Abstract
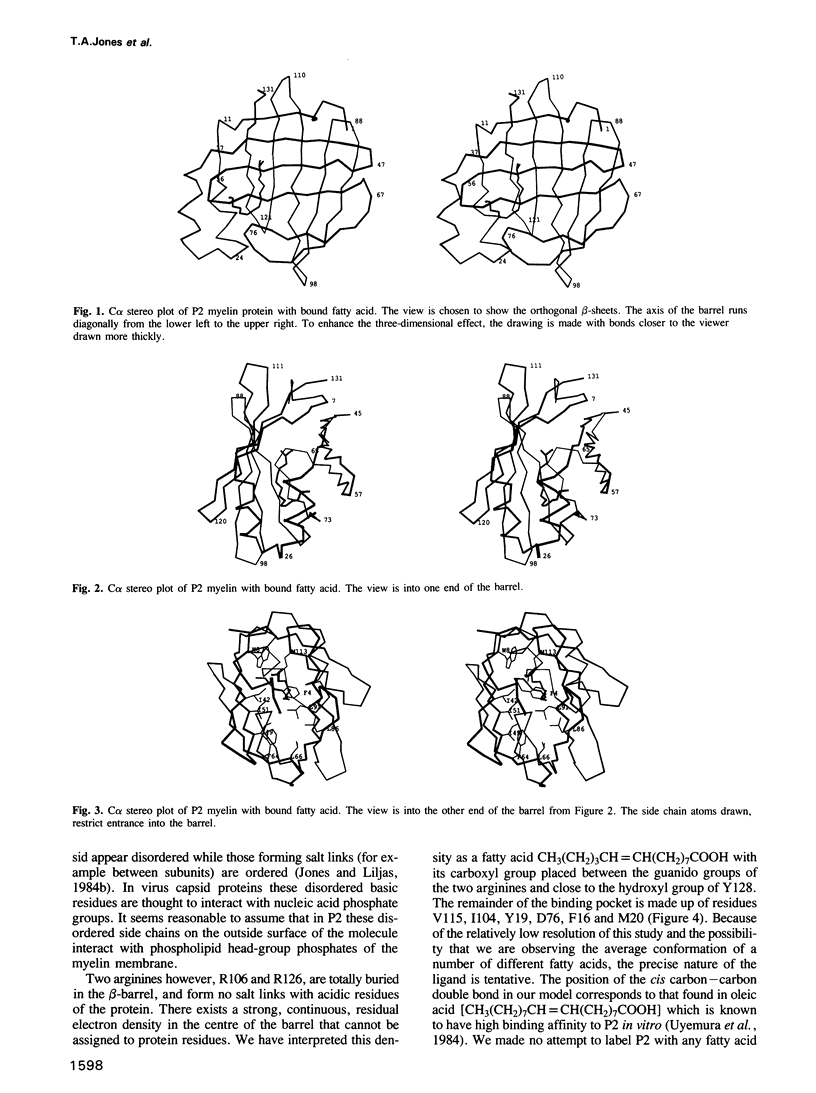
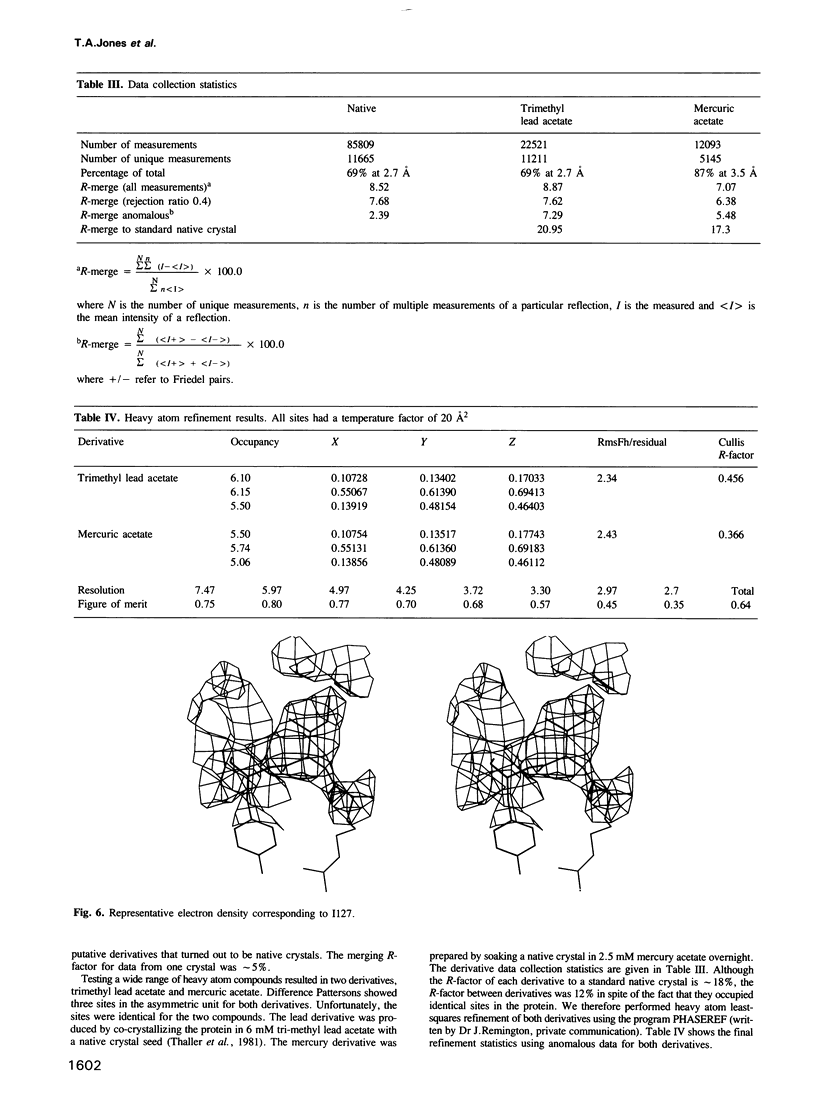
The three-dimensional structure of P2 protein from peripheral nervous system myelin has been determined at 2.7 A resolution by X-ray crystallography. The single isomorphous replacement/anomalous map was interpreted using skeletonized electron density on a computer graphics system. An atomic model was built using fragment fitting. The structure forms a compact 10-stranded up-and-down beta-barrel which encapsulates residual electron density that we interpret as a fatty acid molecule. This beta-barrel shows some similarity to, but is different from, the retinol binding protein family of structures. The relationship of the P2 structure to a family of cytoplasmic, lipid binding proteins is described.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpers D. H., Strauss A. W., Ockner R. K., Bass N. M., Gordon J. I. Cloning of a cDNA encoding rat intestinal fatty acid binding protein. Proc Natl Acad Sci U S A. 1984 Jan;81(2):313–317. doi: 10.1073/pnas.81.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashor M. M., Toft D. O., Chytil F. In vitro binding of retinol to rat-tissue components. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3483–3487. doi: 10.1073/pnas.70.12.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfors T., Sedzik J., Unge T., Fridborg K., Jones T. A., Weise M. Crystallization of P2 myelin protein. J Mol Biol. 1987 Nov 20;198(2):357–358. doi: 10.1016/0022-2836(87)90319-6. [DOI] [PubMed] [Google Scholar]
- Bernlohr D. A., Angus C. W., Lane M. D., Bolanowski M. A., Kelly T. J., Jr Expression of specific mRNAs during adipose differentiation: identification of an mRNA encoding a homologue of myelin P2 protein. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5468–5472. doi: 10.1073/pnas.81.17.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley S. K., Petsko G. A. Amino-aromatic interactions in proteins. FEBS Lett. 1986 Jul 28;203(2):139–143. doi: 10.1016/0014-5793(86)80730-x. [DOI] [PubMed] [Google Scholar]
- Chothia C., Janin J. Orthogonal packing of beta-pleated sheets in proteins. Biochemistry. 1982 Aug 17;21(17):3955–3965. doi: 10.1021/bi00260a009. [DOI] [PubMed] [Google Scholar]
- Demmer L. A., Birkenmeier E. H., Sweetser D. A., Levin M. S., Zollman S., Sparkes R. S., Mohandas T., Lusis A. J., Gordon J. I. The cellular retinol binding protein II gene. Sequence analysis of the rat gene, chromosomal localization in mice and humans, and documentation of its close linkage to the cellular retinol binding protein gene. J Biol Chem. 1987 Feb 25;262(6):2458–2467. [PubMed] [Google Scholar]
- Durbin R. M., Burns R., Moulai J., Metcalf P., Freymann D., Blum M., Anderson J. E., Harrison S. C., Wiley D. C. Protein, DNA, and virus crystallography with a focused imaging proportional counter. Science. 1986 May 30;232(4754):1127–1132. doi: 10.1126/science.3704639. [DOI] [PubMed] [Google Scholar]
- Greenfield S., Brostoff S., Eylar E. H., Morell P. Protein composition of myelin of the peripheral nervous system. J Neurochem. 1973 Apr;20(4):1207–1216. doi: 10.1111/j.1471-4159.1973.tb00089.x. [DOI] [PubMed] [Google Scholar]
- Hase J., Kobashi K., Nakai N., Onosaka S. Binding of retinol-binding protein obtained from human urine with vitamin A derivatives and terpenoids. J Biochem. 1976 Feb;79(2):373–380. doi: 10.1093/oxfordjournals.jbchem.a131080. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Rypniewski W. R., Law J. H., Rayment I. The molecular structure of insecticyanin from the tobacco hornworm Manduca sexta L. at 2.6 A resolution. EMBO J. 1987 Jun;6(6):1565–1570. doi: 10.1002/j.1460-2075.1987.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J., Heller J. Properties of the chromophore binding site of retinol-binding protein from human plasma. J Biol Chem. 1974 Aug 10;249(15):4712–4719. [PubMed] [Google Scholar]
- Huber R., Schneider M., Epp O., Mayr I., Messerschmidt A., Pflugrath J., Kayser H. Crystallization, crystal structure analysis and preliminary molecular model of the bilin binding protein from the insect Pieris brassicae. J Mol Biol. 1987 May 20;195(2):423–434. doi: 10.1016/0022-2836(87)90661-9. [DOI] [PubMed] [Google Scholar]
- Hunt C. R., Ro J. H., Dobson D. E., Min H. Y., Spiegelman B. M. Adipocyte P2 gene: developmental expression and homology of 5'-flanking sequences among fat cell-specific genes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3786–3790. doi: 10.1073/pnas.83.11.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaque A., Hofmann T., Eylar E. H. The complete amino acid sequence of the rabbit P2 protein. J Biol Chem. 1982 Jan 25;257(2):592–595. [PubMed] [Google Scholar]
- Jones T. A., Liljas L. Structure of satellite tobacco necrosis virus after crystallographic refinement at 2.5 A resolution. J Mol Biol. 1984 Aug 25;177(4):735–767. doi: 10.1016/0022-2836(84)90047-0. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadlubowski M., Hughes R. A. Identification of the neuritogen for experimental allergic neuritis. Nature. 1979 Jan 11;277(5692):140–141. doi: 10.1038/277140a0. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Suzuki M., Suzuki A., Uyemura K. The complete amino acid sequence of the P2 protein in bovine peripheral nerve myelin. FEBS Lett. 1980 Jun 16;115(1):27–30. doi: 10.1016/0014-5793(80)80719-8. [DOI] [PubMed] [Google Scholar]
- Li E., Demmer L. A., Sweetser D. A., Ong D. E., Gordon J. I. Rat cellular retinol-binding protein II: use of a cloned cDNA to define its primary structure, tissue-specific expression, and developmental regulation. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5779–5783. doi: 10.1073/pnas.83.16.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer M. E., Jones T. A., Aqvist J., Sundelin J., Eriksson U., Rask L., Peterson P. A. The three-dimensional structure of retinol-binding protein. EMBO J. 1984 Jul;3(7):1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer M. E., Liljas A., Eriksson U., Sundelin J., Rask L., Peterson P. A. Crystallization of and preliminary X-ray data for an intracellular vitamin A-binding protein from rat liver. J Biol Chem. 1981 Aug 10;256(15):8162–8163. [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F., Brecher P. Characterization of a fatty acid-binding protein from rat heart. J Biol Chem. 1986 Apr 25;261(12):5584–5589. [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Cellular retinoic acid-binding protein from rat testis. Purification and characterization. J Biol Chem. 1978 Jul 10;253(13):4551–4554. [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Cellular retinol-binding protein from rat liver. Purification and characterization. J Biol Chem. 1978 Feb 10;253(3):828–832. [PubMed] [Google Scholar]
- Ong D. E., Chytil F. Retinoic acid-binding protein in rat tissue. Partial purification and comparison to rat tissue retinol-binding protein. J Biol Chem. 1975 Aug 10;250(15):6113–6117. [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Petsko G. A. Preparation of isomorphous heavy-atom derivatives. Methods Enzymol. 1985;114:147–156. doi: 10.1016/0076-6879(85)14015-2. [DOI] [PubMed] [Google Scholar]
- Pähler A., Maslowska M., Parge H. E., Schneider M., Steifa M., Saenger W., Keuper H. J., Spener F. X-ray studies on triclinic crystals of fatty acid binding protein. Examples of an extremely X-ray-resistant protein. FEBS Lett. 1985 May 20;184(2):185–187. doi: 10.1016/0014-5793(85)80603-7. [DOI] [PubMed] [Google Scholar]
- Sacchettini J. C., Meininger T. A., Lowe J. B., Gordon J. I., Banaszak L. J. Crystallization of rat intestinal fatty acid binding protein. Preliminary X-ray data obtained from protein expressed in Escherichia coli. J Biol Chem. 1987 Apr 15;262(11):5428–5430. [PubMed] [Google Scholar]
- Sacchettini J. C., Said B., Schulz H., Gordon J. I. Rat heart fatty acid-binding protein is highly homologous to the murine adipocyte 422 protein and the P2 protein of peripheral nerve myelin. J Biol Chem. 1986 Jun 25;261(18):8218–8223. [PubMed] [Google Scholar]
- Sacchettini J. C., Stockhausen D., Li E., Banaszak L. J., Gordon J. I. Crystallization of rat cellular retinol binding protein II. Preliminary X-ray data obtained from the apoprotein expressed in Escherichia coli. J Biol Chem. 1987 Nov 15;262(32):15756–15758. [PubMed] [Google Scholar]
- Sawyer L. Protein structure. One fold among many. 1987 Jun 25-Jul 1Nature. 327(6124):659–659. doi: 10.1038/327659a0. [DOI] [PubMed] [Google Scholar]
- Shubeita H. E., Sambrook J. F., McCormick A. M. Molecular cloning and analysis of functional cDNA and genomic clones encoding bovine cellular retinoic acid-binding protein. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5645–5649. doi: 10.1073/pnas.84.16.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelin J., Anundi H., Trägårdh L., Eriksson U., Lind P., Ronne H., Peterson P. A., Rask L. The primary structure of rat liver cellular retinol-binding protein. J Biol Chem. 1985 May 25;260(10):6488–6493. [PubMed] [Google Scholar]
- Sundelin J., Das S. R., Eriksson U., Rask L., Peterson P. A. The primary structure of bovine cellular retinoic acid-binding protein. J Biol Chem. 1985 May 25;260(10):6494–6499. [PubMed] [Google Scholar]
- Sundelin J., Eriksson U., Melhus H., Nilsson M., Lundvall J., Båvik C. O., Hansson E., Laurent B., Peterson P. A. Cellular retinoid binding proteins. Chem Phys Lipids. 1985 Aug 30;38(1-2):175–185. doi: 10.1016/0009-3084(85)90065-9. [DOI] [PubMed] [Google Scholar]
- Suzuki M., Kitamura K., Sakamoto Y., Uyemura K. The complete amino acid sequence of human P2 protein. J Neurochem. 1982 Dec;39(6):1759–1762. doi: 10.1111/j.1471-4159.1982.tb08017.x. [DOI] [PubMed] [Google Scholar]
- Sweetser D. A., Lowe J. B., Gordon J. I. The nucleotide sequence of the rat liver fatty acid-binding protein gene. Evidence that exon 1 encodes an oligopeptide domain shared by a family of proteins which bind hydrophobic ligands. J Biol Chem. 1986 Apr 25;261(12):5553–5561. [PubMed] [Google Scholar]
- Takahashi K., Odani S., Ono T. A close structural relationship of rat liver Z-protein to cellular retinoid binding proteins and peripheral nerve myelin P2 protein. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1099–1105. doi: 10.1016/0006-291x(82)91225-6. [DOI] [PubMed] [Google Scholar]
- Thaller C., Weaver L. H., Eichele G., Wilson E., Karlsson R., Jansonius J. N. Repeated seeding technique for growing large single crystals of proteins. J Mol Biol. 1981 Apr 15;147(3):465–469. doi: 10.1016/0022-2836(81)90496-4. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Dubois-Dalcq M., Quarles R. H. Ultrastructural localization of P2 protein in actively myelinating rat Schwann cells. J Neurochem. 1984 Oct;43(4):944–948. doi: 10.1111/j.1471-4159.1984.tb12828.x. [DOI] [PubMed] [Google Scholar]
- Uyemura K., Suzuki M., Kitamura K., Horie K., Ogawa Y., Matsuyama H., Nozaki S., Muramatsu I. Neuritogenic determinant of bovine P2 protein in peripheral nerve myelin. J Neurochem. 1982 Sep;39(3):895–898. doi: 10.1111/j.1471-4159.1982.tb07979.x. [DOI] [PubMed] [Google Scholar]
- Uyemura K., Yoshimura K., Suzuki M., Kitamura K. Lipid binding activities of the P2 protein in peripheral nerve myelin. Neurochem Res. 1984 Oct;9(10):1509–1514. doi: 10.1007/BF00964676. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]



