Abstract
CXCR3 is a G-protein coupled receptor which binds to ELR-negative CXC chemokines that have been found to impact immune responses, vascular develop, and wound repair. More recently, CXCR3 has been examined in the context of cancer and increased expression in many human tumors has been correlated with poor prognosis in breast, melanoma, colon and renal cancer patients. Three variants of CXCR3 are identified so far (CXCR3-A, CXCR3-B and CXCR3-alt) with the two primary ones, CXCR3-A and CXCR3-B, considered to induce opposite physiological functions. Generally, CXCR3-A, the predominant form in hematopoietic cells, appears to mediate tumor “go” signaling via promoting cell proliferation, survival, chemotaxis, invasion and metastasis; while CXCR3-B, the main form on formed elements including epithelial cells, appears to mediate tumor “stop” signaling via promoting growth suppression, apoptosis and vascular involution. Thus, aberrant expression of the isoforms CXCR3-A and CXCR3-B could affect tumor progression. In this review, we have discussed the profiles of CXCR3 variants and related signaling, as well as the role of CXCR3 variants in cancer.
Keywords: chemokines, cancer, CXCR3, CXCR3-A, CXCR3-B
Introduction
Chemokines, or chemotactic cytokines, are a superfamily of approximately 50 soluble cytokines with low molecular weight (8–15KD) that were initially defined as proteins which recruit leukocytes to inflammatory sites and to secondary lymphoid organs (Moser & Loetscher, 2001). Chemokines are not simply immune regulators as they have been shown to play important roles in development, angiogenesis, hematopoiesis, atherosclerosis, inflammation, immunity diseases and cancer progression (Luster, 1998; Romagnani et al., 2004; Vandercappellen et al., 2008; Singh et al., 2011). Chemokines are divided into 4 subgroups according to the number and positioning of conserved cysteines in the amino-terminal domains: C, CC, CXC and CX3C. The CXC chemokines are further divided into whether they have glutamic acid-leucine-arginine sequence (“ELR” motif); the effects of the chemokines on angiogenesis are opposite depending on ELR motif presence.
CXCR3, a receptor which binds to the members of so-called angiostatic ELR-negative CXC chemokine subfamily, including CXCL9/MIG, CXCL10/IP10, CXCL11/ITAC/IP9, CXCL4/PF4 and its variant CXCL4L1/PF4V1, has been found to be up-regulated in many human tumors; the increased levels correlate with poor prognosis for breast, melanoma, renal and colon cancer patients (Billottet et al., 2013). Like the other chemokine receptors, CXCR3 is a seven transmembrane pass G protein-coupled receptor (GPCR) whose ligandation triggers several downstream pathways e.g. MAPKs, Src and PI3K signaling upon ligands binding and activation via classical heterotrimeric G proteins.
CXCR3 was cloned in 1996 and renamed as CXCR3-A, after an alternative spliced isoform CXCR3-B was found. Another splice variant, CXCR-alt, was identified in 2004 (Ehlert et al., 2004), but little has been discerned about this isoform. CXCR3-A and CXCR3-B mediate disparate signaling events to promote different cellular responses. Generally, CXCR3-A appears to promote proliferation, cell survival, chemotaxis and invasion, while CXCR3-B appears to mediate growth suppression, apoptosis and angiostatic. Almost all human cells express both CXCR3-A and CXCR3-B, except for primary cultured human mesangial cells (HMC) only expressing CXCR3-A and human microvascular endothelial cell (HMvEC) only expressing CXCR3-B (Lasagni et al., 2003). However, the predominant isoform differs by cell types. In hematopoietically-derived cells, CXCR3A represents essentially all the receptor, whereas on differentiated epithelial cells and fibroblasts, CXCR3B predominates.
In the tumor organ, CXCR3 and its ligands are expressed on the tumor cells, stromal cells, vessels and recruited leukocytes, with most all of these cells also producing various ligands. Consequently, CXCR3 is involved in tumor progression directly or indirectly by regulating tumor outgrowth, migration, invasion, angiogenesis and immunity. In light of complexity of CXCR3 and ligands expression and activation in tumor microenvironment, we chose to focus our discussion on the divergent role of human CXCR3 isoforms, specifically in human tumor biology per se, instead of tumor angiogenesis or tumor immunity.
Gene and protein structures
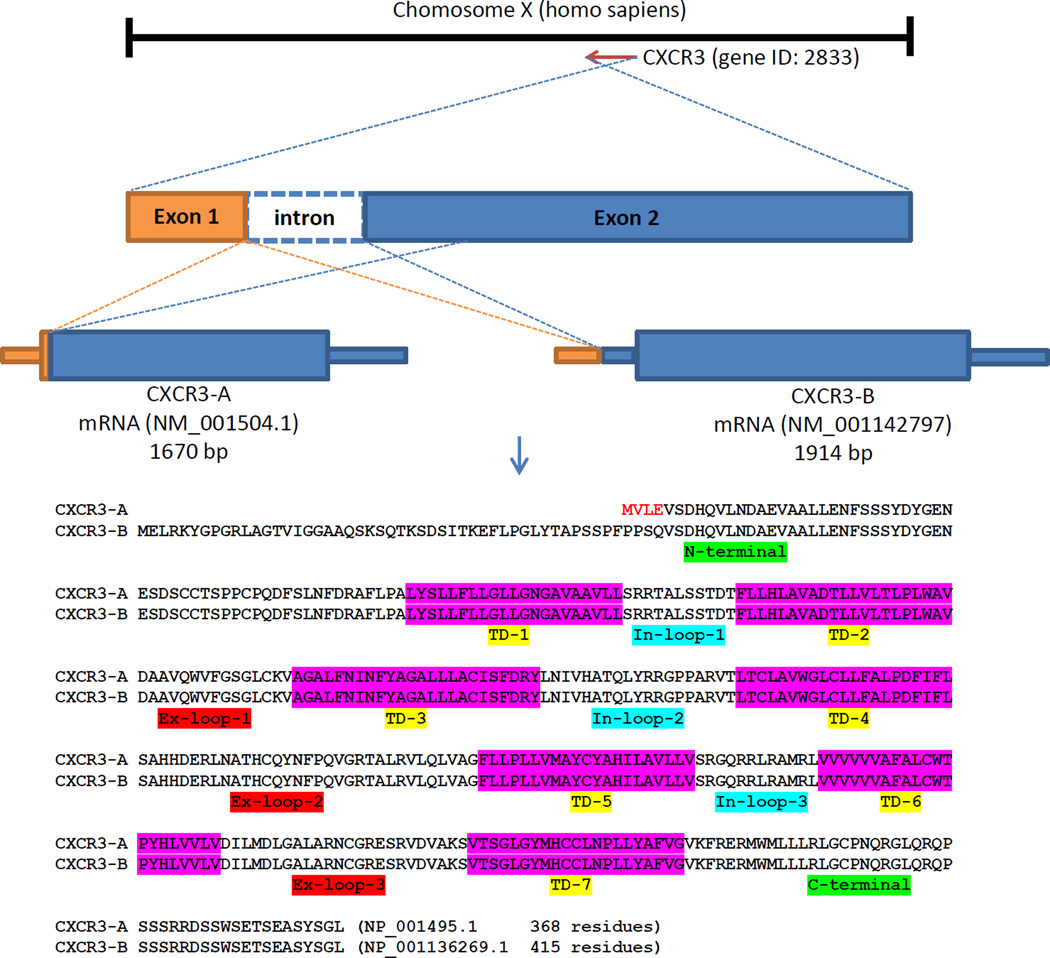
The CXCR3 gene was cloned and characterized initially as the selective receptor for CXCL9 and CXCL10 (Loetscher et al., 1996), and was later mapped as a single-copy gene on chromosome Xq13 (Loetscher et al., 1998). The CXCR3 mRNA species originally denoted, was renamed to CXCR3-A after an alternative spliced isoform CXCR3-B was identified and characterized (Lasagni et al., 2003). CXCR3-A mRNA encodes a protein of 368 amino acids with a molecular mass of 40,659 Daltons when unmodified. CXCR3-B has 415 residues which contains a longer extracellular domain at the N-terminus. Therefore, isolated detection of CXCR3-A is difficult due to almost complete overlap with CXCR3B (Figure 1). Both isoforms are predicted to have seven transmembrane domains. The electrophoresis shift of mildly reduced CXCR3 indicated post-translational modification or the homo/hetero-dimers (Ehlert et al., 2004).
Figure 1.
Schematic of CXCR3 isoforms gene and protein sequence. CXCR3 gene mapped on human chromosome X with two exons and one intron. CXCR3-B transcript variant uses an alternate acceptor splice site at the 3’ terminal exon compared to CXCR3-A variant. This and an alternative in-frame ATG start codon result in CXCR3-B with a longer and distinct N-terminus compared to CXCR3-A. Both isoforms encode a seven-transmembrane G-protein coupling protein. TD: transmembrane domain; In-loop: intracellular loop; Ex-loop: extracellular loop.
Another spliced variant CXCR3-alt, a drastically altered C-terminal protein sequence compared to CXCR3-A, has a predicted four- or five-transmembrane domain structure, differing from all known functional chemokine receptors. CXCR3-alt has 267 residues with the predicted size of 28,715 Daltons and displays a well-focusing band at ~33kDa on western blot analyses due to potential N-glycosylation on the extracellular parts of the receptor. Despite severe structural changes, CXCR3-alt still localizes to the cell surface and mediates functional activity in the presence of CXCL11 (Ehlert et al., 2004). RT-PCR products from colorectal cancer cell lines showed an additional PCR band other than CXCR3-A, CXCR3-B or CXCR3-alt (Zipin-Roitman et al., 2007). However, this last altered CXCR3 isoforms has not been properly studied similar to the very few studies on CXCR3-alt; thus we will limit our discussion only on CXCR3-A and CXCR3-B.
CXCR3 ligands and isoforms binding
CXC chemokines such as CXCL9, CXCL10, CXCL11, CXCL4 and its non-allelic variant CXCL4L1 are the members of ELR-negative CXC chemokine subfamily and all bind to the CXCR3 chemokine receptors. However, the ligands bind to the CXCR3 isoforms with different affinities (Fulton, 2009; Billottet et al., 2013). CXCL9, CXCL10 and CXCL11 bind to both CXCR3-A and CXCR3-B, with all three chemokines having higher affinity for CXCR3-A (Loetscher et al., 1996; Lasagni et al., 2003). Another ligand, CXCL4, shows high affinity for CXCR3-B but not CXCR3-A (Lasagni et al., 2003). Of note, it was later found the higher concentration of CXCL4 (micromolar) could also induce signaling and T-lymphocytes migration, likely via CXCR3-A (Mueller et al., 2008). CXCL4L1, a variant of CXCL4 with only 3 amino acid residues substitution in the C-terminus, can bind to both isoforms (Struyf et al., 2010), whereas CXCL4L1 appears more angiostatic than CXCL4 (Struyf et al., 2004). Human CCL21, in the absence of its primary receptor CCR7, has been reported as a functional ligand for CXCR3 inducing chemotaxis in adult microglial cells where CXCR3 is expressed predominantly (Dijkstra et al., 2004), though this has not been separately reported.
The residues and domains in CXCR3 receptor have been studied to identify their roles in ligands binding and receptor activation. It has been demonstrated that chemokine activation of CXCR3 involved both high-affinity ligand-binding interactions with negatively charged residues in the extracellular domains of CXCR3 and a lower-affinity receptor-activating interaction in the second extracellular loop. The sulfation of CXCR3 on its N terminus (Y27 or/and Y29) is required for binding and activation by CXCL9, CXCL10 and CXCL11 (Colvin et al., 2006). The first 16 amino acids and the first extracellular loop of CXCR3 are important for maximal CXCL10 and CXCL11 binding and activation but are dispensable for CXCL9 binding (Xanthou et al., 2003). A D112A (within 1st extracellular loop) mutation dramatically reduced CXCR3 function. Arginine 216 in the second extracellular loop is required for CXCR3 activation upon CXCL9–11 binding, but plays a minimal role in ligand binding or ligand-induced receptor internalization (Colvin et al., 2006). The third extracellular loop of CXCR3 is important only for CXCL9- and CXCL10-induced chemotaxis but not CXCL11 (Xanthou et al., 2003). On the signaling side, CXCL11-induced cell migration is regulated by the CXCR3 membrane proximal carboxyl terminus (Dagan-Berger et al., 2006).
Subcellular localization
The cell surface exposure of CXCR3 is regulated and associated with specific cell conditions i.e. replicating or malignant cells. For example, human bronchial epithelial cells (HBECs) were found to express CXCR3-B primarily, but the majority of the cells (>80%) expressed intracellular CXCR3, and only a minority (<40%) expressed it on cell surface and most of these cells were in the late S to G2/M phases of the cell cycle (Aksoy et al., 2006). We previously published that CXCR3 was predominantly expressed on the cell membrane of normal prostate tissues and primary prostate tumors. However, in metastatic prostate cancer, most of the receptors were detected on in the whole cell analyses, indicating that the receptor was internalized or down-regulated following metastatic transformation based on autocrine or paracrine signaling (Wu et al., 2012). In breast cancer cell lines, cell surface expression of CXCR3 could only be detected in a sub-population of the cells, but most of cells expressed cytosolic CXCR3 (Datta et al., 2006; Walser et al., 2006).
Receptor trafficking
CXCR3 internalization occurs in different ways depending on different ligands stimulation and cell type. CXCR3(+) T cells are themselves a source of IFN-gamma, which potently induces the expression of CXCR3 ligands, thus the activities of CXCR3 in T-lymphocytes are tightly controlled. CXCR3 replenishment on the cell surface is much slower than most other chemokine receptors due to dependency on de novo mRNA and protein synthesis and protein trafficking to the cell surface (Meiser et al., 2008). CXCR3 can be efficiently internalized in the absence of ligand, a process involving a YXXL motif at the extreme of the C terminus (Meiser et al., 2008). Out of the three ligands, CXCL11 is the most potent and physiologic inducer of CXCR3 internalization (Colvin et al., 2004). CXCL11-induced CXCR3 down-regulation occurs in a rapid, dose-dependent manner, and is dynamin and β-arrestin independent. However, CXCL10- and CXCL9-induced internalization proceeds through the dynamin/β-arrestin 1 pathway. Additionally, CXCL10- and CXCL9-induced CXCR3 internalization requires the C terminus of CXCR3, while CXCL11-induced CXCR3 internalization is independent of the C terminus instead requiring the third intracellular loop of CXCR3 (Colvin et al., 2004). Internalized CXCR3 receptor is degraded by lysosomes and proteasomes independent of phosphorylation, ubiquitination status or a conserved LL motif (Meiser et al., 2008).
In cancer this may be deranged. CXCR3 expression in breast cancer cell lines was found not down-regulated by exposure to high concentrations (500ng/ml) of its ligand, CXCL10, but rather was enhanced, with increased de novo protein synthesis (Goldberg-Bittman et al., 2004). Additionally, overexpression of CXCL10 in prostate localized cancer cell line LNCap increased CXCR3 expression significantly, which is another evidence of CXCL10 induced receptor upregulation (Nagpal et al., 2006).
Signal transduction
CXCR3-A and CXCR3-B mediate distinct signaling cascades that depend on specific G protein coupling, different binding affinity of the ligands and cell types (Lasagni et al., 2003; Kouroumalis et al., 2005). In general, CXCR3-A signaling promotes cell proliferation and chemotaxis, whereas CXCR3-B suppresses proliferation and migration and sensitizes to apoptosis. HMvEC transfected with CXCR3-A exhibited a rapid, dose-dependent intracellular calcium flux in response to CXCL9–11; CXCR3-B transfectants had much higher basal cAMP levels compared to mock transfectants and this was further increased upon CXCL9–11 or CXCL4 stimulation indicating receptor coupling with Gαs protein. CXCR3-A induced proliferation was pertussis toxic (PTX)-sensitive which indicates signaling via a Gαi/o protein (Lasagni et al., 2003). A study using mice deficient in the Gαi2 and Gαi3 found that knocking out Gαi2 subunits abrogated CXCR3-induced lymphocytes chemotaxis, whereas knocking out Gαi3 increased lymphocytes migration and GTPγS binding (Thompson et al., 2007). These results suggest that Gαi2 subunits are required for CXCR3-mediated signaling and Gαi3 subunits inhibit CXCR3 signaling in mouse T-lymphocytes, though the translation to other cell types remain uncertain.
CXCR3B has been noted as angiostatic. The angiostatic activity of CXCL10 is mediated through PKA-dependent inhibition of m-calpain that prevent rear-end retraction for endothelial cells motility (Bodnar et al., 2006). In addition, the angiostatic effect follows from the activation of p38/MAPK activity induced by CXCL4/CXCL10 binding to the CXCR3-B isoform (Petrai et al., 2008). In keratinocyte, on the other hand, CXCL11 induces cell motility via signaling through PLC-β3, resulting in the activation of µ-calpain to allow for partial cell de-adhesion from the substratum (Satish et al., 2005). These findings were confirmed in prostate cancer cells where we found that CXCL4 and CXCL10 promoted cell motility and invasiveness of DU145 and PC3 cells through PLC-β3 and µ-calpain whereas in normal prostate cells, RWPE-1 PKA-mediated signaling to block m-calpain reduced cell migration (Wu et al., 2012). The activation of µ-calpain by CXCR3 ligandation in endothelial cells results in anoikis due to cleavage of the intracellular tail of the β 3 integrin (Bodnar et al., 2009). Of interest, pericytes produce CXCR3 ligands that then trigger anoikis in the immature vessels via the same µ-calpain pathway as noted above (Bodnar et al., 2013). Thus, CXCR3 signaling would limit angiogenesis even in tumors.
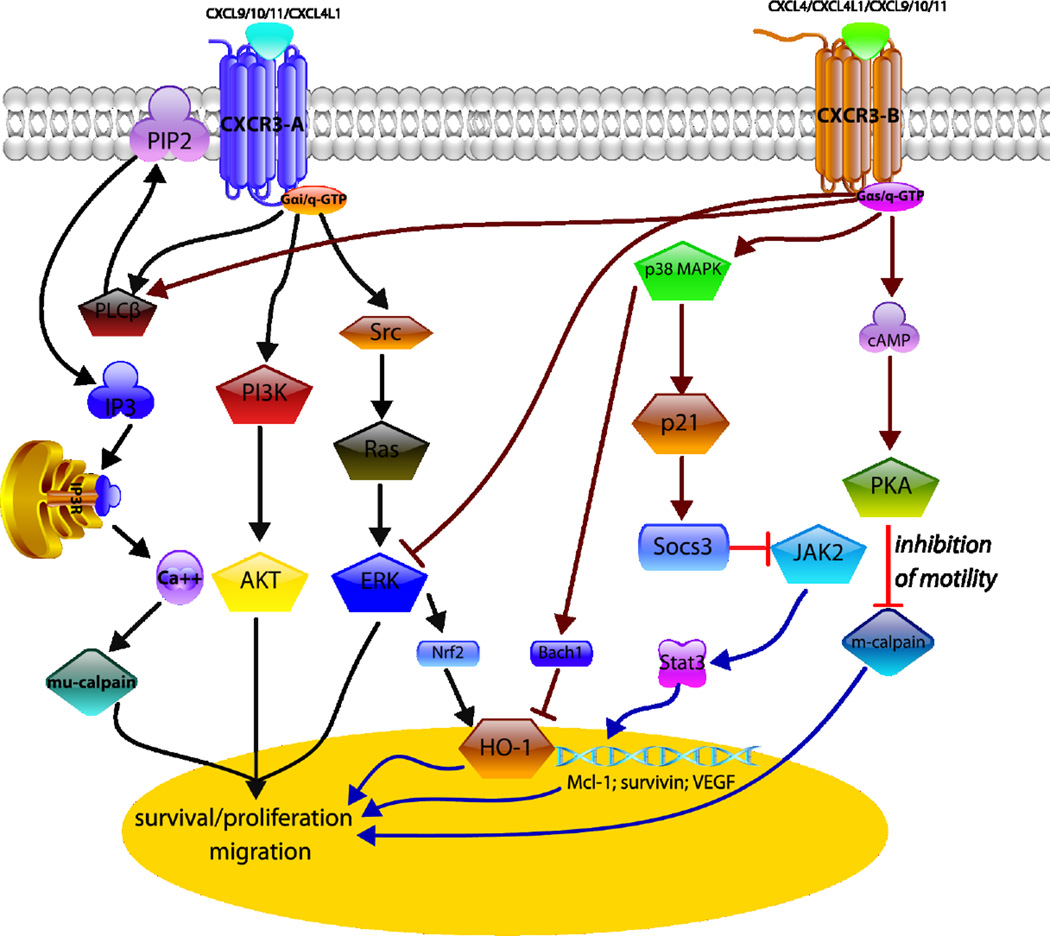
Finally, it was reported CXCR3-B can mediate growth-inhibitory signals in human renal cancer cells and breast cancer cells by downregulating the expression of heme oxygenase-1 and by modulating Bach-1 and Nrf2 nuclear translocation, respectively and both studies indicated that growth inhibition was due to p38/MAPK activation (Datta et al., 2010; Balan & Pal, 2013). Overall, distinct signaling cascades mediated by CXCR3 are cell type specific and results in various cellular responses including cell motility, invasion, apoptosis and proliferation (Figure 2).
Figure 2.
CXCR3 Isoforms play functional roles in seemingly opposing directions. CXCR3A signaling mainly via Gαi or Gαq subunits activates phospholipase C-beta (PLC-β) to initiate calcium influx; mu-calpain (calpain-1) is subsequently activated to facilitate motility or even cause anoikis in endothelial cells (via β-3 integrin cleavage). Gα protein also activates PI3K-AKT and Src-Ras-ERK signaling pathways to promote cell migration and survival/proliferation. CXCR3B signaling via Gαs or Gαq subunits to trigger protein kinase A (PKA) to inhibit m-calpain (calpain 2) results in the inhibition of migration. CXCR3-B activates p38 MAPK-p21-Socs3 pathway to prevent JAK2-stats3 activation. In breast cancer, CXCR3-B activation promotes the activation of p38 MAPK and inhibition of ERK1/2 that associates with an increased nuclear localization of Bach-2 and nuclear export of Nrf2. The modulation of Bach-1/Nfr2 nuclear localization down-regulates HO-1, and these events can promote increased apoptosis and reduced proliferation of breast cancer cells.
Regulation of CXCR3 Isoforms Expression
Although the differences in the expression and signaling cascades of the two isoforms are well established, the modulation of alternative splicing for the differential expression of these isoforms has not been extensively studied. There are several possible mechanisms for the alternative splicing. Datta et al showed that Ras could down-regulate the CXCR3-B isoform in breast cancer cells. Specifically, these authors found that activated Ras, Ha-Ras(12v), could up-regulate CXCL10 gene expression as transfection with CXCL10 promoter-luciferase construct led to Ha-Ras(12v) dose-dependent luciferase activity and this up-regulation was coupled with Ha-Ras(12v) dose dependent down-regulation of the growth-inhibitory isoform, CXCR3-B, resulted in enhanced breast cancer cells proliferation (Datta et al., 2006). However, this study did not explicitly determine the transcription factors involved in inducing CXCL10 or the factors responsible for CXCR3-B down-regulation.
Another possible mechanism for CXCR3 alternative splicing is through epigenetic changes. We previously published that CXCR3-A was highly expressed in metastatic prostate cancer cells when compared to normal prostate cells. We did not find significant mutation in CXCR3 gene in cancer cells and concluded that nucleotide substitution was not a differentiating factor for CXCR3 in normal and malignant prostate cell (Kumar, 2013). Moreover, both receptors are functional in normal and malignant prostate cell lines (Wu et al., 2012). Therefore, in the absence of DNA mutation in both normal and malignant cells, other mechanism(s) might be the cause for changes in the level of CXCR3 isoform and we theorized that epigenetic regulation might play roles in CXCR3 alternative splicing. We found that CXCR3 gene promoter was highly methylated in RWPE-1 (normal prostate cells), PC-3 and DU145 (cancer cells) with no appreciable differences (Kumar, 2013). This justifies our observations that the CXCR3 gene expression did not change between normal and metastatic cells; rather, the cells differ only in the level of the isoforms. Intragenic DNA methylation was observed to be high in included alternatively spliced exons and serve as the binding sites for methyl-CpG-binding protein (MeCP2) that supports RNA polymerase II during transcription elongation (Maunakea et al., 2013). Interestingly, we found that the normal cells and prostate cancer cells have different intragenic methylation profile with the cancer cells showed lacked of several CpG methylation in the intron flanking the splice junction, suggesting that these unmethylated CpG sites might be responsible for exon skipping resulting in higher CXCR3-A in the cancer cells (Kumar, Ma, Wells, unpublished observations). However, the contribution of these specific CpG sites and the contribution of intron methylation in alternative splicing remain to be validated.
CXCR3 in cancer
Metastatic cancers are responsible for about 90% of cancer-related mortality (Mehlen & Puisieux, 2006). The initial steps for tumor dissemination are ‘escape’ from the solid tumor and then migration to other sites via hematogenous route. Next, disseminated cancer cells need to seed, survive and colonize the ectopic sites. Disseminated cancers are usually more refractory to prior cancer treatments, therefore an ideal strategy is to prevent metastasis by limiting initial dissemination and preventing secondary spread (Wells et al., 2013). A potential approach to limit dissemination is to re-instate the physiological ‘stop’ signals that keep normal and dysplastic epithelial cells localized. One aspect of this field has mainly focused on carcinoma cells switching between epithelial and mesenchymal (epithelial-mesenchymal transition and mesenchymal-epithelial reverting transition) phenotypes to facilitate migration, survival and colonization at the ectopic site (Chao, Wu, Acquafondata et al., 2011; Chao, Wu, Shepard et al., 2011; Ma & Wells, 2014; Taylor et al., 2014). More recently, paracrine signals have been recognized as providing additional inhibitions to migration. Consequently, CXCR3 has been examined in the context of cancer as increasing evidence shows that CXCR3 is expressed and functional in almost all cells, and is crucial in terminating migration during wound repair (Yates et al., 2008; Huen & Wells, 2012) and angiogenesis (Bodnar et al., 2006; Bodnar et al., 2009; Bodnar et al., 2013). CXCR3 was found upregulated in many primary and metastatic tumors such as breast, prostate, colon, colorectal, melanoma and ovarian cancer (Table 1). Moreover, CXCR3 has been linked with poor prognosis in breast, melanoma and colon cancer patients.
Table 1.
CXCR in cancer cell lines
| Cancer type | cell lines | tumor | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CXCR3 | A | B | CXCR3 | A | B | ||||
| prostate | RWPE1# | √ | low | high | normal | √ | √ | √ | (Wu et al., 2012) |
| DU145 | √ | ↑** | ↓** | primary | ↑** | ↑** | ↓** | ||
| PC3 | √ | ↑** | ↓** | Mets | ↑** | ↑** | ↓* | ||
| LNCap | √ | ↑** | →** | ||||||
| breast | MCF-10A# | low | primary | ↑** | (Li et al., 2011) | ||||
| MCF-7 | √ | LN Mets | ↑* | (Ma et al., 2009) | |||||
| T-47D | √ | (Walser et al., 2006) | |||||||
| MDA-MB-231 | √ | (Datta et al., 2006) | |||||||
| MDA-MB-468 | √ | (Goldberg-Bittman et al., 2004) | |||||||
| MDA-MB-435 | √ | √ | √ | ||||||
| colon/rectum | SW620 | √ | × | primary | √ | (Kawada et al., 2007) | |||
| HT29 | √ | low | LN Mets | ↑* | (Cambien et al., 2009) | ||||
| HCT116 | √ | low | Liver Mets | ↑* | (Du et al., 2014) | ||||
| SW480 | √ | × | (Zipin-Roitman et al., 2007) | ||||||
| DLD-1 | × | low | (Murakami et al., 2012) | ||||||
| colo205 | √ | ||||||||
| WiDr | √ | ||||||||
| RKO | √ | ||||||||
| LS174T | √ | ||||||||
| Caco2 | × | ||||||||
| HCT15 | × | ||||||||
| C26 | √ | ||||||||
| KM12C | √ | ||||||||
| KM12SM | √ | ||||||||
| melanoma | C32TG | √ | (Kawada et al., 2004) | ||||||
| G361 | √ | ||||||||
| HMV-1 | √ | ||||||||
| SK-Mel28 | low | ||||||||
| ovarian | normal | √ | √ | √ | (Furuya et al., 2007) | ||||
| endometriosis | ↑** | ||||||||
| primary | ↑** | ↓*** | |||||||
| Mets | ↑* | ||||||||
| melanoma | IPC-298 | √ | primary | √ | (Pinto et al., 2014) | ||||
| Mel-Juso | √ | (Monteagudo et al., 2007) | |||||||
| Mel-HO | √ | ||||||||
| IGR-39 | √ | ||||||||
| WM-115 | √ | ||||||||
| A-375 | low | ||||||||
| MeWo | low | ||||||||
| SK-Mel28 | low | ||||||||
| Malme-3 M | √ | ||||||||
| SK-Mel 2 | √ | ||||||||
| WM-266.4 | √ | ||||||||
| IGR-37 | √ | ||||||||
| Mel-RC08 | √ | ||||||||
| renal | ACHN | √ | √ | √ | normal | √ | √ | √ | (Utsumi et al., 2014) |
| Caki-1 | √ | √ | √ | primary | ↑** | ↑** | ↓** | (Datta et al., 2008) | |
| 786-O | √ | √ | √ | Mets | ↑* | ↑* | ↓** | ||
compare to primary tumor
compare to normal tissue/cell line
compare to endometriosis tissue
immortalized normal cell line
CXCR3 in prostate cancer
The differential expression pattern of CXCR3 isoforms was found to correlate with the progression of prostate cancer. Previously, we published that CXCR3 expression was elevated in prostate cancer when compared to normal tissues (Wu et al., 2012). Specifically, CXCR3-A mRNA was upregulated in prostate cancer specimens while CXCR3-B mRNA was downregulated in these specimens. In all cell lines, CXCR3-B was predominantly expressed except for DU145 and total CXCR3 mRNA was sustained. However, the ratio of CXCR3-A/CXCR3-B mRNA levels was increased in the invasive and metastatic DU145 and PC3 prostate cancer cells compared to RWPE1, but not in the localized LNCaP cells. These resulted in CXCL10 and CXCL4-promoted cell motility and invasiveness in both DU-145 and PC-3 cells instead of inhibiting cell migration as in RWPE-1 cells. We also found that ectopic expression of CXCR3-B in DU-145 cells decreased cell movement and invasion. It was previously reported that overexpression of CXCL10 in LNCaP cells, where CXCR3-B is the dominant isoform, inhibited cell proliferation and PSA production (Nagpal et al., 2006). These two findings indicate that CXCR3-B functions as anti-growth and anti-migratory isoform in prostate cancer. As mentioned before, CXCR3 is found primarily on the membrane of the normal cells but internalized in the cancer cells. It was postulated that intracellular expression might indicate progression into highly aggressive phenotype and induce intracellular tumorigenesis signaling (Engl et al., 2006). In all, these results suggest that a change from low to high ratio CXCR3-A/CXCR3-B promotes prostate cancer metastasis and stimulates cell migration and invasion (Wu et al., 2012).
CXCR3 and ligands in breast cancer
Both CXCR3 isoforms were found expressed in breast cancer cell lines (Goldberg-Bittman et al., 2004; Datta et al., 2006; Liet al., 2011). Ma et al examined CXCR3 protein expression in a series of 75 primary breast tumors from women with stage I or II disease at diagnosis. They detected CXCR3 in the cytoplasm and membrane of malignant cells from every patient, whereas normal ducts were negative or weakly positive and found that CXCR3 is associated with the poor survival of breast cancer patients (Ma et al., 2009). Additionally, it was reported that activated form of Ras, HA-Ras(12V), promoted CXCL10 transcriptional activation and downregulated the anti-growth isoform CXCR3-B in two human breast cancer cell lines, MDA-MB-435 and MCF-7, with the combination of these two events resulting in enhanced breast cancer cells proliferation (Datta et al., 2006).
Several ways of preventing breast oncogenesis and metastasis have been proposed. It was reported that prostaglandin E2 (PGE2) could repress CXCL9 and CXCL10 secretion in MCF-7 and MDA-MB231 cells and PGE2 repression could be inhibited by cyclooxygenase inhibitor to enhance intratumoral immune infiltration (Bronger et al., 2012). Other methods include CXCR3 gene silencing and small molecule inhibitor of CXCR3 signaling, AMG487. Both methods were effective in inhibiting lung metastases but did not affect the growth of local breast cancer in mouse model highlighting the role of CXCR3 in promoting breast cancer metastasis but not incidence (Walser et al., 2006; Ma et al., 2009). In all, it was found that signaling via CXCR3-A promotes breast cancer proliferation and CXCR3-B prevents cancer growth. Several therapeutic strategies have been explored including the use of COX2 inhibitor to promote tumor infiltrating immune cells and the roles of gene silencing and small molecule inhibitor AMG487 to prevent metastasis.
CXCR3 in colo-rectal cancer
In colon cancer, 18–34% of the patient specimens showed intense CXCR3 staining; most of these CXCR3-positive patients were also diagnosed with lymph node metastases (Kawada et al., 2007; Du et al., 2014). Kawada et al reported that, similar to breast cancer, patients with CXCR3 expression presented with poorer prognosis than those without CXCR3, or those expressing CXCR4 or CCR7. They found that some human colon cancer cell lines express CXCR3 constitutively but both CXCR3 expressing and non-expressing cells metastasized to the lymph nodes at similar rate. Exogenously expressing CXCR3 in colon cancer cells resulted in greater tumor growth at 4 weeks when introduced into susceptible mice and that more mice showed macroscopic metastasis in para-aortic lymph node at 6 weeks (59% vs 14%, P<0.05). In contrast to lymph nodes, metastasis to the liver or lung was rare, and unaffected by CXCR3 expression (Kawada et al., 2007).
In clinical colorectal cancer (CRC) samples, CXCR3 expression level is significantly higher in metastatic foci within the lymph nodes (LNs) and liver compared to primary tumors. Some human CRC cell lines constitutively express all three known CXCR3 variants (Zipin-Roitman et al., 2007; Rubie et al., 2008; Murakami et al., 2012). Similarly, CXCR3 activation in vitro and in vivo promoted cancer migration and growth, with CXCR3 inhibition by AMG487 abrogating both responses. In the mice, however, CXCR3 antagonism only prevented lung metastases but not in the liver as CXCR3 was only increased in lung nodules when compared to liver nodules (Cambien et al., 2009). However, in vivo metastatic activity of CXCR3 knockdown in SW620 CRC cell line significantly reduced metastasis to the LNs, liver and lungs in a mouse rectal transplantation model with greater suppression on LNs metastases (Murakami et al., 2012).
CXCR3 and ligands in lung cancer
In lung cancer, CXCR3 promotes cancer progression via modulating receptor expression level in the inflammatory cells or modulating ligands expression level in the tumor cells. Unlike other cancers, in non-small cell lung cancer clinical samples, the tumor cells and vessels are mainly negative, but the infiltrating immune cells stain strongly for CXCR3. Increased expression of CXCR3 in the tumor islets and stroma was correlated with extended survival indicating that inflammatory cells were recruited to the tumor islets and stroma for tumor killing (Ohri et al., 2010). Moreover, administration of IL-7 decreased tumor burden and was associated with increased CXCR3 expression on tumor associated T-cells, further corroborating previous observation showing the recruitment of immune cells to the tumor (Andersson et al., 2009; Andersson et al., 2011). In comparison to Calu-1, a squamous cell carcinoma cell line that produces high level of angiostatic CXCL10, human lung adenocarcinoma epithelial cell line A549 has reduced CXCL10 secretion and restoration of CXCL10 in A549 led to inhibition of tumorigenesis without increased leukocyte infiltration (Arenberg et al., 1997). It is thus thought that in lung cancer, generally, CXCR3 and its ligands play roles in tumor killing via the recruitment of immune cells or inflammatory cells and the inhibition of tumor angiogenesis as opposed to direct effects on the tumor cells.
CXCR3 and ligands in ovarian cancer
Furuya et al have demonstrated differential expressions of CXCR3 variants in endometriosis and ovarian cancers and CXCL4/CXCL4L1 expressions in the tumor associated macrophages (TAMs). CXCR3-A was found elevated in both ovarian cancer and endometriosis samples when compared to normal ovary while CXCR3-alt and CXCR3-B were up-regulated and down-regulated in ovarian cancer, respectively when compared to endometriosis samples. CXCR3-A was mainly expressed on the cancer cells and infiltrating lymphocytes whereas CXCR3-B and CXCR3-alt were detected in the microvessels (Furuya et al., 2011). CXCL4 and CXCL4L1 in endometriosis-associated ovarian cancers (EAOCs) were significantly downregulated compared with those in endometriosis. Specifically, CXCL4 and CXCL4L1 were lower in cancer lesions when compared to corresponding endometriosis lesions found within the same cysts. Further analyses showed that CXCL4 was strongly expressed in the CD68+ macrophages in the endometriosis but CD68+ macrophages in ovarian cancer lesions were negative for CXCL4 suggesting different functions of these cells (Furuya et al., 2007; Furuya et al., 2011; Furuya et al., 2012). These studies suggest that CXCR3-A contributes to ovarian cancer tumorigenesis, similar to our findings with prostate cancer (Wu et al., 2012), and taken together, the two studies show that lower expression of anti-growth CXCR3-B isoform and angiostatic CXCL4 in ovarian tumor leads to impaired tumor angiogenesis.
CXCR3 in renal cancer
CXCR3 was found up-regulated in renal cancer and this was correlated to poor prognosis (Johrer et al., 2005; Suyama et al., 2005; Klatte et al., 2008). CXCR3-A/CXCR3-B ratio was found higher in renal cell carcinoma samples than in normal kidney samples, and total CXCR3 and CXCR3-A expression was significantly higher in metastatic than in nonmetastatic carcinoma samples (Utsumi et al., 2014). Calcineurin inhibitors (CNI), used to limit inflammation and allograft rejection, promoted the development and recurrence of several cancers. In renal cancer, specifically, CNI may mediate the progression of human renal cancer by downregulating CXCR3-B and by promoting proliferation through CXCR3-A (Datta et al., 2008). A separate study found that CXCR3-B overexpression significantly down-regulated the expression of anti-apoptotic heme oxygenase-1 (HO-1) in human renal cancer cells. Additionally, human renal cancer tissues expressing low amounts of CXCR3-B significantly overexpress HO-1 at both mRNA and protein level (Datta et al., 2010). CXCR3-B acts as anti-tumor isoform in renal cancer, similar to prostate and breast cancers.
CXCR3 and ligands in melanoma
Melanocytes present an epithelial phenotype transitions to a mesenchymal one as melanoma develops. As such, one might consider the same ‘stop’ and ‘go’ signals as in carcinomas. The expression of CXCR3 has been evaluated in patients with primary invasive cutaneous melanomas and there is a significant association of CXCR3-positive tumor cell immunoreactivity with tumor thickness of >1mm, or invasive, lethal melanoma (Monteagudo et al., 2007). Another study assessed the expression of CXCR3 and its ligands in thirteen human melanoma cell lines from primary tumors and eight cell lines established from metastasis from different tissues. All cell lines expressed CXCR3 mainly in the cytosol but a small subpopulation (<2%) of the cells in six cell lines showed positive surface staining. Additionally, most cell lines expressed high levels of CXCL9/CXCL11 but not CXCL10. These results suggest that surface expression of CXCR3 is tightly regulated and intracellular receptor expression might be related to metastasis and poor prognosis (Pinto et al., 2014). Accumulating evidence has shown a positive correlation of CXCR3 with melanoma invasion and metastasis. Knock down of CXCR3 in mouse melanoma B16F10 cells markedly reduced metastatic frequency compared with the parental cells (Kawada et al., 2004). In a highly invasive melanoma cell line BLM, CXCL9 triggered cell chemotaxis (Robledo et al., 2001). Lastly, it has been shown that tumor endothelial cells (ECs) secrete high levels of CXCL9 and CXCL10 in melanoma metastases, which induce spontaneous migration of melanoma cells and disrupts the endothelial barrier, resulting in an accelerated transendothelial migration (Amatschek et al., 2010). In sum, the CXCR3 signaling conspires to promote melanoma invasion and dissemination.
Conclusions
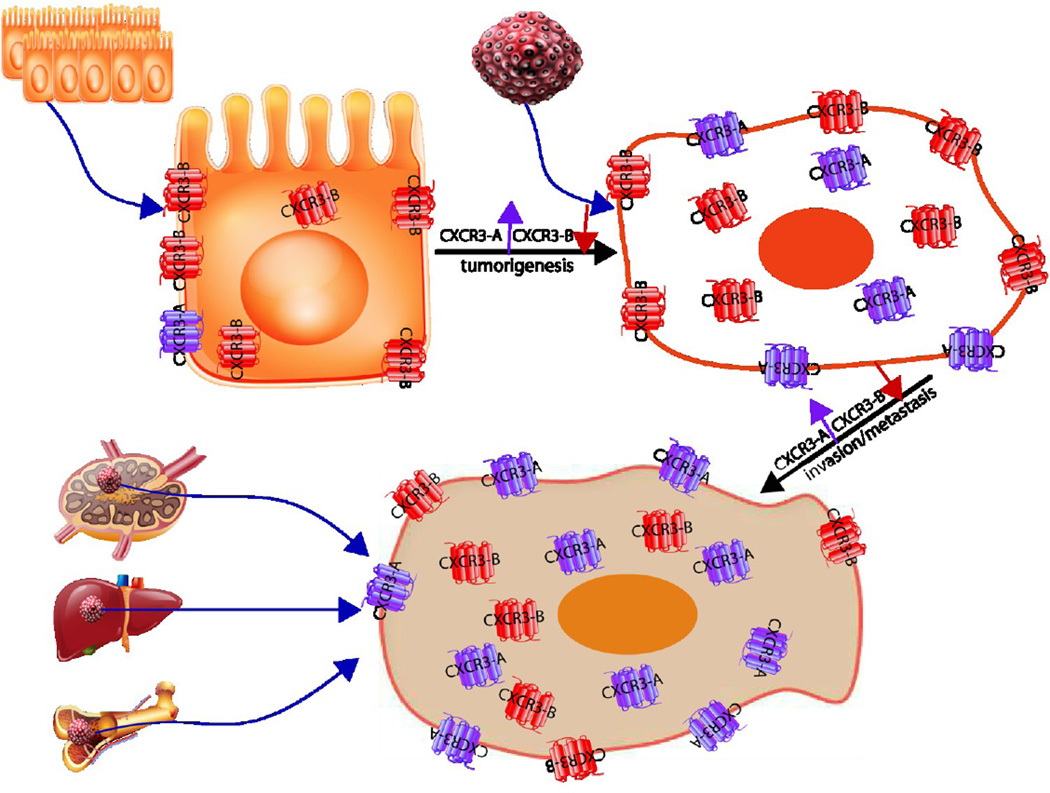
In the tumor microenvironment, all the cells including tumor epithelial cells, inflammatory cells and endothelial cells express CXCR3 and secrete its cognate ligands. Thus, the responses of the tumor organ could be quite complex, but the overall direction seems to be one of a shift from normally suppressive signals to positive, tumor progression promoting signals. Differential expression of CXCR3 isoforms contributes to the divergent physiological functions as the tumor cells shift from predominantly CXCR3-B to CXCR3-A, with much of the receptor being left in the cytosol (Figure 3).
Figure 3.
Schematic modeling of CXCR3 isoforms expression during tumor progression. In non-transformed cells CXCR3-B is the predominant isoform. CXCR3 expression level is elevated in primary and metastatic tumors. With progression, the ratio shifts towards more CXCR3-A with most of CXCR3 translocated to the cell cytosol in the malignant cells.
The role of CXCR3 on tumor progression involves intricate interaction with stromal cells and results in complex signaling cascades. Therefore, this review might not cover all of the aspects involving CXCR3 but there are several general conclusions can be derived. 1) total CXCR3 is upregulated in almost all kinds of tumors; 2) the ratio of CXCR3-A/CXCR3-B is higher in tumor compare to normal tissue or cell line, and this ratio is further elevated in metastatic tumor compared to primary tumor due to increased CXCR3-A or decreased CXCR3-B, or both; 3) CXCR3-A promotes tumor progression by promoting cell migration; 4) CXCR3-B inhibits tumor progression by limiting tumor migration/invasion as well as preventing tumor angiogenesis; 5) a shift of CXCR3 from membrane to cytoplasm plays role in tumor progression as a means to limit CXCR3-B signaling.
Although several laboratories investigated and discussed differential expression of CXCR3-A and CXCR3-B, the CXCR3 isoforms in cancer still need to be explored further. Due to overlapping CXCR-A and CXCR3-B protein sequence, specific antibodies targeting CXCR3-A cannot be constructed and pose a hindrance in studying the specific role CXCR3-A in cancer. The antibodies against different protein 3-D structures of CXCR3 isoforms are needed urgently. Moreover, most CXCR3 was found in the cytoplasm of tumor cells than on the cell surface, but the role of CXCR3 in the cytoplasm is unknown. Interestingly, forced overexpression of CXCR3-B in some cell lines is technically challenging including the cells with predominant CXCR3-B (unpublished data in our lab and (Datta et al., 2010; Balan & Pal, 2013)). This suggests that high level CXCR3-B expression can lead to activation and cells quiescence/senescence or apoptosis.
In summary, CXCR3 expression appears to be linked to tumor progression. Basic investigations have provided mechanisms by which this may support lethal developments. Based on this, disruption of CXCR3 signaling may be beneficial for limiting progression. However, this simple approach is confounded by CXCR3-B signaling limiting tumor angiogenesis and CXCR3-A signaling increasing immune cell infiltration. Thus, the therapies would need to be targeted to the tumor cells specifically.
Acknowledgements
This study was supported by grants from the VA Merit Program and the DoD CDMRP in Breast and Prostate Cancer, and the NIH UH2 TR000496.
Abbreviations
- MIG
monokine induced by gamma-interferon
- IP10
interferon gamma-induced protein 10
- ITAC
interferon inducible T-Cell alpha chemoattractant
- PF4
platelet factor 4
- PKA
protein kinase A
- PKC
protein kinase C
- PLCβ
phospholipase C β
- IFN-γ
interferon γ
- PGE2
prostaglandin E2
References
- Aksoy MO, Yang Y, Ji R, Reddy PJ, Shahabuddin S, Litvin J, Rogers TJ, Kelsen SG. Cxcr3 surface expression in human airway epithelial cells: Cell cycle dependence and effect on cell proliferation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L909–L918. doi: 10.1152/ajplung.00430.2005. [DOI] [PubMed] [Google Scholar]
- Amatschek S, Lucas R, Eger A, Pflueger M, Hundsberger H, Knoll C, Grosse-Kracht S, Schuett W, Koszik F, Maurer D, Wiesner C. Cxcl9 induces chemotaxis, chemorepulsion and endothelial barrier disruption through cxcr3-mediated activation of melanoma cells. Br. J. Cancer. 2010;104:469–479. doi: 10.1038/sj.bjc.6606056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A, Srivastava MK, Harris-White M, Huang M, Zhu L, Elashoff D, Strieter RM, Dubinett SM, Sharma S. Role of cxcr3 ligands in il-7/il-7r alpha-fc-mediated antitumor activity in lung cancer. Clin. Cancer Res. 2011;17:3660–3672. doi: 10.1158/1078-0432.CCR-10-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A, Yang SC, Huang M, Zhu L, Kar UK, Batra RK, Elashoff D, Strieter RM, Dubinett SM, Sharma S. Il-7 promotes cxcr3 ligand-dependent t cell antitumor reactivity in lung cancer. J. Immunol. 2009;182:6951–6958. doi: 10.4049/jimmunol.0803340. [DOI] [PubMed] [Google Scholar]
- Arenberg DA, Polverini PJ, Kunkel SL, Shanafelt A, Hesselgesser J, Horuk R, Strieter RM. The role of cxc chemokines in the regulation of angiogenesis in non-small cell lung cancer. J. Leukoc. Biol. 1997;62:554–562. doi: 10.1002/jlb.62.5.554. [DOI] [PubMed] [Google Scholar]
- Balan M, Pal S. A novel cxcr3-b chemokine receptor-induced growth-inhibitory signal in cancer cells is mediated through the regulation of bach-1 protein and nrf2 protein nuclear translocation. J. Biol. Chem. 2013;289:3126–3137. doi: 10.1074/jbc.M113.508044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billottet C, Quemener C, Bikfalvi A. Cxcr3, a double-edged sword in tumor progression and angiogenesis. Biochim. Biophys. Acta. 2013;1836:287–295. doi: 10.1016/j.bbcan.2013.08.002. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Yates CC, Wells A. Ip-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006;98:617–625. doi: 10.1161/01.RES.0000209968.66606.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Rodgers ME, Chen WC, Wells A. Pericyte regulation of vascular remodeling through the cxc receptor 3. Arterioscler Thromb. Vasc. Biol. 2013;33:2818–2829. doi: 10.1161/ATVBAHA.113.302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. Ip-10 induces dissociation of newly formed blood vessels. J. Cell Sci. 2009;122:2064–2077. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronger H, Kraeft S, Schwarz-Boeger U, Cerny C, Stockel A, Avril S, Kiechle M, Schmitt M. Modulation of cxcr3 ligand secretion by prostaglandin e2 and cyclooxygenase inhibitors in human breast cancer. Breast Cancer Res. 2012;14:R30. doi: 10.1186/bcr3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambien B, Karimdjee BF, Richard-Fiardo P, Bziouech H, Barthel R, Millet MA, Martini V, Birnbaum D, Scoazec JY, Abello J, Al Saati T, Johnson MG, Sullivan TJ, Medina JC, Collins TL, Schmid-Alliana A, Schmid-Antomarchi H. Organ-specific inhibition of metastatic colon carcinoma by cxcr3 antagonism. Br. J. Cancer. 2009;100:1755–1764. doi: 10.1038/sj.bjc.6605078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Wu Q, Shepard C, Wells A. Hepatocyte induced re-expression of e-cadherin in breast and prostate cancer cells increases chemoresistance. Clin. Exp. Metastasis. 2011;29:39–50. doi: 10.1007/s10585-011-9427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Y, Wu Q, Acquafondata M, Dhir R, Wells A. Partial mesenchymal to epithelial reverting transition in breast and prostate cancer metastases. Cancer Microenviron. 2011;5:19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of cxcr3 that mediate cxcl9, cxcl10, and cxcl11 function. J. Biol. Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Manice LA, Luster AD. Cxcr3 requires tyrosine sulfation for ligand binding and a second extracellular loop arginine residue for ligand-induced chemotaxis. Mo.l Cell. Biol. 2006;26:5838–5849. doi: 10.1128/MCB.00556-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan-Berger M, Feniger-Barish R, Avniel S, Wald H, Galun E, Grabovsky V, Alon R, Nagler A, Ben-Baruch A, Peled A. Role of cxcr3 carboxyl terminus and third intracellular loop in receptor-mediated migration, adhesion and internalization in response to cxcl11. Blood. 2006;107:3821–3831. doi: 10.1182/blood-2004-01-0214. [DOI] [PubMed] [Google Scholar]
- Datta D, Banerjee P, Gasser M, Waaga-Gasser AM, Pal S. Cxcr3-b can mediate growth-inhibitory signals in human renal cancer cells by down-regulating the expression of heme oxygenase-1. J. Biol. Chem. 2010;285:36842–36848. doi: 10.1074/jbc.M110.170324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Contreras AG, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Calcineurin inhibitors modulate cxcr3 splice variant expression and mediate renal cancer progression. J. Am. Soc. Nephrol. 2008;19:2437–2446. doi: 10.1681/ASN.2008040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D, Flaxenburg JA, Laxmanan S, Geehan C, Grimm M, Waaga-Gasser AM, Briscoe DM, Pal S. Ras-induced modulation of cxcl10 and its receptor splice variant cxcr3-b in mda-mb-435 and mcf-7 cells: Relevance for the development of human breast cancer. Cancer Res. 2006;66:9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]
- Dijkstra IM, Hulshof S, van der Valk P, Boddeke HW, Biber K. Cutting edge: Activity of human adult microglia in response to cc chemokine ligand 21. J. Immunol. 2004;172:2744–2747. doi: 10.4049/jimmunol.172.5.2744. [DOI] [PubMed] [Google Scholar]
- Du C, Yao Y, Xue W, Zhu WG, Peng Y, Gu J. The expression of chemokine receptors cxcr3 and cxcr4 in predicting postoperative tumour progression in stages i–ii colon cancer: A retrospective study. BMJ Open. 2014;4:e005012. doi: 10.1136/bmjopen-2014-005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlert JE, Addison CA, Burdick MD, Kunkel SL, Strieter RM. Identification and partial characterization of a variant of human cxcr3 generated by posttranscriptional exon skipping. J. Immunol. 2004;173:6234–6240. doi: 10.4049/jimmunol.173.10.6234. [DOI] [PubMed] [Google Scholar]
- Engl T, Relja B, Blumenberg C, Muller I, Ringel EM, Beecken WD, Jonas D, Blaheta RA. Prostate tumor cxc-chemokine profile correlates with cell adhesion to endothelium and extracellular matrix. Life Sci. 2006;78:1784–1793. doi: 10.1016/j.lfs.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Fulton AM. The chemokine receptors cxcr4 and cxcr3 in cancer. Curr Oncol Rep. 2009;11:125–131. doi: 10.1007/s11912-009-0019-1. [DOI] [PubMed] [Google Scholar]
- Furuya M, Suyama T, Usui H, Kasuya Y, Nishiyama M, Tanaka N, Ishiwata I, Nagai Y, Shozu M, Kimura S. Up-regulation of cxc chemokines and their receptors: Implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum. Pathol. 2007;38:1676–1687. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- Furuya M, Yoneyama T, Miyagi E, Tanaka R, Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y, Aoki I. Differential expression patterns of cxcr3 variants and corresponding cxc chemokines in clear cell ovarian cancers and endometriosis. Gynecol. Oncol. 2011;122:648–655. doi: 10.1016/j.ygyno.2011.05.034. [DOI] [PubMed] [Google Scholar]
- Furuya M, Tanaka R, Miyagi E, Kami D, Nagahama K, Miyagi Y, Nagashima Y, Hirahara F, Inayama Y, Aoki I. Impaired cxcl4 expression in tumor-associated macrophages (tams) of ovarian cancers arising in endometriosis. Cancer Biol. Ther. 2012;13:671–680. doi: 10.4161/cbt.20084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg-Bittman L, Neumark E, Sagi-Assif O, Azenshtein E, Meshel T, Witz IP, Ben-Baruch A. The expression of the chemokine receptor cxcr3 and its ligand, cxcl10, in human breast adenocarcinoma cell lines. Immunol. Lett. 2004;92:171–178. doi: 10.1016/j.imlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Huen AC, Wells A. The beginning of the end: Cxcr3 signaling in late-stage wound healing. Adv. Wound Care (New Rochelle) 2012;1:244–248. doi: 10.1089/wound.2011.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johrer K, Zelle-Rieser C, Perathoner A, Moser P, Hager M, Ramoner R, Gander H, Holtl L, Bartsch G, Greil R, Thurnher M. Up-regulation of functional chemokine receptor ccr3 in human renal cell carcinoma. Clin. Cancer Res. 2005;11:2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of cxcr3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–4017. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- Kawada K, Hosogi H, Sonoshita M, Sakashita H, Manabe T, Shimahara Y, Sakai Y, Takabayashi A, Oshima M, Taketo MM. Chemokine receptor cxcr3 promotes colon cancer metastasis to lymph nodes. Oncogene. 2007;26:4679–4688. doi: 10.1038/sj.onc.1210267. [DOI] [PubMed] [Google Scholar]
- Klatte T, Seligson DB, Leppert JT, Riggs SB, Yu H, Zomorodian N, Kabbinavar FF, Strieter RM, Belldegrun AS, Pantuck AJ. The chemokine receptor cxcr3 is an independent prognostic factor in patients with localized clear cell renal cell carcinoma. J. Urol. 2008;179:61–66. doi: 10.1016/j.juro.2007.08.148. [DOI] [PubMed] [Google Scholar]
- Kouroumalis A, Nibbs RJ, Aptel H, Wright KL, Kolios G, Ward SG. The chemokines cxcl9, cxcl10, and cxcl11 differentially stimulate g alpha i-independent signaling and actin responses in human intestinal myofibroblasts. J. Immunol. 2005;175:5403–5411. doi: 10.4049/jimmunol.175.8.5403. [DOI] [PubMed] [Google Scholar]
- Kumar DSS. Ph.D. thesis. Graduate school of public health, University of pittsburgh; 2013. Epigenetic regulation of alternative splicing in cancer; p. 130. [Google Scholar]
- Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P. An alternatively spliced variant of cxcr3 mediates the inhibition of endothelial cell growth induced by ip-10, mig, and i-tac, and acts as functional receptor for platelet factor 4. J. Exp. Med. 2003;197:1537–1549. doi: 10.1084/jem.20021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen J, Lu ZH, Yu SN, Luo YF, Zhao WG, Ma YH, Jia CW. Significance of chemokine receptor cxcr3 expression in breast cancer. Zhonghua Bing Li Xue Za Zhi. 2011;40:85–88. [PubMed] [Google Scholar]
- Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor cxcr3: Regulation, chemokine binding and gene localization. Eur. J. Immunol. 1998;28:3696–3705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for ip10 and mig: Structure, function, and expression in activated t-lymphocytes. J. Exp. Med. 1996;184:963–969. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Ma B, Wells A. The mitogen-activated protein (map) kinases p38 and extracellular signal-regulated kinase (erk) are involved in hepatocyte-mediated phenotypic switching in prostate cancer cells. J. Biol. Chem. 2014;289:11153–11161. doi: 10.1074/jbc.M113.540237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Norsworthy K, Kundu N, Rodgers WH, Gimotty PA, Goloubeva O, Lipsky M, Li Y, Holt D, Fulton A. Cxcr3 expression is associated with poor survival in breast cancer and promotes metastasis in a murine model. Mol. Cancer. Ther. 2009;8:490–498. doi: 10.1158/1535-7163.MCT-08-0485. [DOI] [PubMed] [Google Scholar]
- Maunakea AK, Chepelev I, Cui K, Zhao K. Intragenic DNA methylation modulates alternative splicing by recruiting mecp2 to promote exon recognition. Cell Res. 2013;23:1256–1269. doi: 10.1038/cr.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: A question of life or death. Nat. Rev. Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Meiser A, Mueller A, Wise EL, McDonagh EM, Petit SJ, Saran N, Clark PC, Williams TJ, Pease JE. The chemokine receptor cxcr3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. J. Immunol. 2008;180:6713–6724. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo C, Martin JM, Jorda E, Llombart-Bosch A. Cxcr3 chemokine receptor immunoreactivity in primary cutaneous malignant melanoma: Correlation with clinicopathological prognostic factors. J. Clin. Pathol. 2007;60:596–599. doi: 10.1136/jcp.2005.032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- Mueller A, Meiser A, McDonagh EM, Fox JM, Petit SJ, Xanthou G, Williams TJ, Pease JE. Cxcl4-induced migration of activated t lymphocytes is mediated by the chemokine receptor cxcr3. J. Leukoc. Biol. 2008;83:875–882. doi: 10.1189/jlb.1006645. [DOI] [PubMed] [Google Scholar]
- Murakami T, Kawada K, Iwamoto M, Akagami M, Hida K, Nakanishi Y, Kanda K, Kawada M, Seno H, Taketo MM, Sakai Y. The role of cxcr3 and cxcr4 in colorectal cancer metastasis. Int. J. Cancer. 2012;132:276–287. doi: 10.1002/ijc.27670. [DOI] [PubMed] [Google Scholar]
- Nagpal ML, Davis J, Lin T. Overexpression of cxcl10 in human prostate lncap cells activates its receptor (cxcr3) expression and inhibits cell proliferation. Biochim. Biophys. Acta. 2006;1762:811–818. doi: 10.1016/j.bbadis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Ohri CM, Shikotra A, Green RH, Waller DA, Bradding P. Chemokine receptor expression in tumour islets and stroma in non-small cell lung cancer. BMC Cancer. 2010;10:172. doi: 10.1186/1471-2407-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrai I, Rombouts K, Lasagni L, Annunziato F, Cosmi L, Romanelli RG, Sagrinati C, Mazzinghi B, Pinzani M, Romagnani S, Romagnani P, Marra F. Activation of p38(mapk) mediates the angiostatic effect of the chemokine receptor cxcr3-b. Int. J. Biochem. Cell Biol. 2008;40:1764–1774. doi: 10.1016/j.biocel.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Pinto S, Martinez-Romero A, O'Connor JE, Gil-Benso R, San-Miguel T, Terradez L, Monteagudo C, Callaghan RC. Intracellular coexpression of cxc- and cc- chemokine receptors and their ligands in human melanoma cell lines and dynamic variations after xenotransplantation. BMC Cancer. 2014;14:118. doi: 10.1186/1471-2407-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo MM, Bartolome RA, Longo N, Rodriguez-Frade JM, Mellado M, Longo I, van Muijen GN, Sanchez-Mateos P, Teixido J. Expression of functional chemokine receptors cxcr3 and cxcr4 on human melanoma cells. J. Biol. Chem. 2001;276:45098–45105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. Cxc chemokines: The regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rubie C, Kollmar O, Frick VO, Wagner M, Brittner B, Graber S, Schilling MK. Differential cxc receptor expression in colorectal carcinomas. Scand. J. Immunol. 2008;68:635–644. doi: 10.1111/j.1365-3083.2008.02163.x. [DOI] [PubMed] [Google Scholar]
- Satish L, Blair HC, Glading A, Wells A. Interferon-inducible protein 9 (cxcl11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of mu-calpain. Mol. Cell. Biol. 2005;25:1922–1941. doi: 10.1128/MCB.25.5.1922-1941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Lillard JW, Jr, Singh S. Chemokines: Key players in cancer progression and metastasis. Front Biosci. (Schol Ed) 2011;3:1569–1582. doi: 10.2741/246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struyf S, Burdick MD, Proost P, Van Damme J, Strieter RM. Platelets release cxcl4l1, a nonallelic variant of the chemokine platelet factor-4/cxcl4 and potent inhibitor of angiogenesis. Circ. Res. 2004;95:855–857. doi: 10.1161/01.RES.0000146674.38319.07. [DOI] [PubMed] [Google Scholar]
- Struyf S, Salogni L, Burdick MD, Vandercappellen J, Gouwy M, Noppen S, Proost P, Opdenakker G, Parmentier M, Gerard C, Sozzani S, Strieter RM, Van Damme J. Angiostatic and chemotactic activities of the cxc chemokine cxcl4l1 (platelet factor-4 variant) are mediated by cxcr3. Blood. 2010;117:480–488. doi: 10.1182/blood-2009-11-253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama T, Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ichikawa T, Ueda T, Nikaido T, Ito H, Ishikura H. Up-regulation of the interferon gamma (ifn-gamma)-inducible chemokines ifn-inducible t-cell alpha chemoattractant and monokine induced by ifn-gamma and of their receptor cxc receptor 3 in human renal cell carcinoma. Cancer. 2005;103:258–267. doi: 10.1002/cncr.20747. [DOI] [PubMed] [Google Scholar]
- Taylor DP, Clark A, Wheeler S, Wells A. Hepatic nonparenchymal cells drive metastatic breast cancer outgrowth and partial epithelial to mesenchymal transition. Breast Cancer Res. Treat. 2014;144:551–560. doi: 10.1007/s10549-014-2875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BD, Jin Y, Wu KH, Colvin RA, Luster AD, Birnbaumer L, Wu MX. Inhibition of g alpha i2 activation by g alpha i3 in cxcr3-mediated signaling. J. Biol. Chem. 2007;282:9547–9555. doi: 10.1074/jbc.M610931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi T, Suyama T, Imamura Y, Fuse M, Sakamoto S, Nihei N, Ueda T, Suzuki H, Seki N, Ichikawa T. The association of cxcr3 and renal cell carcinoma metastasis. J. Urol. 2014;192:567–574. doi: 10.1016/j.juro.2014.01.100. [DOI] [PubMed] [Google Scholar]
- Vandercappellen J, Van Damme J, Struyf S. The role of cxc chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM. Antagonism of cxcr3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7707. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- Wells A, Grahovac J, Wheeler S, Ma B, Lauffenburger D. Targeting tumor cell motility as a strategy against invasion and metastasis. Trends Pharmacol. Sci. 2013;34:283–289. doi: 10.1016/j.tips.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Dhir R, Wells A. Altered cxcr3 isoform expression regulates prostate cancer cell migration and invasion. Mol. Cancer. 2012;11:3. doi: 10.1186/1476-4598-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthou G, Williams TJ, Pease JE. Molecular characterization of the chemokine receptor cxcr3: Evidence for the involvement of distinct extracellular domains in a multi-step model of ligand binding and receptor activation. Eur. J. Immunol. 2003;33:2927–2936. doi: 10.1002/eji.200324235. [DOI] [PubMed] [Google Scholar]
- Yates CC, Whaley D, A YC, Kulesekaran P, Hebda PA, Wells A. Elr-negative cxc chemokine cxcl11 (ip-9/i-tac) facilitates dermal and epidermal maturation during wound repair. Am. J. Pathol. 2008;173:643–652. doi: 10.2353/ajpath.2008.070990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipin-Roitman A, Meshel T, Sagi-Assif O, Shalmon B, Avivi C, Pfeffer RM, Witz IP, Ben-Baruch A. Cxcl10 promotes invasion-related properties in human colorectal carcinoma cells. Cancer Res. 2007;67:3396–3405. doi: 10.1158/0008-5472.CAN-06-3087. [DOI] [PubMed] [Google Scholar]