Abstract
The hair bundle—the sensory organelle of inner-ear hair cells of vertebrates—exemplifies the ability of a cell to assemble complex, elegant structures. Proper construction of the bundle is required for proper mechanotransduction in response to external forces and to transmit information about sound and movement. Bundles contain tightly controlled numbers of actin-filled stereocilia, which are arranged in defined rows of precise heights. Indeed, many deafness mutations that disable hair-cell cytoskeletal proteins also disrupt bundles. Bundle assembly is a tractable problem in molecular and cellular systems biology; the sequence of structural changes in stereocilia is known, and a modest number of proteins may be involved.
INTRODUCTION
The remarkable structure of the vertebrate hair bundle derives from an equally remarkable assembly process. Actin-rich stereocilia form, lengthen, and widen in a precisely determined order (Tilney et al., 1992), giving the bundle its asymmetric, staircase appearance (Figure 1). The importance of bundle assembly and structure is highlighted by the number of deafness-causing mutations, a subset of which are in genes that encode actin or actin-associated proteins (for examples, see Table 1). Assembly of the bundle involves multiple overlapping and interacting cellular processes, such as stereocilia lengthening and widening, each of which is responsible for one or more features of the final structure.
FIGURE 1:
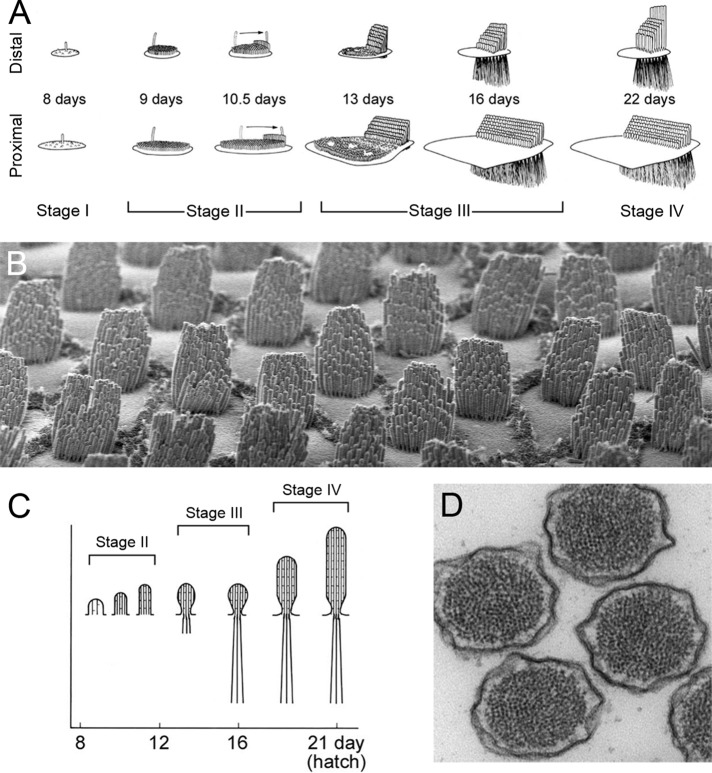
Development of the hair bundle. (A) Progression of hair-bundle morphology in chick cochlea. (B) Chick cochlea hair bundles at hatch. Hair cells imaged are at an intermediate position between the apical and basal extremes. Note the cell-to-cell reproducibility of the numbers of stereocilia, their orientation, and their lengths. Hair bundles are ∼10 μm tall. (C) Progression of stereocilia actin paracrystal development in chick cochlea. (D) Actin paracrystals in mouse vestibular stereocilia. Note the stereocilium-to-stereocilium reproducibility of the size and organization of the actin cores. Stereocilia are ∼200 nm in diameter. A and C are reproduced from Tilney et al. (1992) with permission.
TABLE 1:
Selected key proteins for hair bundles.
| Gene symbol | Gene name | Deafness mutant | Hair-bundle phenotype | Protein location |
|---|---|---|---|---|
| ACTB | Actin beta | Actb knockout | Progressive degeneration of hair bundles | Stereocilia shafts |
| ACTG1 | Actin gamma 1 | DFNA20/26, Actg1 knockout | Progressive degeneration of hair bundles | Stereocilia shafts |
| ANXA5 | Annexin V | Anxa5 knockout | None | Shafts |
| ATP2B2 | ATPase, Ca2+ transporting, plasma membrane 2 | Atp2b2 knockout | Progressive degeneration of hair bundles | Shafts (excluded from taper); concentration toward tips |
| CAPZ | Capping protein (A1, A2, and B genes) | n/a | Unknown | Stereocilia tips |
| CDH23 | Cadherin 23 | USH1D, DFNB12 | Disorganized hair bundles | Tip links; transient lateral links; kinocilial links |
| DFNB31 | Whirlin | USH2D, DFNB31 | Short stereocilia | Tips; ankle links |
| EPS8 | Epidermal growth factor receptor pathway substrate 8 | Eps8 knockout | Short stereocilia | Stereocilia tips |
| EPS8L2 | EPS8-like 2 | Eps8l2 knockout | Progressive degeneration of hair bundles | Stereocilia tips |
| ESPN | Espin | DFNB36 | Short and thin stereocilia; degeneration in the cochlea | Short ESPN splice forms throughout stereocilia shafts; long isoform (ESPN-1) at tips |
| FSCN2 | Fascin 2 | Fscn2R109H | Progressive degeneration of hair bundles | Stereocilia shafts |
| MYO1C | Myosin IC | Myo1cY61G | Slowed adaptation | Toward stereocilia tips |
| MYO1H | Myosin IH | n/a | Unknown | Toward stereocilia tips |
| MYO3A | Myosin IIIA | DFNB30 | Progressive hearing loss | Thimble-like pattern at tips |
| MYO3B | Myosin IIIB | n/a | Unknown | Thimble-like pattern at tips |
| MYO6 | Myosin VI | DFNA22, DFNB37 | Fusion and elongation of stereocilia; apical membrane uplifting | Shafts and taper region |
| MYO7A | Myosin VIIA | USH1B, DFNA11, DFNB2 | Disorganized hair bundles | Tip links; transient lateral links; kinocilial links |
| MYO15A | Myosin XVA | DFNB3 | Short stereocilia | Stereocilia tips |
| PCDH15 | Protocadherin 15 | USH1F, DFNB23 | Disorganized hair bundles | Tip links; transient lateral links; kinocilial links |
| PLS1 | Plastin 1 | Pls1 knockout | Progressive degeneration of hair bundles | Stereocilia shafts |
| PTPRQ | Protein tyrosine phosphatase receptor Q | DFNB84 | Fusion and elongation of stereocilia | Ankle region, stereociliary shafts |
| RDX | Radixin | DFNB24, Rdx knockout | Progressive degeneration of hair bundles | Concentrated near base of stereocilia; activated RDX only found above the taper region |
| USH1C | Harmonin | USH1C, DFNB18 | Disorganized hair bundles | Tip link upper insertion; tips in early development |
| USH1G | Sans | USH1G | Disorganized hair bundles | Tip link upper insertion; tips in early development |
Most of these were initially flagged as being important because mutations in their genes caused deafness.
Tom Pollard has written eloquently on the reductionist-synthetic strategy to characterize complex biological mechanisms (Pollard, 2013). His first step is to frame a good problem—and I believe that hair-bundle assembly fits the bill. Like other subcellular processes, including cytokinesis or cell migration, bundle development is relatively circumscribed mechanistically and may require a modest number of proteins, perhaps 100 or fewer. As a reasonably simple systems-biology problem, it is both amenable to study with present technology and suitable as a model for more complex problems. I suggest that now is the time to use systems and cell biology to study how the bundle is built and that this problem is ideal for young cell biologists looking for a career-defining problem to solve.
Description of hair-bundle assembly
First, I present what is known about molecular mechanisms of bundle assembly. Part of Pollard's first step is to define the problem of interest in enough detail that it can be interrogated using a molecular approach. Lew Tilney's landmark studies of hair-bundle development from the 1980s, using electron microscopy, remain the most comprehensive description of bundle assembly. Focusing on the chick cochlea, he and his colleagues identified four distinct stages of bundle growth (Figure 1A). In stage I, before embryonic day 8 (E8), a hair cell's progenitor undergoes its terminal division, and the hair cell begins to differentiate. In stage II (E8–E13), the kinocilium moves to one side of the bundle, stereocilia adjacent to the kinocilium elongate, tip links appear, and cross-linking of actin filaments increases. By the end of stage II, the bundle has a robust staircase separation of stereocilia length. In stage III (E13–E16), stereocilia lengthening stops, but the stereocilia begin to widen, from ∼100 to as many as 900 filaments per stereocilium. During this time, the anchoring structure for the stereocilia—the cuticular plate—begins to form and the stereocilia bases taper, presumably essential for proper bundle flexibility. Finally, lengthening resumes in stage IV (E16–postnatal day 3, P3), stopping only when stereocilia reach their mature lengths. The cochlea encodes sounds of different frequencies using different hair cells; high frequencies are encoded at the proximal (basal) end, and low frequencies are encoded at the distal (apical end). Indeed, the number and length of stereocilia vary systematically from proximal to distal ends. Nevertheless, adjacent cells control bundle assembly very reproducibly (Figure 1B). The general principles of Tilney's scheme for chick-bundle morphogenesis, especially the temporal and spatial segregation of stages of bundle development, are believed to apply to all hair-cell organs.
Components of the hair bundle
Pollard's second step in characterization of molecular mechanisms is to develop a parts list. The auditory neuroscience field has been engaged in this problem for the last several decades, using both genetics and biochemistry to determine which proteins are present in hair bundles and which are functionally important. “Deafness genes,” which, when mutated, lead to deafness, are good candidates for key molecules, particularly if their protein products are present in bundles (Petit and Richardson, 2009; Dror and Avraham, 2009). Several dozen already identified proteins fit this category, and geneticists continue to slowly add more. Examples of key deafness genes that are expressed in hair bundles are listed in Table 1; locations for many of the proteins are shown in Figure 2. Because the genetic strategy could easily miss many key proteins, including those whose activity can be compensated for by functional paralogues or those with pleiotropic effects, other approaches are needed to identify all functionally important proteins of the bundle.
FIGURE 2:
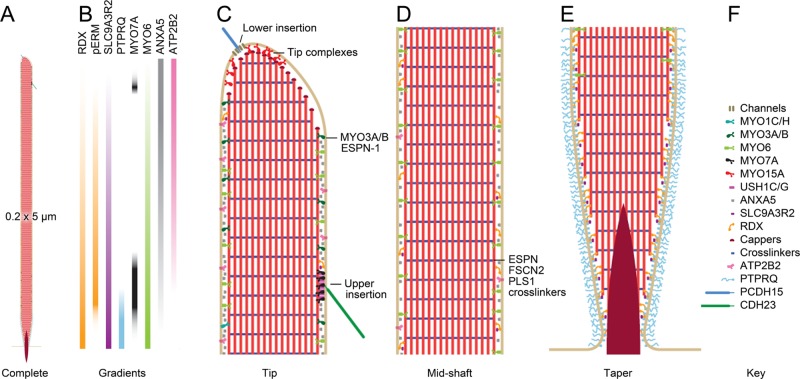
Model chick vestibular stereocilium. Scale model of stereocilium showing selected molecules; these molecules are drawn in at the approximate density for each as determined by mass spectrometry experiments (Shin et al., 2013). (A) Complete stereocilium at low magnification showing dimensions. (B) Selected molecular gradients in the stereocilia. See Shin et al. (2013) for details. (C) High-magnification view of the tip region of the stereocilium. The two insertions of the tip link are highlighted; the lower insertion includes transduction channels, and the upper insertion has USH1C, USH1G, and MYO7A. In addition, the MYO15A tip complex is indicated; DFNB31, EPS8, and perhaps EPS8L2 are part of this complex. MYO3A and MYO3B are found, along with their cargo ESPN-1, in the tip region as well. (D) The stereocilia shaft is made of parallel actin filaments cross-linked by a variety of proteins, most prominently ESPN, FSCN2, and PLS1. Major components of the shaft include the calcium pump ATP2B2, the membrane-associated ANXA5, the actin-to-membrane connector radixin (RDX), and SLC9A3R2, a ligand for RDX. RDX with the activating phosphorylation is found above the taper region. (E) The taper region. Most actin filaments terminate at the membrane, but a few project through into the soma as the rootlet (density). The lipid phosphatase PTPRQ is concentrated in the taper region, as is unactivated RDX. (F) Key to molecules included in the diagram.
Protein biochemistry offers a complementary approach. Development of methods for purification of hair bundles (Shepherd et al., 1989; Gillespie and Hudspeth, 1991) provided enough starting material to specifically examine which proteins are present in bundles. Although initial experiments used one-by-one examination of proteins by immunoblotting, development of modern mass spectrometry methods now allow parallel examination of thousands of proteins. Mass spectrometry analysis of purified bundles has identified hundreds of bundle-specific proteins from vestibular and auditory organs of the chick (Shin et al., 2013; Avenarius et al., 2014). Present challenges include determining which of these proteins are most important for bundle assembly and the features of bundle development in which they participate.
Specification of stereocilia number and position
A close look at hair-bundle assembly with the parts list in mind allows us to associate some proteins with stages of morphogenesis. At early points during hair-cell differentiation, short stereocilia cover the apical surface of the cell; these cilia can nonetheless be distinguished from the shorter microvilli on adjacent supporting cells. The position of the kinocilium—an axonemal cilium (with 9 + 2 microtubule doublets)—may then specify bundle polarity. Stereocilia adjacent to the kinocilium first elongate, followed by succeeding rows (Tilney et al., 1992; Kindt et al., 2012). These results show the essential role of the kinocilium (or the basal body) in establishing subcellular planar polarity (Deans, 2013).
The number and placement of stereocilia can vary systematically along one or more tissue axes. How different cells specify stereocilia number is unknown, but an intriguing suggestion is that they arise as Turing patterns from simple reaction-diffusion mechanisms (Jacobo and Hudspeth, 2014). Stabilization of stereocilia by a Turing mechanism could complement developmental stimulation of stereocilia elongation; together these mechanisms would allow for growth of the rows followed by pruning of unstabilized stereocilia. Candidates for components for control of Turing patterns or stereocilia stabilization include RAC1 and its downstream effector p21-activated kinase (Grimsley-Myers et al., 2009, 2012), both of which are detected in stereocilia (Shin et al., 2013). Upstream control of those mechanisms may be exerted by Sonic hedgehog (SHH); eptopic SHH reduces the numbers of stereocilia in basal hair cells, which typically have many stereocilia (Son et al., 2015).
Different molecules for two stages of stereocilia lengthening
Actin elongation in stereocilia does not appear to depend on the usual suspects, such as the Arp2/3 complex, formins, or the Ena/vasodilator-stimulated phosphoprotein (VASP) family. Although the Arp2/3 complex is present in hair bundles (Shin et al., 2013), this complex forms only branched actin networks. Formins and Ena/VASP proteins do generate parallel bundles of actin (Campellone and Welch, 2010), but neither is found at significant levels in chick (Shin et al., 2013) or mouse (unpublished data) bundles. Other less-well-characterized elongation-promoting proteins, such as Spire and cordon bleu (Campellone and Welch, 2010), are also absent from bundles. This is not to say that none of these proteins is involved in the very earliest stages of development of the stereocilia, when they are growing as microvilli; nevertheless, subsequent lengthening must be promoted by different proteins.
Myosin molecules instead play critical roles in promoting and regulating stereocilia length, presumably by controlling the levels of actin itself or actin-regulating molecules. Key myosin isozymes involved in stereocilia length regulation include myosins IIIA, IIIB, VIIA, and XVA; indeed, length regulation in stereocilia may employ multiple redundant systems, offering substantial robustness.
Myosin XVA (MYO15A) certainly participates in stereocilia length regulation. As part of a complex with DFNB31 (whirlin), EPS8, and perhaps EPS8L2, MYO15A is essential for growth of cochlear stereocilia past ∼0.5 μm (Manor et al., 2011); similarly, vestibular stereocilia of mice lacking MYO15A are only a few micrometers long (Belyantseva et al., 2005). In both auditory and vestibular hair bundles, adjacent stereocilia in a single rank do show increasing lengths, but the staircase spacing is only 100–200 nm (Stepanyan and Frolenkov, 2009). These observations suggest that MYO15A is required for Tilney's first step of stereocilia elongation, that which occurs beyond the initial development of a short staircase.
The cross-linking protein espin (ESPN) is necessary for the second stage of stereocilia lengthening (Sekerkova et al., 2011); lengthening stops prematurely in mice homozygous for the jerker mutation, which disables Espn (Sekerkova et al., 2011). The longest splice form of ESPN, called ESPN-1, may exert its effects after transport to stereocilia tips by myosin IIIA or IIIB (MYO3A or MYO3B; Salles et al., 2009; Merritt et al., 2012). Short forms of ESPN elongate microvilli in cultured cells, however, suggesting that control of stereocilia length might involve multiple ESPN splice forms (Loomis et al., 2003).
Stereocilia widening
In the chick cochlea, widening of stereocilia from ∼50 to >400 filaments occurs as a distinct step, sandwiched in time between the first and last lengthening phases (Figure 1, C and D; Tilney et al., 1980). By contrast, in the mammal, widening occurs concurrently with the second lengthening phase (Kaltenbach et al., 1994; Sekerkova et al., 2011). As with second-phase lengthening, widening during this period is dependent on ESPN; moreover, the tapered stereocilia seen in jerker heterozygotes, with half the normal ESPN levels, suggest that widening occurs from the base of the stereocilium to the tip (Sekerkova et al., 2011). This observation and the relationship between the myosin III paralogues and the ESPN-1 splice form suggests that widening may occur as myosin III motors transport ESPN-1 toward stereocilia tips, with ESPN-1 catalyzing barbed-end growth of new filaments that are nucleated at stereocilia taper regions.
Control of stereocilia length by mechanotransduction
The remarkable precision of stereocilia length in adjacent rows begs for local feedback control within the hair bundle; it would seem impossible for the cell to control lengths so reproducibly from the cytoplasm. Phenotypes of mutant hair bundles for which mechanotransduction is known to be disrupted—for example, in Cdh23 and Ush1g mice—suggest that when mechanotransduction is disrupted, length regulation is altered (Caberlotto et al., 2011).
Possible mechanisms for transduction control of stereocilia length are few. Tip links—the extracellular filaments that gate the transduction channels—are tensioned by myosin motors at the tip-link upper insertion point (LeMasurier and Gillespie, 2005). The upward force on tip links apparently puckers the membrane of a shorter stereocilium enough to allow actin polymerization (Kachar et al., 2000), producing a zone of actin turnover at tips (Zhang et al., 2012; Drummond et al., 2015; Narayanan et al., 2015). Thus tip-link force may elongate stereocilia, at least over a short distance.
There must also be a mechanism that opposes the upward tip-link force, or stereocilia would elongate until the upper insertion point reached the stereocilia tip. Myosin motors that control tip-link tension—probably MYO1C or MYO7A—should be sensitive to Ca2+; perhaps Ca2+ that enters at a stereocilium tip and diffuses down to the upper insertion point of the next tip link could be responsible, providing a constant brake to upward movement of the myosin motors (Gillespie and Müller, 2009). This mechanism could account for differences in spacing of the staircase step, as the reach of Ca2+ will depend not only Ca2+ entry, but also on concentrations of mobile Ca2+ buffers, which can vary substantially from cell to cell (Hackney et al., 2005). Of course, because the tallest stereocilia do not have active transduction channels (Beurg et al., 2009), for this model to be correct, there would need be an alternative route for Ca2+ entry at their tips.
As an alternative model, the force could come from the active turnover of actin filaments at the tips of shorter stereocilia, those associated with the base of a tip link (Zhang et al., 2012; Drummond et al., 2015; Narayanan et al., 2015). Active disassembly of actin there could lead to increased tension in the membrane that would pull the tip link downward, which could counter the upward force of the myosin motors. These two models could be tested by manipulating Ca2+ or actin-depolymerizing molecules in the stereocilium and examining effects on stereocilia length.
A call to arms
Although individual steps of hair-bundle assembly are beginning to be understood, the problem begs for a systematic approach, given the large number of overlapping and partially redundant molecular mechanisms. Modern technology allows for experiments that could only be dreamt of even a few years ago. As a systems-biology problem, assembly of the bundle seems highly tractable. What additional information is needed? Will this information require new approaches?
Pollard argues that three large areas of research—biochemical, cellular, and structural—are needed for complete characterization of a biological problem of interest. One need is for description of the order of expression and appearance in stereocilia of key hair-bundle proteins. Single-cell transcriptomics experiments now allow for a temporal ordering of expression of genes in a cellular process (Durruthy-Durruthy et al., 2014) and can be used to understand bundle development. Complementary experiments showing developmental progression of protein expression are needed. The lack of coordination of hair-cell development in vestibular organs (Goodyear et al., 1999; Burns et al., 2012) suggests that these protein expression experiments will require single-cell mass spectrometry. Although some efforts have been made in this direction (Wu and Singh, 2012), detection of rare proteins in single hair cells will require increasing the sensitivity of mass spectrometry by at least 100-fold. Given the spectacular advances in instrumentation in the last two decades, this goal seems achievable.
We also need to know the activities, protein–protein interactions, and activity-modifying posttranslational modifications of all proteins important for bundle assembly (Pollard, 2013). Activities of many proteins are known or can be inferred from better-characterized paralogues. Because we expect that only a few hundred proteins are needed for bundle assembly, examining, for example, a 100 × 100 matrix of interactions would be straightforward with a variety of techniques, including yeast two-hybrid assays (Bruckner et al., 2009) and direct protein–protein binding assays (Syafrizayanti et al., 2014), as well as with methods for testing the relevance of those interactions in vivo. Systematic characterization of all of these parameters is essential.
Finally, computational biologists need to develop mathematical models that describe hair-bundle assembly. The models need to be of sufficient complexity that meaningful hypothesis-testing experiments can be inferred from them (Pollard, 2013). The advent of CRISPR technology means that these testing experiments can be conducted relatively quickly (Hsu et al., 2014; Incontro et al., 2014).
All of these steps will require the creativity of new investigators approaching the problem of hair-bundle assembly from fresh perspectives. We are on the cusp of the truly exciting experiments: with a description of assembly at the structural level, a parts list that is likely near completion, and systematic experiments imminent, we should be able to soon determine how the hair cell controls stereocilia length, diameter, and spacing to make a functional bundle.
Acknowledgments
Work in my lab is supported by National Institutes of Health Grants R01 DC002368, R01 DC011034, and P30 DC005983. I thank Lori Vaskalis for the original scale stereocilium drawing.
Footnotes
REFERENCES
- Avenarius MR, Saylor KW, Lundeberg MR, Wilmarth PA, Shin JB, Spinelli KJ, Pagana JM, Andrade L, Kachar B, Choi D, et al. Correlation of actin crosslinker and capper expression levels with stereocilia growth phases. Mol Cell Proteomics. 2014;13:606–620. doi: 10.1074/mcp.M113.033704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009;12:553–558. doi: 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner A, Polge C, Lentze N, Auerbach D, Schlattner U. Yeast two-hybrid, a powerful tool for systems biology. Int J Mol Sci. 2009;10:2763–2788. doi: 10.3390/ijms10062763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, On D, Baker W, Collado MS, Corwin JT. Over half the hair cells in the mouse utricle first appear after birth, with significant numbers originating from early postnatal mitotic production in peripheral and striolar growth zones. J Assoc Res Otolaryngol. 2012;13:609–627. doi: 10.1007/s10162-012-0337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E, Michel V, de Monvel JB, Petit C. Coupling of the mechanotransduction machinery and F-actin polymerization in the cochlear hair bundles. Bioarchitecture. 2011;1:169–174. doi: 10.4161/bioa.1.4.17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Welch MD. A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol. 2010;11:237–251. doi: 10.1038/nrm2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR. A balance of form and function: planar polarity and development of the vestibular maculae. Semin Cell Dev Biol. 2013;24:490–498. doi: 10.1016/j.semcdb.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror AA, Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. doi: 10.1146/annurev-genet-102108-134135. [DOI] [PubMed] [Google Scholar]
- Drummond MC, Barzik M, Bird JE, Zhang DS, Lechene CP, Corey DP, Cunningham LL, Friedman TB. Live-cell imaging of actin dynamics reveals mechanisms of stereocilia length regulation in the inner ear. Nat Commun. 2015;6:6873. doi: 10.1038/ncomms7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durruthy-Durruthy R, Gottlieb A, Hartman BH, Waldhaus J, Laske RD, Altman R, Heller S. Reconstruction of the mouse otocyst and early neuroblast lineage at single-cell resolution. Cell. 2014;157:964–978. doi: 10.1016/j.cell.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Hudspeth AJ. High-purity isolation of bullfrog hair bundles and subcellular and topological localization of constituent proteins. J Cell Biol. 1991;112:625–640. doi: 10.1083/jcb.112.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, Müller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139:33–44. doi: 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Gates R, Lukashkin AN, Richardson GP. Hair-cell numbers continue to increase in the utricular macula of the early posthatch chick. J Neurocytol. 1999;28:851–861. doi: 10.1023/a:1007070121751. [DOI] [PubMed] [Google Scholar]
- Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. J Neurosci. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsley-Myers CM, Sipe CW, Wu DK, Lu X. Redundant functions of Rac GTPases in inner ear morphogenesis. Dev Biol. 2012;362:172–186. doi: 10.1016/j.ydbio.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney CM, Mahendrasingam S, Penn A, Fettiplace R. The concentrations of calcium buffering proteins in mammalian cochlear hair cells. J Neurosci. 2005;25:7867–7875. doi: 10.1523/JNEUROSCI.1196-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157:1262–1278. doi: 10.1016/j.cell.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incontro S, Asensio CS, Edwards RH, Nicoll RA. Efficient, complete deletion of synaptic proteins using CRISPR. Neuron. 2014;83:1051–1057. doi: 10.1016/j.neuron.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobo A, Hudspeth AJ. Reaction-diffusion model of hair-bundle morphogenesis. Proc Natl Acad Sci USA. 2014;111:15444–15449. doi: 10.1073/pnas.1417420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Parakkal M, Kurc M, Zhao Y, Gillespie PG. High-resolution structure of hair-cell tip links. Proc Natl Acad Sci USA. 2000;97:13336–13341. doi: 10.1073/pnas.97.24.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Falzarano PR, Simpson TH. Postnatal development of the hamster cochlea. II. Growth and differentiation of stereocilia bundles. J Comp Neurol. 1994;350:187–198. doi: 10.1002/cne.903500204. [DOI] [PubMed] [Google Scholar]
- Kindt KS, Finch G, Nicolson T. Kinocilia mediate mechanosensitivity in developing zebrafish hair cells. Dev Cell. 2012;23:329–341. doi: 10.1016/j.devcel.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasurier M, Gillespie PG. Hair-cell mechanotransduction and cochlear amplification. Neuron. 2005;48:403–415. doi: 10.1016/j.neuron.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Loomis PA, Zheng L, Sekerkova G, Changyaleket B, Mugnaini E, Bartles JR. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol. 2003;163:1045–1055. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, Kachar B. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol. 2011;21:167–172. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RC, Manor U, Salles FT, Grati M, Dose AC, Unrath WC, Quintero OA, Yengo CM, Kachar B. Myosin IIIB uses an actin-binding motif in its espin-1 cargo to reach the tips of actin protrusions. Curr Biol. 2012;22:320–325. doi: 10.1016/j.cub.2011.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan P, Chatterton P, Ikeda A, Ikeda S, Corey DP, Ervasti JM, Perrin BJ. Length regulation of mechanosensitive stereocilia depends on very slow actin dynamics and filament-severing proteins. Nat Commun. 2015;6:6855. doi: 10.1038/ncomms7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C, Richardson GP. Linking genes underlying deafness to hair-bundle development and function. Nat Neurosci. 2009;12:703–710. doi: 10.1038/nn.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. No question about exciting questions in cell biology. PLoS Biol. 2013;11:e1001734. doi: 10.1371/journal.pbio.1001734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salles FT, Merritt RCJ, Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dose AC, Kachar B. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol. 2009;11:443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Richter CP, Bartles JR. Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet. 2011;7:e1002032. doi: 10.1371/journal.pgen.1002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GMG, Barres BA, Corey DP. “Bundle-blot” purification and initial protein characterization of hair cell stereocilia. Proc Natl Acad Sci USA. 1989;86:4973–4977. doi: 10.1073/pnas.86.13.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JB, Krey JF, Hassan A, Metlagel Z, Tauscher AN, Pagana JM, Sherman NE, Jeffery ED, Spinelli KJ, Zhao H, et al. Molecular architecture of the chick vestibular hair bundle. Nat Neurosci. 2013;16:365–374. doi: 10.1038/nn.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son EJ, Ma JH, Ankamreddy H, Shin JO, Choi JY, Wu DK, Bok J. Conserved role of Sonic Hedgehog in tonotopic organization of the avian basilar papilla and mammalian cochlea. Proc Natl Acad Sci USA. 2015;112:3746–3751. doi: 10.1073/pnas.1417856112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanyan R, Frolenkov GI. Fast adaptation and Ca2+ sensitivity of the mechanotransducer require myosin-XVa in inner but not outer cochlear hair cells. J Neurosci. 2009;29:4023–4034. doi: 10.1523/JNEUROSCI.4566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syafrizayanti, Betzen C, Hoheisel JD, Kastelic D. Methods for analyzing and quantifying protein-protein interaction. Expert Rev Proteomics. 2014;11:107–120. doi: 10.1586/14789450.2014.875857. [DOI] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ, Mulroy MJ. The organization of actin filaments in the stereocilia of cochlear hair cells. J Cell Biol. 1980;86:244–259. doi: 10.1083/jcb.86.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol. 1992;8:257–274. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- Wu M, Singh AK. Single-cell protein analysis. Curr Opin Biotechnol. 2012;23:83–88. doi: 10.1016/j.copbio.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DS, Piazza V, Perrin BJ, Rzadzinska AK, Poczatek JC, Wang M, Prosser HM, Ervasti JM, Corey DP, Lechene CP. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature. 2012;481:520–524. doi: 10.1038/nature10745. [DOI] [PMC free article] [PubMed] [Google Scholar]