In the title compound, the Ir3+ cation is coordinated by two C atoms and four N atoms in a slightly distorted octahedral geometry. The asymmetric unit consists of one complex trication, three hexafluoridophosphate anions and three acetonitrile solvent molecules.
Keywords: crystal structure; iridium(III); cyclometalated; 4,4′:2′,2′′:4′′,4′′′-quaterpyridyl ligand
Abstract
In the title compound, [Ir(C11H8N)2(C28H20N8)](PF6)3·3CH3CN or [IrIII(ppy)2{(2-pym)2qpy2+}](PF6)3·3CH3CN (ppy = deprotonated 2-phenylpyridine, pym = pyrimidyl and qpy = 4,4′:2′,2′′:4′′,4′′′-quaterpyridyl), the Ir3+ cation is coordinated by two C atoms and four N atoms in a slightly distorted octahedral geometry. The asymmetric unit consists of one complex trication, three hexafluoridophosphate anions and three acetonitrile solvent molecules. The average Ir—C distance is 2.011 (14) Å, the average Ir—N(ppy) distance is 2.05 (6) Å and the average Ir—N(qpy) distance is longer at 2.132 (10) Å. The dihedral angles within the 4,4′-bipyridyl units are 31.5 (6) and 23.8 (7)°, while those between the 2-pym and attached pyridyl rings are rather smaller, at 11.7 (9) and 7.1 (9)°. The title compound was refined as a two-component inversion twin.
Chemical context
Iridium complexes of cyclometalating ligands have been studied widely, mainly due to their interesting photophysical properties (Flamigni et al., 2007 ▸; You & Nam, 2012 ▸; Ladouceur & Zysman-Colman, 2013 ▸). Complexes of the form [IrIII(ppy)2(N–N)]+ (N–N = 2,2′-bipyridyl or a related α-diimine ligand) are well known, and many examples have been structurally characterized (e.g. Ladouceur et al., 2010 ▸; Zhao et al., 2010 ▸; Constable et al., 2013 ▸; Schneider et al., 2014 ▸).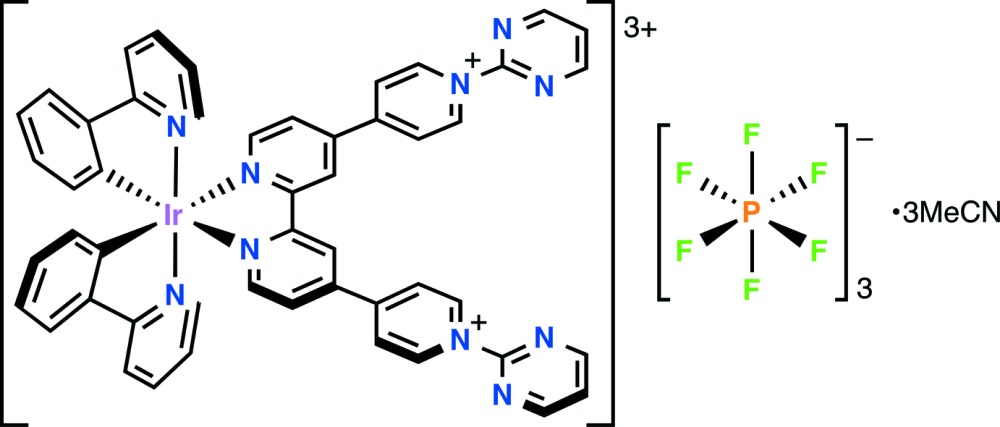
However, such compounds containing ligands with pyridinium substituents are scarce, and the only ones reported to our knowledge are the complex salts [IrIII(C–N)2(Me2qpy2+)][PF6]3 (L–L = ppy or benzo[h]quinoline) (Ahmad et al., 2014 ▸). We report here a related new compound and what appears to be the first X-ray crystal structure determination of an iridium complex containing a qpy-based ligand.
Structural commentary
The molecular structure (Fig. 1 ▸) of the complex cation in [IrIII(ppy)2{(2-pym)2qpy2+}][PF6]3·3CH3CN (I) is as indicated by 1H NMR spectroscopy, with a slightly distorted octahedral coordination geometry. The bite angle of the qpy-based ligand is 76.6 (2)°, while those of the ppy ligands are slightly larger at 80.1 (6) and 80.8 (5)°. As for other related complexes (Ladouceur et al., 2010 ▸; Zhao et al., 2010 ▸; Constable et al., 2013 ▸; Schneider et al., 2014 ▸), the strong trans effects of a σ-bonded phenyl ring (Coe & Glenwright, 2000 ▸) causes these units to adopt a cis orientation, so that the pyridyl rings of the ppy ligands are oriented trans. The structural trans effect of the phenyl rings is shown by the ca 0.08 Å lengthening of the Ir—N(qpy) distances [average = 2.132 (10) Å] with respect to the Ir—N(ppy) ones [average = 2.05 (6) Å]. The Ir—C distances (Table 1 ▸) are shorter still, with an average value of 2.01 (14) Å. All of the geometric parameters around the Ir3+ cation are similar to those reported for related structures.
Figure 1.
View of the molecular components of (I) (50% probability displacement ellipsoids)
Table 1. Selected geometric parameters (, ).
| C35Ir1 | 2.021(16) | Ir1N9 | 2.095(13) |
| C46Ir1 | 2.000(15) | Ir1N1 | 2.125(11) |
| Ir1N10 | 2.011(14) | Ir1N5 | 2.139(11) |
| C46Ir1N10 | 80.8(5) | C35Ir1N1 | 172.7(6) |
| C46Ir1C35 | 86.0(3) | N9Ir1N1 | 93.8(4) |
| N10Ir1C35 | 94.2(6) | C46Ir1N5 | 173.1(6) |
| C46Ir1N9 | 93.9(5) | N10Ir1N5 | 94.4(5) |
| N10Ir1N9 | 172.5(2) | C35Ir1N5 | 99.4(6) |
| C35Ir1N9 | 80.1(6) | N9Ir1N5 | 91.3(4) |
| C46Ir1N1 | 98.4(6) | N1Ir1N5 | 76.6(2) |
| N10Ir1N1 | 92.3(5) |
The dihedral angles within the 4,4′-bipyridyl units in (I) are larger than those [20.8 (6) and 21.0 (5)°] in the only other structurally characterized complex of the (2-pym)2qpy2+ ligand, [RuII(bpy)2{(2-pym)2qpy2+}][PF6]4 (bpy = 2,2′-bipyridyl) (Coe et al., 2011 ▸). On the other hand, the dihedral angles between the 2-pyrimidyl and attached pyridyl rings are closely similar in (I), whereas two quite different such angles are observed in [RuII(bpy)2{(2-pym)2qpy2+}][PF6]4 [6.0 (9) and 20.0 (5)°].
Supramolecular features
The unit cell contains four complex cations with their qpy units aligned approximately parallel (Fig. 2 ▸). There may be a weak π-stacking interaction between a 2-pym ring and one of the rings of the bpy fragment in an adjacent complex, with a centroid-to-centroid distance of 3.854 (8) Å and a dihedral angle of 9.8 (6)°. RuII complexes of (2-pym)2qpy2+ and related ligands show interesting non-linear optical (NLO) properties (Coe et al. 2005 ▸). In this context, crystal packing arrangements are of great importance because macroscopic polarity is necessary for the existence of bulk quadratic NLO effects. The space group Cc adopted by (I) is non-centrosymmetric, potentially affording a polar material that could display such NLO properties. However, the overall orientation of the dipoles formed by the electron-donating IrIII(ppy)2 units and the accepting (2-pym)2qpy2+ ligands is antiparallel (Fig. 3 ▸). Therefore, significant bulk quadratic NLO behaviour is not expected for this particular crystal form.
Figure 2.
Crystal packing diagram, viewed approximately along the b axis, showing the alignment of the qpy fragments. The H atoms, PF6 − anions and acetonitrile solvent molecules have been removed for clarity.
Figure 3.
Crystal packing diagram, viewed approximately along the a axis, showing the antiparallel alignment of the molecular dipoles (represented by arrows for the extreme left and right complexes). The H atoms, PF6 − anions and acetonitrile solvent molecules have been removed for clarity.
Synthesis and crystallization
The new compound (I) was synthesised simply by cleaving the commercial chloride-bridged dimer [IrIII(ppy)2Cl]2 with the proligand salt [(2-pym)2qpy2+]Cl2 (Coe et al., 2011 ▸) in refluxing 2-methoxyethanol/water.
[IrIII(ppy)2Cl]2 (40 mg, 0.037 mmol) and N′′,N′′′-di(2-pyrimidyl)-4,4′:2′,2′′:4′′,4′′′-quaterpyridinium chloride·2.3H2O (47 mg, 0.081 mmol) in argon-sparged 2-methoxyethanol/water (3:1, 10 ml) were heated at reflux for 20 h. After cooling to room temperature, the solvent was removed by rotary evaporation and the residue redissolved in a minimum volume of methanol to which was added an excess of solid NH4PF6. Cold water was added and the precipitate was filtered off and washed with water. The product was purified by column chromatography on silica gel, eluting with 0.1 M NH4PF6 in acetonitrile, to afford a brown–green solid. Yield: 68 mg (65%). Analysis calculated for C50H36F18IrN10P3·H2O: C 42.2, H 2.7, N 9.8%; found: C 42.0, H 2.5, N 9.6%. Spectroscopic analysis: 1H NMR (400 MHz, CD3CN, δ, p.p.m.) 10.12 (4H, dd, J = 7.5, 1.9 Hz), 9.18 (2H, d, J = 1.4 Hz), 9.13 (4H, d, J = 4.9 Hz), 8.68 (4H, dd, J = 7.4, 1.8 Hz), 8.31 (2H, d, J = 5.6 Hz), 8.13 (2H, dt, J = 8.1, 0.8 Hz), 8.05 (2H, dd, J = 5.7, 1.8 Hz), 7.93–7.87 (8H), 7.71 (2H, ddd, J = 5.9, 1.5, 0.7 Hz), 7.14–6.98 (8H), 6.33 (2H, dd, J = 7.6, 0.9 Hz). MALDI–MS m/z = 1405 ({M}+), 1260 ({M – PF6}+), 1115 ({M – 2PF6}+), 970 ({M – 3PF6}+).
Single crystals (amber plates) suitable for X-ray diffraction studies were grown by slow diffusion of diethyl ether vapour into an acetonitrile solution at room temperature.
Other Characterization
The complex salt (I) shows a relatively weak, broad visible absorption band at λmax = 562 nm (∊ = 1,800 M −1 dm3) in acetonitrile. Based on the results of time-dependent density functional theory (TD–DFT) calculations on the related complex [IrIII(ppy)2(Me2qpy2+)]3+ (Peers, 2012 ▸), this absorption is attributable to d→π* metal-to-ligand charge-transfer (MLCT) transitions directed towards the qpy-based ligand, with significant contributions by the ppy ligands to the donor orbitals introducing also ligand-to-ligand CT character. Below 500 nm, absorption increases steadily into the UV region, with another maximum at 378 nm (∊ = 16,600 M −1 dm3), and a shoulder at ca 410 nm. By way of contrast, the lowest energy band for [IrIII(ppy)2(Me2qpy2+)][PF6]3 appears at λmax = 531 nm (∊ = 1,200 M −1 dm3) in acetonitrile (Peers, 2012 ▸). The substantial red-shift of this band on moving to (I) is due to the enhanced electron-accepting ability of the N-(2-pyrimidyl)pyridinium groups. The higher intensity for (I) is a consequence of extended π-conjugation involving the 2-pym rings.
Cyclic voltammetric studies on (I) reveal an irreversible oxidation process at E pa = 1.43 V vs Ag–AgCl {acetonitrile, 0.1 M [N(n-Bu4)]PF6, 2 mm Pt disc working electrode, 100 mV s−1, ferrocene/ferrocenium standard at 0.44 V (ΔE p = 70–90 mV)}. The reductive region shows a reversible wave at E 1/2 = −0.29 V (ΔE p = 80 mV), followed by an irreversible process with E pc = −0.79 V. Based on the relative peak currents, the reversible wave is assigned as a two-electron process involving reduction of both pyridinium units. The redox behaviour of this complex can be rationalized with the aid of DFT results obtained for [IrIII(ppy)2(Me2qpy2+)]3+ (Peers, 2012 ▸). The irreversible oxidation wave corresponds with removing an electron from the HOMO comprising the Ir and ppy ligands. The first and second reductions involve adding electrons to the LUMO based on the (2-pym)2qpy2+ ligand. The oxidation occurs at the same E pa value for (I) and its methylated analogue, [IrIII(ppy)2(Me2qpy2+)][PF6]3, but the first two reductions appear as overlapping reversible waves at E 1/2 = −0.62 V (ΔE p = 70 mV) and E 1/2 = −0.73 V (ΔE p = 60 mV) in the latter compound. These waves can be resolved by using differential pulse voltammetry (potential increment = 2 mV, amplitude = 50 mV, pulse width = 0.01 s). The anodic shift in the reduction waves is consistent with the qpy-based ligand being more electron-deficient, and therefore easier to reduce, in (I). The lack of splitting of these waves in (I) indicates that electronic communication between the pyridyl radicals is diminished with respect to its methyl analogue. Interestingly, for the related compound [RuII(bpy)2{(2-pym)2qpy2+}][PF6]4, the first two reductions are irreversible under the same conditions using a glassy carbon working electrode (Coe et al., 2011 ▸).
Refinement
The structure was solved by direct methods. The two rings of one of the ppy ligands are indistinguishable by bond lengths, and the presented structure gives the lowest R factors. Crystal twinning is present. There is a pseudo-twofold axis that manifests itself as high correlation between parameters during refinement. The non-hydrogen atoms were refined anisotropically, but a rigid bond restraint (RIGU in SHELX) was applied for atoms with pseudo-symmetry-related counterparts. H atoms were included in calculated positions with C—H bond lengths of 0.95 (CH), 0.99 (CH2) and 0.98 (CH3) Å; U ĩso(H) values were fixed at 1.2U eq(C) except for CH3 where it was 1.5U eq(C). Crystal data, data collection and structure refinement details are given in Table 2 ▸.
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | [Ir(C11H8N)2(C28H20N8)](PF6)33C2H3N |
| M r | 1527.16 |
| Crystal system, space group | Monoclinic, C c |
| Temperature (K) | 100 |
| a, b, c () | 22.2647(16), 14.6139(11), 18.6288(14) |
| () | 102.447(1) |
| V (3) | 5918.9(8) |
| Z | 4 |
| Radiation type | Mo K |
| (mm1) | 2.45 |
| Crystal size (mm) | 0.25 0.20 0.03 |
| Data collection | |
| Diffractometer | Bruker SMART CCD area detector |
| Absorption correction | Multi-scan (SADABS; Bruker, 2001 ▸) |
| T min, T max | 0.697, 0.930 |
| No. of measured, independent and observed [I > 2(I)] reflections | 25080, 13146, 11295 |
| R int | 0.037 |
| (sin /)max (1) | 0.669 |
| Refinement | |
| R[F 2 > 2(F 2)], wR(F 2), S | 0.041, 0.093, 1.00 |
| No. of reflections | 13146 |
| No. of parameters | 824 |
| No. of restraints | 434 |
| H-atom treatment | H-atom parameters constrained |
| max, min (e 3) | 1.24, 1.00 |
| Absolute structure | Refined as an inversion twin |
| Absolute structure parameter | 0.384(7) |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015012463/zl2625sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015012463/zl2625Isup2.hkl
CCDC reference: 1409461
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank the BBSRC for support in the form of a PhD studentship (MKP). NSS is supported by an EPSRC Established Career Fellowship (grant EP/J020192/1) and was funded by a Royal Society Wolfson Merit Award.
supplementary crystallographic information
Crystal data
| [Ir(C11H8N)2(C28H20N8)](PF6)3·3C2H3N | F(000) = 3024 |
| Mr = 1527.16 | Dx = 1.714 Mg m−3 |
| Monoclinic, Cc | Mo Kα radiation, λ = 0.71073 Å |
| a = 22.2647 (16) Å | Cell parameters from 5333 reflections |
| b = 14.6139 (11) Å | θ = 2.2–23.6° |
| c = 18.6288 (14) Å | µ = 2.44 mm−1 |
| β = 102.447 (1)° | T = 100 K |
| V = 5918.9 (8) Å3 | Plate, brown |
| Z = 4 | 0.25 × 0.20 × 0.03 mm |
Data collection
| Bruker SMART CCD area-detector diffractometer | 11295 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.037 |
| Absorption correction: multi-scan (SADABS; Bruker, 2001) | θmax = 28.4°, θmin = 1.7° |
| Tmin = 0.697, Tmax = 0.930 | h = −29→29 |
| 25080 measured reflections | k = −19→19 |
| 13146 independent reflections | l = −24→24 |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.041 | w = 1/[σ2(Fo2) + (0.0493P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.093 | (Δ/σ)max < 0.001 |
| S = 1.00 | Δρmax = 1.24 e Å−3 |
| 13146 reflections | Δρmin = −1.00 e Å−3 |
| 824 parameters | Absolute structure: Refined as an inversion twin. |
| 434 restraints | Absolute structure parameter: 0.384 (7) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refined as a 2-component inversion twin. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.2259 (6) | 0.2672 (9) | 0.2388 (7) | 0.016 (2) | |
| C2 | 0.1948 (6) | 0.3457 (9) | 0.2574 (7) | 0.022 (3) | |
| H2 | 0.2117 | 0.4048 | 0.2540 | 0.027* | |
| C3 | 0.1400 (6) | 0.3367 (9) | 0.2803 (7) | 0.022 (3) | |
| C4 | 0.1148 (6) | 0.2499 (9) | 0.2826 (7) | 0.021 (3) | |
| H4 | 0.0773 | 0.2415 | 0.2982 | 0.025* | |
| C5 | 0.1461 (6) | 0.1756 (9) | 0.2613 (8) | 0.022 (3) | |
| H5 | 0.1283 | 0.1165 | 0.2612 | 0.027* | |
| C6 | 0.1124 (8) | 0.4180 (9) | 0.3043 (10) | 0.025 (3) | |
| C7 | 0.1177 (6) | 0.5038 (10) | 0.2731 (7) | 0.029 (3) | |
| H7 | 0.1422 | 0.5094 | 0.2374 | 0.035* | |
| C8 | 0.0887 (6) | 0.5808 (9) | 0.2923 (8) | 0.028 (3) | |
| H8 | 0.0926 | 0.6382 | 0.2699 | 0.033* | |
| C9 | 0.0490 (6) | 0.4917 (9) | 0.3774 (8) | 0.029 (3) | |
| H9 | 0.0257 | 0.4879 | 0.4144 | 0.034* | |
| C10 | 0.0776 (6) | 0.4132 (9) | 0.3577 (8) | 0.022 (2) | |
| H10 | 0.0732 | 0.3564 | 0.3809 | 0.027* | |
| C11 | 0.0220 (6) | 0.6533 (9) | 0.3638 (7) | 0.024 (3) | |
| C12 | −0.0487 (6) | 0.7102 (9) | 0.4174 (8) | 0.045 (4) | |
| H12 | −0.0782 | 0.7039 | 0.4471 | 0.055* | |
| C13 | −0.0393 (7) | 0.7960 (10) | 0.3933 (8) | 0.039 (3) | |
| H13 | −0.0618 | 0.8481 | 0.4030 | 0.047* | |
| C14 | 0.0060 (7) | 0.7995 (10) | 0.3538 (9) | 0.045 (4) | |
| H14 | 0.0163 | 0.8577 | 0.3371 | 0.054* | |
| C15 | 0.2866 (7) | 0.2711 (10) | 0.2175 (9) | 0.029 (3) | |
| C16 | 0.3129 (6) | 0.3483 (9) | 0.2000 (7) | 0.023 (3) | |
| H16 | 0.2934 | 0.4058 | 0.2024 | 0.028* | |
| C17 | 0.3691 (6) | 0.3434 (10) | 0.1782 (8) | 0.025 (3) | |
| C18 | 0.3968 (6) | 0.2579 (10) | 0.1791 (8) | 0.029 (3) | |
| H18 | 0.4362 | 0.2525 | 0.1675 | 0.035* | |
| C19 | 0.3678 (6) | 0.1829 (10) | 0.1964 (7) | 0.027 (3) | |
| H19 | 0.3869 | 0.1249 | 0.1962 | 0.032* | |
| C20 | 0.4021 (8) | 0.4263 (8) | 0.1574 (11) | 0.027 (4) | |
| C21 | 0.3913 (7) | 0.5118 (11) | 0.1809 (10) | 0.049 (5) | |
| H21 | 0.3612 | 0.5207 | 0.2094 | 0.058* | |
| C22 | 0.4228 (9) | 0.5822 (11) | 0.1639 (12) | 0.054 (6) | |
| H22 | 0.4137 | 0.6414 | 0.1799 | 0.065* | |
| C23 | 0.4759 (6) | 0.4923 (9) | 0.0972 (8) | 0.033 (3) | |
| H23 | 0.5045 | 0.4867 | 0.0663 | 0.039* | |
| C24 | 0.4456 (8) | 0.4188 (10) | 0.1122 (10) | 0.043 (4) | |
| H24 | 0.4531 | 0.3610 | 0.0925 | 0.051* | |
| C25 | 0.5026 (5) | 0.6526 (9) | 0.1111 (7) | 0.023 (2) | |
| C26 | 0.5170 (7) | 0.8075 (10) | 0.1180 (8) | 0.039 (3) | |
| H26 | 0.5070 | 0.8670 | 0.1324 | 0.047* | |
| C27 | 0.5625 (7) | 0.7970 (10) | 0.0824 (9) | 0.041 (3) | |
| H27 | 0.5830 | 0.8492 | 0.0688 | 0.049* | |
| C28 | 0.5797 (5) | 0.7123 (9) | 0.0654 (8) | 0.039 (3) | |
| H28 | 0.6146 | 0.7043 | 0.0444 | 0.047* | |
| C29 | 0.3416 (6) | 0.0041 (8) | 0.3583 (7) | 0.021 (2) | |
| C30 | 0.3697 (6) | −0.0094 (10) | 0.4323 (7) | 0.029 (3) | |
| H30 | 0.4010 | −0.0544 | 0.4455 | 0.035* | |
| C31 | 0.3525 (7) | 0.0409 (11) | 0.4848 (8) | 0.029 (3) | |
| H31 | 0.3710 | 0.0319 | 0.5352 | 0.035* | |
| C32 | 0.3089 (7) | 0.1038 (12) | 0.4643 (8) | 0.032 (3) | |
| H32 | 0.2968 | 0.1414 | 0.5002 | 0.038* | |
| C33 | 0.2812 (5) | 0.1147 (11) | 0.3905 (7) | 0.025 (3) | |
| H33 | 0.2495 | 0.1588 | 0.3764 | 0.030* | |
| C34 | 0.3566 (7) | −0.0476 (10) | 0.2978 (8) | 0.025 (3) | |
| C35 | 0.3202 (7) | −0.0283 (11) | 0.2284 (9) | 0.024 (3) | |
| C36 | 0.3307 (8) | −0.0773 (8) | 0.1672 (10) | 0.025 (4) | |
| H36 | 0.3059 | −0.0649 | 0.1200 | 0.030* | |
| C37 | 0.3771 (6) | −0.1442 (9) | 0.1744 (8) | 0.025 (3) | |
| H37 | 0.3836 | −0.1781 | 0.1332 | 0.030* | |
| C38 | 0.4124 (6) | −0.1580 (9) | 0.2432 (7) | 0.026 (3) | |
| H38 | 0.4454 | −0.2006 | 0.2492 | 0.032* | |
| C39 | 0.4022 (6) | −0.1135 (10) | 0.3030 (8) | 0.027 (3) | |
| H39 | 0.4269 | −0.1277 | 0.3499 | 0.032* | |
| C40 | 0.1739 (6) | −0.0114 (9) | 0.1020 (8) | 0.025 (3) | |
| C41 | 0.1474 (6) | −0.0268 (10) | 0.0296 (7) | 0.031 (3) | |
| H41 | 0.1184 | −0.0750 | 0.0171 | 0.037* | |
| C42 | 0.1623 (7) | 0.0272 (11) | −0.0272 (9) | 0.036 (3) | |
| H42 | 0.1429 | 0.0162 | −0.0771 | 0.044* | |
| C43 | 0.2074 (7) | 0.0991 (11) | −0.0083 (8) | 0.026 (3) | |
| H43 | 0.2188 | 0.1363 | −0.0450 | 0.031* | |
| C44 | 0.2327 (6) | 0.1113 (11) | 0.0641 (7) | 0.029 (3) | |
| H44 | 0.2619 | 0.1591 | 0.0770 | 0.034* | |
| C45 | 0.1616 (7) | −0.0586 (8) | 0.1663 (8) | 0.021 (3) | |
| C46 | 0.1983 (7) | −0.0308 (10) | 0.2348 (8) | 0.020 (3) | |
| C47 | 0.1897 (7) | −0.0784 (9) | 0.2961 (9) | 0.026 (4) | |
| H47 | 0.2142 | −0.0643 | 0.3432 | 0.031* | |
| C48 | 0.1464 (7) | −0.1454 (10) | 0.2891 (8) | 0.033 (4) | |
| H48 | 0.1405 | −0.1745 | 0.3326 | 0.040* | |
| C49 | 0.1101 (6) | −0.1740 (9) | 0.2227 (7) | 0.032 (3) | |
| H49 | 0.0807 | −0.2216 | 0.2205 | 0.038* | |
| C50 | 0.1188 (6) | −0.1296 (10) | 0.1591 (7) | 0.033 (3) | |
| H50 | 0.0960 | −0.1474 | 0.1120 | 0.039* | |
| C51 | 0.2934 (8) | 0.7144 (9) | 0.3228 (9) | 0.083 (5) | |
| H51A | 0.2700 | 0.7210 | 0.3614 | 0.125* | |
| H51B | 0.2848 | 0.7665 | 0.2890 | 0.125* | |
| H51C | 0.3375 | 0.7127 | 0.3452 | 0.125* | |
| C52 | 0.2755 (5) | 0.6290 (7) | 0.2821 (7) | 0.050 (3) | |
| C53 | 0.2087 (8) | 0.6642 (11) | 0.0818 (8) | 0.084 (5) | |
| H53A | 0.2270 | 0.7128 | 0.1157 | 0.126* | |
| H53B | 0.2356 | 0.6104 | 0.0892 | 0.126* | |
| H53C | 0.1684 | 0.6477 | 0.0911 | 0.126* | |
| C54 | 0.2017 (6) | 0.6948 (9) | 0.0098 (7) | 0.059 (3) | |
| C55 | 0.3156 (8) | 0.4400 (11) | 0.4141 (8) | 0.079 (4) | |
| H55A | 0.3164 | 0.4092 | 0.3676 | 0.118* | |
| H55B | 0.3409 | 0.4955 | 0.4184 | 0.118* | |
| H55C | 0.2732 | 0.4565 | 0.4152 | 0.118* | |
| C56 | 0.3386 (7) | 0.3822 (11) | 0.4716 (8) | 0.057 (4) | |
| F1 | 0.5218 (5) | 0.5503 (8) | 0.3177 (7) | 0.107 (4) | |
| F2 | 0.4542 (7) | 0.6128 (10) | 0.4395 (6) | 0.140 (5) | |
| F3 | 0.4492 (7) | 0.6520 (8) | 0.3254 (6) | 0.158 (6) | |
| F4 | 0.5169 (5) | 0.5071 (8) | 0.4329 (5) | 0.095 (4) | |
| F5 | 0.5426 (7) | 0.6515 (8) | 0.4094 (6) | 0.129 (5) | |
| F6 | 0.4350 (4) | 0.5105 (8) | 0.3415 (5) | 0.101 (3) | |
| F7 | 0.2000 (3) | 0.6676 (4) | 0.5720 (3) | 0.0510 (16) | |
| F8 | 0.2549 (3) | 0.5503 (5) | 0.5384 (4) | 0.062 (2) | |
| F9 | 0.2081 (4) | 0.5770 (5) | 0.4199 (4) | 0.068 (2) | |
| F10 | 0.1541 (4) | 0.6900 (7) | 0.4542 (5) | 0.063 (2) | |
| F11 | 0.2579 (3) | 0.6905 (5) | 0.4913 (4) | 0.066 (2) | |
| F12 | 0.1508 (3) | 0.5525 (5) | 0.5020 (5) | 0.068 (2) | |
| F13 | −0.0032 (5) | 0.6054 (9) | 0.1681 (5) | 0.109 (4) | |
| F14 | 0.0776 (3) | 0.5921 (8) | 0.1137 (5) | 0.079 (3) | |
| F15 | 0.0179 (4) | 0.6271 (6) | 0.0040 (5) | 0.072 (2) | |
| F16 | −0.0633 (3) | 0.6303 (5) | 0.0573 (4) | 0.062 (2) | |
| F17 | 0.0169 (3) | 0.7190 (5) | 0.0984 (4) | 0.065 (2) | |
| F18 | −0.0041 (4) | 0.5076 (5) | 0.0676 (7) | 0.102 (4) | |
| Ir1 | 0.25717 (3) | 0.07100 (2) | 0.22916 (3) | 0.01852 (8) | |
| N1 | 0.1991 (5) | 0.1828 (8) | 0.2413 (6) | 0.018 (2) | |
| N2 | 0.0540 (7) | 0.5722 (7) | 0.3447 (8) | 0.028 (3) | |
| N3 | −0.0202 (5) | 0.6343 (8) | 0.4028 (6) | 0.041 (3) | |
| N4 | 0.0357 (6) | 0.7290 (9) | 0.3376 (8) | 0.041 (3) | |
| N5 | 0.3110 (5) | 0.1880 (8) | 0.2146 (7) | 0.024 (3) | |
| N6 | 0.4664 (6) | 0.5746 (6) | 0.1255 (8) | 0.025 (3) | |
| N7 | 0.4839 (6) | 0.7316 (8) | 0.1344 (7) | 0.036 (3) | |
| N8 | 0.5457 (5) | 0.6371 (7) | 0.0792 (6) | 0.032 (2) | |
| N9 | 0.2980 (6) | 0.0656 (6) | 0.3415 (7) | 0.020 (3) | |
| N10 | 0.2188 (6) | 0.0582 (7) | 0.1215 (7) | 0.023 (3) | |
| N11 | 0.2633 (5) | 0.5614 (6) | 0.2520 (5) | 0.047 (3) | |
| N12 | 0.1991 (8) | 0.7186 (11) | −0.0486 (7) | 0.109 (6) | |
| N13 | 0.3612 (8) | 0.3294 (13) | 0.5157 (10) | 0.093 (6) | |
| P1 | 0.4866 (2) | 0.5830 (3) | 0.3762 (3) | 0.0604 (11) | |
| P2 | 0.20527 (14) | 0.6201 (2) | 0.49735 (18) | 0.0384 (7) | |
| P3 | 0.00657 (16) | 0.6120 (3) | 0.0852 (2) | 0.0445 (8) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.019 (4) | 0.016 (5) | 0.009 (5) | −0.002 (4) | 0.000 (3) | 0.002 (4) |
| C2 | 0.027 (5) | 0.019 (5) | 0.021 (6) | −0.001 (4) | 0.004 (4) | −0.001 (4) |
| C3 | 0.025 (5) | 0.023 (5) | 0.018 (6) | −0.001 (4) | 0.006 (4) | −0.001 (4) |
| C4 | 0.017 (5) | 0.024 (5) | 0.020 (6) | −0.003 (4) | 0.003 (4) | −0.001 (4) |
| C5 | 0.023 (5) | 0.015 (5) | 0.032 (8) | −0.005 (4) | 0.012 (5) | −0.005 (5) |
| C6 | 0.026 (7) | 0.022 (5) | 0.026 (7) | −0.004 (4) | 0.006 (6) | −0.001 (4) |
| C7 | 0.031 (6) | 0.033 (5) | 0.027 (6) | 0.001 (4) | 0.013 (5) | 0.003 (4) |
| C8 | 0.025 (6) | 0.031 (6) | 0.027 (5) | 0.002 (4) | 0.007 (4) | 0.003 (4) |
| C9 | 0.025 (5) | 0.031 (5) | 0.032 (5) | −0.002 (4) | 0.010 (4) | −0.002 (4) |
| C10 | 0.024 (5) | 0.019 (5) | 0.024 (5) | 0.003 (4) | 0.005 (4) | 0.002 (4) |
| C11 | 0.018 (5) | 0.029 (5) | 0.025 (6) | −0.001 (3) | 0.003 (4) | −0.004 (4) |
| C12 | 0.035 (7) | 0.031 (5) | 0.082 (10) | 0.005 (4) | 0.038 (7) | 0.003 (5) |
| C13 | 0.043 (6) | 0.028 (5) | 0.050 (7) | 0.000 (4) | 0.020 (6) | −0.006 (5) |
| C14 | 0.042 (7) | 0.021 (5) | 0.080 (10) | 0.009 (4) | 0.031 (7) | 0.015 (5) |
| C15 | 0.029 (5) | 0.028 (5) | 0.034 (8) | 0.000 (4) | 0.014 (5) | −0.001 (5) |
| C16 | 0.016 (5) | 0.027 (6) | 0.030 (7) | 0.001 (4) | 0.013 (4) | −0.001 (5) |
| C17 | 0.019 (5) | 0.026 (5) | 0.032 (8) | 0.000 (4) | 0.010 (5) | −0.001 (4) |
| C18 | 0.019 (6) | 0.028 (6) | 0.043 (9) | 0.001 (4) | 0.015 (5) | −0.001 (5) |
| C19 | 0.022 (5) | 0.035 (6) | 0.024 (7) | −0.003 (4) | 0.005 (5) | −0.001 (5) |
| C20 | 0.022 (7) | 0.026 (6) | 0.034 (8) | −0.005 (4) | 0.008 (6) | 0.000 (4) |
| C21 | 0.044 (8) | 0.029 (6) | 0.085 (11) | −0.007 (5) | 0.043 (8) | −0.011 (6) |
| C22 | 0.060 (10) | 0.029 (7) | 0.093 (13) | −0.012 (6) | 0.059 (10) | −0.017 (6) |
| C23 | 0.028 (6) | 0.021 (5) | 0.054 (8) | 0.005 (4) | 0.022 (6) | 0.002 (4) |
| C24 | 0.041 (8) | 0.024 (6) | 0.072 (10) | 0.004 (5) | 0.032 (7) | 0.006 (5) |
| C25 | 0.018 (5) | 0.027 (4) | 0.024 (6) | −0.001 (3) | 0.001 (4) | 0.002 (4) |
| C26 | 0.047 (6) | 0.020 (5) | 0.052 (7) | −0.015 (4) | 0.017 (5) | −0.003 (5) |
| C27 | 0.045 (7) | 0.025 (5) | 0.056 (8) | −0.011 (4) | 0.022 (6) | 0.002 (5) |
| C28 | 0.021 (5) | 0.042 (5) | 0.057 (8) | −0.007 (4) | 0.012 (5) | 0.008 (5) |
| C29 | 0.024 (5) | 0.015 (5) | 0.021 (4) | −0.004 (4) | 0.002 (3) | 0.000 (3) |
| C30 | 0.032 (6) | 0.033 (6) | 0.020 (5) | 0.002 (4) | −0.001 (4) | −0.002 (4) |
| C31 | 0.034 (7) | 0.036 (6) | 0.016 (5) | 0.000 (5) | 0.002 (4) | 0.003 (4) |
| C32 | 0.036 (7) | 0.040 (7) | 0.017 (6) | 0.002 (6) | 0.001 (5) | −0.003 (5) |
| C33 | 0.020 (6) | 0.033 (7) | 0.020 (5) | −0.004 (5) | 0.005 (4) | 0.003 (4) |
| C34 | 0.024 (6) | 0.027 (5) | 0.021 (5) | −0.001 (5) | 0.000 (4) | −0.001 (4) |
| C35 | 0.021 (6) | 0.023 (7) | 0.027 (5) | −0.002 (5) | 0.003 (4) | −0.003 (5) |
| C36 | 0.030 (7) | 0.020 (7) | 0.025 (6) | 0.005 (5) | 0.003 (5) | 0.003 (4) |
| C37 | 0.026 (5) | 0.021 (6) | 0.032 (6) | 0.003 (4) | 0.014 (4) | 0.003 (5) |
| C38 | 0.025 (5) | 0.020 (6) | 0.032 (5) | 0.006 (4) | 0.003 (4) | −0.001 (4) |
| C39 | 0.024 (5) | 0.029 (5) | 0.027 (5) | 0.001 (4) | 0.007 (4) | −0.001 (4) |
| C40 | 0.025 (5) | 0.019 (5) | 0.033 (5) | −0.003 (4) | 0.010 (4) | 0.001 (4) |
| C41 | 0.031 (6) | 0.032 (6) | 0.029 (5) | −0.009 (5) | 0.007 (4) | 0.005 (4) |
| C42 | 0.042 (7) | 0.044 (7) | 0.024 (6) | −0.008 (6) | 0.008 (5) | −0.001 (5) |
| C43 | 0.027 (6) | 0.028 (6) | 0.025 (6) | 0.006 (5) | 0.010 (5) | 0.003 (5) |
| C44 | 0.039 (8) | 0.022 (6) | 0.027 (6) | −0.004 (6) | 0.010 (5) | 0.006 (5) |
| C45 | 0.026 (6) | 0.020 (6) | 0.021 (6) | −0.001 (4) | 0.009 (5) | 0.005 (4) |
| C46 | 0.022 (7) | 0.020 (7) | 0.021 (6) | 0.003 (5) | 0.011 (5) | −0.002 (5) |
| C47 | 0.020 (6) | 0.036 (9) | 0.023 (7) | −0.004 (5) | 0.005 (5) | 0.007 (5) |
| C48 | 0.036 (7) | 0.042 (9) | 0.021 (6) | −0.003 (6) | 0.006 (5) | 0.006 (6) |
| C49 | 0.040 (7) | 0.017 (6) | 0.041 (7) | −0.009 (5) | 0.011 (5) | 0.004 (5) |
| C50 | 0.042 (7) | 0.030 (7) | 0.024 (6) | −0.018 (5) | 0.003 (5) | −0.008 (5) |
| C51 | 0.105 (12) | 0.045 (8) | 0.122 (13) | −0.019 (8) | 0.076 (11) | −0.025 (8) |
| C52 | 0.058 (7) | 0.033 (6) | 0.073 (8) | 0.000 (5) | 0.044 (6) | −0.001 (6) |
| C53 | 0.110 (12) | 0.086 (11) | 0.066 (7) | 0.013 (9) | 0.041 (7) | 0.010 (7) |
| C54 | 0.064 (8) | 0.058 (8) | 0.061 (6) | −0.007 (6) | 0.023 (6) | 0.010 (6) |
| C55 | 0.079 (11) | 0.088 (10) | 0.076 (9) | 0.018 (8) | 0.032 (8) | 0.003 (7) |
| C56 | 0.071 (10) | 0.060 (8) | 0.041 (7) | 0.001 (8) | 0.016 (7) | −0.019 (5) |
| F1 | 0.093 (7) | 0.144 (10) | 0.097 (9) | −0.042 (7) | 0.048 (7) | −0.016 (7) |
| F2 | 0.207 (14) | 0.163 (11) | 0.066 (7) | 0.032 (12) | 0.062 (8) | 0.009 (7) |
| F3 | 0.286 (17) | 0.090 (8) | 0.071 (7) | 0.108 (10) | −0.018 (8) | 0.007 (6) |
| F4 | 0.101 (7) | 0.099 (8) | 0.069 (6) | −0.028 (6) | −0.018 (5) | 0.019 (5) |
| F5 | 0.200 (13) | 0.091 (8) | 0.085 (7) | −0.057 (9) | 0.009 (9) | −0.017 (6) |
| F6 | 0.073 (6) | 0.112 (8) | 0.098 (7) | −0.013 (6) | −0.024 (5) | 0.024 (6) |
| F7 | 0.038 (3) | 0.067 (4) | 0.046 (3) | 0.011 (3) | 0.005 (3) | −0.020 (3) |
| F8 | 0.058 (4) | 0.057 (4) | 0.060 (4) | 0.024 (3) | −0.010 (3) | −0.011 (3) |
| F9 | 0.082 (5) | 0.077 (5) | 0.046 (4) | 0.021 (4) | 0.014 (4) | −0.023 (3) |
| F10 | 0.056 (5) | 0.064 (5) | 0.065 (5) | 0.025 (4) | 0.003 (4) | 0.000 (4) |
| F11 | 0.043 (4) | 0.068 (5) | 0.096 (6) | −0.005 (3) | 0.031 (4) | −0.009 (4) |
| F12 | 0.055 (5) | 0.070 (5) | 0.076 (5) | −0.022 (4) | 0.009 (4) | −0.011 (4) |
| F13 | 0.083 (6) | 0.194 (11) | 0.063 (6) | 0.087 (8) | 0.045 (5) | 0.054 (7) |
| F14 | 0.030 (4) | 0.139 (9) | 0.067 (5) | 0.016 (4) | 0.010 (4) | −0.012 (5) |
| F15 | 0.090 (6) | 0.071 (5) | 0.062 (5) | 0.023 (5) | 0.033 (4) | −0.004 (4) |
| F16 | 0.054 (4) | 0.065 (5) | 0.068 (5) | −0.011 (4) | 0.014 (4) | −0.039 (4) |
| F17 | 0.053 (4) | 0.058 (4) | 0.094 (5) | −0.021 (3) | 0.036 (4) | −0.041 (4) |
| F18 | 0.080 (6) | 0.024 (4) | 0.202 (13) | 0.002 (4) | 0.031 (8) | 0.002 (6) |
| Ir1 | 0.01925 (12) | 0.01816 (12) | 0.01878 (12) | 0.0000 (3) | 0.00550 (8) | 0.0008 (3) |
| N1 | 0.019 (4) | 0.017 (5) | 0.015 (5) | −0.005 (3) | −0.002 (4) | 0.003 (4) |
| N2 | 0.027 (5) | 0.030 (5) | 0.030 (5) | 0.008 (4) | 0.013 (4) | 0.004 (3) |
| N3 | 0.049 (6) | 0.029 (5) | 0.060 (7) | 0.001 (4) | 0.041 (5) | 0.001 (4) |
| N4 | 0.030 (5) | 0.034 (5) | 0.062 (7) | 0.002 (4) | 0.021 (5) | 0.004 (4) |
| N5 | 0.029 (5) | 0.020 (5) | 0.027 (6) | −0.005 (4) | 0.013 (5) | 0.003 (4) |
| N6 | 0.021 (5) | 0.021 (4) | 0.034 (6) | 0.003 (3) | 0.010 (4) | −0.002 (3) |
| N7 | 0.043 (6) | 0.021 (4) | 0.053 (6) | −0.006 (4) | 0.028 (5) | −0.005 (4) |
| N8 | 0.029 (4) | 0.030 (5) | 0.042 (6) | −0.001 (3) | 0.019 (4) | 0.005 (4) |
| N9 | 0.018 (5) | 0.020 (5) | 0.020 (5) | −0.007 (3) | 0.002 (4) | 0.002 (4) |
| N10 | 0.027 (6) | 0.019 (5) | 0.024 (5) | −0.001 (4) | 0.007 (4) | 0.005 (4) |
| N11 | 0.054 (6) | 0.036 (5) | 0.056 (7) | 0.004 (4) | 0.026 (6) | −0.006 (4) |
| N12 | 0.136 (13) | 0.121 (12) | 0.063 (7) | −0.068 (11) | 0.008 (7) | 0.025 (7) |
| N13 | 0.078 (11) | 0.098 (11) | 0.095 (11) | 0.003 (8) | 0.000 (9) | 0.015 (9) |
| P1 | 0.059 (3) | 0.055 (3) | 0.065 (2) | 0.0109 (19) | 0.010 (2) | 0.0109 (19) |
| P2 | 0.0353 (17) | 0.0401 (17) | 0.0383 (16) | 0.0025 (14) | 0.0047 (13) | −0.0089 (13) |
| P3 | 0.0349 (19) | 0.051 (2) | 0.0489 (19) | −0.0018 (15) | 0.0122 (15) | −0.0149 (17) |
Geometric parameters (Å, º)
| C1—N1 | 1.375 (16) | C33—H33 | 0.9500 |
| C1—C2 | 1.421 (18) | C34—C35 | 1.40 (2) |
| C1—C15 | 1.489 (9) | C34—C39 | 1.39 (2) |
| C2—C3 | 1.382 (17) | C35—C36 | 1.41 (2) |
| C2—H2 | 0.9500 | C35—Ir1 | 2.021 (16) |
| C3—C4 | 1.392 (18) | C36—C37 | 1.408 (19) |
| C3—C6 | 1.452 (19) | C36—H36 | 0.9500 |
| C4—C5 | 1.394 (17) | C37—C38 | 1.367 (19) |
| C4—H4 | 0.9500 | C37—H37 | 0.9500 |
| C5—N1 | 1.317 (15) | C38—C39 | 1.352 (18) |
| C5—H5 | 0.9500 | C38—H38 | 0.9500 |
| C6—C10 | 1.39 (2) | C39—H39 | 0.9500 |
| C6—C7 | 1.397 (18) | C40—C41 | 1.370 (18) |
| C7—C8 | 1.383 (19) | C40—N10 | 1.418 (16) |
| C7—H7 | 0.9500 | C40—C45 | 1.459 (17) |
| C8—N2 | 1.37 (2) | C41—C42 | 1.414 (19) |
| C8—H8 | 0.9500 | C41—H41 | 0.9500 |
| C9—N2 | 1.341 (16) | C42—C43 | 1.44 (2) |
| C9—C10 | 1.400 (17) | C42—H42 | 0.9500 |
| C9—H9 | 0.9500 | C43—C44 | 1.357 (19) |
| C10—H10 | 0.9500 | C43—H43 | 0.9500 |
| C11—N4 | 1.273 (17) | C44—N10 | 1.409 (18) |
| C11—N3 | 1.334 (14) | C44—H44 | 0.9500 |
| C11—N2 | 1.466 (16) | C45—C50 | 1.396 (18) |
| C12—N3 | 1.335 (15) | C45—C46 | 1.42 (2) |
| C12—C13 | 1.362 (18) | C46—C47 | 1.39 (2) |
| C12—H12 | 0.9500 | C46—Ir1 | 2.000 (15) |
| C13—C14 | 1.372 (18) | C47—C48 | 1.361 (19) |
| C13—H13 | 0.9500 | C47—H47 | 0.9500 |
| C14—N4 | 1.294 (18) | C48—C49 | 1.388 (19) |
| C14—H14 | 0.9500 | C48—H48 | 0.9500 |
| C15—N5 | 1.336 (18) | C49—C50 | 1.401 (17) |
| C15—C16 | 1.343 (19) | C49—H49 | 0.9500 |
| C16—C17 | 1.400 (16) | C50—H50 | 0.9500 |
| C16—H16 | 0.9500 | C51—C52 | 1.470 (17) |
| C17—C18 | 1.392 (19) | C51—H51A | 0.9800 |
| C17—C20 | 1.509 (19) | C51—H51B | 0.9800 |
| C18—C19 | 1.347 (19) | C51—H51C | 0.9800 |
| C18—H18 | 0.9500 | C52—N11 | 1.140 (13) |
| C19—N5 | 1.379 (16) | C53—C54 | 1.390 (18) |
| C19—H19 | 0.9500 | C53—H53A | 0.9800 |
| C20—C21 | 1.362 (19) | C53—H53B | 0.9800 |
| C20—C24 | 1.42 (2) | C53—H53C | 0.9800 |
| C21—C22 | 1.32 (2) | C54—N12 | 1.132 (16) |
| C21—H21 | 0.9500 | C55—C56 | 1.37 (2) |
| C22—N6 | 1.33 (2) | C55—H55A | 0.9800 |
| C22—H22 | 0.9500 | C55—H55B | 0.9800 |
| C23—C24 | 1.330 (19) | C55—H55C | 0.9800 |
| C23—N6 | 1.349 (15) | C56—N13 | 1.16 (2) |
| C23—H23 | 0.9500 | F1—P1 | 1.549 (12) |
| C24—H24 | 0.9500 | F2—P1 | 1.571 (12) |
| C25—N8 | 1.256 (14) | F3—P1 | 1.505 (10) |
| C25—N7 | 1.331 (16) | F4—P1 | 1.579 (11) |
| C25—N6 | 1.453 (16) | F5—P1 | 1.614 (12) |
| C26—C27 | 1.336 (17) | F6—P1 | 1.594 (10) |
| C26—N7 | 1.402 (16) | F7—P2 | 1.581 (6) |
| C26—H26 | 0.9500 | F8—P2 | 1.577 (7) |
| C27—C28 | 1.352 (19) | F9—P2 | 1.589 (7) |
| C27—H27 | 0.9500 | F10—P2 | 1.611 (9) |
| C28—N8 | 1.388 (15) | F11—P2 | 1.582 (8) |
| C28—H28 | 0.9500 | F12—P2 | 1.582 (8) |
| C29—N9 | 1.311 (16) | F13—P3 | 1.608 (9) |
| C29—C30 | 1.400 (16) | F14—P3 | 1.582 (8) |
| C29—C34 | 1.453 (19) | F15—P3 | 1.602 (9) |
| C30—C31 | 1.343 (18) | F16—P3 | 1.553 (8) |
| C30—H30 | 0.9500 | F17—P3 | 1.592 (8) |
| C31—C32 | 1.33 (2) | F18—P3 | 1.568 (9) |
| C31—H31 | 0.9500 | Ir1—N10 | 2.011 (14) |
| C32—C33 | 1.388 (18) | Ir1—N9 | 2.095 (13) |
| C32—H32 | 0.9500 | Ir1—N1 | 2.125 (11) |
| C33—N9 | 1.280 (18) | Ir1—N5 | 2.139 (11) |
| N1—C1—C2 | 118.6 (12) | C44—C43—H43 | 121.5 |
| N1—C1—C15 | 117.9 (14) | C42—C43—H43 | 121.5 |
| C2—C1—C15 | 123.5 (15) | C43—C44—N10 | 124.9 (14) |
| C3—C2—C1 | 120.5 (12) | C43—C44—H44 | 117.6 |
| C3—C2—H2 | 119.7 | N10—C44—H44 | 117.6 |
| C1—C2—H2 | 119.7 | C50—C45—C46 | 123.3 (12) |
| C4—C3—C2 | 119.0 (13) | C50—C45—C40 | 121.2 (12) |
| C4—C3—C6 | 122.4 (12) | C46—C45—C40 | 115.4 (12) |
| C2—C3—C6 | 118.5 (13) | C47—C46—C45 | 116.3 (14) |
| C3—C4—C5 | 118.1 (12) | C47—C46—Ir1 | 128.7 (12) |
| C3—C4—H4 | 120.9 | C45—C46—Ir1 | 115.0 (10) |
| C5—C4—H4 | 120.9 | C46—C47—C48 | 120.2 (15) |
| N1—C5—C4 | 123.5 (12) | C46—C47—H47 | 119.9 |
| N1—C5—H5 | 118.3 | C48—C47—H47 | 119.9 |
| C4—C5—H5 | 118.3 | C47—C48—C49 | 124.6 (15) |
| C10—C6—C7 | 117.0 (14) | C47—C48—H48 | 117.7 |
| C10—C6—C3 | 121.2 (12) | C49—C48—H48 | 117.7 |
| C7—C6—C3 | 121.8 (16) | C48—C49—C50 | 117.0 (12) |
| C8—C7—C6 | 122.3 (14) | C48—C49—H49 | 121.5 |
| C8—C7—H7 | 118.8 | C50—C49—H49 | 121.5 |
| C6—C7—H7 | 118.8 | C49—C50—C45 | 118.6 (12) |
| N2—C8—C7 | 118.4 (13) | C49—C50—H50 | 120.7 |
| N2—C8—H8 | 120.8 | C45—C50—H50 | 120.7 |
| C7—C8—H8 | 120.8 | C52—C51—H51A | 109.5 |
| N2—C9—C10 | 120.8 (13) | C52—C51—H51B | 109.5 |
| N2—C9—H9 | 119.6 | H51A—C51—H51B | 109.5 |
| C10—C9—H9 | 119.6 | C52—C51—H51C | 109.5 |
| C6—C10—C9 | 120.1 (13) | H51A—C51—H51C | 109.5 |
| C6—C10—H10 | 119.9 | H51B—C51—H51C | 109.5 |
| C9—C10—H10 | 119.9 | N11—C52—C51 | 177.7 (15) |
| N4—C11—N3 | 130.1 (13) | C54—C53—H53A | 109.5 |
| N4—C11—N2 | 116.1 (12) | C54—C53—H53B | 109.5 |
| N3—C11—N2 | 113.6 (12) | H53A—C53—H53B | 109.5 |
| N3—C12—C13 | 125.7 (13) | C54—C53—H53C | 109.5 |
| N3—C12—H12 | 117.2 | H53A—C53—H53C | 109.5 |
| C13—C12—H12 | 117.2 | H53B—C53—H53C | 109.5 |
| C12—C13—C14 | 113.3 (14) | N12—C54—C53 | 176.4 (18) |
| C12—C13—H13 | 123.4 | C56—C55—H55A | 109.5 |
| C14—C13—H13 | 123.4 | C56—C55—H55B | 109.5 |
| N4—C14—C13 | 124.4 (14) | H55A—C55—H55B | 109.5 |
| N4—C14—H14 | 117.8 | C56—C55—H55C | 109.5 |
| C13—C14—H14 | 117.8 | H55A—C55—H55C | 109.5 |
| N5—C15—C16 | 123.5 (14) | H55B—C55—H55C | 109.5 |
| N5—C15—C1 | 112.1 (15) | N13—C56—C55 | 173.4 (18) |
| C16—C15—C1 | 124.4 (16) | C46—Ir1—N10 | 80.8 (5) |
| C15—C16—C17 | 119.4 (13) | C46—Ir1—C35 | 86.0 (3) |
| C15—C16—H16 | 120.3 | N10—Ir1—C35 | 94.2 (6) |
| C17—C16—H16 | 120.3 | C46—Ir1—N9 | 93.9 (5) |
| C16—C17—C18 | 117.7 (13) | N10—Ir1—N9 | 172.5 (2) |
| C16—C17—C20 | 123.3 (13) | C35—Ir1—N9 | 80.1 (6) |
| C18—C17—C20 | 119.0 (12) | C46—Ir1—N1 | 98.4 (6) |
| C19—C18—C17 | 119.9 (13) | N10—Ir1—N1 | 92.3 (5) |
| C19—C18—H18 | 120.0 | C35—Ir1—N1 | 172.7 (6) |
| C17—C18—H18 | 120.0 | N9—Ir1—N1 | 93.8 (4) |
| C18—C19—N5 | 121.9 (14) | C46—Ir1—N5 | 173.1 (6) |
| C18—C19—H19 | 119.1 | N10—Ir1—N5 | 94.4 (5) |
| N5—C19—H19 | 119.1 | C35—Ir1—N5 | 99.4 (6) |
| C21—C20—C24 | 116.8 (14) | N9—Ir1—N5 | 91.3 (4) |
| C21—C20—C17 | 121.7 (16) | N1—Ir1—N5 | 76.6 (2) |
| C24—C20—C17 | 121.5 (13) | C5—N1—C1 | 120.2 (12) |
| C22—C21—C20 | 119.9 (16) | C5—N1—Ir1 | 124.8 (9) |
| C22—C21—H21 | 120.0 | C1—N1—Ir1 | 114.1 (8) |
| C20—C21—H21 | 120.0 | C9—N2—C8 | 121.2 (12) |
| C21—C22—N6 | 123.4 (15) | C9—N2—C11 | 120.4 (13) |
| C21—C22—H22 | 118.3 | C8—N2—C11 | 118.5 (11) |
| N6—C22—H22 | 118.3 | C12—N3—C11 | 111.1 (11) |
| C24—C23—N6 | 120.5 (14) | C11—N4—C14 | 115.3 (13) |
| C24—C23—H23 | 119.7 | C15—N5—C19 | 117.5 (12) |
| N6—C23—H23 | 119.7 | C15—N5—Ir1 | 118.5 (10) |
| C23—C24—C20 | 120.4 (14) | C19—N5—Ir1 | 123.8 (10) |
| C23—C24—H24 | 119.8 | C22—N6—C23 | 118.7 (12) |
| C20—C24—H24 | 119.8 | C22—N6—C25 | 122.1 (11) |
| N8—C25—N7 | 129.5 (12) | C23—N6—C25 | 119.2 (13) |
| N8—C25—N6 | 117.2 (12) | C25—N7—C26 | 113.6 (12) |
| N7—C25—N6 | 113.3 (11) | C25—N8—C28 | 116.6 (12) |
| C27—C26—N7 | 120.5 (14) | C33—N9—C29 | 122.0 (13) |
| C27—C26—H26 | 119.7 | C33—N9—Ir1 | 124.1 (10) |
| N7—C26—H26 | 119.7 | C29—N9—Ir1 | 113.8 (10) |
| C26—C27—C28 | 120.3 (14) | C44—N10—C40 | 117.2 (12) |
| C26—C27—H27 | 119.8 | C44—N10—Ir1 | 126.2 (10) |
| C28—C27—H27 | 119.8 | C40—N10—Ir1 | 116.6 (9) |
| C27—C28—N8 | 119.3 (12) | F3—P1—F1 | 93.4 (8) |
| C27—C28—H28 | 120.4 | F3—P1—F2 | 90.3 (8) |
| N8—C28—H28 | 120.4 | F1—P1—F2 | 176.2 (8) |
| N9—C29—C30 | 118.8 (13) | F3—P1—F4 | 171.8 (9) |
| N9—C29—C34 | 116.9 (12) | F1—P1—F4 | 92.6 (7) |
| C30—C29—C34 | 124.2 (12) | F2—P1—F4 | 83.8 (7) |
| C31—C30—C29 | 120.2 (13) | F3—P1—F6 | 86.4 (7) |
| C31—C30—H30 | 119.9 | F1—P1—F6 | 86.5 (6) |
| C29—C30—H30 | 119.9 | F2—P1—F6 | 94.7 (8) |
| C32—C31—C30 | 118.3 (13) | F4—P1—F6 | 88.4 (5) |
| C32—C31—H31 | 120.8 | F3—P1—F5 | 95.9 (8) |
| C30—C31—H31 | 120.8 | F1—P1—F5 | 89.7 (7) |
| C31—C32—C33 | 120.3 (15) | F2—P1—F5 | 88.9 (8) |
| C31—C32—H32 | 119.9 | F4—P1—F5 | 89.7 (6) |
| C33—C32—H32 | 119.9 | F6—P1—F5 | 175.7 (7) |
| N9—C33—C32 | 120.4 (14) | F8—P2—F12 | 91.9 (4) |
| N9—C33—H33 | 119.8 | F8—P2—F7 | 92.0 (4) |
| C32—C33—H33 | 119.8 | F12—P2—F7 | 91.3 (4) |
| C35—C34—C39 | 118.3 (14) | F8—P2—F11 | 90.2 (4) |
| C35—C34—C29 | 115.3 (13) | F12—P2—F11 | 177.9 (5) |
| C39—C34—C29 | 126.4 (13) | F7—P2—F11 | 88.5 (4) |
| C34—C35—C36 | 118.8 (15) | F8—P2—F9 | 91.3 (4) |
| C34—C35—Ir1 | 113.8 (12) | F12—P2—F9 | 89.0 (5) |
| C36—C35—Ir1 | 127.4 (12) | F7—P2—F9 | 176.7 (4) |
| C37—C36—C35 | 121.5 (16) | F11—P2—F9 | 91.0 (4) |
| C37—C36—H36 | 119.2 | F8—P2—F10 | 178.9 (5) |
| C35—C36—H36 | 119.2 | F12—P2—F10 | 87.5 (5) |
| C38—C37—C36 | 117.0 (14) | F7—P2—F10 | 89.0 (4) |
| C38—C37—H37 | 121.5 | F11—P2—F10 | 90.4 (5) |
| C36—C37—H37 | 121.5 | F9—P2—F10 | 87.7 (5) |
| C39—C38—C37 | 122.5 (13) | F16—P3—F18 | 90.1 (5) |
| C39—C38—H38 | 118.7 | F16—P3—F14 | 179.3 (6) |
| C37—C38—H38 | 118.7 | F18—P3—F14 | 89.2 (6) |
| C38—C39—C34 | 121.8 (13) | F16—P3—F17 | 89.0 (4) |
| C38—C39—H39 | 119.1 | F18—P3—F17 | 176.9 (6) |
| C34—C39—H39 | 119.1 | F14—P3—F17 | 91.7 (5) |
| C41—C40—N10 | 120.1 (12) | F16—P3—F15 | 90.6 (5) |
| C41—C40—C45 | 127.9 (12) | F18—P3—F15 | 89.1 (6) |
| N10—C40—C45 | 112.0 (12) | F14—P3—F15 | 89.6 (5) |
| C40—C41—C42 | 121.6 (13) | F17—P3—F15 | 87.9 (5) |
| C40—C41—H41 | 119.2 | F16—P3—F13 | 89.9 (5) |
| C42—C41—H41 | 119.2 | F18—P3—F13 | 95.6 (7) |
| C41—C42—C43 | 119.1 (13) | F14—P3—F13 | 90.0 (5) |
| C41—C42—H42 | 120.4 | F17—P3—F13 | 87.4 (6) |
| C43—C42—H42 | 120.4 | F15—P3—F13 | 175.3 (7) |
| C44—C43—C42 | 117.1 (14) | ||
| N1—C1—C2—C3 | 2.6 (18) | C41—C40—C45—C50 | 1 (2) |
| C15—C1—C2—C3 | −176.6 (10) | N10—C40—C45—C50 | −180.0 (13) |
| C1—C2—C3—C4 | −2.1 (19) | C41—C40—C45—C46 | 177.1 (14) |
| C1—C2—C3—C6 | 175.2 (13) | N10—C40—C45—C46 | −3.4 (18) |
| C2—C3—C4—C5 | −0.2 (19) | C50—C45—C46—C47 | 0 (2) |
| C6—C3—C4—C5 | −177.3 (14) | C40—C45—C46—C47 | −176.5 (13) |
| C3—C4—C5—N1 | 2 (2) | C50—C45—C46—Ir1 | 178.8 (12) |
| C4—C3—C6—C10 | 28 (2) | C40—C45—C46—Ir1 | 2.3 (17) |
| C2—C3—C6—C10 | −149.1 (15) | C45—C46—C47—C48 | −2 (2) |
| C4—C3—C6—C7 | −149.6 (14) | Ir1—C46—C47—C48 | 179.1 (12) |
| C2—C3—C6—C7 | 33 (2) | C46—C47—C48—C49 | 3 (2) |
| C10—C6—C7—C8 | −2 (2) | C47—C48—C49—C50 | −1 (2) |
| C3—C6—C7—C8 | 176.1 (14) | C48—C49—C50—C45 | −2 (2) |
| C6—C7—C8—N2 | 1 (2) | C46—C45—C50—C49 | 2 (2) |
| C7—C6—C10—C9 | 1 (2) | C40—C45—C50—C49 | 178.3 (13) |
| C3—C6—C10—C9 | −176.9 (14) | C4—C5—N1—C1 | −2 (2) |
| N2—C9—C10—C6 | 1 (2) | C4—C5—N1—Ir1 | 167.1 (10) |
| N3—C12—C13—C14 | −3 (2) | C2—C1—N1—C5 | −0.7 (18) |
| C12—C13—C14—N4 | 3 (3) | C15—C1—N1—C5 | 178.5 (10) |
| N1—C1—C15—N5 | −9.7 (9) | C2—C1—N1—Ir1 | −170.6 (9) |
| C2—C1—C15—N5 | 169.5 (15) | C15—C1—N1—Ir1 | 8.7 (10) |
| N1—C1—C15—C16 | 167.5 (16) | C10—C9—N2—C8 | −2 (2) |
| C2—C1—C15—C16 | −13.3 (11) | C10—C9—N2—C11 | 177.7 (13) |
| N5—C15—C16—C17 | −1 (2) | C7—C8—N2—C9 | 1 (2) |
| C1—C15—C16—C17 | −177.7 (10) | C7—C8—N2—C11 | −178.5 (12) |
| C15—C16—C17—C18 | −3 (2) | N4—C11—N2—C9 | 171.0 (14) |
| C15—C16—C17—C20 | 180.0 (15) | N3—C11—N2—C9 | −12.9 (19) |
| C16—C17—C18—C19 | 4 (2) | N4—C11—N2—C8 | −10 (2) |
| C20—C17—C18—C19 | −179.1 (14) | N3—C11—N2—C8 | 166.6 (13) |
| C17—C18—C19—N5 | −1 (2) | C13—C12—N3—C11 | 3 (2) |
| C16—C17—C20—C21 | 22 (3) | N4—C11—N3—C12 | −3 (2) |
| C18—C17—C20—C21 | −154.8 (16) | N2—C11—N3—C12 | −178.8 (12) |
| C16—C17—C20—C24 | −157.4 (17) | N3—C11—N4—C14 | 3 (2) |
| C18—C17—C20—C24 | 26 (2) | N2—C11—N4—C14 | 178.6 (14) |
| C24—C20—C21—C22 | −3 (3) | C13—C14—N4—C11 | −3 (3) |
| C17—C20—C21—C22 | 177.5 (18) | C16—C15—N5—C19 | 4 (2) |
| C20—C21—C22—N6 | −1 (3) | C1—C15—N5—C19 | −179.1 (9) |
| N6—C23—C24—C20 | 1 (3) | C16—C15—N5—Ir1 | −171.3 (12) |
| C21—C20—C24—C23 | 3 (3) | C1—C15—N5—Ir1 | 6.0 (13) |
| C17—C20—C24—C23 | −177.2 (15) | C18—C19—N5—C15 | −3 (2) |
| N7—C26—C27—C28 | −4 (2) | C18—C19—N5—Ir1 | 171.8 (11) |
| C26—C27—C28—N8 | 6 (2) | C21—C22—N6—C23 | 6 (3) |
| N9—C29—C30—C31 | −1 (2) | C21—C22—N6—C25 | −176.5 (18) |
| C34—C29—C30—C31 | −179.4 (14) | C24—C23—N6—C22 | −5 (2) |
| C29—C30—C31—C32 | −1 (2) | C24—C23—N6—C25 | 176.9 (14) |
| C30—C31—C32—C33 | 2 (3) | N8—C25—N6—C22 | 174.8 (17) |
| C31—C32—C33—N9 | −1 (2) | N7—C25—N6—C22 | −5 (2) |
| N9—C29—C34—C35 | −3 (2) | N8—C25—N6—C23 | −7.3 (19) |
| C30—C29—C34—C35 | 175.5 (13) | N7—C25—N6—C23 | 172.4 (13) |
| N9—C29—C34—C39 | 177.9 (14) | N8—C25—N7—C26 | 2 (2) |
| C30—C29—C34—C39 | −4 (2) | N6—C25—N7—C26 | −177.5 (12) |
| C39—C34—C35—C36 | 1 (2) | C27—C26—N7—C25 | 0 (2) |
| C29—C34—C35—C36 | −178.2 (13) | N7—C25—N8—C28 | 0 (2) |
| C39—C34—C35—Ir1 | −178.7 (12) | N6—C25—N8—C28 | 179.7 (12) |
| C29—C34—C35—Ir1 | 2.1 (18) | C27—C28—N8—C25 | −4.2 (19) |
| C34—C35—C36—C37 | −1 (2) | C32—C33—N9—C29 | 0 (2) |
| Ir1—C35—C36—C37 | 179.0 (11) | C32—C33—N9—Ir1 | 177.0 (11) |
| C35—C36—C37—C38 | −1 (2) | C30—C29—N9—C33 | 1 (2) |
| C36—C37—C38—C39 | 3 (2) | C34—C29—N9—C33 | 179.8 (13) |
| C37—C38—C39—C34 | −3 (2) | C30—C29—N9—Ir1 | −176.2 (10) |
| C35—C34—C39—C38 | 1 (2) | C34—C29—N9—Ir1 | 2.4 (16) |
| C29—C34—C39—C38 | 179.8 (13) | C43—C44—N10—C40 | −2 (2) |
| N10—C40—C41—C42 | −2 (2) | C43—C44—N10—Ir1 | 177.9 (12) |
| C45—C40—C41—C42 | 177.4 (15) | C41—C40—N10—C44 | 2 (2) |
| C40—C41—C42—C43 | 1 (2) | C45—C40—N10—C44 | −177.3 (12) |
| C41—C42—C43—C44 | −1 (2) | C41—C40—N10—Ir1 | −177.4 (10) |
| C42—C43—C44—N10 | 1 (2) | C45—C40—N10—Ir1 | 3.0 (15) |
References
- Ahmad, H., Wragg, A., Cullen, W., Wombwell, C., Meijer, A. J. H. M. & Thomas, J. A. (2014). Chem. Eur. J. 20, 3089–3096. [DOI] [PubMed]
- Bruker (2001). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2003). SMART and SAINT-Plus. Bruker AXS Inc., Madison, Wisconsin, USA.
- Coe, B. J. & Glenwright, S. J. (2000). Coord. Chem. Rev. 203, 5–80.
- Coe, B. J., Harper, E. C., Helliwell, M. & Ta, Y. T. (2011). Polyhedron, 30, 1830–1841.
- Coe, B. J., Harris, J. A., Jones, L. A., Brunschwig, B. S., Song, K., Clays, K., Garín, J., Orduna, J., Coles, S. J. & Hursthouse, M. B. (2005). J. Am. Chem. Soc. 127, 4845–4859. [DOI] [PubMed]
- Constable, E. C., Housecroft, C. E., Kopecky, P., Martin, C. J., Wright, I. A., Zampese, J. A., Bolink, H. J. & Pertegás, A. (2013). Dalton Trans. 42, 8086–8103. [DOI] [PubMed]
- Flamigni, L., Barbieri, A., Sabatini, C., Ventura, B. & Barigelletti, F. (2007). Top. Curr. Chem. 281, 143–203.
- Ladouceur, S., Fortin, D. & Zysman-Colman, E. (2010). Inorg. Chem. 49, 5625–5641. [DOI] [PubMed]
- Ladouceur, S. & Zysman-Colman, E. (2013). Eur. J. Inorg. Chem. pp. 2985–3007.
- Peers, M. K. (2012). PhD thesis, The University of Manchester, England.
- Schneider, G. E., Pertegás, A., Constable, E. C., Housecroft, C. E., Hostettler, N., Morris, C. D., Zampese, J. A., Bolink, H. J., Junquera-Hernández, J. M., Ortí, E. & Sessolo, M. (2014). J. Mater. Chem. C. 2, 7047–7055.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- You, Y.-M. & Nam, W.-W. (2012). Chem. Soc. Rev. 41, 7061–7084.
- Zhao, N., Wu, Y.-H., Shi, L.-X., Lin, Q.-P. & Chen, Z.-N. (2010). Dalton Trans. 39, 8288–8295. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989015012463/zl2625sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989015012463/zl2625Isup2.hkl
CCDC reference: 1409461
Additional supporting information: crystallographic information; 3D view; checkCIF report





