Abstract
The title compound [systematic name: [(1α,14α,16β)-20-ethyl-8,9,10-trihydroxy-1,14,16-trimethoxyaconitan-4-yl 2-aminobenzoate], C30H42N2O8, a natural C19-diterpenoid alkaloid, possesses an aconitane carbon skeleton with four six-membered rings and two five-membered rings. The fused ring system contains two chair, one boat, one twist-boat and two envelope conformations. Intramolecular N—H⋯O hydrogen bonds are observed between the amino and carbonyl groups. The molecules are linked together via O—H⋯O hydrogen bonds, forming a three-dimensional framework.
Keywords: crystal structure, C19-diterpenoid alkaloid, hydrogen bonding
Related literature
For the synthesis of the title compound, see: Wei et al. (1996 ▸). The absolute configuration of the title compound has been assigned to be the same as that reported for typical natural C19-diterpenoid alkaloids, see: Wang et al. (2007 ▸); He et al. (2008 ▸). The six-ring rigid-frame structure of the title compound is identical to that of lappaconitine and mesaconitine (Wang et al., 2007 ▸; He et al., 2008 ▸).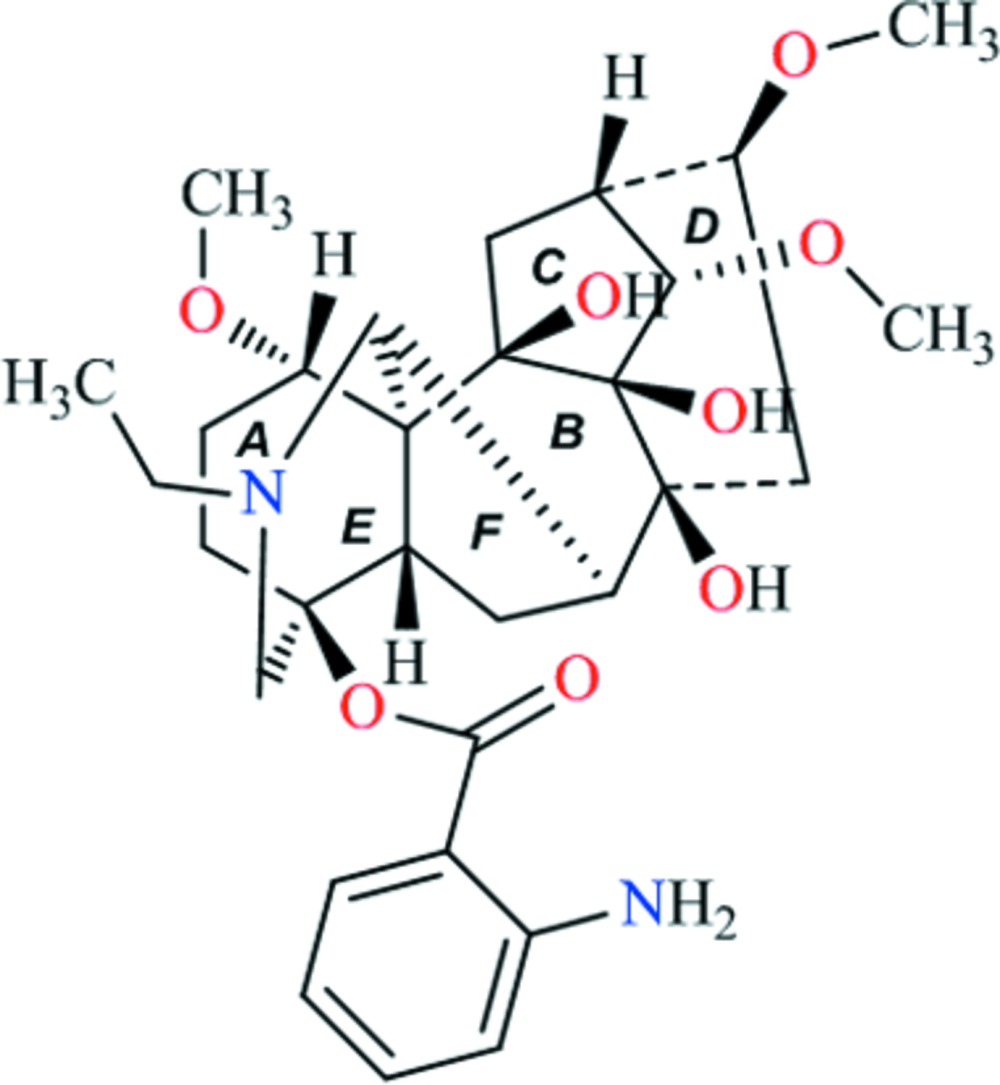
Experimental
Crystal data
C30H42N2O8
M r = 558.66
Orthorhombic,
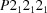
a = 9.6917 (5) Å
b = 16.0510 (7) Å
c = 18.3549 (7) Å
V = 2855.3 (2) Å3
Z = 4
Cu Kα radiation
μ = 0.77 mm−1
T = 173 K
0.32 × 0.32 × 0.28 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2002 ▸) T min = 0.791, T max = 0.813
8125 measured reflections
4528 independent reflections
3831 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.046
wR(F 2) = 0.126
S = 1.05
4528 reflections
388 parameters
60 restraints
H-atom parameters constrained
Δρmax = 0.46 e Å−3
Δρmin = −0.19 e Å−3
Data collection: SMART (Bruker, 2002 ▸); cell refinement: SAINT (Bruker, 2002 ▸); data reduction: SAINT; program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▸); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S205698901501258X/hg5447sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901501258X/hg5447Isup2.hkl
Supporting information file. DOI: 10.1107/S205698901501258X/hg5447Isup4.cdx
ORTEPII . DOI: 10.1107/S205698901501258X/hg5447fig1.tif
ORTEPII drawing of sepaconitine (I) with the atomic numbering scheme. Displacement ellipsoids are plotted at the 50% probability level.
c . DOI: 10.1107/S205698901501258X/hg5447fig2.tif
The packing of molecules in the crystal structure of sepaconitine (I), viewed along the c direction (Hydrogen bonds are shown as dashed lines).
CCDC reference: 1409635
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (, ).
| DHA | DH | HA | D A | DHA |
|---|---|---|---|---|
| O5H5AO6 | 0.84 | 2.34 | 2.909(3) | 126 |
| O4H4O5 | 0.84 | 2.27 | 2.684(3) | 111 |
| O4H4O3i | 0.84 | 1.94 | 2.713(3) | 153 |
| O3H3O4 | 0.84 | 1.99 | 2.524(3) | 121 |
| N1H1BO1 | 0.88 | 2.00 | 2.666(4) | 131 |
| N1H1AO8ii | 0.88 | 2.19 | 2.999(3) | 152 |
Symmetry codes: (i) 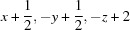 ; (ii)
; (ii) 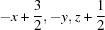 .
.
Acknowledgments
This project was supported by the Science and Technology Research and Development Projects of Shaanxi Province (grant No. 2013KJXX-74), the National Natural Science Foundation of China (grant No. 31200257) and the Science and Technology Program of Shaanxi Academy of Sciences (grant No. 2012k-04).
supplementary crystallographic information
S1. Comment
The title compound was isolated from the roots of Aconitum sinomontanum Nakai, collected in Taibai mountain of the Qinling area, Shaanxi province, People's Republic of China. the crystal structure determination of sepaconitine was carried out and the result reported here.
The molecular structure is shown in Fig. 1. The molecule has a rigid structure consisting of six main rings (A—F), which is identical with that of lappaconitine and mesaconitine (Wang et al., 2007; He et al., 2008). The six-membered rings A (C1/C2/C3/C4/C5/C11) and N-containing heterocyclic ring E (C4/C5/C11/C17/N2/C18) adopt chair conformations; The six-membered ring D (C8/C9/C14/C13/C16/C15) displays a boat conformation, but B (C7/C8/C9/C10/C11/C17) adopts a twist-boat conformation; the five-membered rings C (C9/C10/C12/C13/C14) and F (C5/C6/C7/C17/C11) adopt C14- and C 17-envelope conformations, respectively. Two cis-fused ring junctions involve rings A/E and also B/C. Two trans-fused ring junctions are observed between rings A/B and between E/F. Ring E is slightly flattened at C5 due to the presence of an ethyl-substituted N atom in the ring. The benzoate moiety attached to C4 is almost planar. The OCH3 group attached to C16 is disordered into two positions with site occupancies Factor of 0.5.
The crystal structure has an intra-molecular N—H···O hydrogen bond between the amino group and carbonyl O atom (Table 1). Inter-molecular N—H···O hydrogen bonds are observed in the crystal. The molecules are linked together via O—H```O hydrogen bonds in the c direction (Table 1, Fig. 2).
S2. Experimental
The title compound was isolated from the roots of Aconitum sinomontanum, using a method described previously (Wei et al., 1996). Colourless crystals were grown from methanol at room temperature by slow evaporation.
S3. Refinement
The hydrogen atoms were placed in calculated positions and refined as riding with Uiso(H) = 1.2 Ueq (C) or 1.5Ueq(C, O). The positions of methyl and hydroxy hydrogens were rotationally optimized. The absolute configuration of the title compound, sepaconitine, has been assigned to be the same as that reported for typical natural C19-diterpenoid alkaloids (Wang et al., 2007; He et al., 2008).
Figures
Fig. 1.
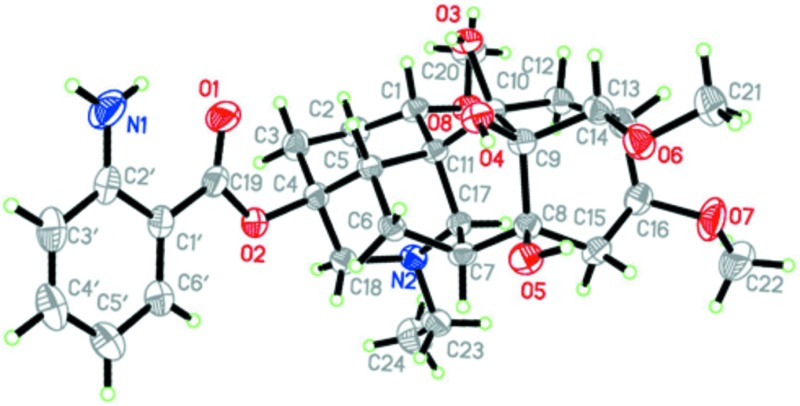
ORTEPII drawing of sepaconitine (I) with the atomic numbering scheme. Displacement ellipsoids are plotted at the 50% probability level.
Fig. 2.
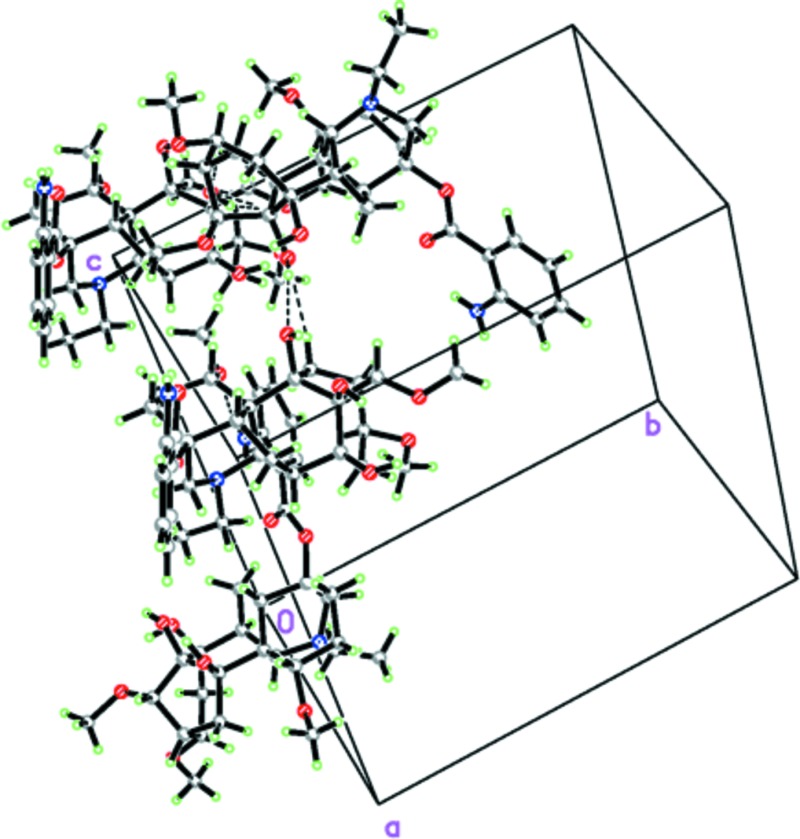
The packing of molecules in the crystal structure of sepaconitine (I), viewed along the c direction (Hydrogen bonds are shown as dashed lines).
Crystal data
| C30H42N2O8 | Dx = 1.300 Mg m−3 |
| Mr = 558.66 | Melting point = 250–252 K |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 2628 reflections |
| a = 9.6917 (5) Å | θ = 3.7–64.2° |
| b = 16.0510 (7) Å | µ = 0.77 mm−1 |
| c = 18.3549 (7) Å | T = 173 K |
| V = 2855.3 (2) Å3 | Block, colorless |
| Z = 4 | 0.32 × 0.32 × 0.28 mm |
| F(000) = 1200 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 4528 independent reflections |
| Radiation source: fine-focus sealed tube | 3831 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.029 |
| phi and ω scans | θmax = 65.0°, θmin = 3.7° |
| Absorption correction: multi-scan (SADABS; Bruker, 2002) | h = −7→11 |
| Tmin = 0.791, Tmax = 0.813 | k = −18→18 |
| 8125 measured reflections | l = −21→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.046 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.126 | H-atom parameters constrained |
| S = 1.05 | w = 1/[σ2(Fo2) + (0.0752P)2 + 0.0411P] where P = (Fo2 + 2Fc2)/3 |
| 4528 reflections | (Δ/σ)max = 0.001 |
| 388 parameters | Δρmax = 0.46 e Å−3 |
| 60 restraints | Δρmin = −0.19 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.5519 (3) | 0.04441 (18) | 0.93627 (14) | 0.0459 (6) | |
| H1 | 0.5224 | 0.0713 | 0.9829 | 0.055* | |
| C2 | 0.5401 (3) | −0.04952 (19) | 0.94729 (17) | 0.0574 (7) | |
| H2A | 0.4494 | −0.0626 | 0.9693 | 0.069* | |
| H2B | 0.5451 | −0.0777 | 0.8994 | 0.069* | |
| C3 | 0.6545 (3) | −0.08242 (19) | 0.99638 (17) | 0.0587 (7) | |
| H3A | 0.6440 | −0.0598 | 1.0462 | 0.070* | |
| H3B | 0.6503 | −0.1440 | 0.9990 | 0.070* | |
| C4 | 0.7912 (3) | −0.05507 (17) | 0.96421 (14) | 0.0472 (6) | |
| C5 | 0.8106 (3) | 0.03991 (16) | 0.96718 (13) | 0.0417 (6) | |
| H5 | 0.8054 | 0.0615 | 1.0182 | 0.050* | |
| C6 | 0.9497 (3) | 0.06134 (17) | 0.93102 (14) | 0.0457 (6) | |
| H6A | 1.0101 | 0.0117 | 0.9287 | 0.055* | |
| H6B | 0.9979 | 0.1059 | 0.9583 | 0.055* | |
| C7 | 0.9109 (3) | 0.09122 (16) | 0.85379 (13) | 0.0443 (6) | |
| H7 | 0.9740 | 0.0659 | 0.8168 | 0.053* | |
| C8 | 0.9157 (3) | 0.18622 (16) | 0.84910 (14) | 0.0445 (6) | |
| C9 | 0.8267 (3) | 0.22508 (16) | 0.91195 (13) | 0.0416 (6) | |
| C10 | 0.6911 (3) | 0.17703 (16) | 0.92456 (12) | 0.0405 (6) | |
| C11 | 0.6968 (3) | 0.07934 (16) | 0.91761 (13) | 0.0399 (5) | |
| C12 | 0.5904 (3) | 0.22027 (18) | 0.87023 (15) | 0.0496 (6) | |
| H12A | 0.5568 | 0.1796 | 0.8338 | 0.060* | |
| H12B | 0.5098 | 0.2431 | 0.8967 | 0.060* | |
| C13 | 0.6700 (3) | 0.29100 (18) | 0.83222 (15) | 0.0527 (7) | |
| H13 | 0.6084 | 0.3399 | 0.8230 | 0.063* | |
| C14 | 0.7783 (3) | 0.31184 (17) | 0.88965 (15) | 0.0512 (7) | |
| H14 | 0.7319 | 0.3389 | 0.9322 | 0.061* | |
| C15 | 0.8737 (3) | 0.2179 (2) | 0.77238 (14) | 0.0565 (7) | |
| H15A | 0.9459 | 0.2574 | 0.7560 | 0.068* | |
| H15B | 0.8767 | 0.1696 | 0.7389 | 0.068* | |
| C16 | 0.7352 (4) | 0.2605 (2) | 0.76183 (15) | 0.0604 (8) | |
| H16 | 0.6702 | 0.2225 | 0.7356 | 0.073* | |
| C17 | 0.7620 (3) | 0.05823 (16) | 0.84348 (12) | 0.0415 (6) | |
| H17 | 0.7146 | 0.0900 | 0.8039 | 0.050* | |
| C18 | 0.8127 (3) | −0.08526 (17) | 0.88536 (14) | 0.0515 (7) | |
| H18A | 0.9130 | −0.0893 | 0.8758 | 0.062* | |
| H18B | 0.7734 | −0.1419 | 0.8806 | 0.062* | |
| C19 | 0.9371 (3) | −0.08098 (19) | 1.07202 (15) | 0.0556 (7) | |
| C20 | 0.3182 (3) | 0.0800 (3) | 0.9099 (2) | 0.0738 (10) | |
| H20A | 0.2839 | 0.0276 | 0.9305 | 0.111* | |
| H20B | 0.2567 | 0.0982 | 0.8706 | 0.111* | |
| H20C | 0.3209 | 0.1227 | 0.9480 | 0.111* | |
| C21 | 0.8619 (4) | 0.4468 (2) | 0.8583 (2) | 0.0807 (10) | |
| H21A | 0.8080 | 0.4534 | 0.8135 | 0.121* | |
| H21B | 0.9477 | 0.4788 | 0.8543 | 0.121* | |
| H21C | 0.8081 | 0.4672 | 0.8999 | 0.121* | |
| C22 | 0.7499 (12) | 0.3113 (7) | 0.6432 (4) | 0.114 (3) | 0.578 (12) |
| H22A | 0.8162 | 0.2697 | 0.6260 | 0.170* | 0.578 (12) |
| H22B | 0.7575 | 0.3617 | 0.6134 | 0.170* | 0.578 (12) |
| H22C | 0.6561 | 0.2888 | 0.6393 | 0.170* | 0.578 (12) |
| C22' | 0.6743 (13) | 0.3573 (9) | 0.6707 (7) | 0.104 (4) | 0.422 (12) |
| H22D | 0.6386 | 0.3124 | 0.6398 | 0.155* | 0.422 (12) |
| H22E | 0.7364 | 0.3927 | 0.6421 | 0.155* | 0.422 (12) |
| H22F | 0.5972 | 0.3909 | 0.6890 | 0.155* | 0.422 (12) |
| C23 | 0.7964 (4) | −0.0559 (2) | 0.75690 (15) | 0.0625 (8) | |
| H23A | 0.8964 | −0.0683 | 0.7583 | 0.075* | |
| H23B | 0.7822 | −0.0086 | 0.7230 | 0.075* | |
| C24 | 0.7206 (6) | −0.1305 (2) | 0.7287 (2) | 0.0994 (14) | |
| H24A | 0.7516 | −0.1803 | 0.7548 | 0.149* | |
| H24B | 0.7394 | −0.1371 | 0.6765 | 0.149* | |
| H24C | 0.6213 | −0.1229 | 0.7362 | 0.149* | |
| C1' | 1.0692 (3) | −0.11619 (17) | 1.09396 (15) | 0.0525 (7) | |
| C2' | 1.1153 (4) | −0.10849 (19) | 1.16724 (16) | 0.0600 (8) | |
| C3' | 1.2446 (4) | −0.1426 (2) | 1.1845 (2) | 0.0732 (10) | |
| H3' | 1.2769 | −0.1389 | 1.2333 | 0.088* | |
| C4' | 1.3246 (4) | −0.1804 (2) | 1.1341 (2) | 0.0786 (11) | |
| H4' | 1.4116 | −0.2025 | 1.1480 | 0.094* | |
| C5' | 1.2809 (4) | −0.1874 (2) | 1.0619 (2) | 0.0739 (9) | |
| H5' | 1.3379 | −0.2134 | 1.0265 | 0.089* | |
| C6' | 1.1544 (4) | −0.15597 (19) | 1.04293 (17) | 0.0624 (8) | |
| H6' | 1.1236 | −0.1613 | 0.9940 | 0.075* | |
| N1 | 1.0397 (4) | −0.0685 (2) | 1.21841 (14) | 0.0858 (10) | |
| H1A | 1.0716 | −0.0636 | 1.2631 | 0.103* | |
| H1B | 0.9588 | −0.0473 | 1.2070 | 0.103* | |
| N2 | 0.7503 (2) | −0.03157 (13) | 0.82988 (10) | 0.0461 (5) | |
| O1 | 0.8590 (3) | −0.04165 (18) | 1.11148 (11) | 0.0792 (7) | |
| O2 | 0.9070 (2) | −0.09711 (12) | 1.00172 (10) | 0.0533 (5) | |
| O3 | 0.64582 (19) | 0.19706 (12) | 0.99729 (9) | 0.0492 (4) | |
| H3 | 0.7146 | 0.2082 | 1.0234 | 0.074* | |
| O4 | 0.8988 (2) | 0.22811 (12) | 0.97916 (9) | 0.0493 (5) | |
| H4 | 0.9790 | 0.2463 | 0.9721 | 0.074* | |
| O5 | 1.05780 (19) | 0.20907 (13) | 0.86056 (11) | 0.0567 (5) | |
| H5A | 1.0672 | 0.2605 | 0.8540 | 0.085* | |
| O6 | 0.8938 (2) | 0.36055 (12) | 0.86885 (12) | 0.0613 (5) | |
| O7 | 0.7770 (13) | 0.3303 (12) | 0.7134 (9) | 0.092 (3) | 0.578 (12) |
| O7' | 0.7307 (16) | 0.3306 (16) | 0.7166 (13) | 0.084 (3) | 0.422 (12) |
| O8 | 0.45273 (18) | 0.06771 (13) | 0.88177 (10) | 0.0525 (5) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0340 (14) | 0.0631 (16) | 0.0404 (12) | −0.0009 (13) | 0.0003 (11) | −0.0027 (11) |
| C2 | 0.0407 (16) | 0.0666 (18) | 0.0650 (17) | −0.0101 (15) | 0.0019 (13) | 0.0052 (14) |
| C3 | 0.0552 (19) | 0.0600 (16) | 0.0609 (17) | −0.0055 (15) | 0.0027 (15) | 0.0118 (13) |
| C4 | 0.0425 (15) | 0.0532 (15) | 0.0457 (14) | 0.0050 (13) | −0.0030 (11) | 0.0054 (11) |
| C5 | 0.0368 (14) | 0.0518 (14) | 0.0365 (12) | 0.0057 (12) | −0.0025 (10) | 0.0001 (10) |
| C6 | 0.0378 (14) | 0.0488 (14) | 0.0506 (14) | 0.0084 (12) | −0.0013 (12) | 0.0003 (11) |
| C7 | 0.0367 (14) | 0.0537 (14) | 0.0424 (13) | 0.0037 (12) | 0.0040 (11) | −0.0013 (11) |
| C8 | 0.0348 (14) | 0.0524 (14) | 0.0462 (13) | −0.0005 (12) | 0.0019 (11) | 0.0017 (11) |
| C9 | 0.0351 (13) | 0.0507 (13) | 0.0390 (12) | 0.0015 (12) | −0.0043 (10) | −0.0035 (10) |
| C10 | 0.0356 (14) | 0.0515 (14) | 0.0344 (12) | 0.0059 (11) | −0.0004 (10) | −0.0047 (10) |
| C11 | 0.0297 (13) | 0.0502 (13) | 0.0399 (12) | −0.0002 (11) | 0.0002 (10) | −0.0014 (10) |
| C12 | 0.0364 (14) | 0.0590 (16) | 0.0535 (15) | 0.0064 (13) | −0.0080 (12) | −0.0011 (12) |
| C13 | 0.0502 (17) | 0.0558 (15) | 0.0522 (15) | 0.0080 (14) | −0.0063 (13) | 0.0050 (11) |
| C14 | 0.0480 (16) | 0.0524 (15) | 0.0532 (15) | 0.0039 (13) | −0.0008 (13) | −0.0009 (12) |
| C15 | 0.0641 (19) | 0.0610 (16) | 0.0442 (14) | −0.0007 (15) | 0.0094 (13) | 0.0038 (12) |
| C16 | 0.069 (2) | 0.0665 (18) | 0.0457 (14) | 0.0031 (16) | −0.0044 (14) | 0.0074 (13) |
| C17 | 0.0373 (14) | 0.0496 (14) | 0.0376 (12) | 0.0042 (12) | −0.0020 (10) | 0.0008 (10) |
| C18 | 0.0553 (17) | 0.0493 (14) | 0.0499 (14) | 0.0053 (13) | 0.0003 (13) | −0.0020 (11) |
| C19 | 0.0615 (19) | 0.0633 (17) | 0.0420 (14) | 0.0028 (16) | −0.0019 (14) | 0.0099 (12) |
| C20 | 0.0339 (16) | 0.106 (3) | 0.082 (2) | 0.0081 (18) | 0.0051 (15) | −0.0039 (19) |
| C21 | 0.101 (3) | 0.0491 (16) | 0.092 (2) | 0.0004 (19) | 0.003 (2) | 0.0063 (16) |
| C22 | 0.135 (6) | 0.138 (6) | 0.067 (4) | 0.031 (5) | 0.013 (4) | 0.034 (4) |
| C22' | 0.097 (6) | 0.126 (6) | 0.088 (6) | 0.041 (5) | 0.003 (5) | 0.037 (5) |
| C23 | 0.072 (2) | 0.0711 (19) | 0.0441 (14) | 0.0178 (17) | −0.0071 (14) | −0.0102 (13) |
| C24 | 0.149 (4) | 0.077 (2) | 0.072 (2) | 0.017 (3) | −0.021 (3) | −0.0308 (19) |
| C1' | 0.0545 (17) | 0.0534 (15) | 0.0496 (14) | −0.0008 (14) | −0.0054 (13) | 0.0126 (12) |
| C2' | 0.067 (2) | 0.0612 (17) | 0.0519 (16) | −0.0053 (16) | −0.0100 (15) | 0.0187 (13) |
| C3' | 0.080 (3) | 0.069 (2) | 0.071 (2) | −0.004 (2) | −0.031 (2) | 0.0131 (17) |
| C4' | 0.069 (2) | 0.0639 (19) | 0.103 (3) | 0.0035 (19) | −0.032 (2) | 0.0146 (19) |
| C5' | 0.061 (2) | 0.069 (2) | 0.091 (2) | 0.0112 (18) | −0.0127 (18) | 0.0022 (17) |
| C6' | 0.064 (2) | 0.0612 (17) | 0.0618 (18) | 0.0054 (17) | −0.0093 (16) | 0.0075 (14) |
| N1 | 0.089 (2) | 0.125 (3) | 0.0431 (13) | 0.005 (2) | −0.0066 (14) | 0.0039 (15) |
| N2 | 0.0471 (13) | 0.0501 (12) | 0.0412 (11) | 0.0055 (11) | −0.0047 (10) | −0.0058 (8) |
| O1 | 0.0785 (16) | 0.1133 (18) | 0.0457 (11) | 0.0293 (15) | −0.0017 (11) | −0.0006 (11) |
| O2 | 0.0560 (12) | 0.0576 (11) | 0.0462 (10) | 0.0098 (9) | −0.0034 (9) | 0.0067 (8) |
| O3 | 0.0427 (11) | 0.0645 (11) | 0.0404 (9) | 0.0048 (9) | 0.0031 (8) | −0.0097 (8) |
| O4 | 0.0418 (11) | 0.0626 (11) | 0.0436 (9) | −0.0016 (9) | −0.0073 (8) | −0.0034 (8) |
| O5 | 0.0392 (11) | 0.0613 (11) | 0.0695 (12) | −0.0060 (9) | 0.0066 (9) | 0.0040 (10) |
| O6 | 0.0618 (13) | 0.0485 (10) | 0.0737 (13) | −0.0011 (10) | 0.0018 (10) | 0.0024 (9) |
| O7 | 0.104 (6) | 0.104 (3) | 0.069 (3) | 0.011 (6) | −0.007 (5) | 0.047 (3) |
| O7' | 0.087 (7) | 0.096 (4) | 0.067 (4) | 0.013 (6) | 0.001 (6) | 0.036 (3) |
| O8 | 0.0320 (10) | 0.0767 (12) | 0.0488 (10) | 0.0033 (9) | −0.0025 (8) | −0.0018 (9) |
Geometric parameters (Å, º)
| C1—O8 | 1.437 (3) | C17—H17 | 1.0000 |
| C1—C2 | 1.525 (4) | C18—N2 | 1.465 (3) |
| C1—C11 | 1.551 (3) | C18—H18A | 0.9900 |
| C1—H1 | 1.0000 | C18—H18B | 0.9900 |
| C2—C3 | 1.523 (4) | C19—O1 | 1.223 (4) |
| C2—H2A | 0.9900 | C19—O2 | 1.348 (3) |
| C2—H2B | 0.9900 | C19—C1' | 1.456 (4) |
| C3—C4 | 1.515 (4) | C20—O8 | 1.416 (3) |
| C3—H3A | 0.9900 | C20—H20A | 0.9800 |
| C3—H3B | 0.9900 | C20—H20B | 0.9800 |
| C4—O2 | 1.479 (3) | C20—H20C | 0.9800 |
| C4—C5 | 1.537 (4) | C21—O6 | 1.431 (4) |
| C4—C18 | 1.540 (4) | C21—H21A | 0.9800 |
| C5—C6 | 1.542 (4) | C21—H21B | 0.9800 |
| C5—C11 | 1.563 (3) | C21—H21C | 0.9800 |
| C5—H5 | 1.0000 | C22—O7 | 1.350 (19) |
| C6—C7 | 1.543 (3) | C22—H22A | 0.9800 |
| C6—H6A | 0.9900 | C22—H22B | 0.9800 |
| C6—H6B | 0.9900 | C22—H22C | 0.9800 |
| C7—C8 | 1.528 (4) | C22'—O7' | 1.09 (2) |
| C7—C17 | 1.549 (4) | C22'—H22D | 0.9800 |
| C7—H7 | 1.0000 | C22'—H22E | 0.9800 |
| C8—O5 | 1.440 (3) | C22'—H22F | 0.9800 |
| C8—C15 | 1.552 (4) | C23—N2 | 1.465 (3) |
| C8—C9 | 1.570 (4) | C23—C24 | 1.497 (5) |
| C9—O4 | 1.419 (3) | C23—H23A | 0.9900 |
| C9—C14 | 1.525 (4) | C23—H23B | 0.9900 |
| C9—C10 | 1.542 (4) | C24—H24A | 0.9800 |
| C10—O3 | 1.442 (3) | C24—H24B | 0.9800 |
| C10—C12 | 1.558 (3) | C24—H24C | 0.9800 |
| C10—C11 | 1.574 (4) | C1'—C6' | 1.402 (4) |
| C11—C17 | 1.538 (3) | C1'—C2' | 1.423 (4) |
| C12—C13 | 1.540 (4) | C2'—N1 | 1.353 (4) |
| C12—H12A | 0.9900 | C2'—C3' | 1.404 (5) |
| C12—H12B | 0.9900 | C3'—C4' | 1.352 (5) |
| C13—C16 | 1.520 (4) | C3'—H3' | 0.9500 |
| C13—C14 | 1.525 (4) | C4'—C5' | 1.397 (5) |
| C13—H13 | 1.0000 | C4'—H4' | 0.9500 |
| C14—O6 | 1.418 (4) | C5'—C6' | 1.371 (5) |
| C14—H14 | 1.0000 | C5'—H5' | 0.9500 |
| C15—C16 | 1.518 (5) | C6'—H6' | 0.9500 |
| C15—H15A | 0.9900 | N1—H1A | 0.8800 |
| C15—H15B | 0.9900 | N1—H1B | 0.8800 |
| C16—O7' | 1.40 (2) | O3—H3 | 0.8400 |
| C16—O7 | 1.487 (17) | O4—H4 | 0.8400 |
| C16—H16 | 1.0000 | O5—H5A | 0.8400 |
| C17—N2 | 1.467 (3) | ||
| O8—C1—C2 | 107.4 (2) | C8—C15—H15B | 107.4 |
| O8—C1—C11 | 111.0 (2) | H15A—C15—H15B | 106.9 |
| C2—C1—C11 | 117.0 (2) | O7'—C16—C15 | 117.7 (7) |
| O8—C1—H1 | 107.0 | O7—C16—C15 | 100.0 (5) |
| C2—C1—H1 | 107.0 | O7'—C16—C13 | 103.4 (9) |
| C11—C1—H1 | 107.0 | O7—C16—C13 | 112.2 (7) |
| C3—C2—C1 | 111.5 (2) | C15—C16—C13 | 113.8 (2) |
| C3—C2—H2A | 109.3 | O7'—C16—H16 | 100.7 |
| C1—C2—H2A | 109.3 | O7—C16—H16 | 110.1 |
| C3—C2—H2B | 109.3 | C15—C16—H16 | 110.1 |
| C1—C2—H2B | 109.3 | C13—C16—H16 | 110.1 |
| H2A—C2—H2B | 108.0 | N2—C17—C11 | 109.6 (2) |
| C4—C3—C2 | 107.8 (2) | N2—C17—C7 | 115.4 (2) |
| C4—C3—H3A | 110.1 | C11—C17—C7 | 101.48 (18) |
| C2—C3—H3A | 110.1 | N2—C17—H17 | 110.0 |
| C4—C3—H3B | 110.1 | C11—C17—H17 | 110.0 |
| C2—C3—H3B | 110.1 | C7—C17—H17 | 110.0 |
| H3A—C3—H3B | 108.5 | N2—C18—C4 | 114.3 (2) |
| O2—C4—C3 | 110.5 (2) | N2—C18—H18A | 108.7 |
| O2—C4—C5 | 110.1 (2) | C4—C18—H18A | 108.7 |
| C3—C4—C5 | 112.4 (2) | N2—C18—H18B | 108.7 |
| O2—C4—C18 | 101.0 (2) | C4—C18—H18B | 108.7 |
| C3—C4—C18 | 113.2 (2) | H18A—C18—H18B | 107.6 |
| C5—C4—C18 | 109.2 (2) | O1—C19—O2 | 122.1 (3) |
| C4—C5—C6 | 108.2 (2) | O1—C19—C1' | 125.5 (3) |
| C4—C5—C11 | 107.1 (2) | O2—C19—C1' | 112.4 (3) |
| C6—C5—C11 | 106.03 (19) | O8—C20—H20A | 109.5 |
| C4—C5—H5 | 111.7 | O8—C20—H20B | 109.5 |
| C6—C5—H5 | 111.7 | H20A—C20—H20B | 109.5 |
| C11—C5—H5 | 111.7 | O8—C20—H20C | 109.5 |
| C5—C6—C7 | 104.6 (2) | H20A—C20—H20C | 109.5 |
| C5—C6—H6A | 110.8 | H20B—C20—H20C | 109.5 |
| C7—C6—H6A | 110.8 | O6—C21—H21A | 109.5 |
| C5—C6—H6B | 110.8 | O6—C21—H21B | 109.5 |
| C7—C6—H6B | 110.8 | H21A—C21—H21B | 109.5 |
| H6A—C6—H6B | 108.9 | O6—C21—H21C | 109.5 |
| C8—C7—C6 | 110.8 (2) | H21A—C21—H21C | 109.5 |
| C8—C7—C17 | 111.3 (2) | H21B—C21—H21C | 109.5 |
| C6—C7—C17 | 103.5 (2) | O7'—C22'—H22D | 109.5 |
| C8—C7—H7 | 110.4 | O7'—C22'—H22E | 109.5 |
| C6—C7—H7 | 110.4 | H22D—C22'—H22E | 109.5 |
| C17—C7—H7 | 110.4 | O7'—C22'—H22F | 109.5 |
| O5—C8—C7 | 106.0 (2) | H22D—C22'—H22F | 109.5 |
| O5—C8—C15 | 107.5 (2) | H22E—C22'—H22F | 109.5 |
| C7—C8—C15 | 111.7 (2) | N2—C23—C24 | 112.3 (3) |
| O5—C8—C9 | 108.5 (2) | N2—C23—H23A | 109.1 |
| C7—C8—C9 | 109.8 (2) | C24—C23—H23A | 109.1 |
| C15—C8—C9 | 113.1 (2) | N2—C23—H23B | 109.1 |
| O4—C9—C14 | 110.7 (2) | C24—C23—H23B | 109.1 |
| O4—C9—C10 | 107.86 (19) | H23A—C23—H23B | 107.9 |
| C14—C9—C10 | 103.6 (2) | C23—C24—H24A | 109.5 |
| O4—C9—C8 | 112.5 (2) | C23—C24—H24B | 109.5 |
| C14—C9—C8 | 109.5 (2) | H24A—C24—H24B | 109.5 |
| C10—C9—C8 | 112.3 (2) | C23—C24—H24C | 109.5 |
| O3—C10—C9 | 106.68 (19) | H24A—C24—H24C | 109.5 |
| O3—C10—C12 | 107.6 (2) | H24B—C24—H24C | 109.5 |
| C9—C10—C12 | 102.42 (19) | C6'—C1'—C2' | 119.1 (3) |
| O3—C10—C11 | 107.93 (19) | C6'—C1'—C19 | 120.6 (3) |
| C9—C10—C11 | 117.1 (2) | C2'—C1'—C19 | 120.3 (3) |
| C12—C10—C11 | 114.4 (2) | N1—C2'—C3' | 120.7 (3) |
| C17—C11—C1 | 119.2 (2) | N1—C2'—C1' | 121.8 (3) |
| C17—C11—C5 | 97.83 (19) | C3'—C2'—C1' | 117.4 (3) |
| C1—C11—C5 | 111.34 (19) | C4'—C3'—C2' | 122.2 (3) |
| C17—C11—C10 | 107.79 (19) | C4'—C3'—H3' | 118.9 |
| C1—C11—C10 | 108.1 (2) | C2'—C3'—H3' | 118.9 |
| C5—C11—C10 | 112.4 (2) | C3'—C4'—C5' | 120.8 (3) |
| C13—C12—C10 | 107.7 (2) | C3'—C4'—H4' | 119.6 |
| C13—C12—H12A | 110.2 | C5'—C4'—H4' | 119.6 |
| C10—C12—H12A | 110.2 | C6'—C5'—C4' | 118.8 (3) |
| C13—C12—H12B | 110.2 | C6'—C5'—H5' | 120.6 |
| C10—C12—H12B | 110.2 | C4'—C5'—H5' | 120.6 |
| H12A—C12—H12B | 108.5 | C5'—C6'—C1' | 121.7 (3) |
| C16—C13—C14 | 111.9 (3) | C5'—C6'—H6' | 119.2 |
| C16—C13—C12 | 110.8 (2) | C1'—C6'—H6' | 119.2 |
| C14—C13—C12 | 101.2 (2) | C2'—N1—H1A | 120.0 |
| C16—C13—H13 | 110.9 | C2'—N1—H1B | 120.0 |
| C14—C13—H13 | 110.9 | H1A—N1—H1B | 120.0 |
| C12—C13—H13 | 110.9 | C18—N2—C23 | 110.7 (2) |
| O6—C14—C13 | 118.6 (2) | C18—N2—C17 | 115.3 (2) |
| O6—C14—C9 | 109.4 (2) | C23—N2—C17 | 113.2 (2) |
| C13—C14—C9 | 101.4 (2) | C19—O2—C4 | 121.5 (2) |
| O6—C14—H14 | 109.0 | C10—O3—H3 | 109.5 |
| C13—C14—H14 | 109.0 | C9—O4—H4 | 109.5 |
| C9—C14—H14 | 109.0 | C8—O5—H5A | 109.5 |
| C16—C15—C8 | 119.7 (2) | C14—O6—C21 | 113.5 (3) |
| C16—C15—H15A | 107.4 | C22—O7—C16 | 110.3 (13) |
| C8—C15—H15A | 107.4 | C22'—O7'—C16 | 142 (2) |
| C16—C15—H15B | 107.4 | C20—O8—C1 | 113.5 (2) |
| O8—C1—C2—C3 | 172.1 (2) | O4—C9—C14—O6 | 70.7 (3) |
| C11—C1—C2—C3 | 46.5 (3) | C10—C9—C14—O6 | −173.9 (2) |
| C1—C2—C3—C4 | −54.4 (3) | C8—C9—C14—O6 | −53.9 (3) |
| C2—C3—C4—O2 | −170.1 (2) | O4—C9—C14—C13 | −163.2 (2) |
| C2—C3—C4—C5 | 66.6 (3) | C10—C9—C14—C13 | −47.9 (2) |
| C2—C3—C4—C18 | −57.7 (3) | C8—C9—C14—C13 | 72.2 (3) |
| O2—C4—C5—C6 | 58.3 (2) | O5—C8—C15—C16 | −136.5 (3) |
| C3—C4—C5—C6 | −178.2 (2) | C7—C8—C15—C16 | 107.7 (3) |
| C18—C4—C5—C6 | −51.7 (3) | C9—C8—C15—C16 | −16.8 (4) |
| O2—C4—C5—C11 | 172.24 (18) | C8—C15—C16—O7' | 139.0 (12) |
| C3—C4—C5—C11 | −64.2 (3) | C8—C15—C16—O7 | 137.6 (8) |
| C18—C4—C5—C11 | 62.2 (3) | C8—C15—C16—C13 | 17.7 (4) |
| C4—C5—C6—C7 | 100.5 (2) | C14—C13—C16—O7' | −100.8 (8) |
| C11—C5—C6—C7 | −14.2 (3) | C12—C13—C16—O7' | 147.2 (8) |
| C5—C6—C7—C8 | 101.8 (2) | C14—C13—C16—O7 | −84.6 (6) |
| C5—C6—C7—C17 | −17.6 (2) | C12—C13—C16—O7 | 163.3 (6) |
| C6—C7—C8—O5 | 64.6 (3) | C14—C13—C16—C15 | 28.1 (3) |
| C17—C7—C8—O5 | 179.14 (19) | C12—C13—C16—C15 | −83.9 (3) |
| C6—C7—C8—C15 | −178.7 (2) | C1—C11—C17—N2 | −48.0 (3) |
| C17—C7—C8—C15 | −64.1 (3) | C5—C11—C17—N2 | 71.9 (2) |
| C6—C7—C8—C9 | −52.4 (3) | C10—C11—C17—N2 | −171.5 (2) |
| C17—C7—C8—C9 | 62.2 (3) | C1—C11—C17—C7 | −170.4 (2) |
| O5—C8—C9—O4 | −33.9 (3) | C5—C11—C17—C7 | −50.5 (2) |
| C7—C8—C9—O4 | 81.5 (3) | C10—C11—C17—C7 | 66.1 (2) |
| C15—C8—C9—O4 | −153.0 (2) | C8—C7—C17—N2 | 166.20 (19) |
| O5—C8—C9—C14 | 89.7 (2) | C6—C7—C17—N2 | −74.8 (2) |
| C7—C8—C9—C14 | −155.0 (2) | C8—C7—C17—C11 | −75.4 (2) |
| C15—C8—C9—C14 | −29.4 (3) | C6—C7—C17—C11 | 43.5 (2) |
| O5—C8—C9—C10 | −155.8 (2) | O2—C4—C18—N2 | −157.5 (2) |
| C7—C8—C9—C10 | −40.4 (3) | C3—C4—C18—N2 | 84.4 (3) |
| C15—C8—C9—C10 | 85.1 (3) | C5—C4—C18—N2 | −41.5 (3) |
| O4—C9—C10—O3 | 34.5 (3) | O1—C19—C1'—C6' | −176.2 (3) |
| C14—C9—C10—O3 | −82.8 (2) | O2—C19—C1'—C6' | 4.5 (4) |
| C8—C9—C10—O3 | 159.04 (19) | O1—C19—C1'—C2' | 2.0 (5) |
| O4—C9—C10—C12 | 147.5 (2) | O2—C19—C1'—C2' | −177.3 (3) |
| C14—C9—C10—C12 | 30.1 (2) | C6'—C1'—C2'—N1 | 177.9 (3) |
| C8—C9—C10—C12 | −88.0 (2) | C19—C1'—C2'—N1 | −0.3 (5) |
| O4—C9—C10—C11 | −86.4 (2) | C6'—C1'—C2'—C3' | −0.7 (4) |
| C14—C9—C10—C11 | 156.2 (2) | C19—C1'—C2'—C3' | −178.9 (3) |
| C8—C9—C10—C11 | 38.1 (3) | N1—C2'—C3'—C4' | −177.7 (3) |
| O8—C1—C11—C17 | −55.8 (3) | C1'—C2'—C3'—C4' | 0.9 (5) |
| C2—C1—C11—C17 | 68.0 (3) | C2'—C3'—C4'—C5' | −0.2 (5) |
| O8—C1—C11—C5 | −168.5 (2) | C3'—C4'—C5'—C6' | −0.8 (5) |
| C2—C1—C11—C5 | −44.7 (3) | C4'—C5'—C6'—C1' | 0.9 (5) |
| O8—C1—C11—C10 | 67.6 (3) | C2'—C1'—C6'—C5' | −0.2 (5) |
| C2—C1—C11—C10 | −168.6 (2) | C19—C1'—C6'—C5' | 178.0 (3) |
| C4—C5—C11—C17 | −75.3 (2) | C4—C18—N2—C23 | 169.7 (2) |
| C6—C5—C11—C17 | 40.1 (2) | C4—C18—N2—C17 | 39.6 (3) |
| C4—C5—C11—C1 | 50.3 (3) | C24—C23—N2—C18 | 79.2 (3) |
| C6—C5—C11—C1 | 165.7 (2) | C24—C23—N2—C17 | −149.5 (3) |
| C4—C5—C11—C10 | 171.7 (2) | C11—C17—N2—C18 | −57.3 (3) |
| C6—C5—C11—C10 | −72.9 (2) | C7—C17—N2—C18 | 56.4 (3) |
| O3—C10—C11—C17 | −173.14 (18) | C11—C17—N2—C23 | 173.8 (2) |
| C9—C10—C11—C17 | −52.8 (3) | C7—C17—N2—C23 | −72.5 (3) |
| C12—C10—C11—C17 | 67.1 (3) | O1—C19—O2—C4 | 11.8 (4) |
| O3—C10—C11—C1 | 56.8 (2) | C1'—C19—O2—C4 | −168.9 (2) |
| C9—C10—C11—C1 | 177.13 (19) | C3—C4—O2—C19 | −67.1 (3) |
| C12—C10—C11—C1 | −63.0 (2) | C5—C4—O2—C19 | 57.5 (3) |
| O3—C10—C11—C5 | −66.5 (2) | C18—C4—O2—C19 | 172.8 (2) |
| C9—C10—C11—C5 | 53.9 (3) | C13—C14—O6—C21 | 74.1 (3) |
| C12—C10—C11—C5 | 173.8 (2) | C9—C14—O6—C21 | −170.5 (2) |
| O3—C10—C12—C13 | 110.3 (2) | O7'—C16—O7—C22 | −78 (5) |
| C9—C10—C12—C13 | −1.9 (2) | C15—C16—O7—C22 | 98.3 (9) |
| C11—C10—C12—C13 | −129.7 (2) | C13—C16—O7—C22 | −140.7 (8) |
| C10—C12—C13—C16 | 92.0 (3) | O7—C16—O7'—C22' | 135 (8) |
| C10—C12—C13—C14 | −26.7 (3) | C15—C16—O7'—C22' | 130 (3) |
| C16—C13—C14—O6 | 46.8 (3) | C13—C16—O7'—C22' | −103 (3) |
| C12—C13—C14—O6 | 164.9 (2) | C2—C1—O8—C20 | 83.5 (3) |
| C16—C13—C14—C9 | −72.9 (3) | C11—C1—O8—C20 | −147.4 (3) |
| C12—C13—C14—C9 | 45.1 (3) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H5A···O6 | 0.84 | 2.34 | 2.909 (3) | 126 |
| O4—H4···O5 | 0.84 | 2.27 | 2.684 (3) | 111 |
| O4—H4···O3i | 0.84 | 1.94 | 2.713 (3) | 153 |
| O3—H3···O4 | 0.84 | 1.99 | 2.524 (3) | 121 |
| N1—H1B···O1 | 0.88 | 2.00 | 2.666 (4) | 131 |
| N1—H1A···O8ii | 0.88 | 2.19 | 2.999 (3) | 152 |
Symmetry codes: (i) x+1/2, −y+1/2, −z+2; (ii) −x+3/2, −y, z+1/2.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: HG5447).
References
- Bruker (2002). SADABS, SAINT and SMART. Bruker AXS Inc., Madison, Wisconsin, USA.
- He, D.-H., Zhu, Y.-C. & Hu, A.-X. (2008). Acta Cryst. E64, o1033–o1034. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wang, Y.-P., Sun, W.-X., Zhang, J., Liu, H.-S. & Wen, H.-H. (2007). Acta Cryst. E63, o1645–o1647.
- Wei, X.-Y., Wei, B.-Y. & Zhang, J. (1996). Acta Botanica Sin. 38, 995–997.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S205698901501258X/hg5447sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S205698901501258X/hg5447Isup2.hkl
Supporting information file. DOI: 10.1107/S205698901501258X/hg5447Isup4.cdx
ORTEPII . DOI: 10.1107/S205698901501258X/hg5447fig1.tif
ORTEPII drawing of sepaconitine (I) with the atomic numbering scheme. Displacement ellipsoids are plotted at the 50% probability level.
c . DOI: 10.1107/S205698901501258X/hg5447fig2.tif
The packing of molecules in the crystal structure of sepaconitine (I), viewed along the c direction (Hydrogen bonds are shown as dashed lines).
CCDC reference: 1409635
Additional supporting information: crystallographic information; 3D view; checkCIF report


