutons, which was performed at 22°C or after heating to 30°C. Dynamin inhibition at the elevated temperature in these flies was verified by the block of depolarization-evoked FM4-64 (Molecular Probes, Eugene, OR) uptake into SSVs (Ramaswami et al., 1994).
Statistics
Data are presented as mean ± SEM. Pairwise statistics were calculated with the Student's t test.
Acknowledgments
We thank David Deitcher (Cornell University, Ithaca, NY) for experimental advice and Dinara Bulgari (University of Pittsburgh, Pittsburgh, PA) for comments on the manuscript. This research was supported by National Institutes of Health Grant R01 NS32385 to E.S.L.
Abbreviations used:
- ANF
atrial natriuretic factor
- DCV
dense-core vesicle
- Dilp2
Drosophila insulin-like peptide 2
- NMJ
neuromuscular junction
- shi
shibire
- SPAIM
simultaneous photobleaching and imaging.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E15-01-0002) on April 22, 2015.
*Present address: Department of Neurobiology, Harvard Medical School, Boston, MA 02115.
The authors declare no competing financial interests.
REFERENCES
- Anantharam A, Bittner MA, Aikman RL, Stuenkel EL, Schmid SL, Axelrod D, Holz RW. A new role for the dynamin GTPase in the regulation of fusion pore expansion. Mol Biol Cell. 2011;22:1907–1918. doi: 10.1091/mbc.E11-02-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Halpern ME, Keshishian H. Identification of the neuropeptide transmitter proctolin in Drosophila larvae: characterization of muscle fiber-specific neuromuscular endings. J Neurosci. 1988;8:242–255. doi: 10.1523/JNEUROSCI.08-01-00242.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg S, Olofsson CS, Schriever-Abeln J, Wendt A, Gebre-Medhin S, Renström E, Rorsman P. Delay between fusion pore opening and peptide release from large dense-core vesicles in neuroendocrine cells. Neuron. 2002;33:287–299. doi: 10.1016/s0896-6273(02)00563-9. [DOI] [PubMed] [Google Scholar]
- Bulgari D, Zhou C, Hewes RS, Deitcher DL, Levitan ES. Vesicle capture, not delivery, scales up neuropeptide storage in neuroendocrine terminals. Proc Natl Acad Sci USA. 2014;111:3597–3601. doi: 10.1073/pnas.1322170111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke NV, Han W, Li D, Takimoto K, Watkins SC, Levitan ES. Neuronal peptide release is limited by secretory granule mobility. Neuron. 1997;19:1095–1102. doi: 10.1016/s0896-6273(00)80400-6. [DOI] [PubMed] [Google Scholar]
- de Wit J, Toonen RF, Verhage M. Matrix-dependent local retention of secretory vesicle cargo in cortical neurons. J Neurosci. 2009;29:23–37. doi: 10.1523/JNEUROSCI.3931-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan RR, Greaves J, Wiegand UK, Matskevich I, Bodammer G, Apps DK, Shipston MJ, Chow RH. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Fejtova A, Viesti M, Stephan A, Sonderegger P. Activity-induced synaptic capture and exocytosis of the neuronal serine protease neurotrypsin. J Neurosci. 2008;28:1568–1579. doi: 10.1523/JNEUROSCI.3398-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Doreian B, Smith C. Dynamin I plays dual roles in the activity-dependent shift in exocytic mode in mouse adrenal chromaffin cells. Arch Biochem Biophys. 2008;477:146–154. doi: 10.1016/j.abb.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Jamett AM, Momboisse F, Guerra MJ, Ory S, Báez-Matus X, Barraza N, Calco V, Houy S, Couve E, Neely A, et al. Dynamin-2 regulates fusion pore expansion and quantal release through a mechanism that involves actin dynamics in neuroendocrine chromaffin cells. PLoS One. 2013;8:e70638. doi: 10.1371/journal.pone.0070638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ME, O'Callaghan DW, McMahon HT, Burgoyne RD. Dynamin-dependent and dynamin-independent processes contribute to the regulation of single vesicle release kinetics and quantal size. Proc Natl Acad Sci USA. 2002;99:7124–7129. doi: 10.1073/pnas.102645099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero G, Reiff DF, Agarwal G, Ball RW, Borst A, Goodman CS, Isacoff EY. Heterogeneity in synaptic transmission along a Drosophila larval motor axon. Nat Neurosci. 2005;8:1188–1196. doi: 10.1038/nn1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Ng YK, Axelrod D, Levitan ES. Neuropeptide release by efficient recruitment of diffusing cytoplasmic secretory vesicles. Proc Natl Acad Sci USA. 1999;96:14577–14582. doi: 10.1073/pnas.96.25.14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RE, Hoover KM, Bulgari D, McLaughlin CN, Wilson CG, Wharton KM, Levitan ES, Broihier HT. Crimpy enables discrimination of pre and postsynaptic pools of a BMP at the Drosophila NMJ. Dev Cell. 2014;31:1–13. doi: 10.1016/j.devcel.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Kuznetsov IA, Kuznetsov AV. A compartmental model of neuropeptide circulation and capture between the axon soma and nerve terminals. Int J Numer Method Biomed Eng. 2013;29:574–585. doi: 10.1002/cnm.2542. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Lanni F, Shakiryanova D. In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat Protocols. 2007;2:1117–1125. doi: 10.1038/nprot.2007.142. [DOI] [PubMed] [Google Scholar]
- Lnenicka GA, Grizzaffi J, Lee B, Rumpal N. Ca2+ dynamics along identified synaptic terminals in Drosophila larvae. J Neurosci. 2006;26:12283–12293. doi: 10.1523/JNEUROSCI.2665-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner JE, Honigman LS, Grant WF, Gessford SK, Hansen AB, Silverman MA, Scalettar BA. Activity-dependent release of tissue plasminogen activator from the dendritic spines of hippocampal neurons revealed by live-cell imaging. J Neurobiol. 2006;66:564–577. doi: 10.1002/neu.20250. [DOI] [PubMed] [Google Scholar]
- Macleod GT, Marin L, Charlton MP, Atwood HL. Synaptic vesicles: test for a role in presynaptic calcium regulation. J Neurosci. 2004;24:2496–2505. doi: 10.1523/JNEUROSCI.5372-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min L, Leung YM, Tomas A, Watson RT, Gaisano HY, Halban PA, Pessin JE, Hou JC. Dynamin is functionally coupled to insulin granule exocytosis. J Biol Chem. 2007;282:33530–33536. doi: 10.1074/jbc.M703402200. [DOI] [PubMed] [Google Scholar]
- Ng YK, Lu X, Gulacsi A, Han W, Saxton MJ, Levitan ES. Unexpected mobility variation among individual secretory vesicles produces an apparent refractory neuropeptide pool. Biophys J. 2003;84:4127–4134. doi: 10.1016/S0006-3495(03)75137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Rao S, Lang C, Levitan ES, Deitcher DL. Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J Neurobiol. 2001;49:159–172. doi: 10.1002/neu.1072. [DOI] [PubMed] [Google Scholar]
- Scalettar BA, Shaver D, Kaech S, Lochner JE. Super-resolution imaging of neuronal dense-core vesicles. J Vis Exp. 2014;2014:e51394. doi: 10.3791/51394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Klose MK, Zhou Y, Gu T, Deitcher DL, Atwood HL, Hewes RS, Levitan ES. Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J Neurosci. 2007;27:7799–7806. doi: 10.1523/JNEUROSCI.1879-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Hewes RS, Deitcher DL, Levitan ES. Activity-dependent liberation of synaptic neuropeptide vesicles. Nat Neurosci. 2005;8:173–178. doi: 10.1038/nn1377. [DOI] [PubMed] [Google Scholar]
- Shakiryanova D, Tully A, Levitan ES. Activity-dependent synaptic capture of transiting peptidergic vesicles. Nat Neurosci. 2006;9:896–900. doi: 10.1038/nn1719. [DOI] [PubMed] [Google Scholar]
- Sturman DA, Shakiryanova D, Hewes RS, Deitcher DL, Levitan ES. Nearly neutral secretory vesicles in Drosophila nerve terminals. Biophys J. 2006;90:L45–L47. doi: 10.1529/biophysj.106.080978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillon R, Ewing AG. Amperometric measurements at cells support a role for dynamin in the dilation of the fusion pore during exocytosis. Chemphyschem. 2013;14:2295–2301. doi: 10.1002/cphc.201300319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, McMahon HT, Rutter GA. Mechanisms of dense core vesicle recapture following “kiss and run” (“cavicapture”) exocytosis in insulin-secreting cells. J Biol Chem. 2004;279:47115–47124. doi: 10.1074/jbc.M408179200. [DOI] [PubMed] [Google Scholar]
- Weiss AN, Anantharam A, Bittner MA, Axelrod D, Holz RW. Lumenal protein within secretory granules affects fusion pore expansion. Biophys J. 2014;107:26–33. doi: 10.1016/j.bpj.2014.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MY, Zhou C, Shakiryanova D, Lloyd TE, Deitcher DL, Levitan ES. Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell. 2012;148:1029–1038. doi: 10.1016/j.cell.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Hamid E, Shin W, Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014;76:301–331. doi: 10.1146/annurev-physiol-021113-170305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Lessmann V, Martin TF. Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J Cell Sci. 2009;122:75–82. doi: 10.1242/jcs.034603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupanc GK. Peptidergic transmission: from morphological correlates to functional implications. Micron. 2006;27:35–91. doi: 10.1016/0968-4328(95)00028-3. [DOI] [PubMed] [Google Scholar]