Abstract
2-Amino-1-methylimidazo[4,5-b]pyridine (PhIP) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) are carcinogenic heterocyclic aromatic amines (HAAs) formed in well-done cooked meats. Chemicals that induce cytochrome P450 (P450) 1A2, a major enzyme involved in the bioactivation of HAAs, also form in cooked meat. Therefore, well-done cooked meat may pose an increase in cancer risk because it contains both inducers of P450 1A2 and procarcinogenic HAAs. We examined the influence of components in meat to modulate P450 1A2 activity and the metabolism of PhIP and MeIQx in volunteers during a 4 week feeding study of well-done cooked beef. The mean P450 1A2 activity, assessed by caffeine metabolic phenotyping, ranged from 6.3 to 7.1 before the feeding study commenced and from 9.6 to 10.4 during the meat feeding period: the difference in means was significant (P < 0.001). Unaltered PhIP, MeIQx, and their P450 1A2 metabolites, N2-(β-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine (HON-PhIP-N2-Gl); N3-(β-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine (HON-PhIP-N3-Gl); 2-amino-3-methylimidazo-[4,5-f]quinoxaline-8-carboxylic acid (IQx-8-COOH); and 2-amino-8-(hydroxymethyl)-3-methylimidazo[4,5-f]quinoxaline (8-CH2OH-IQx) were measured in urine during days 2, 14, and 28 days of the meat diet. Significant correlations were observed on these days between the levels of the unaltered HAAs and their oxidized metabolites, when expressed as percent of dose ingested or as metabolic ratios. However, there was no statistically significant correlation between the caffeine P450 1A2 phenotype and any urinary HAA biomarker. Although the P450 1A2 activity varied by greater than 20-fold among the subjects, there was a large intra-individual variation of the P450 1A2 phenotype and inconsistent responses to inducers of P450 1A2. The coefficient of variation of the P450 1A2 phenotype within-individual ranged between 1 to 112% (median=40%) during the entire course of the study. The caffeine metabolic phenotype for P450 1A2 was a poor predictor of oxidative urinary metabolites of PhIP and MeIQx and may not be a reliable measure to assess the role of HAAs in cancer risk.
Introduction
Heterocyclic aromatic amines (HAAs) are formed during the cooking of meats, fish, and poultry.1,2 HAAs are experimental animal carcinogens and thought to contribute to some common forms of human cancers for individuals who frequently consume well-done cooked meats.1,3,4 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) are two of the most mass-abundant carcinogenic HAAs formed in cooked meats: concentrations can range from less than 1 part per billion (ppb) to >100 ppb in meats prepared under common household cooking conditions.1,2
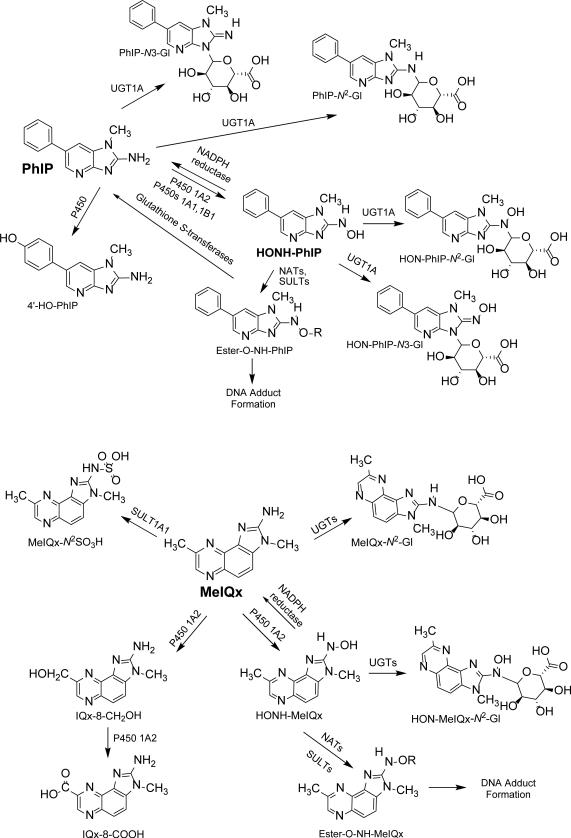
HAAs must undergo metabolism in order to exert their genotoxic effects. The biotransformation pathways of PhIP and MeIQx have been characterized in experimental laboratory animal models,5 human liver microsomes6-8 and cytosolic samples,9 recombinant phase I and II enzymes,10,11 mammalian cell lines,12,13 and human hepatocytes.14 The liver is the most active organ in the oxidative metabolism of PhIP and MeIQx. HAAs are activated to carcinogenic intermediates by N-oxidation, which is catalyzed principally by cytochrome P450 1A2 expressed in the liver,7,8 and by P450 1A1 and P450 1B1 in extrahepatic organs.15 One paradigm has been put forth where high P450 1A2 activity is a proposed susceptibility factor for HAA-induced cancer (Figure 1).16-19 In this paradigm, the activated N-hydroxylated HAA metabolites produced by P450 1A2 in the liver are exported to extrahepatic tissues where they are further activated by N-acetyltransferases or sulfotransferases to form reactive esters that bind to DNA.20 However, competing pathways of HAA metabolism may obscure the relationship between P450 1A2 activity and bioactivation of HAAs. For example, an NADH-dependent reductase in human liver rapidly converts N-hydroxy-HAA metabolites back to the parent amines.21 Several Glutathione S-transferases also catalyze the reduction of HONH-PhIP and its esters back to PhIP.22 P450 1A2 is also the major P450 that catalyzes the oxidation of the C8-methyl group of MeIQx to form 2-amino-3-methyl-8-hydroxymethylimidazo[4,5-f]quinoxaline (8-CH2OH-IQx) and its oxidation product 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid (IQx-8-COOH),23 the major detoxication metabolite of MeIQx in humans (Figure 1).24,25
Figure 1.
Major pathways of metabolism of PhIP and MeIQx in human liver.
Molecular epidemiological studies have examined the role of meat intake, HAA exposure, and genetic polymorphisms in P450 1A2 and other metabolizing enzymes in cancer risk. Collectively, the data from the studies have been inconsistent with no clear association found for any genetic polymorphisms in carcinogen metabolism enzymes and cancer risk.26-31 A major limiting factor in the risk assessment is the uncertainty in quantitative estimates of chronic exposure to HAAs.32 Some of the inconsistency also may be that the different genotypes in metabolizing enzymes do not accurately reflect appreciable changes in the level of protein expression or the phenotypic activity of the enzymes.28,33 Alternatively, a change in phenotype of more than one metabolism enzyme may be required to manifest elevated or diminished cancer risk. Two epidemiologic studies have reported that both rapid P450 1A2 and NAT2 phenotypes were required to increase the risk of colorectal cancer or to induce colon polyps in smokers who frequently consumed well-done cooked meat as a surrogate of HAA exposure.4,34 In support of this observation, many HAAs are bioactivated by P450 1A2 and NAT2 to form the N-acetoxy-HAA metabolites that bind to DNA to form adducts, which can lead to mutations.35 However, the joint effect of rapid P450 1A2 and NAT2 phenotypes in elevated colorectal cancer risk was not observed in a third study.36
The development of biomarkers associated with P450 1A2 activity has been sought to understand the role of this enzyme and HAAs in cancer risk. Caffeine undergoes extensive metabolism by P450 1A2 and the urinary metabolic ratio (MR) of caffeine and several of its metabolites is used to assess an individual's P450 1A2 metabolic activity in vivo.37,38 However, the relationship between P450 1A2 genotype or phenotype activity of caffeine and HAA biomarkers has only been examined in a few human studies,39-42 and the role of different genotypes or phenotypes of P450 1A2 (or other enzymes) in HAA metabolism, DNA adduct formation, and cancer risk is unclear.32,43 Genetic, environmental and life-style factors, such as tobacco smoke, cooked meat, cruciferous vegetables, medications, and even vigorous exercise can influence the inducibility of the P450 1A2 enzyme and the level of protein expression.44-50 There are also chemicals in the diet that serve as competitive inhibitors of P450 1A2.50,51 The impact of these factors on the metabolic pathways of HAAs is unknown. Of particular interest, well-done meat consumption leads to an increase in P450 1A2 activity,46,47 and thus, may increase the genotoxicity of HAAs by higher conversion of HAAs to their N-oxidized metabolites,8,39 which can react with DNA directly, or undergo further activation by conjugation reactions to form DNA adducts.32 The understanding of the role of P450 1A2 and conjugating enzymes in the metabolism of HAAs and ensuing DNA damage is important for molecular epidemiology studies that seek to assess the risk of consumption of well-done cooked meat containing HAAs in the etiology of dietary-associated cancers.
Urine is a useful biological matrix for the measurement of HAAs and their metabolites, since large quantities can be obtained noninvasively. Though the measurement of PhIP, MeIQx, and their metabolites in urine does not shed light on DNA damage, these urinary biomarkers may reflect the metabolic capacity of an individual to bioactivate or detoxicate these procarcinogens. The analysis of biomarkers of HAAs in human urine is an analytical challenge, because usually only ~1 μg to several micrograms of each HAA is ingested per day, for individuals eating well-done cooked meat, and the concentrations of the urinary biomarkers are often well below the part per billion (ppb) level in urine. Until the recent development of our solid-phase extraction (SPE) method,52,53 no method could simultaneously measure PhIP, MeIQx, and their major P450 1A2-oxidative metabolites in urine.41,42,54-56 Correlations between the urinary C8-oxidation metabolites of MeIQx, the major pathway of MeIQx metabolism in humans,24,25,53 and caffeine P450 1A2 phenotype have not been examined previously; nor have the correlations between the major oxidative metabolites of MeIQx and PhIP been investigated. In this study, we report on the effect of consumption of well-done cooked meat containing inducers of P450 1A2 on the metabolism of caffeine, and correlate the changes in P450 1A2 phenotype activity of caffeine to the metabolism of MeIQx and PhIP, by the measurement of their major P450 1A2-catalyzed oxidative metabolites excreted in urine over a 4 week semi-controlled meat-feeding study.
Materials and Methods
Chemicals
PhIP, MeIQx, 2-amino-1-trideuteromethylimidazo[4, 5-b]pyridine ([2H3C]-PhIP), and 2-amino-3-trideutromethyl-8-methylimidazo[4,5-f]quinoxaline ([2H3C]-MeIQx) (>99% isotopic purity) were purchased from Toronto Research Chemicals (Toronto, ON, Canada). The metabolites of PhIP and 1-[2H3C]-PhIP, N2-(ß-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine (HON-PhIP-N2-Gl), and N3-(ß-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine (HON-PhIP-N3-Gl) were prepared biosynthetically from human or rat liver microsomes fortified with UDPGA and 2-hydroxyamino-1-methylimidaoz[4,5-b]pyridine (HONH-PhIP).52,57 The C8-methyl oxidation products of MeIQx, IQx-8-COOH and 8-CH2OH-IQx, were prepared by oxidation of MeIQx with selenium dioxide as previously described.25
Study Subjects
A group of N = 44 (82% males, 18% females, and 45% White, 44% Asian/Pacific Islander, 9% African American, 2% Hispanic Non-White) healthy, non-smoking volunteers were recruited among University of Hawaii students and staff on the Manoa campus. Inclusion criteria included: regular beef eater; age >18; no use of hair dyes; taking no prescribed or over the counter medication except an occasional analgesic; no history of gastrointestinal tract disorders; having a weight not less than 90% or greater than 130% of 1983 Metropolitan Life Insurance desirable weights; no weight change of more than 10 pounds in the past 12 months; no special diet (e.g., vegetarian, macrobiotic, weight loss, diabetic, etc.); fruit and vegetable intake ≤7 servings/day; fiber intake less than 22 g/d, alcohol intake no greater than 2 drinks per day; caffeine intake no greater than 2 caffeinated drinks per day. The dietary variables were assessed using a food frequency questionnaire and a three-day food record. Recruitment was carried out through advertising on the University of Hawaii Manoa campus in Honolulu. The feeding study was divided into three phases. In phase I, a three week pre-feeding period, subjects refrained from eating pan-fried, grilled or oven-broiled meats, poultry or fish, cooked well-done. Thereafter, in phase II, volunteers ate dinner, five days a week, for four weeks at a study site on the University of Hawaii Manoa campus. As part of this meal, subjects were fed a ground beef patty (150 or 200 g) grilled well-done. The rest of the meal was varied on a five day rotating basis but was low in dietary fiber and included a starch (rice, potato or pasta), a vegetable, a fruit, dessert, and a drink. For the remainder of their daily meals and on Saturdays and Sundays, the participants followed their normal diets, except that they were asked to avoid eating any well-done meat or fish. After this 4-week feeding period, in phase III, the volunteers went back to their regular diet but refrained from eating meats that were grilled well done for four weeks. This study was run in groups of 10-20 subjects. The meat portion size was adjusted (150 or 200 g) to decrease the variation in dosing of PhIP per serving. All subjects provided informed consent and the study was approved by the Institutional Review Boards at the Wadsworth Center and the University of Hawaii.
Preparation of cooked meat
Ground beef (15% fat) was cooked as half pound patties on a commercial flat top griddle for an average of 10 minutes per side. The surface temperature of the griddle was monitored and ranged from 440 °F to 600 °F. The patties were flipped only once. After cooking, the patties were refrigerated overnight. They were then minced and homogenized with a food processor, and frozen until needed. On each day of feeding, the meat was re-heated in a warmer so no additional HAAs would be formed. Several samples of the cooked meat were analyzed for PhIP and MeIQx to estimate the dose provided to each study group. The amounts of PhIP given per serving to the subjects were on average (μg PhIP/serving): 1.2 (study group 1), 3.0 (study group 2), 11.7 (study group 3), and 8.0 (study group 4). The amounts of MeIQx given per serving to the subjects were on average: (μg MeIQx/serving): 0.77 (study group 1), 0.98 (study group 2), 0.97 (study group 3), and 1.3 (study group 4). The HAAs were isolated from cooked meat by tandem solid-phase extraction (SPE). Quantitative mass spectrometric measurements were done as previously described employing the stable isotopic dilution method58.
Biological specimens
Urine was collected for the 12 h period immediately after dinner time until rising the following morning and kept on blue ice in a cooler until aliquoting and freezing at −80 °C. During phase II, subjects collected urine samples on days 21, 23, 35, and 49 (0, 2, 14, and 21 days following the start of consumption of well-done meat). During phase III, subjects provided 12-hour overnight urine samples on days 70 and 77 (the end of the 3rd and 4th week of cessation of consumption of well-done meat). The time of collection of urine following consumption of meat was chosen based on previous studies designed to examine the metabolism of MeIQx and PhIP,39-42 which showed that between 80 – 90% of the dose of these HAAs and their metabolites eliminated in urine occurred within 12 h of ingestion of cooked meat. Urine was stored at −80 °C until assayed.
Caffeine phenotyping for P450 1A2 activity
Our caffeine test protocol has been described in detail.59 We provided two packets of Maxwell House Instant Coffee to the participants and requested that they drink two cups of coffee, made with these two packets, upon rising. Each cup provides approximately 50 mg of caffeine. Participants were also instructed not to use cream, milk or non-dairy creamer and to fast for the subsequent two hours in order to standardize intestinal absorption. Subjects fasted for 10 hours, then consumed 2 cups of coffee upon rising, maintained fasting for another 2 hours, abstained from other caffeine consumption, and collected their urine during the fifth hour after dosage. Caffeine metabolites (1,7-dimethyluric acid (17U); 1,7-dimethylxanthine (17X); and caffeine (137X)) were analyzed by high-performance liquid chromatography (HPLC) with a photodiode array detector to assess metabolic phenotypes.59 The urinary molar ratio of [17X + 17U]/137X obtained at 5 h after caffeine dosage has been shown to accurately reflect caffeine 3-demethylatioin activity in vivo.37 The coefficient of variation (CV) for the caffeine metabolite ratio based on 69 blind duplicate pairs analyzed with the study samples was 2.6%. Participants were asked to limit their coffee consumption to two caffeinated beverages per day. Data reported in the literature show that caffeine does not induce P450 1A2 in primary human hepatocytes at a concentration attained by ordinary coffee drinking and imply that caffeine contained in coffee does not induce P450 1A2 expression in humans.60 Thus, the increased P450 1A2 activity during the feeding phase with well-done meat is unlikely to be due to caffeine intake. During the semi-controlled meat-feeding phase, the caffeine phenotyping was done on the morning after the 12-h collection of urine for HAA measurements.
Solid-Phase Extraction (SPE) of MeIQx and PhIP and their Metabolites from Urine
The enrichment procedure was previously reported.52,57 Urine samples (1.0 mL) were spiked with isotopically labeled internal standards 100 pg of [2H3C]-MeIQx and [2H3C]-PhIP, 300 pg of [2H3C]-8-CH2OH-IQx and IQx-8-COOH, and 1000 pg each of [2H3C]-PhIP-N2-Gl, [2H3C]-PhIP-N3-Gl, [2H3C]-HON-PhIP-N2-Gl, and [2H3C]-HON-PhIP-N3-Gl. The samples were acidified with HCO2H (88% (v/v), 20 μL) and then placed on ice for 30 min, before being centrifuged at 15000g for 2 min to remove particulates. The supernatants were applied to ThermoFisher HyperSep Retain CX (30 mg resin) cartridges that had been prewashed with CH3OH containing 5% NH4OH (1 mL), followed by 2% HCO2H in H2O (1 mL). The resins were attached to a vacuum manifold, under slight positive pressure (~5 inches of Hg), to achieve a flow rate of the eluent of approximately 1 mL/min. After application of the samples, the cartridges were washed with 2% HCO2H in H2O (1 mL), followed by 2% HCO2H in CH3OH (1 mL), H2O (1 mL) and 5% NH4OH (1 mL). The resin was allowed to run to dryness. Next, the biomarkers were eluted from the resin with CH3OH containing 1% NH4OH (1.5 mL). The extracts were concentrated to approximately 0.1 mL, by vacuum centrifugation. Then, the samples were transferred into silylated glass conical vials (0.35 mL volume) from MicroLiter Analytical Supplies, Inc. (Suwanee, GA) and evaporated to dryness by vacuum centriguation. The samples were resuspended in 1:1 H2O:DMSO (20 μL).
Ultraperformance liquid chromatography/tandem mass spectrometry (UPLC/MS2) Analyses
Chromatography was performed with a NanoAcquity UPLC system (Waters Corp., Milford, MA) equipped with a Michrom C18 AQ column (0.3 mm × 150 mm, 3 μm particle size, Michrom Bioresources Inc., Auburn, CA). Analytes were separated by a gradient. The A solvent was 0.01% HCO2H in H2O, and the B solvent contained 0.01% HCO2H and 5%H2O in CH3CN. The flow rate was set at 6 μL/min, starting at 100% A increased by a linear gradient to 65% B in 13 min and then to 100% B at 14 min holding for 1 min. The gradient was reversed to 100% A over 1 min, and held with a postrun time of 3 min for reequilibration. The mass-spectral data were acquired on a Finnigan Quantum Ultra Triple Stage Quadrupole MS (Thermo Fisher, San Jose, CA) and processed with Xcalibur version 2.07 software. Analyses were conducted in the positive electrospray ionization mode and employed an Advance CaptiveSpray source from Michrom Bioresources. The spray voltage was set at 1400 V, the in-source fragmentation was −5 V, and the capillary temperature was 200 °C. There was no sheath or auxiliary gas. The peak widths (in Q1 and Q3) were set at 0.7 Da. The measurement of analytes was done by selected reaction monitoring (SRM). The following transitions and collision energies were used for the quantification: MeIQx and [2H3C]-MeIQx, 214.1 → 199.1, 131.1 and 217.1 → 199.1, 131.1 at 30 eV; 8-CH2OH-IQx and [2H3C]-8-CH2OH-IQx, 230.1 → 197.1 and 233.1 →197.1, at 35 eV; IQx-8-COOH and [2H3C]-IQx-8-COOH, 244.1 → 183.1 and 247.1 → 183.1, at 38 eV; PhIP and [2H3C]-PhIP, 225.1 → 210.1 and 228.1 → 210.1 at 33 eV; HON-PhIP-N2-Gl, and [2H3C]-HON-PhIP-N2-Gl: 417.1 → 225.1 and 223.1 and 420.1 → 228.1 and 225.1 at 34 eV; HON-PhIP-N3-Gl and [2H3C]-HON-PhIP-N3-Gl: 417.1 → 225.1 and 224.1 and 420.1 → 228.1 and 227.1 at 34 eV. The dwell time for each transition was 10 m. Argon was used as the collision gas and was set at 1.5 mTorr.
Statistical Analyses
The biomarkers of PhIP and MeIQx in the urine and caffeine metabolic phenotypes were analyzed using linear mixed regression models, with person included as a random effect and study day as a set of fixed-effect indicators. The values were log-transformed as ln(x + 1) to meet model assumptions. Geometric means and 95% confidence intervals (CI) were computed across study days as the antilog of the covariate-adjusted means and their 95% CIs. Spearman rank correlation coefficients (r) were used to assess associations between PhIP and MeIQ biomarkers by study day; partial correlations were created to determine the aggregate association for the entire feeding period, adjusted for collection day. Additionally, to test whether increasing dosage (μg PhIP/serving) was associated with higher levels of PhIP and its metabolites (pg/mL) during feeding, simple linear regression of the log-transformed mean PhIP levels of the three feeding days on dosage was performed, using dosage as a continuous and also as a categorical variable (with the lowest dose of 1.2 μg as the reference). The estimates of PhIP, MeIQx and their metabolites are reported as the percent of the ingested dose, and metabolic ratios were determined by converting the amount of urinary biomarker (pg/mL) to pmol and dividing by the amount of unmetabolized PhIP or MeIQx (pmol) in urine. Seventeen out of the 44 subjects had levels of PhIP below the limit of quantification (LOQ) value for at least one or more of the days of feeding cooked meat. Six out of the 44 subjects had levels of MeIQx below the LOQ during one out of the 3 days of meat feeding and one subject had levels below the LOQ in two out of the 3 days of meat feeding and urine collection. A value of 2.5 pg, one-half the LOQ value, was assigned when HAAs were below the LOQ.
Results
UPLC/MS2 analyses and estimates of PhIP, MeIQx, and their oxidized metabolites in urine
A facile solid-phase extraction method, employing a mixed-mode reverse-phase cation exchange resin, was established to simultaneously isolate PhIP, its major N-oxidized metabolites, the N2- and N3-glucuronide conjugates of HONH-PhIP, MeIQx and its major C8-methyl oxidized metabolites, 8-CH2OH-IQx and IQx-8-COOH, all of which are produced by P450 1A2,14,23,24 in urine. The intraday and interday precisions of the estimates of PhIP, MeIQx, and their metabolites, reported as the coefficients of variation, were <10%. The limit of quantification (LOQ) values for PhIP and MeIQx were about 5 pg/mL, whereas the LOQ values of their metabolites ranged from 10 to 40 pg/mL.53
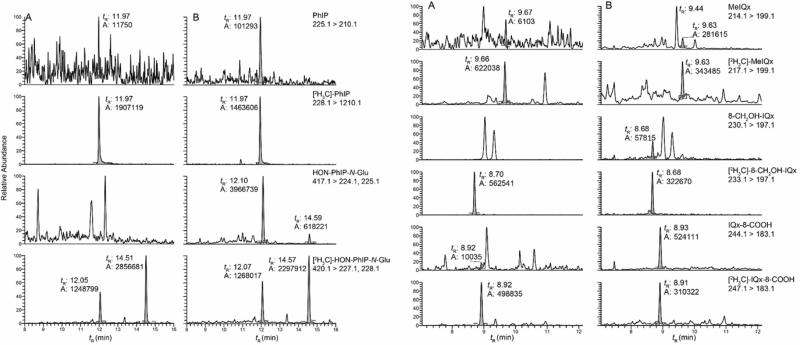
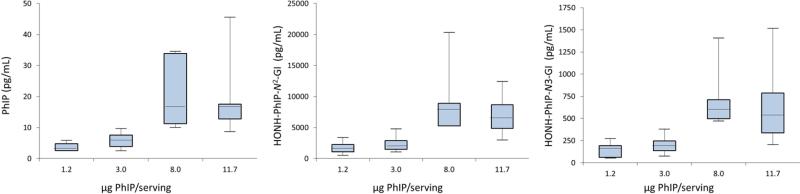
Representative UPLC/MS2 chromatograms of PhIP, MeIQx, and their metabolites in urine during the pre-feeding and cooked meat feeding phases are depicted in Figure 2. The adjusted geometric means (95% CIs) of urinary HAA metabolite biomarkers (pg/mL and % dose) are summarized in Table 1. During phases I and III of the study, these urinary HAA biomarkers were infrequently detected or occurred at trace levels. The exposure data confirm that the subjects adhered to a diet containing very little well-done cooked meat during these phases. In phase II, when subjects ate well-done cooked meat, the levels of unaltered PhIP in urine ranged from below the LOQ value up to 51 pg/mL, and the levels of MeIQx ranged from below the LOQ value up to 105 pg/mL. The major oxidative urinary metabolite of PhIP was HONH-PhIP-N2-Gl, and it accounted for up to 91% of the dose. The major metabolite of MeIQx was IQx-8-COOH, and it accounted for up to 59% of the dose. These findings are in agreement to other studies measuring these urinary biomarkers.24,40-43,56 The levels of all HAA biomarkers in urine during pre- and post-feeding at days 21, 70 and 77 are statistically significantly different from the levels present in urine at days 23, 35 and 49, during feeding with cooked meat (Table 1) (p-value < 0.001). The average amount of PhIP ingested by volunteers in study groups 3 and 4 was 4.7-fold greater than the average amount ingested by volunteers in study groups 1 and 2 (9.9 vs 2.1 μg), while the average amount of MeIQx ingested by volunteers in study groups 3 and 4 was 1.1-fold greater than the average amount ingested by members of study groups 1 and 2 (1.1 vs 0.9 μg). There was a proportional increase in the amount of PhIP and its metabolites excreted in urine (pg/mL) as a function of dose (1.2, 3.0, 8.0, 11.7 μg PhIP/serving), p < 0.001 for PhIP, HONH-PhIP-N2-GI, and HONH-PhIP-N3-GI; however, there were no significant differences in these levels when comparing the two highest doses (8.0 and 11.7 μg PhIP/serving), p = 0.55 for PhIP, p = 0.27 for HONH-PhIP-N2-GI, and p = 0.36 for HONH-PhIP-N3-Gl (Figure 3).
Figure 2.
UPLC/MS2 analysis of PhIP and MeIQx metabolites in urine (A) before and (B) during semi-controlled meat feeding study. The peak detected at tR 9.4 min with the transition of 214.1 > 199.1 is 2-amino-1,7-dimethylimidazo[4,5-g]quinoxaline, an isomer of MeIQx.78 HON-PhIP-N2-Gl elutes at tR 21.10 min and HON-PhIP-N3-Gl elutes at tR 14.59 min.
Table 1.
| Phase I (pre-feeding) | Phase II (meat-feeding) | Phase III (post-feeding) | p-value (phases I & III vs II) | |||||
|---|---|---|---|---|---|---|---|---|
| Biomarkers | Unit | Day 21 n=42 | Day 23b n=44 | Day 35b n=44 | Day 49b n=44 | Day 70 n=44 | Day 77 n=24 | |
| MelQx | pg/mL | 0.37 (0.19, 0.58) | 23.7 (18.9, 29.7) | 22.1 (17.6, 27.8) | 24.0 (17.5, 32.8) | 0.58 (0.37, 0.83) | 0.55 (0.28, 0.87) | <0.001 |
| % dose | 0.05 (0.01, 0.08) | 2.69 (2.30, 3.11) | 1.88 (1.60, 2.20) | 2.24 (1.80, 2.75) | 0.06 (0.04, 0.09) | 0.07 (0.02, 0.11) | <0.001 | |
| 8-CH2OH-IQX | pg/mL | 0.40 (0.16, 0.69) | 39.8 (30.5, 51.9) | 41.9 (32.8, 53.4) | 44.4 (33.5, 58.7) | 0.63 (0.31, 1.03) | 0.73 (0.29, 1.31) | <0.001 |
| % dose | 0.06 (0.01, 0.11) | 3.77 (3.15, 4.49) | 3.11 (2.57, 3.72) | 3.43 (2.79, 4.17) | 0.07 (0.03, 0.11) | 0.08 (0.02, 0.13) | <0.001 | |
| IQx-8-COOH | pg/mL | 1.42 (0.54, 2.80) | 253 (185, 346) | 352 (271, 458) | 349 (249, 490) | 0.82 (0.27, 1.62) | 2.83 (1.50, 4.87) | <0.001 |
| % dose | 0.31 (0.08, 0.59) | 17.6 (13.5, 22.9) | 22.9 (18.4, 28.4) | 22.1 (16.9, 28.7) | 0.17 (0.04, 0.32) | 0.32 (0.11, 0.56) | <0.001 | |
| PhIP | pg/mL | 0.27 (0.11, 0.46) | 8.43 (6.59, 10.7) | 9.19 (7.04, 11.9) | 9.80 (7.67, 12.5) | 0.29 (0.11, 0.51) | 0.51 (0.30, 0.75) | <0.001 |
| % dose | 0.003 (0, 0.01) | 0.16 (0.14, 0.18) | 0.15 (0.12, 0.18) | 0.17 (0.14, 0.20) | 0.005 (0, 0.01) | 0.01 (0, 0.02) | <0.001 | |
| HONH-PhIP-N2-GI | pg/mL | 4.09 (1.96, 7.76) | 2793 (2083, 3746) | 3648 (2717, 4897) | 3411 (2447, 4755) | 3.09 (1.37, 6.06) | 4.62 (1.79, 10.3) | <0.001 |
| % dose | 0.13 (0.04, 0.22) | 21.8 (18.2, 26.2) | 28.0 (23.3, 33.6) | 25.1 (19.5, 32.1) | 0.17 (0.04, 0.31) | 0.17 (0.02, 0.35) | <0.001 | |
| HONH-PhIP-N3-GI | pg/mL | 0.20 (0, 0.48) | 247 (187, 324) | 321 (233, 442) | 307 (215, 438) | 0.26 (0, 0.67) | 0.11 (0, 0.40) | <0.001 |
| % dose | 0.003 (0, 0.11) | 2.07 (1.77, 2.41) | 2.48 (2.14, 2.86) | 2.49 (2.15, 2.88) | 0.02 (0, 0.13) | 0.01 (0, 0.12) | <0.001 | |
The collection of urine samples commenced after 3 weeks on a low meat diet. Samples were collected on Day 0, 2, 14, and 21 days (Days 21, 23, 35, and 49) following the start of consumption of well-done meat, and 3 and 4 weeks (Days 70 and 77) following cessation of consumption of cooked meat.
Geometric mean and 95% CI based on mixed linear model.
Comparing the values of days 21, 70, and 71 to values of days 23, 35, and 49 based on an F test with (1, 43) degrees of freedom.
Figure 3.
Box and whisker plots showing the amounts of PhIP, HON-PhIP-N2-Glu and HON-PhI-N3-Glu eliminated in urine as a function of PhIP serving. The length of the boxes represents the 25th to 75th percentiles, and the horizontal lines within the box represent the median values. The whiskers show the minimum and maximum values.
Correlations among Caffeine P450 1A2 metabolic phenotype, unmetabolized urinary levels of PhIP, MeIQx, and their P450 1A2-catalyzed metabolites
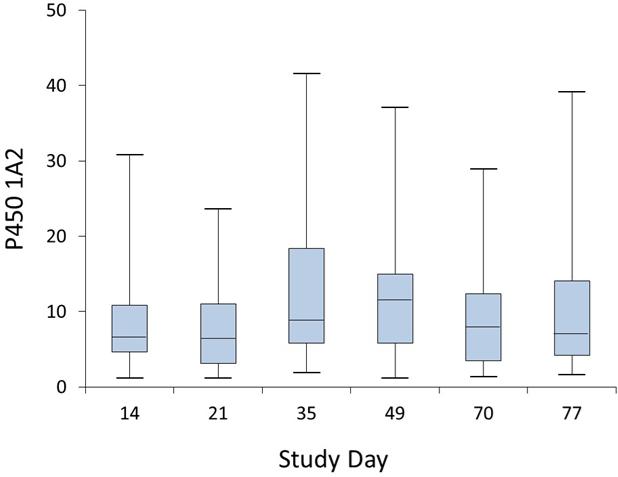
P450 1A2 is the major P450 isoform involved in the metabolism of caffeine, the N-oxidation of PhIP and MeIQx, and the C8-methyl oxidation of MeIQx,6,7,16,23 which are the major pathways of metabolism of these HAAs in humans.14,23,24,41-43,56 The consumption of well-done red meat during 4 weeks induced P450 1A2 activity. The geometric mean level of P450 1A2, as assessed by the urinary metabolic ratio (MR) of the caffeine metabolites 1,7-dimethyluric acid (17U) and 1,7-dimethylxanthine (17X) / caffeine (137X), varied from 6.3 to 7.1 before meat consumption, from 9.6 to 10.4 during the meat feeding period, and from 7.1 to 7.2 during the post-feeding period; the difference in means was significantly different between feeding and non-feeding periods (P < 0.001). P450 1A2 activity ranged from 1.18 to 23.6 on day 21, before the onset of the cooked meat feeding, from 1.9 to 41.6 at day 35 (following 2 weeks of meat feeding) and from 1.17 to 37.1 at day 49 (following 4 weeks of meat feeding). The caffeine P450 1A2 phenotype data measured over the entire study are depicted in the box and whisker plots, which show the interquartile range from the 25th to the 75th percentile, displayed by the length of the box, the median value, represented by the horizontal line within the box, and the lowest and highest values, shown by the whiskers (Figure 4). The positive effect of grilled meat consumption on the induction of P450 1A2 activity is consistent with findings from a previous study.49
Figure 4.
Box and whisker plots showing the distributions of the caffeine P450 1A2 phenotype data measured over the 77 day feeding study. The length of the boxes represents the 25th to 75th percentiles, and the horizontal lines within the box represent the median values. The whiskers show the minimum and maximum values. Subjects refrained from eating well-done cooked meats from days 1 – 21 (pre-feeding phase) and following day 49 (post-meat feeding phase).
We postulated that the induction of P450 1A2 activity would lead to increased metabolism of PhIP and MeIQx and produce more N-oxidized metabolites of PhIP and C8 oxidation products of MeIQx during the course of the meat feeding study.39,49 We sought to determine if: 1) the levels of unmetabolized PhIP and MeIQx, and the levels of N2 and N3 glucuronides of HONH-PhIP, and 8-CH2OH-IQx and IQx-8-COOH, were correlated within individuals; 2) the levels of these oxidative HAA metabolites were correlated to P450 1A2 activity based on the the urinary ratio (17U +17X)/137X; and 3) the levels of urinary HAAs and their oxidative metabolites were changed during the course of the four week meat feeding study and correlated to the induction of caffeine P450 1A2 activity.
The correlations of PhIP and MeIQx urinary biomarkers, measured as % dose or metabolic ratio (pmol metabolite/pmol PhIP or MeIQx), are tabulated in Table 2. The percent of dose eliminated as PhIP and MeIQx in urine on days 23 and 49 was highly correlated within subjects (p < 0.001), and the correlation approached borderline significance at day 35 (p < 0.07) (Table 2). The correlation between the unmetabolized dose of PhIP and MeIQx eliminated in urine, adjusted for the collection day in the feeding phase (days 23, 35 and 49) was r = 0.59, p < 0.001. However, the percent of the dose eliminated as unaltered PhIP did not change during feeding (geometric means of 0.16, 0.15, 0.17 for days 23, 35, 49, respectively, p=0.38), while the percent of the dose eliminated as MeIQx did change marginally (geometric means of 2.69, 1.88, 2.24 for days 23, 35, 49, respectively, p=0.002). The percentage of the dose eliminated in urine as PhIP and MeIQx continually decreased only in 3 and 7 of the 44 subjects, respectively, at days 35 and 49 in comparison to day 23, whereas the mean level of caffeine P450 1A2 activity increased by 1.7-fold by day 35 of the feeding study. Thus, the induction of P450 1A2, the major human P450 involved in the metabolism of PhIP and MeIQx in vitro,7,8,15 did not appreciably alter the levels of either HAA in urine during the course of the 4 week semi-controlled feeding study.
Table 2.
Correlations of PhIP and MeIQx biomarkers as a function of % dose or metabolic ratio (pmol metabolite/pmol PhIP or pmol MeIQx) in urine during meat-feeding phase
| Biomarkers | Spearman correlations |
|||||||
|---|---|---|---|---|---|---|---|---|
| Day 23 (n=44) |
p-value | Day 35 (n=44) |
p-value | Day 49 (n=44) |
p-value | Adjusted for Days 23,35,49 (n=132) |
p-value | |
| % Dose | ||||||||
| PhIP & MeIQx | 0.71 | <0.001 | 0.28 | 0.069 | 0.68 | <0.001 | 0.59 | <0.001 |
| IQx-8-COOH & HONH-PhIP-N2-GI | 0.31 | 0.041 | 0.01 | 0.97 | 0.51 | <0.001 | 0.31 | <0.001 |
| IQx-8-COOH & HONH-PhIP-N3-GI | 0.33 | 0.028 | 0.18 | 0.25 | 0.53 | <0.001 | 0.37 | <0.001 |
| HONH-PhIP-N2-GI & HONH-PhIP-N3-GI | 0.85 | <0.001 | 0.72 | <0.001 | 0.88 | <0.001 | 0.82 | <0.001 |
| IQx-8-COOH & 8-CH2OH-IQx | 0.73 | <0.001 | 0.60 | <0.001 | 0.81 | <0.001 | 0.72 | <0.001 |
| 8-CH2OH-IQx & HONH-PhIP-N2-GI | 0.46 | 0.002 | 0.16 | 0.29 | 0.52 | <0.001 | 0.37 | <0.001 |
| 8-CH2OH-IQx & HONH-PhIP-N3-GI | 0.54 | <0.001 | 0.31 | 0.040 | 0.60 | <0.001 | 0.49 | <0.001 |
| Metabolic Ratio | ||||||||
| IQx-8-COOH & HONH-PhIP-N2-GI | 0.28 | 0.066 | 0.14 | 0.36 | 0.37 | 0.013 | 0.28 | 0.001 |
| IQx-8-COOH & HONH-PhIP-N3-GI | 0.38 | 0.010 | 0.07 | 0.66 | 0.59 | <0.001 | 0.38 | <0.001 |
| HONH-PhIP-N2-GI & HONH-PhIP-N3-GI | 0.74 | <0.001 | 0.63 | <0.001 | 0.54 | <0.001 | 0.63 | <0.001 |
| IQx-8-COOH & 8-CH2OH-IQx | 0.52 | <0.001 | 0.33 | 0.027 | 0.59 | <0.001 | 0.49 | <0.001 |
| 8-CH2OH-IQx & HONH-PhIP-N2-GI | 0.18 | 0.24 | −0.04 | 0.82 | 0.07 | 0.64 | 0.07 | 0.41 |
| 8-CH2OH-IQx & HONH-PhIP-N3-GI | 0.29 | 0.054 | 0.03 | 0.83 | 0.44 | 0.003 | 0.25 | 0.004 |
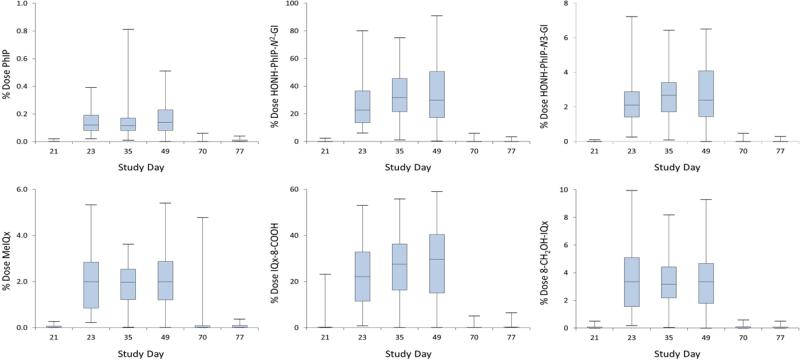
The amount of HONH-PhIP-N2-Gl eliminated in urine was highly variable and accounted for <1% to 91% of the ingested dose. The amount of HONH-PhIP-N3-Gl ranged from <1% to 7.2% of the ingested dose. IQx-8-COOH accounted for <1% up to 59% of the ingested dose, and IQx-8-CH2OH accounted for <1% up to 9.9% of the ingested dose. The mean of the % doses eliminated as HONH-PhIP-N2-Gl, HONH-PhIP-N3-Gl, IQx-8-COOH in urine increased by 15 to 30% at days 35 and 49 relative to day 23. Fourteen and six of the 44 subjects, respectively, had a continually increasing percent of the dose eliminated as HON-PhIP-N2-Gl and HON-PhIP-N3-Gl at days 35 and 49 versus day 23, whereas, five and fifteen of the 44 subjects showed an increasing percentage of the dose eliminated, respectively, as 8-CH2OH-IQx and IQx-8-COOH at days 35 and 49 versus day 23. The differences between geometric means of these metabolites were greater at day 49 versus day 23 than the differences between day 35 vs day 23. There was a strong partial correlation within subjects between the levels of HON-PhIP-N2-Gl and HON-PhIP-N3-Gl eliminated in urine during the days 23, 35 and 49 of the feeding phase with cooked meat with r = 0.82, p < 0.001, when expressed as % dose (Table 2). Similarly, the percentages of the dose eliminated in urine as 8-CH2OH-IQx and IQx-8-COOH over the course of the meat feeding study were strongly correlated with partial r = 0.72, p < 0.001. The percentages of the doses of the N-oxidized metabolites of PhIP and C8 oxidation metabolites of MeIQx in urine also were correlated within subjects (Table 2). The partial correlation between the two major oxidized metabolites of PhIP and MeIQx eliminated in urine HON-PhIP-N2-Gl and IQx-8-COOH was weak but significant (r = 0.31, p < 0.001). Statistically significant correlations also were observed between HON-PhIP-N3-Gl and IQx-8-COOH, and between both N-glucuronide conjugates of HONH-PhIP and IQx-8-CH2OH (Table 2). The correlations varied across the days of the feeding phase. The correlations were uniformly strong for days 23 and 49, but were much weaker for day 35. On day 35, statistically significant correlations were only observed between the individual C8-oxidation metabolites of MeIQx and between the individual N-oxidation PhIP urinary biomarkers; however, there was a poor cross-correlation between the C8-oxidation metabolites of MeIQx and the N-oxidation metabolites of PhIP. We investigated this phenomenon and found that the pattern of the HAA urinary biomarkers over time during Phase II for all subjects was to increase, reach a zenith and then either plateau or decline somewhat. It appears that there was more variability in the metabolite values near the beginning (day 23) and the end (day 49) of the feeding period than at day 35, where the zenith occurred for almost all subjects, which led to a squashing of the variability. In Figure 5 clearly shows this reduction in variability at day 35, where the length of the boxes, representing the variability measure of interquartile range, are uniformly less at day 35 than days 23 and 49. This phenomenon is an interesting artifactual consequence of human metabolism and is not a reflection of no correlation between analytes overall.
Figure 5.
Box and whisker plots showing the % of the dose of PhIP, MeIQx, and their oxidation metabolites eliminated urine during the study. The length of the boxes represents the 25th to 75th percentiles, and the horizontal lines within the box represent the median values. The whiskers show the minimum and maximum values.
Many of the MR of PhIP and MeIQx were also correlated; however, for the most part, the overall associations were weaker than those observed with the percentage of dose (Table 2). The weaker correlations of the MRs may be attributed to lower precision in the denominator measurements of the urinary levels of PhIP and MeIQx in many subjects where the levels of these HAAs approached or occurred below the LOQ value.
The correlations between P450 1A2 activity, based on the caffeine phenotype, and the levels of PhIP, MeIQx and their oxidized metabolites in urine as a function of the % dose or MR are summarized in Table 3. We did not observe significant correlations between P450 1A2 activity, based on the caffeine phenotype, and the levels of any HAA urinary biomarker across the feeding period.
Table 3.
Correlation between Caffeine P450 1A2 Phenotype Activity and % of Dose of Urinary HAA Biomarker or Caffeine P450 1A2 Phenotype Activity and HAA Metabolic Ratio
| Biomarkers | Spearman correlations |
|||||
|---|---|---|---|---|---|---|
| Day 35 (n=44) |
p-value | Day 49 (n=44) |
p-value | Adjusted for Days 35, 49 (n=88) |
p-value | |
| P450 1A2 & % Dose PhIP | −0.25 | 0.10 | 0.20 | 0.20 | −0.02 | 0.85 |
| P450 1A2 & % Dose MeIQx | −0.29 | 0.061 | 0.07 | 0.63 | −0.08 | 0.49 |
| P450 1A2 & % Dose IQx-8-COOH | −0.11 | 0.46 | 0.23 | 0.14 | 0.08 | 0.46 |
| P450 1A2 & % Dose 8-CH2OH-IQx | −0.38 | 0.012 | 0.11 | 0.49 | −0.10 | 0.34 |
| P450 1A2 & % Dose HONH-PhIP-N2-GI | −0.19 | 0.23 | 0.33 | 0.031 | 0.11 | 0.30 |
| P450 1A2 & % Dose HONH-PhIP-N3-GI | −0.20 | 0.18 | 0.23 | 0.14 | 0.03 | 0.80 |
| P450 1A2 & MR IQx-8-COOH | 0.09 | 0.55 | 0.22 | 0.15 | 0.14 | 0.20 |
| P450 1A2 & MR 8-CH2OH-IQx | −0.22 | 0.15 | 0.02 | 0.87 | −0.09 | 0.39 |
| P450 1A2 & MR HONH-PhIP-N2-GI | 0.09 | 0.55 | 0.09 | 0.57 | 0.09 | 0.42 |
| P450 1A2 & MR HONH-PhIP-N3-GI | 0.03 | 0.83 | 0.01 | 0.97 | 0.01 | 0.96 |
Discussion
P450 1A2 is thought to be the major P450 involved in the metabolism of PhIP and MeIQx based on metabolic studies conducted with recombinant P450s,11,61 human liver microsomal samples7,8 and human hepatocytes14 employing selective inhibitors of P450 1A2. The most compelling evidence for a role of P450 1A2 in the metabolism of PhIP and MeIQx in vivo is from a pharmacokinetic study conducted with human volunteers pretreated with furafylline,62 a mechanism-based inhibitor of P450 1A2.63 The pretreatment of subjects with furafylline increased the excretion of unchanged MeIQx by 14.3-fold, and that of unchanged PhIP increased by 4.1-fold in urine. P450 1A2 was estimated to contribute to 91% of the elimination of ingested MeIQx and 70% of ingested PhIP, following consumption of a meal of fried beef.62
Although we observed a significant increase in the P450 1A2 phenotype, based on the caffeine MR, at the population level in our feeding study of cooked meat, we did not observe a correlation between P450 1A2 phenotype and any of the HAA urinary biomarkers. However, there was a large intra- and inter-individual variation in response to the inducers of P450 1A2 over the 4 week feeding study of cooked meat: eleven out of the 44 subjects did not show an increase in P450 1A2 activity on day 35 versus day 21; 13 out of 44 subjects did not show an increase of P450 1A2 activity on day 49 versus day 21; and only 11 out of the 44 subjects showed a continual increase in P450 1A2 activity on both days 35 and 49 versus day 21 (Figure 4). The variation in P450 1A2 activity is not attributed to the method of measurement of caffeine metabolites, which is robust with a CV of 2.6%. The high level of P450 1A2 phenotypic variation may well explain the lack of correlation between P450 1A2 activity and HAA urinary biomarkers.
The role of rapid P450 1A2 genotype or phenotype, based on the caffeine MR, in the susceptibility of cancer risk by consumption of well-done cooked meats containing putative HAAs is unclear.26-28,30,64 The expression of hepatic P450 1A2 is highly variable in humans. Both genetic and environmental factors influence the inducibility of the enzyme and the level of protein expression.45,46,49,50 In one study, the weekly intra-individual variability in the urinary [17X + 17U]/137X MR, reported as the coefficients of variation (%CV, SD/mean × 100%) ranged from 23.0% to 48.0% in subjects on a free-choice diet who did not smoke and ranged from 13.5% to 27.4% in smokers.37 We previously reported a mean intra-individual %CV in the weekly caffeine MR measured over 4 weeks at 23.2% for non-smoking subjects on a free-choice diet (range 10.8% – 39.0%; both genders).59 Nakajima and colleagues reported a mean intra-individual %CV in the caffeine MR ranging from 0.7% to 84.5% in non-smokers and 6.9 – 37.6% of both genders (the time interval between measurements was not reported).65 Kashuba reported a range of 9.5 – 49.3 %CV in the caffeine MR in women; and of 4.5 – 45.3 in men measured over 3 months, employing another urinary MR of caffeine and its metabolites as an index of P450 1A2 activity.66 In our current study, the %CV of P450 1A2 activity within individuals prior to and post-feeding of meat ranged from 2% to 112% (median=40%) and the %CV ranged from 1% to 103% (median=40%) during the 3 weeks of feeding with well-done cooked meat (Figure 4). Thus, the within individual variation in P450 1A2 phenotype can be considerable over time, when employing caffeine as the probe. This large weekly intra-individual variation of P450 1A2 activity can explain the poor correlation between the caffeine MR and urinary HAA biomarkers.
We and others have reported that the level of P450 1A2 protein varies by greater than 60-fold in human liver microsomal preparations.8,46,67 The levels of P450 1A2 protein expression in human liver microsomes were highly correlated to the rates of N-oxidation of PhIP and MeQx in vitro, when employing high concentrations of these HAAs.8 The data on the correlations between P450 1A2 genotypes or phenotypes and HAA biomarkers in humans are limited. In one controlled meat feeding study, only a weak association was observed between caffeine P450 1A2 phenotype and the levels of urinary N-glucuronide conjugates of HONH-PhIP;42 in the same study an association was not observed between the N2-glucuronide conjugate of 2-hydroxyamino-3,8-dimethylimidazo[4,5-f]quinoxaline (HONH-MeIQx) and P450 1A2 activity, or between the urinary levels of the N-glucuronide conjugate of HONH-PhIP and HONH-MeIQx.41 An inverse association between P450 1A2 activity and the levels of unmetabolized MeIQx in urine of the same study group was found,39 but, this association was not observed for PhIP.40 Moreover, even though the range in P450 1A2 activity varied by 14-fold, the difference in the amount of unmetabolized MeIQx excreted in urine of the subjects with the highest and lowest rapid P4501A2 phenotype was only 2-fold.39
The relationship between P450 1A2 activity and HON-PhIP-N2-Gl and HONH-PhIP-N3-Gl levels may be obscured by the variable levels of UGT expression within individuals; there are at least 4 UGTs that contribute to the N-glucuronidation of HONH-PhIP.68-71 Our SPE method does not recover the N2-glucuronide of HONH-MeIQx, which was reported to range from 2.2 to 17.1% of the ingested dose,41 and thus we could not correlate the levels of the N2-glucuronide of HONH-MeIQx, to the isomeric N-glucuronide conjugates of HONH-PhIP in urine or caffeine P450 1A2 phenotype. However, the amounts of the urinary C8-methyl oxidation metabolites of MeIQx, 8-CH2OH-IQx and IQx-8-COOH, which are predominantly produced by P450 1A2 and do not undergo further biotransformation by conjugating enzymes,23,24 were correlated to the amounts of the urinary N-glucuronide metabolites of HONH-PhIP. Nevertheless, 8-CH2OH-IQx and IQx-8-COOH were not correlated to the caffeine P450 1A2 phenotype (Table 2). The correlations between the C8-oxidation metabolites of MeIQx and between the N-oxidation PhIP urinary biomarkers were stronger than the cross correlations between individual C8-oxidized metabolites of MeIQx and individual N-oxidized metabolites of PhIP, which may be explained by variable levels of UGT activities or other enzymes that bioactivate or detoxicate HONH-PhIP (Figure 1). The partial correlations among these oxidative metabolites of MeIQx and PhIP, and the correlation between the levels of unmetabolized PhIP and MeIQx in urine, imply a common pathway of P450-mediated oxidation occurs in vivo.
The correlation between the P450 1A2 phenotype and urinary HAA biomarkers also may be weakened by large differences between the dose of caffeine and HAAs, competing xenobiotic metabolic pathways, and pharmacokinetic parameters. The dose of caffeine is 10,000 to 100,000-fold greater than the amounts of PhIP or MeIQx ingested in cooked meat.1,2 Caffeine is commonly given as a bolus dose of 100 mg per coffee beverage after the subjects have fasted for 10 hours, and urinary metabolic ratios are measured 5 h following dosing.37 In contrast, PhIP and MeIQx are consumed as part of the diet containing cooked meat and biomarkers are measured in urine collected over a 12 h interval following a meal.39-42 The elimination of caffeine, PhIP, and MeIQx follow first order kinetics in humans.37,62 The half-life of caffeine is about 5.5 hours,37,38 and the estimated half-lives of PhIP and MeIQx are, respectively, 4.5 and 3.4 hours.62 The catalytic efficiency (kcat/Km) of the C8-oxidative metabolites of MeIQx and N-oxidative metabolites of MeIQx and PhIP in human liver microsomes are 90 to 370-fold greater than 17X formation from caffeine.7,8,72 The superior catalytic efficiency of P450 1A2 for the metabolism of PhIP and MeIQx may not allow us to distinguish between rapid and slow metabolizers by measurement of urine samples that were collected over a 12 h interval, where greater than 80% of the dose of HAAs and their metabolites eliminated in urine has occurred.39-42 Thus, despite the large interindividual differences in expression of hepatic P450 1A2 activity, even subjects with slow caffeine P450 1A2 phenotypes may have sufficient activity to efficiently metabolize PhIP and MeIQx in vivo.
Other studies on HAA urinary biomarkers and P450 1A2 phenotype have been reported. One study examined the effect of consumption of well-done cooked chicken for 10 consecutive days on the extent of PhIP eliminated in urine in 71 female volunteers.73 The authors reported a significant association between the P450 1A2-164A→C (P450 1A2*1F) polymorphism, a reported slow oxidizer,74 and higher urinary PhIP excretion levels. However, the association was driven by a small number of subjects possessing the homozygous variant. When the C/C genotypes were excluded from the analysis, the correlation was no longer found to be significant and no relationship was found between differential urinary PhIP excretion levels and phenotypes for caffeine. A study by Kim reported that subjects harboring P450 1A2/-2467delT had a significantly higher caffeine MR than the P450 1A2/-2464T/T or P450 1A2/-2464-/T genotypes (P<0.05),75 but paradoxically showed significantly higher levels of unmetabolized PhIP in urine than those subjects with T/T and -/T genotypes (P<0.05). This study also failed to show a relationship between the urinary PhIP levels and phenotypes of P450 1A2*1F polymorphism. In a study in the United Kingdom, there was no detectable association between any of the six major P450 1A2 genotypes and caffeine phenotype in vivo. Moreover, P450 1A2 activity was lower in colorectal cancer patients than in controls.28 The genotype(s) responsible for the 60-fold inter-individual differences in human hepatic P450 1A2 constitutive expression and P450 1A2 activity has not been identified.44 These findings highlight the challenges in employing genotypes or phenotypic biomarkers as measures of metabolic activity of HAAs in vivo.
We also previously reported that the levels of unmetabolized PhIP accrued in hair of the volunteers of this 4 week semi-controlled meat feeding study were not correlated to P450 1A2 activity, as assessed by the caffeine phenotype.76 The amount of unmetabolized PhIP in the bloodstream that reaches the hair follicle, following first-pass metabolism by hepatic P450 1A2, was expected to differ among individuals, and the phenotypic activity P450 1A2 was considered to be an important factor influencing the level of PhIP accrued in hair: subjects with rapid P450 1A2 phenotypes were expected to have the lowest levels of PhIP. Our findings suggest that the hepatic P450 1A2 expression, even in subjects with slow P450 1A2 phenotypes, may be at levels sufficient to significantly reduce the concentration of PhIP during first-pass metabolism before it reaches systemic circulation. Collectively, the urinary and hair biomarker data reinforce the notion that the caffeine P450 1A2 phenotype does not accurately predict the metabolic processing of PhIP and MeIQx in vivo.
In summary, differences in P450 1A2 activity alter the metabolism of caffeine and therapeutic drugs and influence the clinical outcome of some these compounds;51,77 however, the relationship between P450 1A2 polymorphisms and the metabolism and biodisposition of HAAs present at parts per billion levels in cooked meats is unclear. The large, unpredictable intra-individual variation of the caffeine P450 1A2 phenotype over time contributes to the poor correlation with oxidative urinary metabolites of MeIQx and PhIP. The direct measurement of urinary biomarkers of HAAs combined with measurements of their DNA or protein adducts, as a measure of the biologically effective dose, may provide a clearer picture of the interindividual differences in metabolism and cancer susceptibilities due to HAAs.
Acknowledgement
The technical work of Mr. Lin Liu, Wadsworth Center, New York State Department of Health, Albany, NY, on sample workup of urinary HAA biomarkers is greatly appreciated. The authors also thank the University of Hawaii Cancer Center Analytical Chemistry Share Resource and Dr. Adrian Franke for assaying the study samples for caffeine metabolites.
Funding
This research was supported by Grant 2R01 CA122320 (R.J.T.) and in part by National Cancer Institute Cancer Center Support Grant CA-77598 (R.J.T.)
ABBREVIATIONS
- PhIP
2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
- HONH-PhIP
2-hydroxyamino-1-methylimidaoz[4,5-b]pyridine; N2-(β-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine
- HON-PhIP-N3-Gl
N3-(β-1-glucosiduronyl-2-(hydroxyamino)-1-methyl-6-phenylimidazo[4,5-b]pyridine)
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- IQx-8-COOH
2-amino-3-methylimidazo-[4,5-f]quinoxaline-8-carboxylic acid
- 8-CH2OH-IQx
2-amino-8-(hydroxymethyl)-3-methylimidazo[4,5-f]quinoxaline
- 137X
caffeine
- 17U
1,7-dimethyluric acid
- 17X
1,7-dimethylxanthine
- LOQ
limit of quantitation
- CV
coefficient of variation
- MR
metabolic ratio
- SPE
solid phase extraction
- SRM
selected reaction monitoring
- UPLC/MS2
Ultraperformance liquid chromatography/mass spectrometry
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felton JS, Jagerstad M, Knize MG, Skog K, Wakabayashi K. Contents in foods, beverages and tobacco. In: Nagao M, Sugimura T, editors. In Food Borne Carcinogens Heterocyclic Amines. John Wiley & Sons Ltd.; Chichester, England: 2000. pp. 31–71. [Google Scholar]
- 3.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat. Res. 2002;506-507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 4.Le Marchand L, Hankin JH, Pierce LM, Sinha R, Nerurkar PV, Franke AA, Wilkens LR, Kolonel LN, Donlon T, Seifried A, Custer LJ, Lum-Jones A, Chang W. Well-done red meat, metabolic phenotypes and colorectal cancer in Hawaii. Mutat. Res. 2002;506-507:205–214. doi: 10.1016/s0027-5107(02)00167-7. [DOI] [PubMed] [Google Scholar]
- 5.Kato R, Yamazoe Y. Metabolic activation and covalent binding to nucleic acids of carcinogenic heterocyclic amines from cooked foods and amino acid pyrolysates. Jpn. J. Cancer Res. 1987;78:297–311. [PubMed] [Google Scholar]
- 6.Butler MA, Iwasaki M, Guengerich FP, Kadlubar FF. Human cytochrome P-450 PA (P450IA2), the phenacetin O -deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao K, Murray S, Davies DS, Boobis AR, Gooderham NJ. Metabolism of the food derived mutagen and carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5- b ]pyridine (PhIP) by human liver microsomes. Carcinogenesis. 1994;15:1285–1288. doi: 10.1093/carcin/15.6.1285. [DOI] [PubMed] [Google Scholar]
- 8.Turesky RJ, Constable A, Richoz J, Varga N, Markovic J, Martin MV, Guengerich FP. Activation of heterocyclic aromatic amines by rat and human liver microsomes and by purified rat and human cytochrome P450 1A2. Chem. Res. Toxicol. 1998;11:925–936. doi: 10.1021/tx980022n. [DOI] [PubMed] [Google Scholar]
- 9.Yamazoe Y, Nagata K. In vitro metabolism. In: Sugimura T, Nagao M, editors. Food Borne Carcinogens Heterocyclic Amines. John Wiley & Sons Ltd.; Chichester, England: 2000. pp. 74–89. [Google Scholar]
- 10.Shimada T, Iwasaki M, Martin MV, Guengerich FP. Human liver microsomal cytochrome P-450 enzymes involved in the bioactivation of procarcinogens detected by umu gene response in Salmonella typhimurium TA 1535/pSK1002. Cancer Res. 1989;49:3218–3228. [PubMed] [Google Scholar]
- 11.Shimada T, Guengerich FP. Activation of amino-à-carboline, 2-amino-1-methyl-6-phenylimidazo[4,5- b ]pyridine, and a copper phthalocyanine cellulose extract of cigarette smoke condensate by cytochrome P-450 enzymes in rat and human liver microsomes. Cancer Res. 1991;51:5284–5291. [PubMed] [Google Scholar]
- 12.Hein DW, Rustan TD, Bucher KD, Furman EJ, Martin WJ. Extrahepatic expression of the N-acetylation polymorphism toward arylamine carcinogens in tumor target organs of an inbred rat model. J.Pharmacol.Exp.Ther. 1991;258:232–236. [PubMed] [Google Scholar]
- 13.Metry KJ, Zhao S, Neale JR, Doll MA, States JC, McGregor WG, Pierce WM, Jr., Hein DW. 2-Amino-1-methyl-6-phenylimidazo [4,5-b] pyridine-induced DNA adducts and genotoxicity in Chinese hamster ovary (CHO) cells expressing human CYP1A2 and rapid or slow acetylator N-acetyltransferase 2. Mol.Carcinog. 2007;46:553–563. doi: 10.1002/mc.20302. [DOI] [PubMed] [Google Scholar]
- 14.Turesky RJ, Guengerich FP, Guillouzo A, Langouet S. Metabolism of heterocyclic aromatic amines by human hepatocytes and cytochrome P4501A2. Mutat. Res. 506. 2002;507:187–195. doi: 10.1016/s0027-5107(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 15.Crofts FG, Sutter TR, Strickland PT. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis. 1998;19:1969–1973. doi: 10.1093/carcin/19.11.1969. [DOI] [PubMed] [Google Scholar]
- 16.Turesky RJ, Lang NP, Butler MA, Teitel CH, Kadlubar FF. Metabolic activation of carcinogenic heterocyclic aromatic amines by human liver and colon. Carcinogenesis. 1991;12:1839–1845. doi: 10.1093/carcin/12.10.1839. [DOI] [PubMed] [Google Scholar]
- 17.Kadlubar FF, Butler MS, Kaderlik KR, Chou HC, Lang NP. Polymorphisms for aromatic amines metabolism in humans: relevance for human carcinogenesis. Environ. Health Perspect. 1992;98:69–74. doi: 10.1289/ehp.929869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadlubar FF, Kaderlik KR, Mulder GJ, Lin DX, Butler MA, Teitel CH, Minchin RF, Ilett KF, Friesen MD, Bartsch H, Nagao M, Esumi E, Sugimura T, Lang NP. Metabolic activation and DNA adduct detection of PhIP in dogs, rats, and humans in relation to urinary bladder and colon carcinogenesis. In: Adamson RH, Gustafsson JA, Ito N, Nagao M, Sugimura T, Wakabayashi K, Yamazoe Y, editors. Heterocyclic Amines in Cooked Foods: Possible Human Carcinogens; 23rd Proceedings of the Princess Takamatusu Cancer Society; Princeton, NJ.: Princeton Scientific Publishing Co., Inc.; 1995. pp. 207–213. [PubMed] [Google Scholar]
- 19.Gooderham NJ, Murray S, Lynch AM, Yadollahi-Farsani M, Zhao K, Boobis AR, Davies DS. Food-derived heterocyclic amine mutagens: variable metabolism and significance to humans. Drug Metab.Dispos. 2001;29:529–534. [PubMed] [Google Scholar]
- 20.Kaderlik KR, Minchin RF, Mulder GJ, Ilett KF, Daugaard-Jenson M, Teitel CH, Kadlubar FF. Metabolic activation pathway for the formation of DNA adducts of the carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rat extrahepatic tissues. Carcinogenesis. 1994;15:1703–1709. doi: 10.1093/carcin/15.8.1703. [DOI] [PubMed] [Google Scholar]
- 21.King RS, Teitel CH, Shaddock JG, Casciano DA, Kadlubar FF. Detoxification of carcinogenic aromatic and heterocyclic amines by enzymatic reduction of the N-hydroxy derivative. Cancer Lett. 1999;143:167–171. doi: 10.1016/s0304-3835(99)00119-6. [DOI] [PubMed] [Google Scholar]
- 22.Lin DX, Meyer DJ, Ketterer B, Lang NP, Kadlubar FF. Effects of human and rat glutathione-S-transferase on the covalent binding of the N -acetoxy derivatives of heterocyclic amine carcinogens in vitro : a possible mechanism of organ specificity in their carcinogensis. Cancer Res. 1994;54:4920–4926. [PubMed] [Google Scholar]
- 23.Turesky RJ, Parisod V, Huynh-Ba T, Langou‰t S, Guengerich FP. Regioselective differences in C(8)- and N-oxidation of 2-amino-3,8-dimethylimidazo[4,5- f ]quinoxaline by human and rat liver microsomes and cytochromes P450 1A2. Chem. Res. Toxicol. 2001;14:901–911. doi: 10.1021/tx010035s. [DOI] [PubMed] [Google Scholar]
- 24.Turesky RJ, Garner RC, Welti DH, Richoz J, Leveson SH, Dingley KH, Turteltaub KW, Fay LB. Metabolism of the food-borne mutagen 2-amino-3,8-dimethylimidazo[4,5- f ]quinoxaline in humans. Chem. Res. Toxicol. 1998;11:217–225. doi: 10.1021/tx9701891. [DOI] [PubMed] [Google Scholar]
- 25.Langouët S, Welti DH, Kerriguy N, Fay LB, Huynh-Ba T, Markovic J, Guengerich FP, Guillouzo A, Turesky RJ. Metabolism of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in human hepatocytes: 2-amino-3-methylimidazo[4,5-f]quinoxaline-8-carboxylic acid is a major detoxification pathway catalyzed by cytochrome P450 1A2. Chem. Res. Toxicol. 2001;14:211–221. doi: 10.1021/tx000176e. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Chen ZX, Rewuti A, Ma YS, Wang XF, Xia Q, Fu D, Han YS. quantitative assessment of the influence of cytochrome P450 1A2 gene polymorphism and colorectal cancer risk. PLoS One. 2013;8:e71481. doi: 10.1371/journal.pone.0071481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sachse C, Smith G, Wilkie MJ, Barrett JH, Waxman R, Sullivan F, Forman D, Bishop DT, Wolf CR. A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis. 2002;23:1839–1849. doi: 10.1093/carcin/23.11.1839. [DOI] [PubMed] [Google Scholar]
- 28.Sachse C, Bhambra U, Smith G, Lightfoot TJ, Barrett JH, Scollay J, Garner RC, Boobis AR, Wolf CR, Gooderham NJ. Polymorphisms in the cytochrome P450 CYP1A2 gene (CYP1A2) in colorectal cancer patients and controls: allele frequencies, linkage disequilibrium and influence on caffeine metabolism. Br.J.Clin.Pharmacol. 2003;55:68–76. doi: 10.1046/j.1365-2125.2003.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Zhang Z, Han S, Lu Y, Feng F, Yuan J. CYP1A2 rs762551 polymorphism contributes to cancer susceptibility: a meta-analysis from 19 case-control studies. BMC.Cancer. 2012;12:528. doi: 10.1186/1471-2407-12-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters U, Sinha R, Bell DA, Rothman N, Grant DJ, Watson MA, Kulldorff M, Brooks LR, Warren SH, DeMarini DM. Urinary mutagenesis and fried red meat intake: Influence of cooking temperature, phenotype, and genotype of metabolizing enzymes in a controlled feeding study. Environ. Mol. Mutagen. 2004;43:53–74. doi: 10.1002/em.10205. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Joshi AD, Corral R, Siegmund KD, Marchand LL, Martinez ME, Haile RW, Ahnen DJ, Sandler RS, Lance P, Stern MC. Carcinogen metabolism genes, red meat and poultry intake, and colorectal cancer risk. Int. J. Cancer. 2012;130:1898–1907. doi: 10.1002/ijc.26199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem. Res. Toxicol. 2011;24:1169–1214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hein DW. N-acetyltransferase 2 genetic polymorphism: effects of carcinogen and haplotype on urinary bladder cancer risk. Oncogene. 2006;25:1649–1658. doi: 10.1038/sj.onc.1209374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang NP, Butler MA, Massengill JP, Lawson M, Stotts RC, Hauer-Jensen M, Kadlubar FF. Rapid metabolic phenotypes for acetyltransferase and cytochrome P4501A2 and putative exposure to food-borne heterocyclic amines increase the risk for colorectal cancer or polyps. Cancer Epidemiol. Biomarkers Prev. 1994;3:675–682. [PubMed] [Google Scholar]
- 35.Schut HA, Snyderwine EG. DNA adducts of heterocyclic amine food mutagens: implications for mutagenesis and carcinogenesis. Carcinogenesis. 1999;20:353–368. doi: 10.1093/carcin/20.3.353. [DOI] [PubMed] [Google Scholar]
- 36.Barbir A, Linseisen J, Hermann S, Kaaks R, Teucher B, Eichholzer M, Rohrmann S. Effects of phenotypes in heterocyclic aromatic amine (HCA) metabolism-related genes on the association of HCA intake with the risk of colorectal adenomas. Cancer Causes Control. 2012;23:1429–1442. doi: 10.1007/s10552-012-0017-8. [DOI] [PubMed] [Google Scholar]
- 37.Butler MA, Lang NP, Young JF, Caporaso NE, Vineis P, Hayes RB, Teitel CH, Massengill JP, Lawsen MF, Kadlubar FF. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992;2:116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Kalow W, Tang BK. Use of caffeine metabolite ratios to explore CYP1A2 and xanthine oxidase ratios. Clin.Pharmacol.Ther. 1991;50:508–519. doi: 10.1038/clpt.1991.176. [DOI] [PubMed] [Google Scholar]
- 39.Sinha R, Rothman N, Mark SD, Murray S, Brown ED, Levander OA, Davies DS, Lang NP, Kadlubar FF, Hoover RN. Lower levels of urinary 2-amino-3,8-dimethylimidazo[4,5-f]-quinoxaline (MeIQx) in humans with higher CYP1A2 activity. Carcinogenesis. 1995;16:2859–2861. doi: 10.1093/carcin/16.11.2859. [DOI] [PubMed] [Google Scholar]
- 40.Stillwell WG, Kidd LC, Kim SB, Wishnok JW, Tannenbaum SR, Sinha R. Urinary excretion of unmetabolized and phase II conjugates of 2-amino-1-methyl-6-phenylimidazo[4,5- b ]pyridine and 2-amino-3,8-dimethylimidazo[4,5- f ]quinoxaline in humans: Relationship to cytochrome P450 1A2 and N -acetyltransferase actvity. Cancer Res. 1997;57:3457–3464. [PubMed] [Google Scholar]
- 41.Stillwell WG, Turesky RJ, Sinha R, Tannenbaum SR. N-oxidative metabolism of 2-amino-3,8-dimethylimidazo[4,5- f ]quinoxaline (MeIQx) in humans: excretion of the N 2 -glucuronide conjugate of 2-hydroxyamino-MeIQx in urine. Cancer Res. 1999;59:5154–5159. [PubMed] [Google Scholar]
- 42.Stillwell WG, Sinha R, Tannenbaum SR. Excretion of the N 2 - glucuronide conjugate of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5-b]pyridine in urine and its relationship to CYP1A2 and NAT2 activity levels in humans. Carcinogenesis. 2002;23:831–838. doi: 10.1093/carcin/23.5.831. [DOI] [PubMed] [Google Scholar]
- 43.Malfatti MA, Dingley KH, Nowell-Kadlubar S, Ubick EA, Mulakken N, Nelson D, Lang NP, Felton JS, Turteltaub KW. The urinary metabolite profile of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine is predictive of colon DNA adducts after a low-dose exposure in humans. Cancer Res. 2006;66:10541–10547. doi: 10.1158/0008-5472.CAN-06-1573. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Z, Dragin N, Jorge-Nebert LF, Martin MV, Guengerich FP, Aklillu E, Ingelman-Sundberg M, Hammons GJ, Lyn-Cook BD, Kadlubar FF, Saldana SN, Sorter M, Vinks AA, Nassr N, von, Richter O, Jin L, Nebert DW. Search for an association between the human CYP1A2 genotype and CYP1A2 metabolic phenotype. Pharmacogenet.Genomics. 2006;16:359–367. doi: 10.1097/01.fpc.0000204994.99429.46. [DOI] [PubMed] [Google Scholar]
- 45.Conney AH. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982;42:4875–4917. [PubMed] [Google Scholar]
- 46.Sesardic D, Boobis AR, Edwards RJ, Davies DS. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-deethylation of phenacetin and is inducible by cigarette smoking. Br.J.clin.Pharmac. 1988;26:363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pantuck EJ, Hsiao KC, Conney AH, Garland WA, Kappas A, Anderson KE, Alvares AP. Effect of charcoal-broiled beef on phenacetin metabolism in man. Science. 1976;194:1055–1057. doi: 10.1126/science.982059. [DOI] [PubMed] [Google Scholar]
- 48.Pantuck EJ, Pantuck CB, Garland WA, Min BH, Wattenberg LW, Anderson KE, Kappas A, Conney AH. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin.Pharmacol.Ther. 1979;25:88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- 49.Sinha R, Rothman N, Brown ED, Mark SD, Hoover RN, Caporaso NE, Levander OA, Knize MG, Lang NP, Kadlubar FF. Pan-fried meat containing high levels of heterocyclic aromatic amines but low levels of polycyclic aromatic hydrocarbons induces cytochrome P4501A2 activity in humans. Cancer Res. 1994;54:6154–6159. [PubMed] [Google Scholar]
- 50.Vistisen K, Poulsen HE, Loft S. Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis. 1992;13:1561–1568. doi: 10.1093/carcin/13.9.1561. [DOI] [PubMed] [Google Scholar]
- 51.Zhou SF, Wang B, Yang LP, Liu JP. Structure, function, regulation and polymorphism and the clinical significance of human cytochrome P450 1A2. Drug Metab. Rev. 2010;42:268–354. doi: 10.3109/03602530903286476. [DOI] [PubMed] [Google Scholar]
- 52.Gu D, McNaughton L, LeMaster D, Lake BG, Gooderham NJ, Kadlubar FF, Turesky RJ. A comprehensive approach to the profiling of the cooked meat carcinogens 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, and their metabolites in human urine. Chem. Res. Toxicol. 2010;23:788–801. doi: 10.1021/tx900436m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu D, Raymundo MM, Kadlubar FF, Turesky RJ. Ultraperformance liquid chromatography-tandem mass spectrometry method for biomonitoring cooked meat carcinogens and their metabolites in human urine. Anal. Chem. 2011;83:1093–1101. doi: 10.1021/ac102918b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murray S, Gooderham NJ, Boobis AR, Davies DS. Detection and measurement of MeIQx in human urine after ingestion of a cooked meat meal. Carcinogenesis. 1989;10:763–765. doi: 10.1093/carcin/10.4.763. [DOI] [PubMed] [Google Scholar]
- 55.Lynch AM, Knize MG, Boobis AR, Gooderham N, Davies DS, Murray S. Intra- and interindividual variability in systemic exposure in humans to 2-amino-3,8-dimethylimidazo[4,5- f ]quinoxaline and 2-amino-1-methyl-6-phenylimidazo[4,5- b ]pyridine, carcinogens present in food. Cancer Res. 1992;52:6216–6223. [PubMed] [Google Scholar]
- 56.Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG. Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in humans. Carcinogenesis. 2004;25:1659–1669. doi: 10.1093/carcin/bgh164. [DOI] [PubMed] [Google Scholar]
- 57.Fede JM, Thakur AP, Gooderham NJ, Turesky RJ. Biomonitoring of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and its carcinogenic metabolites in urine. Chem. Res. Toxicol. 2009;22:1096–1105. doi: 10.1021/tx900052c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ni W, McNaughton L, LeMaster DM, Sinha R, Turesky RJ. Quantitation of 13 heterocyclic aromatic amines in cooked beef, pork, and chicken by liquid chromatography-electrospray ionization/tandem mass spectrometry. J. Agric. Food Chem. 2008;56:68–78. doi: 10.1021/jf072461a. [DOI] [PubMed] [Google Scholar]
- 59.Le Marchand L, Franke AA, Custer L, Wilkens LR, Cooney RV. Lifestyle and nutritional correlates of cytochrome CYP1A2 activity: inverse associations with plasma lutein and alpha-tocopherol. Pharmacogenetics. 1997;7:11–19. doi: 10.1097/00008571-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Vaynshteyn D, Jeong H. Caffeine induces CYP1A2 expression in rat hepatocytes but not in human hepatocytes. Drug Metab Lett. 2012;6:116–119. [PMC free article] [PubMed] [Google Scholar]
- 61.Shimada T, Gillam EMJ, Sandhu P, Guo Z, Tukey RH, Guengerich FP. Activation of procarcinogens by human cytochrome P450 enzymes expressed in Escherichia coli. Simplified bacterial systems for gentoxicity assays. Carcinogenesis. 1994;15:2523–2529. doi: 10.1093/carcin/15.11.2523. [DOI] [PubMed] [Google Scholar]
- 62.Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farr M, Segura J, Gooderham NJ, Davies DS. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994;54:89–94. [PubMed] [Google Scholar]
- 63.Kunze KL, Trager WF. Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline. Chem. Res. Toxicol. 1993;6:649–656. doi: 10.1021/tx00035a009. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Joshi AD, Corral R, Siegmund KD, Marchand LL, Martinez ME, Haile RW, Ahnen DJ, Sandler RS, Lance P, Stern MC. Carcinogen metabolism genes, red meat and poultry intake, and colorectal cancer risk. Int.J.Cancer. 2012;130:1898–1907. doi: 10.1002/ijc.26199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakajima M, Yokoi T, Mizutani M, Shin S, Kadlubar FF, Kamataki T. Phenotyping of CYP1A2 in Japanese population by analysis of caffeine urinary metabolites: absence of mutation prescribing the phenotype in the CYP1A2 gene. Cancer Epidemiol. Biomarkers Prev. 1994;3:413–412. [PubMed] [Google Scholar]
- 66.Kashuba AD, Bertino JS, Jr., Kearns GL, Leeder JS, James AW, Gotschall R, Nafziger AN. Quantitation of three-month intraindividual variability and influence of sex and menstrual cycle phase on CYP1A2, N-acetyltransferase-2, and xanthine oxidase activity determined with caffeine phenotyping. Clinical pharmacology and therapeutics. 1998;63:540–551. doi: 10.1016/S0009-9236(98)90105-9. [DOI] [PubMed] [Google Scholar]
- 67.Belloc C, Baird S, Cosme J, Lecoeur S, Gautier JC, Challine D, de Waziers I, Flinois JP, Beaune PH. Human cytochrome P450 expressed in Escherichia coli : production of specific antibodies. Toxicology. 1996;106:207–219. doi: 10.1016/0300-483x(95)03178-i. [DOI] [PubMed] [Google Scholar]
- 68.Nowell SA, Massengill JS, Williams S, Radominska-Pandya A, Tephly TR, Cheng Z, Strassburg CP, Tukey RH, MacLeod SL, Lang NP, Kadlubar FF. Glucuronidation of 2-hydroxyamino-1-methyl-6-phenylimidazo[4,5- b ]pyridine by human microsomal UDP-glucuronosyltransferases: identification of specific UGT1A family isoforms involved. Carcinogenesis. 1999;20:1107–1114. doi: 10.1093/carcin/20.6.1107. [DOI] [PubMed] [Google Scholar]
- 69.Malfatti MA, Ubick EA, Felton JS. The impact of glucuronidation on the bioactivation and DNA adduction of the cooked-food carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in vivo. Carcinogenesis. 2005;26:2019–2028. doi: 10.1093/carcin/bgi151. [DOI] [PubMed] [Google Scholar]
- 70.Dellinger RW, Chen G, Blevins-Primeau AS, Krzeminski J, Amin S, Lazarus P. Glucuronidation of PhIP and N-OH-PhIP by UDP-glucuronosyltransferase 1A10. Carcinogenesis. 2007;28:2412–2418. doi: 10.1093/carcin/bgm164. [DOI] [PubMed] [Google Scholar]
- 71.Girard H, Thibaudeau J, Court MH, Fortier LC, Villeneuve L, Caron P, Hao Q, von Moltke LL, Greenblatt DJ, Guillemette C. UGT1A1 polymorphisms are important determinants of dietary carcinogen detoxification in the liver. Hepatology. 2005;42:448–457. doi: 10.1002/hep.20770. [DOI] [PubMed] [Google Scholar]
- 72.Labedzki A, Buters J, Jabrane W, Fuhr U. Differences in caffeine and paraxanthine metabolism between human and murine CYP1A2. Biochem. Pharmacol. 2002;63:2159–2167. doi: 10.1016/s0006-2952(02)01019-5. [DOI] [PubMed] [Google Scholar]
- 73.Moonen HJ, Moonen EJ, Maas L, Dallinga JW, Kleinjans JC, De Kok TM. CYP1A2 and NAT2 genotype/phenotype relations and urinary excretion of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in a human dietary intervention study. Food Chem. Toxicol. 2004;42:869–878. doi: 10.1016/j.fct.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Zhou SF, Yang LP, Zhou ZW, Liu YH, Chan E. Insights into the substrate specificity, inhibitors, regulation, and polymorphisms and the clinical impact of human cytochrome P450 1A2. The AAPS journal. 2009;11:481–494. doi: 10.1208/s12248-009-9127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim D, Lee YJ, Ryu HY, Lee JH, Kim HK, Kim E, Moon JD, Chang DD, Yoon HS. Genetic polymorphisms in metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine. J. Appl. Toxicol. 2013;33:63–70. doi: 10.1002/jat.1712. [DOI] [PubMed] [Google Scholar]
- 76.Turesky RJ, Liu L, Gu D, Yonemori KM, White KK, Wilkens LR, Le Marchand L. Biomonitoring the cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in hair: impact of exposure, hair pigmentation, and cytochrome P450 1A2 phenotype. Cancer Epidemiol. Biomarkers Prev. 2013;22:356–364. doi: 10.1158/1055-9965.EPI-12-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9:625–637. doi: 10.2217/14622416.9.5.625. [DOI] [PubMed] [Google Scholar]
- 78.Turesky RJ, Bessette EE, Dunbar D, Liberman RG, Skipper PL. Cytochrome P450-mediated metabolism and DNA binding of 2-amino-1,7-dimethylimidazo[4,5-g]quinoxaline and its carcinogenic isomer 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in mice. Chem. Res. Toxicol. 2012;25:410–421. doi: 10.1021/tx2004536. [DOI] [PMC free article] [PubMed] [Google Scholar]