Abstract
Retinoid X receptor-α (RXRα), a unique member of the nuclear receptor superfamily, represents an intriguing and unusual target for pharmacologic interventions and therapeutic applications in cancer, metabolic disorders and neurodegenerative diseases. Despite the fact that the RXR-based drug Targretin (bexarotene) is currently used for treating human cutaneous T-cell lymphoma and the fact that RXRα ligands (rexinoids) show beneficial effects in the treatment of cancer and diseases, the therapeutic potential of RXRα remains unexplored. In addition to its conventional transcription regulation activity in the nucleus, RXRα can act in the cytoplasm to modulate important biological processes, such as mitochondria-dependent apoptosis, inflammation, and phosphatidylinositol 3-kinase (PI3K)/AKT-mediated cell survival. Recently, new small-molecule-binding sites on the surface of RXRα have been identified, which mediate the regulation of the nongenomic actions of RXRα by a class of small molecules derived from the nonsteroidal anti-inflammatory drug (NSAID) Sulindac. This review discusses the emerging roles of the nongenomic actions of RXRα in the RXRα signaling network, and their possible implications in cancer, metabolic and neurodegenerative disorders, as well as our current understanding of RXRα regulation by targeting alternate binding sites on its surface.
Keywords: RXRα, rexinoid, RXRα modulator, nongenomic action, coregulator site, apoptosis, inflammation, PI3K, NSAID
Introduction
Retinoid X receptor-alpha (RXRα) belongs to a unique RXR subfamily of the nuclear receptor superfamily, which is encoded by 3 distinct genes: RXRα, RXRβ, and RXRγ1,2,3,4,5,6,7,8,9. RXRs, like other nuclear receptors, consist of 3 distinct domains: a disordered N-terminal A/B region, a DNA-binding domain, and a C-terminal ligand-binding domain (LBD). The LBD possesses a canonical ligand-binding pocket (LBP), a transactivation function domain 2, a coregulator-binding surface groove, and a dimerization surface (Figure 1A). RXRs were initially identified as heterodimeric partners of the retinoic acid receptor (RAR), thyroid hormone receptor (T3R) and vitamin D receptor (VDR). Today, about one-third of the 48 human nuclear receptor superfamily members serve as RXR heterodimerization partners, including Nur77, peroxisome proliferator-activated receptors (PPARs), liver X receptor (LXR), and farnesoid X receptor (FXR)1,2,3,4,6,7. In addition, RXRα can form homodimers10 and homotetramers11,12,13 (Figure 1B), suggesting that RXRα may control its own specific signaling pathways. Binding of RXRα by a ligand regulates the ability of the receptor to dimerize and alters the receptor's cofactor-binding surface due to the rearrangement of helices 10, 11, and 12 (Figure 1C). Aside from its role in DNA binding and transactivation, accumulating evidence indicates that RXRα also has extranuclear functions14,15,16,17,18. RXRα resides in the cytoplasm at different stages of development19. It migrates from the nucleus to the cytoplasm in response to differentiation16, survival20,21, apoptosis14, and inflammation17,18,20,21. 9-cis-retinoic acid (RA) was originally identified as a natural RXRα ligand. Subsequently, several dietary fatty acids were found to bind RXRα and to act as natural RXRα ligands (Figure 2). These include docosahexaenoic acid (DHA), oleic acid, and phytanic acid. However, none of these molecules has been proved to be the bona fide endogenous ligand of RXRα22,23. Numerous natural products and synthetic compounds (rexinoids) have been shown to bind to RXRα and to modulate its activities2,3,4,24,25,26. Thus, the heterodimerization capacity of RXRα together with the diversity of its ligands suggests that RXRα is an important regulator of a wide range of cellular pathways.
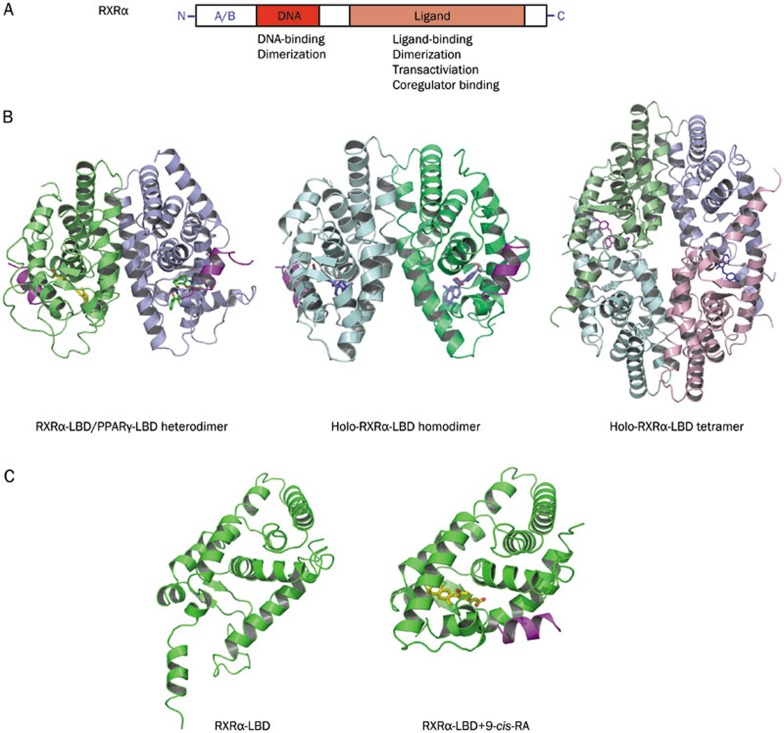
Figure 1.
RXRα structure, homo- and hetero-dimerization, and effect of ligand. (A). Schematic representation of RXRα. (B). Structures of RXRα heterodimer, homodimer, and tetramer. Left, RXRα-LBD/PPARγ heterodimer, PDB code 1FM9. Middle, holo-RXRα-LBD homodimer, PDB code 1MZN. Right, holo-RXRα-LBD tetramer, PDB code 4N8R. (C). Structure of apo-RXRα and holo-RXRα. Left, monomer conformation in the apo-tetramer structure, PDB code 1G1U. Right, RXRα-LBD in complex with agonist CD3254 and coactivator GRIP1, PDB code 3FUG.
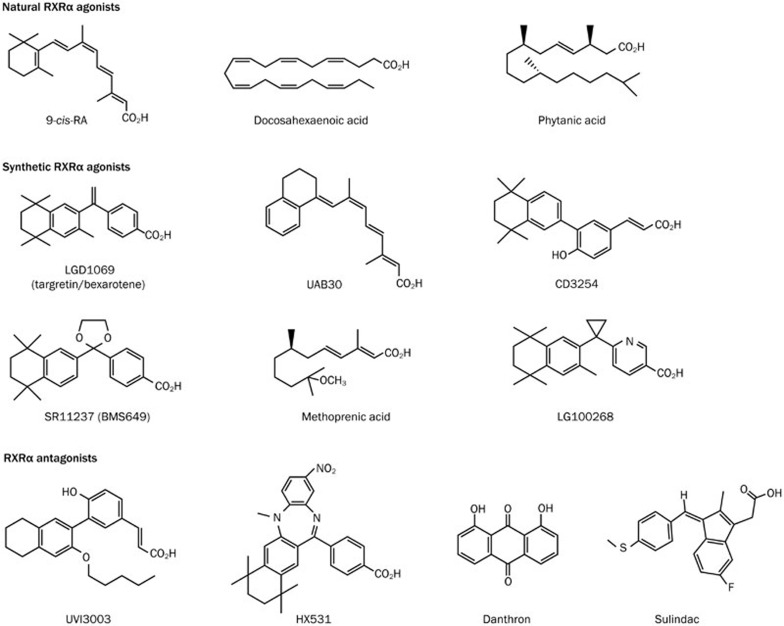
Figure 2.
RXRα ligands.
Genetic analysis demonstrated that RXRα is involved in a plethora of developmental and physiological pathways. A knockout of RXRα was embryonic lethal27. Tissue-specific inactivation of RXRα in hepatocytes28, skin29, prostate30, or adipose tissue31 induces strong phenotypes, indicating a major role of RXRα in these tissues. The phenotypes observed in most RXRα-mutant mice may be related to alterations in pathways regulated by its heterodimerization partners. Structurally, RXRα homodimerization and heterodimerization can be separated by specific amino acid residues at the dimerization interfaces32,33. Ligand-activated RXRα homodimers up-regulate p21 expression through the direct binding of RXRα homodimers to the p21 promoter34. Characterization of mice lacking RXRα in myeloid cells reveals an important role of RXRα homodimers in the innate immune response to inflammatory stimuli35. Rexinoids function as insulin sensitizers and can decrease hyperglycemia and hypertriglyceridemia through an RXRα homodimer-mediated mechanism that is distinct from the one utilized by PPARγ in different mouse models35. Consistent with this, a homodimer-specific RXRα agonist effectively lowers blood glucose in an animal model of insulin-resistant diabetes36. Mechanistically, RXRα homodimers can selectively bind to functional PPAR response elements and induce transactivation in vivo37. These observations underscore the importance of a very intricate RXRα signaling pathway for developing potential therapeutic uses of RXRα-specific modulators.
Altered expression and changes in the function of RXRα have been implicated in the development of a number of cancers and diseases. Although an RXRα knockout fetus dies in the embryonic stage27, targeted disruption of the RXRα gene leads to preneoplastic lesions in the prostate30, alopecia, epidermal interfollicular hyperplasia, keratinocyte hyperproliferation and aberrant terminal differentiation in the skin29, the development of malignant cervical lesions38, alteration of fatty acid oxidation and hepatocyte lifespan in the liver28, and resistance to diet-induced obesity due to impaired adipocyte differentiation in adipose tissue31. Diminished RXRα expression is also associated with the development of certain malignancies, which is largely attributed to proteolytic cleavage of RXRα in tumor cells15,39,40,41,42,43. In addition, alteration of RXRα function by phosphorylation is associated with the development of human cancer44. Intriguingly, several studies have demonstrated that alteration of the subcellular localization of RXRα is implicated in the development of cancer and certain diseases. RXRα is translocated from the nucleus to the cytoplasm in response to endotoxin and other inflammatory mediators to inhibit its transactivation function17,45, while an altered localization of RXRα to the splicing factor compartments occurs in highly malignant human breast cancer cells46. We recently reported that an N-terminally truncated form of RXRα (tRXRα) produced in cancer cells resides in the cytoplasm to promote the growth of tumor cells21. A recent finding that RXRα binding to PML/RARα is required for the development of acute promyelocytic leukemia in transgenic mice47,48 further demonstrates the oncogenic potential of this protein when it functions inappropriately.
The pleiotropic action of RXRα under both physiological and pathophysiological conditions suggests that RXRα is an important target for pharmacologic interventions and therapeutic applications. This is highlighted by the FDA approval of the RXR-based drug Targretin (bexarotene) for treating T-cell lymphoma and its beneficial effects against other indications such as metabolic syndromes and neurodegenerative diseases. Targretin was found to induce a 50% overall inhibitory response in patients with refractory or persistent cutaneous T-cell lymphoma when administered either orally or topically49. The therapeutic use of Targretin has been extended to other cancer types, including breast cancer and lung cancer2,3,4,6. Although a phase III clinical trial of Targretin for non-small cell lung carcinoma did not meet the end points, a subgroup of patients was shown to benefit from Targretin treatment50,51. Numerous studies have also reported the broad impact of rexinoids on metabolic regulation. Rexinoids improve insulin sensitivity, which is similar to the effect of thiazolidinedione (TZD), a PPARγ ligand, and this is likely due to its activation of RXRα/PPARγ as well as a separate RXR signaling pathway. Rexinoids also provoke a very efficient inhibition of cholesterol absorption, and show beneficial effects on the development of atherosclerosis52. Recently, Cramer et al53 reported that Targretin enhances apoE-dependent β-amyloid (Aβ) clearance from the brain and improves neural network function and reversal of behavioral deficits in mouse models of Alzheimer disease. This is exciting because there is currently no cure for Alzheimer disease. The effect of reducing soluble Aβ levels has been confirmed by several studies, although the reduction of Aβ plaques by Targretin remains controversial. Targretin also acts to prevent loss of dopaminergic neurons and restore behavioral function in rodent models of Parkinson's disease54, and it relieved positive symptoms of schizophrenia in a randomized, double-blind, placebo-controlled multicenter trial55. Thus, RXRα-selective modulators are a class of very promising drug candidates for cancer, metabolic syndromes, and neurodegenerative disorders.
The promiscuous nature of RXRα has conferred rexinoids some unwanted side effects2,3,4,6, which has hindered their further development. Thus, there is an urgent need to dissect RXRα signaling pathways and to identify and develop new RXRα modulators that have unique properties and improved therapeutic indexes. In this review, we focus on the nongenomic activity of RXRα and highlight recent advances in this field with an emphasis on tRXRα actions21 and RXRα modulation by targeting alternate binding sites on its surface56,57.
Nongenomic activity of RXR and apoptosis
Apoptosis, programmed cell death, plays a central role both in development and in homeostasis, eliminating redundant cells and ensuring that cells that have migrated to their proper destinations survive58. Abnormal regulation of apoptosis, as a result of either genetic anomalies and/or a persistent disease state, contributes to the establishment and progression of a number of human cancers and diseases, such as autoimmune and neurological disorders, inflammatory diseases, obesity, type 2 diabetes, and atherosclerosis. Apoptosis occurs following either the triggering of cell surface death receptors (the extrinsic pathway) or the perturbation of mitochondria (the intrinsic pathway)58. The intrinsic pathway is initiated by the release of apoptogenic factors such as cytochrome c from mitochondria, while the extrinsic pathway involves the activation of the initiator caspase-8 through stimulation of death receptors of the tumor necrosis factor (TNF) receptor superfamily.
The role of RXRα and RXRα ligands in apoptosis was initially recognized by the finding that 9-cis-RA is a potent negative regulator of activation-induced T-cell apoptosis through its binding of both RXR and RAR59. Subsequent studies demonstrated that rexinoids could either induce or promote apoptosis depending on the nature of the ligands and/or the cellular environment. 9-cis-RA inhibits activation-induced apoptosis in T-cell hybridomas and thymocytes by blocking the expression of Fas ligand following activation. RXRα has a protective role in cellular apoptosis of keratinocytes and melanocytes60, and RXRα antagonist HX531 inhibits the apoptotic effect of 4-para-Nonylphenol in mouse embryonic neuronal cells through an RXR-mediated mitochondria-dependent signaling pathway61. Activation of RXRα induces apoptosis in NB4 acute promyelocytic leukemia cells3. Insulin-like growth factor binding protein (IGFBP)-3 binding to RXRα results in apoptosis of cancer cells62. DHA induces apoptosis of colonocytes63 in an RXRα-dependent manner, while it promotes the survival of rat retina photoreceptors through RXRα-dependent activation of the mitogen-activated protein kinase (MAPK) signaling pathway64. Targretin suppresses the progression of colonic adenomas to adenocarcinomas in animals, which is accompanied by the induction of apoptosis65, while R-etodolac binds to RXRα and induces RXRα-dependent apoptosis of prostate cancer cells in vitro and in animals66. Together, the ability of rexinoids to positively or negatively regulate apoptosis likely contributes to their therapeutic effects in cancer, metabolic disorders, and neurodegenerative diseases.
One way that RXRα and its ligands regulate apoptosis is through their regulation of the Nur77-Bcl-2 apoptotic pathway through RXRα heterodimerization with Nur7767 (Figure 3). In response to several apoptotic stimuli, Nur77 migrates from the nucleus to the cytoplasm, where it targets mitochondria by interacting with Bcl-268, leading to cytochrome c release and apoptosis. Nur77 mitochondrial targeting occurs not only in cancer cells, but also in other cell types such as CD4(+)CD8(+) thymocytes69, cardiomyocytes70, and cerebellar granule neurons71. RXRβ can cotranslocate with Nur77 from the nucleus to the cytoplasm as a heterodimer in PC12 cells in response to nerve growth factor (NGF) treatment16. We reported that RXRα serves as an active partner in shuttling Nur77 from the nucleus to mitochondria in cancer cells14. The shuttling of the Nur77/RXRα heterodimers between the nucleus and the cytoplasm is subject to regulation by RXRα ligands. 9-cis-RA suppresses apoptosis by inhibiting Nur77/RXR mitochondrial targeting14. Such regulation of Nur77 activity by 9-cis-RA may account for its inhibitory effect on apoptosis. 9-cis-RA is known to potently inhibit the activation-induced apoptosis of T cells and thymocytes72, in which Nur77 plays a role. It is worth noting that 9-cis-RA is able to induce RXR-mediated nucleo-cytoplasmic shuttling of Nur77 and its translocation to mitochondria for apoptosis73 and it can relieve the inhibitory effect of RXRα on EGF-induced Nur77 nuclear accumulation74, suggesting that certain RXRα ligands may act to promote RXR nuclear export and apoptosis under certain cellular conditions.
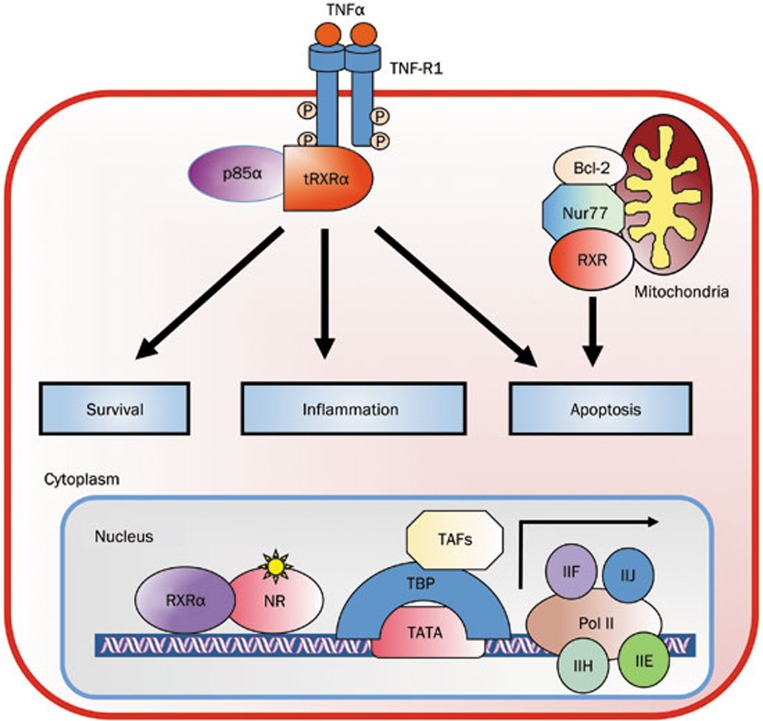
Figure 3.
The nongenomic RXRα actions. The cytoplasmic tRXRα through its interaction with p85α subunit of PI3K or undefined factors associated with TNF-R1 regulates cell survival, inflammation, and apoptosis. In addition, RXRα can target mitochondria through heterodimerization with Nur77 to modulate mitochondria-dependent apoptosis.
RXRα and ligands can also modulate the extrinsic apoptotic pathway. TNFα is a multifunctional cytokine that controls diverse cellular events such as cell survival and death that control the destiny of cancer cells75. The death effect of TNFα is mediated by the recruitment of TNF receptor-associated death domain (TRADD) and Fas-associated death domain (FADD) to TNFα receptor TNF-R1, which then recruits caspase-8, a key initiator of apoptosis. Although less characterized, TNF-R1 also recruits phosphatidylinositol 3-kinase (PI3K) to activate the PI3K/AKT survival pathway76. We found that tRXRα could bind to the p85α regulatory subunit of PI3K in response to TNFα treatment, leading to activation of the PI3K/AKT pathway21. This finding implies that rexinoids could act nongenomically to modulate the TNFα-dependent extrinsic apoptotic pathway. Indeed, inhibition of tRXRα binding to p85α by Sulindac (also called CLINORIL®), a nonsteroidal anti-inflammatory drug (NSAID) currently used for treating pain and inflammation, and analogs, results in caspase-8-dependent apoptosis21. Several natural products, including CF31, can activate this death pathway through direct binding to tRXRα20. Although the apoptotic effect of Sulindac and analogs can be attributed to their inhibition of tRXRα-dependent activation of PI3K/AKT, it remains to be seen whether tRXRα is directly involved in the formation of the TNF-R1-TRADD-FADD apoptosome to modulate the extrinsic apoptotic pathway.
Nongenomic action of RXRα and inflammation
Like other nuclear receptors, RXRα and its ligands regulate diverse aspects of immunity and inflammation. The Karpen laboratory showed that inflammatory mediators decrease the nuclear levels of RXRα and its transactivation in a c-Jun N-terminal kinase (JNK)-dependent manner17, suggesting a role for RXRα and its ligands in inflammation. Accumulating evidence has now revealed their active role in the modulation of inflammatory responses and immunity. Acute challenge with AOM/DSS induces colitis in RXRα heterozygous mice with increased inflammatory maker expression77, and RXRα is highly expressed in macrophages7. Consistent with this, certain anti-inflammatory agents serve as RXRα ligands, implying that RXRα may be an intracellular target that mediates the anti-inflammatory effects of these agents. DHA induces growth inhibition and apoptosis by inhibiting NF-κB activity78 and suppressing cytokine production in macrophages79. The NSAID R-etodolac, which induces RXRα-dependent apoptosis of tumor cells66, decreases constitutive and RANKL-stimulated NF-κB activation in macrophages and suppresses TNFα-induced IKK phosphorylation and subsequent NF-κB activation80. The role of RXRα is further implicated by numerous studies showing that rexinoids are critical regulators of various inflammatory pathways in different cell types, including T cells81, macrophage82, dendritic cells83, and microglia and astrocytes84. Targretin downregulates COX-2 expression in breast cancer cells85, inhibits angiogenesis and metastasis in solid tumors86, reduces the expression of TNFα and IL-1β protein in Apc(Min/+) mice65, and suppresses inflammation in patients with plaque-type psoriasis87. Rexinoids are also protective against colon inflammation that is induced by 2,4,6-trinitrobenzene sulfonic acid88. RXRα antagonists are capable of altering the maturation process from human monocytes to dendritic cells in response to TNFα or lipopolysaccharide (LPS)83, and block T Helper 2 cell differentiation and IL-5 production in T cells89. Thus, the diverse anti-inflammatory effects of RXRα and its ligands in various cell types underscore their function in the prevention and treatment of inflammatory and metabolic disorders, such as cancer, atherosclerosis, insulin resistance, autoimmunity, and neurodegeneration.
The mechanisms by which RXRα and its ligands modulate inflammation and immunity remain an important unanswered question that is currently being actively investigated. Both genomic and nongenomic actions of RXRα could account for its modulation of inflammation in macrophages and other cell types. For genomic action, the most potent anti-inflammatory effects of RXRα appear to result from protein–protein interactions between RXRα and pro-inflammatory transcription factors, particularly NF-κB and AP-1, through the trans-repression mechanism, which has been reviewed elsewhere90. The nongenomic mechanisms of RXRα may involve inhibition of the activation/phosphorylation of JNK and subsequent phosphorylation of c-Jun91. Interestingly, the subcellular localization of RXRα is altered in response to inflammation17,45. LPS alters the subcellular location of RXRα in animals17, while RXRα undergoes rapid nuclear export in response to IL-1β in hepatoma cells18. The effect is rapid, occurring within 30 min of exposure to IL-1β, and is likely due to RXRα phosphorylation by JNK and through a CRM-1-mediated nuclear export process18. IL-1β, IL-6, and TNFα also alter the intracellular distribution of RXR in Schwann cells, which occurs when cells are exposed to cytokine for as little time as 5 minutes45.
Our recent discovery that TNF can induce cytoplasmic localization of tRXRα underscores the significance of tRXRα cytoplasmic action in the regulation of inflammation (Figure 3). As discussed above, TNFα is a cytokine that induces not only the extrinsic apoptotic and PI3K/AKT pathways but also the NF-κB and AP-1 inflammatory pathways. In this regard, TNFα receptor-1 (TNF-R1) recruits TNF receptor-associated factor 2 (TRAF-2) and receptor-interacting protein (RIP) kinase, which results in the initiation of pathways that culminate in the activation of transcription factors NF-κB, c-Jun, c-Fos, and ATF-2 via the activation of various kinases including IκB kinase (IKK) and MAPKs. These pathways control the inducible expression of genes important for inflammation. It remains to be seen whether tRXRα or other forms of RXRα are directly involved in the activation and regulation of the inflammasome. Intriguingly, TNFα induction of tRXRα-dependent responses is inhibited by Sulindac, which is currently used for treating inflammation, implying that the drug may exert its anti-inflammatory effects by targeting tRXRα pathways.
Nongenomic action of RXR and the PI3K/AKT survival pathway
The role of PI3K/AKT activation in oncogenesis and drug resistance has been validated by multiple studies, demonstrating that aberrations in this pathway are potential causes of cell transformation, metabolic disorders, and neurodegenerative diseases, as well as drug resistance92. The pathway has therefore been targeted extensively for the development of therapeutics against cancer and related diseases, and for overcoming drug resistance. However, current targeting strategies that rely on direct inhibition of PI3K/AKT activities have caused profound adverse events and have thus far been confined to preclinical and clinical evaluation due to toxicity and lack of selectivity. Thus, identification of key molecules involved in the aberrant activation of PI3K/AKT pathway will offer new strategies for drug development.
We recently reported that tRXRα, but not the full-length RXRα, could act to mediate TNFα activation of PI3K/AKT in a number of cancer cell lines21 (Figure 3). The tRXRα protein is detected in a variety of cancer cell lines and in primary tumors, but not in tissues surrounding the tumor or in distant normal tissues from the same cancer patients21, suggesting its oncogenic potential. Our finding that tRXRα, but not RXRα, acts nongenomically to interact with p85α indicates that tRXRα acquires a new function that is different from that of the full-length RXRα protein. Such activation or conversion of a protein's phenotype by limited proteolytic cleavage is not without precedent. Limited proteolytic processing of RXRα occurs in many types of cancer cells15,39,40,41,42,43, suggesting that it may represent an important mechanism that regulates the biological activity of RXRα. Regulated proteolysis is a key step in a number of different signaling pathways that respond to developmental cues or external stimuli. Caspase-mediated cleavage of the BH3-only protein Bid into a truncated protein (tBid) and subsequent translocation of tBid to mitochondria is implicated in death receptor signaling93, whereas proteolytic processing of Notch and nuclear translocation of the truncated product is a crucial step in transduction of Notch signaling94. Cleavage of the androgen receptor by calpain produces a truncated receptor protein that may play a role in the development of androgen-independent prostate cancer95. An intriguing question regarding tRXRα-mediated activation of PI3K/AKT relates to the proteases responsible for RXRα cleavage. Our recent study96 identified calpain II as one of the proteases that can cleave RXRα protein in vitro and in vivo. Activation of calpain II by ionomycin enhances the production of tRXRα in cancer cells, which is regulated in a glycogen synthase kinase 3 beta (GSK-3β)-dependent manner96. However, proteases other than calpain II are likely involved in the cleavage of RXRα and remain to be identified.
Many more important questions remain regarding the nongenomic regulation of the PI3K/AKT pathway by RXRα and its ligands. Unlike the full-length RXRα that resides in the nucleus, tRXRα is cytoplasmic and interacts with the p85α subunit of PI3K to activate the PI3K/AKT survival pathway and to induce anchorage-independent cell growth in vitro and cancer cell growth in animals (Figure 3). It is unclear whether the cytoplasmic localization of tRXRα results from its nuclear export or cytoplasmic retention due to its interaction with cytoplasmic proteins such as p85α. In either case, the N-terminal region that is deleted from RXRα is expected to play a critical role in regulating RXR activities. As the N-terminal region of RXRα is subject to regulation by phosphorylation, it remains to be determined whether phosphorylation or other modifications of RXRα are involved in the regulation of RXRα cytoplasmic localization and its interaction with p85α. How tRXRα interacts with p85α is also currently unknown. Several nuclear receptors including RAR, PPAR, and T3R have been shown to interact with p85α, implying the existence of a more general mechanism for their interaction. Nevertheless, our results reveal a nongenomic regulation of the PI3K/AKT signaling pathway by tRXRα, which provides not only an explanation for abnormal activation of the pathway in cancer cells but also new strategies to inhibit the activation of PI3K/AKT in cancer cells by targeting tRXRα. Such tRXRα-based PI3K/AKT inhibitors are likely more specific and tumor selective than conventional PI3K/AKT inhibitors.
TNFα controls diverse cellular events such as cell survival and death that determine the destiny of cancer cells75. Although TNFα is capable of inducing the apoptosis of cancer cells through death receptor-dependent mechanisms, such an effect is often antagonized by TNFα's own survival function through its activation of NF-κB and PI3K/AKT pathways75. Since TNFα is produced by malignant or host cells in the tumor microenvironment but not in normal cells, there has been tremendous interest in developing strategies to shift TNFα signaling from survival to death. Sulindac and its K-80003 and K-8008 analogs can bind to tRXRα to inhibit the TNFα-induced interaction of tRXRα with p85α and the activation of PI3K/AKT, resulting in the activation of the TNFα-dependent apoptotic pathway21. Thus, binding of tRXRα by Sulindac and analogs could convert TNFα signaling from survival to death. It is anticipated that many RXRα modulators exert their therapeutic effect by targeting this pathway.
Novel surface binding sites of RXRα as alternate sites for targeting
Canonical ligands bind to the LBP to directly mediate transcriptional activity, and so identifying and optimizing molecules that bind to RXRα's canonical LBP have so far been the focus of drug discovery efforts targeting RXRα. However, there are key limitations of treatment with rexinoids, including unwanted side effects such as an increase in plasma triglyceride levels, suppression of the thyroid hormone axis, and induction of hepatomegaly. Therefore, targeting alternate sites on RXRα for regulating its activities could become a new strategy for RXRα-based drug discovery. Compounds that bind to alternate sites have been successfully demonstrated for other nuclear receptors97,98,99, including estrogen receptor, androgen receptor, VDR and T3R. Among the reported alternate sites on nuclear receptors, the coregulator-binding site is the most studied. Recently, by employing a docking-based virtual screening approach, we identified some small molecules that bind to the coregulator-binding surface of RXRα, a region where the binding sites of the corepressor and the coactivator overlap (Figure 4A). One of the identified binders, 23, can regulate the biological functions of tRXRα, including inhibition of TNFα-induced interaction of tRXRα with p85α, inhibition of AKT activation in vitro and in animals, and induction of apoptosis56. Compound 23 doesn't bind to the LBP and represents the first example of an RXRα modulator that acts via the coregulator-binding site rather than the classical ligand-binding pocket. Thus, targeting alternate binding sites on the surface of RXRα for therapeutic intervention may become a new paradigm for nuclear receptor-based drug discovery.
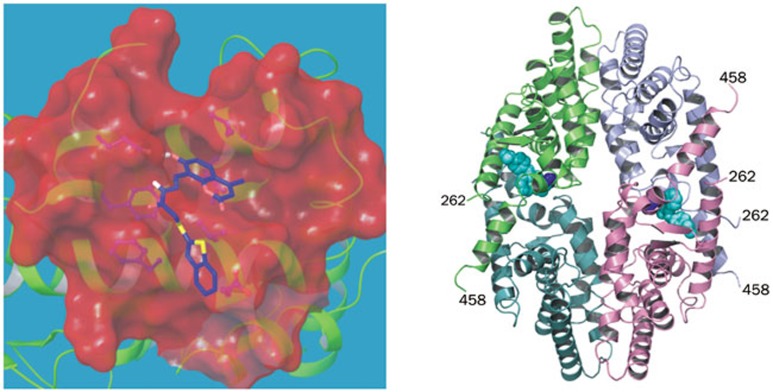
Figure 4.
Alternate sites on the surface of RXRα. (A) The recently identified compound 23 bound to the coregulator-binding groove of RXRα, a docking model. (B) The newly identified site for K-8008 binding, PDB code 4N8R.
In addition to the coregulator-binding groove, another alternate binding site was identified on the surface of RXRα. Our recently determined crystal structure of the RXRα LBD in complex with the Sulindac analogs K-8008 or K-801257 demonstrates the existence of a different binding site. The complex structure exists as a noncrystallographic homo-tetramer similar to the previously reported apo-homotetramer11,13, in which the bottoms of 2 homodimers interface to form a tetramer (Figure 4B). In a tetramer, 2 K-8008 molecules bind to one homotetramer at a hydrophobic region that is near the entry and the edge of the cognate LBP57. The K-8008 binding region is close to the dimer-dimer interface and does not overlap with the binding region of 9-cis-RA. With respect to the monomeric and the dimeric RXRα LBD, the K-8008 binding region is located on the surface of the RXRα molecules. RXRα has been shown to form homo-tetramers in solution, which is transcriptionally silent, but to rapidly dissociate into active homodimers upon binding of agonists or antagonists11,12,13. Therefore, it is intriguing that K-8008, an RXRα antagonist, binds to a novel region and that the binding does not result in dissociation of the tetramer, similar to binding by danthron100. The structural basis of K-8008 binding suggests that RXRα tetramerization represents a key mechanism for the regulation of the nongenomic actions of RXR.
Conclusions and perspectives
It is evident that both genomic and nongenomic mechanisms contribute to the pleiotropic effects of RXRα and its ligands. Recent advances have revealed the roles of the nongenomic actions of RXRα and its ligands in the control of apoptosis, survival, and inflammation, which likely account for their therapeutic effects in cancer and metabolic and neurodegenerative disorders, although their physiological and pathophysiological relevance remains to be fully established. The mechanisms that regulate the nongenomic actions of RXRα need to be further elucidated. Despite the recognition that RXRα is an innovative drug target, development of RXRα-based drugs has been hampered by the side effects associated with targeting its cognate LBP. The findings that RXRα is cleaved in tumor cells and that Sulindac-derived small molecules and others act at the alternate binding sites of the surface of RXRα will provide new rational drug design and screening approaches by targeting functionally important surface-binding sites. Such an approach will likely target tumor- or disease-selective RXRα (ie, tRXRα or RXRα with abnormal modifications) rather than unmodified RXRα and may also circumvent the side effects associated with binding to the cognate RXRα LBP. However, many unanswered questions regarding the production, function, and the underlying mechanisms of tRXRα need to be answered. The binding of Sulindac analogs to the tetrameric form of the RXRα-LBD is interesting. However, little is known about the biological function of the RXRα tetramer with respect to the regulation of the nongenomic function of RXRα. The characterization of the surface binding sites in RXRα and the development of selective inhibitors targeting the surface-binding sites may support a departure from the traditional paradigm of targeting the LBP.
Abbreviations
RXRα, retinoid X receptor-alpha; tRXRα, truncated retinoid X receptor-alpha; PI3K, phosphatidylinositol 3-kinase; NSAID, nonsteroidal anti-inflammatory drug; LBD, ligand-binding domain; LBP, ligand-binding pocket; RAR, retinoic acid receptor; T3R, thyroid hormone receptor; VDR, vitamin D receptor; PPAR, peroxisome proliferator-activated receptor; LXR, liver X receptor; FXR, farnesoid X receptor; RA, retinoic acid; DHA, docosahexaenoic acid; TZD, thiazolidinedione; Aβ, β-amyloid; TNF, tumor necrosis factor; IGFBP, insulin-like growth factor binding protein; MAPK, mitogen-activated protein kinase; NGF, nerve growth factor; TRADD, TNF receptor-associated death domain; FADD, Fas-associated death domain; TNF-R1, TNFα receptor-1; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; GSK-3β, glycogen synthase kinase 3 beta.
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (NSFC-91129302), the fund from the Ministry of Education of China, the Fundamental Research Funds for the Central Universities (2013121038), the 985 Project from Xiamen University, and Fujian Natural Science Foundation Program (2013J01385), and grants from the US. Army Medical Research and Material Command (W81XWH-11-1- 0677), the National Institutes of Health (CA140980, GM089927, CA179379), the California Breast Cancer Research Program (20IB-0138).
References
- Evans RM, Mangelsdorf DJ. Nuclear Receptors, RXR, and the Big Bang. Cell 2014; 157: 255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto A, Narkar V, Shen Q, Uray IP, Davies PJ, Nagy L. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ 2004; 11: S126–43. [DOI] [PubMed] [Google Scholar]
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov 2007; 6: 793–810. [DOI] [PubMed] [Google Scholar]
- Dawson MI, Xia Z. The retinoid X receptors and their ligands. Biochim Biophys Acta 2012; 1821: 21–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MI, Zhang XK. Discovery and design of retinoic acid receptor and retinoid X receptor class- and subtype-selective synthetic analogs of all-trans-retinoic acid and 9-cis-retinoic acid. Curr Med Chem 2002; 9: 623–37. [DOI] [PubMed] [Google Scholar]
- Desvergne B. RXR: from partnership to leadership in metabolic regulations. Vitam Horm 2007; 75: 1–32. [DOI] [PubMed] [Google Scholar]
- Roszer T, Menendez-Gutierrez MP, Cedenilla M, Ricote M. Retinoid X receptors in macrophage biology. Trends Endocrinol Metab 2013; 24: 460–8. [DOI] [PubMed] [Google Scholar]
- Ahuja HS, Szanto A, Nagy L, Davies PJ. The retinoid X receptor and its ligands: versatile regulators of metabolic function, cell differentiation and cell death. J Biol Regul Homeost Agents 2003; 17: 29–45. [PubMed] [Google Scholar]
- Tanaka T, De Luca LM. Therapeutic potential of “rexinoids” in cancer prevention and treatment. Cancer Res 2009; 69: 4945–7. [DOI] [PubMed] [Google Scholar]
- Zhang XK, Lehmann J, Hoffmann B, Dawson MI, Cameron J, Graupner G, et al. Homodimer formation of retinoid X receptor induced by 9-cis retinoic acid. Nature 1992; 358: 587–91. [DOI] [PubMed] [Google Scholar]
- Gampe RT Jr, Montana VG, Lambert MH, Wisely GB, Milburn MV, Xu HE. Structural basis for autorepression of retinoid X receptor by tetramer formation and the AF-2 helix. Genes Dev 2000; 14: 2229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten S, Reczek PR, Noy N. The tetramerization region of the retinoid X receptor is important for transcriptional activation by the receptor. J Biol Chem 1997; 272: 29759–68. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen L, Chen J, Jiang H, Shen X. Structural basis for retinoic X receptor repression on the tetramer. J Biol Chem 2011; 286: 24593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, et al. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol 2004; 24: 9705–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas F, Daury L, Grandemange S, Busson M, Seyer P, Hatier R, et al. Endocrine regulation of mitochondrial activity: involvement of truncated RXRalpha and c-Erb Aalpha1 proteins. FASEB J 2003; 17: 426–36. [DOI] [PubMed] [Google Scholar]
- Katagiri Y, Takeda K, Yu ZX, Ferrans VJ, Ozato K, Guroff G. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol 2000; 2: 435–40. [DOI] [PubMed] [Google Scholar]
- Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept 2004; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman TL, Thevananther S, Ghose R, Burns AR, Karpen SJ. Nuclear export of retinoid X receptor alpha in response to interleukin-1beta-mediated cell signaling: roles for JNK and SER260. J Biol Chem 2006; 281: 15434–40. [DOI] [PubMed] [Google Scholar]
- Fukunaka K, Saito T, Wataba K, Ashihara K, Ito E, Kudo R. Changes in expression and subcellular localization of nuclear retinoic acid receptors in human endometrial epithelium during the menstrual cycle. Mol Hum Reprod 2001; 7: 437–46. [DOI] [PubMed] [Google Scholar]
- Wang GH, Jiang FQ, Duan YH, Zeng ZP, Chen F, Dai Y, et al. Targeting truncated retinoid X receptor-alpha by CF31 induces TNF-alpha-dependent apoptosis. Cancer Res 2013; 73: 307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Liu W, Su Y, Wei Z, Liu J, Kolluri SK, et al. NSAID sulindac and its analog bind RXRalpha and inhibit RXRalpha-dependent AKT signaling. Cancer Cell 2010; 17: 560–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calleja C, Messaddeq N, Chapellier B, Yang H, Krezel W, Li M, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev 2006; 20: 1525–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev 2006; 64: 532–8. [DOI] [PubMed] [Google Scholar]
- Kagechika H. Novel synthetic retinoids and separation of the pleiotropic retinoidal activities. Curr Med Chem 2002; 9: 591–608. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Chandraratna RA. Recent developments in receptor-selective retinoids. Curr Pharm Des 2000; 6: 919–31. [DOI] [PubMed] [Google Scholar]
- Perez E, Bourguet W, Gronemeyer H, de Lera AR. Modulation of RXR function through ligand design. Biochim Biophys Acta 2012; 1821: 57–69. [DOI] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal 2009; 7: e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, et al. Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol 2000; 20: 4436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Indra AK, Warot X, Brocard J, Messaddeq N, Kato S, et al. Skin abnormalities generated by temporally controlled RXRalpha mutations in mouse epidermis. Nature 2000; 407: 633–6. [DOI] [PubMed] [Google Scholar]
- Huang J, Powell WC, Khodavirdi AC, Wu J, Makita T, Cardiff RD, et al. Prostatic intraepithelial neoplasia in mice with conditional disruption of the retinoid X receptor alpha allele in the prostate epithelium. Cancer Res 2002; 62: 4812–9. [PubMed] [Google Scholar]
- Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc Natl Acad Sci U S A 2001; 98: 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivat-Hannah V, Bourguet W, Gottardis M, Gronemeyer H. Separation of retinoid X receptor homo- and heterodimerization functions. Mol Cell Biol 2003; 23: 7678–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XK, Salbert G, Lee MO, Pfahl M. Mutations that alter ligand-induced switches and dimerization activities in the retinoid X receptor. Mol Cell Biol 1994; 14: 4311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Suh KS, Lo AM, De Luca LM. p21WAF1/CIP1 is a common transcriptional target of retinoid receptors: pleiotropic regulatory mechanism through retinoic acid receptor (RAR)/retinoid X receptor (RXR) heterodimer and RXR/RXR homodimer. J Biol Chem 2007; 282: 29987–97. [DOI] [PubMed] [Google Scholar]
- Nunez V, Alameda D, Rico D, Mota R, Gonzalo P, Cedenilla M, et al. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proc Natl Acad Sci U S A 2010; 107: 10626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hansen PA, Xi L, Chandraratna RA, Burant CF. Distinct mechanisms of glucose lowering by specific agonists for peroxisomal proliferator activated receptor gamma and retinoic acid X receptors. J Biol Chem 2005; 280: 38317–27. [DOI] [PubMed] [Google Scholar]
- IJ penberg A, Tan NS, Gelman L, Kersten S, Seydoux J, Xu J, et al. In vivo activation of PPAR target genes by RXR homodimers. The EMBO J 2004; 23: 2083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocadiz-Delgado R, Castaneda-Saucedo E, Indra AK, Hernandez-Pando R, Gariglio P. Impaired cervical homeostasis upon selective ablation of RXRalpha in epithelial cells. Genesis 2008; 46: 19–28. [DOI] [PubMed] [Google Scholar]
- Takiyama Y, Miyokawa N, Sugawara A, Kato S, Ito K, Sato K, et al. Decreased expression of retinoid X receptor isoforms in human thyroid carcinomas. J Clin Endocrinol Metab 2004; 89: 5851–61. [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Shidoji Y, Nishiwaki S, Moriwaki H, Muto Y. Limited degradation of retinoid X receptor by calpain. Biochem Biophys Res Commun 1996; 225: 946–51. [DOI] [PubMed] [Google Scholar]
- Nagaya T, Murata Y, Yamaguchi S, Nomura Y, Ohmori S, Fujieda M, et al. Intracellular proteolytic cleavage of 9-cis-retinoic acid receptor alpha by cathepsin L-type protease is a potential mechanism for modulating thyroid hormone action. J Biol Chem 1998; 273: 33166–73. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Nagaya T, Yamaguchi S, Katunuma N, Seo H. Cleavage of RXRalpha by a lysosomal enzyme, cathepsin L-type protease. Biochem Biophys Res Commun 1999; 254: 388–94. [DOI] [PubMed] [Google Scholar]
- Zhong C, Yang S, Huang J, Cohen MB, Roy-Burman P. Aberration in the expression of the retinoid receptor, RXRalpha, in prostate cancer. Cancer Biol Ther 2003; 2: 179–84. [DOI] [PubMed] [Google Scholar]
- Matsushima-Nishiwaki R, Okuno M, Adachi S, Sano T, Akita K, Moriwaki H, et al. Phosphorylation of retinoid X receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res 2001; 61: 7675–82. [PubMed] [Google Scholar]
- Mey J, Schrage K, Wessels I, Vollpracht-Crijns I. Effects of inflammatory cytokines IL-1beta, IL-6, and TNFalpha on the intracellular localization of retinoid receptors in Schwann cells. Glia 2007; 55: 152–64. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Dancheck BL, Trifiletti LC, Birnkrant RE, Taylor BJ, Garfield SH, et al. Altered localization of retinoid X receptor alpha coincides with loss of retinoid responsiveness in human breast cancer MDA-MB-231 cells. Mol Cell Biol 2004; 24: 3972–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisig BB, Kwok C, Zelent A, Shankaranarayanan P, Gronemeyer H, Dong S, et al. Recruitment of RXR by homotetrameric RARalpha fusion proteins is essential for transformation. Cancer Cell 2007; 12: 36–51. [DOI] [PubMed] [Google Scholar]
- Zhu J, Nasr R, Peres L, Riaucoux-Lormiere F, Honore N, Berthier C, et al. RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell 2007; 12: 23–35. [DOI] [PubMed] [Google Scholar]
- Querfeld C, Nagelli LV, Rosen ST, Kuzel TM, Guitart J. Bexarotene in the treatment of cutaneous T-cell lymphoma. Expert Opin Pharmacother 2006; 7: 907–15. [DOI] [PubMed] [Google Scholar]
- Blumenschein GR Jr, Khuri FR, von Pawel J, Gatzemeier U, Miller WH Jr, Jotte RM, et al. Phase III trial comparing carboplatin, paclitaxel, and bexarotene with carboplatin and paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT II. J Clin Oncol 2008; 26: 1879–85. [DOI] [PubMed] [Google Scholar]
- Ramlau R, Zatloukal P, Jassem J, Schwarzenberger P, Orlov SV, Gottfried M, et al. Randomized phase III trial comparing bexarotene (L1069-49)/cisplatin/vinorelbine with cisplatin/vinorelbine in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT I. J Clin Oncol 2008; 26: 1886–92. [DOI] [PubMed] [Google Scholar]
- Claudel T, Leibowitz MD, Fievet C, Tailleux A, Wagner B, Repa JJ, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci U S A 2001; 98: 2610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, Lee CY, Karlo JC, Zinn AE, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science 2012; 335: 1503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Spalding TA, Hubbard D, Ma JN, Olsson R, Burstein ES. Low dose bexarotene treatment rescues dopamine neurons and restores behavioral function in models of Parkinson's disease. ACS Chem Neurosci 2013; 4: 1430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner V, Miodownik C, Gibel A, Sirota P, Bush I, Elliot H, et al. The retinoid X receptor agonist bexarotene relieves positive symptoms of schizophrenia: a 6-week, randomized, double-blind, placebo-controlled multicenter trial. J Clin Psychiatry 2013; 74: 1224–32. [DOI] [PubMed] [Google Scholar]
- Chen F, Liu J, Huang M, Hu M, Su Y, Zhang XK. Identification of a new RXRalpha antagonist targeting the coregulator-binding site. ACS Med Chem Lett 2014; 5: 736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang ZG, Aleshin AE, Chen F, Chen J, Jiang F, et al. Sulindac-derived RXRalpha modulators inhibit cancer cell growth by binding to a novel site. Chem Biol 2014; 21: 596–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov 2002; 1: 111–21. [DOI] [PubMed] [Google Scholar]
- Yang Y, Minucci S, Ozato K, Heyman RA, Ashwell JD. Efficient inhibition of activation-induced Fas ligand up-regulation and T cell apoptosis by retinoids requires occupancy of both retinoid X receptors and retinoic acid receptors. J Biol Chem 1995; 270: 18672–7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Coleman DJ, Bajaj G, Liang X, Ganguli-Indra G, Indra AK. RXRalpha ablation in epidermal keratinocytes enhances UVR-induced DNA damage, apoptosis, and proliferation of keratinocytes and melanocytes. J Invest Dermatol 2011; 131: 177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwa E, Rzemieniec J, Wnuk A, Lason W, Krzeptowski W, Kajta M. Apoptotic and neurotoxic actions of 4-para-nonylphenol are accompanied by activation of retinoid X receptor and impairment of classical estrogen receptor signaling. J Steroid Biochem Mol Biol 2014; 144: 334–47. [DOI] [PubMed] [Google Scholar]
- Liu B, Lee HY, Weinzimer SA, Powell DR, Clifford JL, Kurie JM, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J Biol Chem 2000; 275: 33607–13. [DOI] [PubMed] [Google Scholar]
- Fan YY, Spencer TE, Wang N, Moyer MP, Chapkin RS. Chemopreventive n-3 fatty acids activate RXRalpha in colonocytes. Carcinogenesis 2003; 24: 1541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German OL, Monaco S, Agnolazza DL, Rotstein NP, Politi LE. Retinoid X receptor activation is essential for docosahexaenoic acid protection of retina photoreceptors. J Lipid Res 2013; 54: 2236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janakiram NB, Mohammed A, Qian L, Choi CI, Steele VE, Rao CV. Chemopreventive effects of RXR-selective rexinoid bexarotene on intestinal neoplasia of Apc(Min/+) mice. Neoplasia 2012; 14: 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri SK, Corr M, James SY, Bernasconi M, Lu D, Liu W, et al. The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. Proc Natl Acad Sci U S A 2005; 102: 2525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene 2006; 25: 4725–43. [DOI] [PubMed] [Google Scholar]
- Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 2000; 289: 1159–64. [DOI] [PubMed] [Google Scholar]
- Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. J Exp Med 2008; 205: 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Volkers M, Din S, Avitabile D, Khan M, Gude N, et al. Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J 2011; 32: 2179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs CM, Boldingh KA, Slagsvold HH, Thoresen GH, Paulsen RE. ERK2 prohibits apoptosis-induced subcellular translocation of orphan nuclear receptor NGFI-B/TR3. J Biol Chem 2004; 279: 50097–101. [DOI] [PubMed] [Google Scholar]
- Yang Y, Bailey J, Vacchio MS, Yarchoan R, Ashwell JD. Retinoic acid inhibition of ex vivo human immunodeficiency virus- associated apoptosis of peripheral blood cells. Proc Natl Acad Sci U S A 1995; 92: 3051–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin XF, Zhao BX, Chen HZ, Ye XF, Yang CY, Zhou HY, et al. RXRalpha acts as a carrier for TR3 nuclear export in a 9-cis retinoic acid-dependent manner in gastric cancer cells. J Cell Sci 2004; 117: 5609–21. [DOI] [PubMed] [Google Scholar]
- Jacobs CM, Paulsen RE. Crosstalk between ERK2 and RXR regulates nuclear import of transcription factor NGFI-B. Biochem Biophys Res Commun 2005; 336: 646–52. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 2009; 9: 361–71. [DOI] [PubMed] [Google Scholar]
- Pincheira R, Castro AF, Ozes ON, Idumalla PS, Donner DB. Type 1 TNF receptor forms a complex with and uses Jak2 and c-Src to selectively engage signaling pathways that regulate transcription factor activity. J Immunol 2008; 181: 1288–98. [DOI] [PubMed] [Google Scholar]
- Knackstedt R, Shaoli S, Moseley V, Wargovich M. The importance of the retinoid X receptor alpha in modulating inflammatory signaling in acute murine colitis. Dig Dis Sci 2014; 59: 753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NK, Narayanan BA, Reddy BS. A combination of docosahexaenoic acid and celecoxib prevents prostate cancer cell growth in vitro and is associated with modulation of nuclear factor-kappaB, and steroid hormone receptors. Int J Oncol 2005; 26: 785–92. [PubMed] [Google Scholar]
- Weldon SM, Mullen AC, Loscher CE, Hurley LA, Roche HM. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem 2007; 18: 250–8. [DOI] [PubMed] [Google Scholar]
- Feng R, Anderson G, Xiao G, Elliott G, Leoni L, Mapara MY, et al. SDX-308, a nonsteroidal anti-inflammatory agent, inhibits NF-kappaB activity, resulting in strong inhibition of osteoclast formation/activity and multiple myeloma cell growth. Blood 2007; 109: 2130–8. [DOI] [PubMed] [Google Scholar]
- Bissonnette RP, Brunner T, Lazarchik SB, Yoo NJ, Boehm MF, Green DR, et al. 9-cis retinoic acid inhibition of activation-induced apoptosis is mediated via regulation of fas ligand and requires retinoic acid receptor and retinoid X receptor activation. Mol Cell Biol 1995; 15: 5576–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Kawada T, Han IS, Kim BS, Goto T, Takahashi N, et al. Capsaicin inhibits the production of tumor necrosis factor alpha by LPS-stimulated murine macrophages, RAW 264.7: a PPARgamma ligand-like action as a novel mechanism. FEBS Lett 2004; 572: 266–70. [DOI] [PubMed] [Google Scholar]
- Zapata-Gonzalez F, Rueda F, Petriz J, Domingo P, Villarroya F, de Madariaga A, et al. 9-cis-Retinoic acid (9cRA), a retinoid X receptor (RXR) ligand, exerts immunosuppressive effects on dendritic cells by RXR-dependent activation: inhibition of peroxisome proliferator-activated receptor gamma blocks some of the 9cRA activities, and precludes them to mature phenotype development. J Immunol 2007; 178: 6130–9. [DOI] [PubMed] [Google Scholar]
- Zhang-Gandhi CX, Drew PD. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J Neuroimmunol 2007; 183: 50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Kim HT, Wu K, DeNardo D, Hilsenbeck SG, Xu XC, et al. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: implications for molecular-based chemoprevention. Cancer Res 2005; 65: 3462–9. [DOI] [PubMed] [Google Scholar]
- Yen WC, Prudente RY, Corpuz MR, Negro-Vilar A, Lamph WW. A selective retinoid X receptor agonist bexarotene (LGD1069, targretin) inhibits angiogenesis and metastasis in solid tumours. Br J Cancer 2006; 94: 654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit JV, de Jong EM, van Hooijdonk CA, Otero ME, Boezeman JB, van de Kerkhof PC. Systemic treatment of psoriatic patients with bexarotene decreases epidermal proliferation and parameters for inflammation, and improves differentiation in lesional skin. J Am Acad Dermatol 2004; 51: 257–64. [DOI] [PubMed] [Google Scholar]
- Desreumaux P, Dubuquoy L, Nutten S, Peuchmaur M, Englaro W, Schoonjans K, et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor gamma (PPARgamma) heterodimer. A basis for new therapeutic strategies. J Exp Med 2001; 193: 827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh R, Gho A, di Lucia P, Klaus M, Bollag W, Ho IC, et al. Cutting Edge: Inhibition of the retinoid X receptor (RXR) blocks T helper 2 differentiation and prevents allergic lung inflammation. J Immunol 2006; 176: 5161–6. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol 2010; 10: 365–76. [DOI] [PubMed] [Google Scholar]
- Caelles C, Gonzalez-Sancho JM, Munoz A. Nuclear hormone receptor antagonism with AP-1 by inhibition of the JNK pathway. Genes Dev 1997; 11: 3351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta 2008; 1784: 159–85. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94: 491–501. [DOI] [PubMed] [Google Scholar]
- Fortini ME. Notch and presenilin: a proteolytic mechanism emerges. Curr Opin Cell Biol 2001; 13: 627–34. [DOI] [PubMed] [Google Scholar]
- Libertini SJ, Tepper CG, Rodriguez V, Asmuth DM, Kung HJ, Mudryj M. Evidence for calpain-mediated androgen receptor cleavage as a mechanism for androgen independence. Cancer Res 2007; 67: 9001–5. [DOI] [PubMed] [Google Scholar]
- Gao W, Liu J, Hu M, Huang M, Cai S, Zeng Z, et al. Regulation of proteolytic cleavage of retinoid X receptor-alpha by GSK-3beta. Carcinogenesis 2013; 34: 1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzon V, Carbo LR, Estruch SB, Fletterick RJ, Estebanez-Perpina E. A conserved surface on the ligand binding domain of nuclear receptors for allosteric control. Mol Cell Endocrinol 2012; 348: 394–402. [DOI] [PubMed] [Google Scholar]
- Moore TW, Mayne CG, Katzenellenbogen JA. Minireview: Not picking pockets: nuclear receptor alternate-site modulators (NRAMs). Mol Endocrinol 2010; 24: 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboni L, Lloyd DG. Beyond the ligand-binding pocket: targeting alternate sites in nuclear receptors. Med Res Rev 2013; 33: 1081–118. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhou R, Li L, Chen J, Chen L, Li C, et al. Danthron functions as a retinoic X receptor antagonist by stabilizing tetramers of the receptor. J Biol Chem 2011; 286: 1868–75. [DOI] [PMC free article] [PubMed] [Google Scholar]