Abstract
The extracellular matrix (ECM) of the prostate, which is comprised primarily of collagen, becomes increasingly disorganized with age, a property that may influence the development of hyperplasia and cancer. Collageous ECM extracted from the tails of aged mice exhibits many characteristics of collagen in aged tissues, including the prostate. When polymerized into a 3-dimensional (3D) gel, these collagen extracts can serve as models for the study of specific cell-ECM interactions. In the present study, we examined the behaviors of human prostatic epithelial cell lines representing normal prostate epithelial cells (PEC), benign prostatic hyperplasia (BPH-1), and adenocarcinoma (LNCaP) cultured in contact with 3D gels made from collagen extracts of young and aged mice. We found that proliferation of PEC, BPH-1, and LNCaP cells were all increased by culture on aged collagen gels relative to young collagen gels. In examining age-associated differences in the composition of the collagen extracts, we found that aged and young collagen had a similar amount of several collagen-associated ECM components, but aged collagen had a much greater content of the glycosaminoglycan hyaluronan (HA) than young collagen. The addition of HA (of similar size and concentration to that found in aged collagen extracts) to cells placed in young collagen elicited significantly increased proliferation in BPH-1 cells, but not in PEC or LNCaP cells, relative to controls not exposed to HA. Of note, histochemical analyses of human prostatic tissues showed significantly higher expression of HA in BPH and prostate cancer stroma relative to stroma of normal prostate. Collectively, these results suggest that changes in ECM involving increased levels of HA contribute to the growth of prostatic epithelium with aging.
Keywords: Aging, Benign prostatic hypertrophy, Collagen gel, 3-Dimensional, Extracellularmatrix, In vitro
Introduction
It is increasingly appreciated that changes in the extracellular matrix (ECM) with age confer an increased risk of disease. One such example is the prostate, where aging is the greatest risk factor for both benign and malignant pathology (Grönberg et al. 1994; Saad et al. 2008). This is due, in part, to interactions between components of the ECM and prostate epithelial cells (Sprenger et al. 2010). Prostatic ECM is comprised primarily of the fibrillar protein type I collagen, the predominant ECM component in skin and many other organs. Type I collagen in aged prostates exhibits many of the features of aged collagen in other tissues, including decreased organization and density (Sadoun and Reed 2003; Bianchi-Frias et al. 2010).
Studies of the influence of collagenous ECM on cell behavior can be facilitated by culturing cells in contact with 3-dimensional (3D) gels of polymerized type I collagen, which simulate ECM environments in vivo better than rigid substrates, such as ECM-coated or tissue culture plastic (Vernon and Sage 1999; Cukierman et al. 2001; Lee et al. 2007; Shi et al. 2011; Petrie et al. 2012). Of note, collagen is easily and reproducibly extracted from native tissues, and the resulting solutions of monomeric collagen are homogenous, retain collagen-associated ECM components, and are free of growth factors, cytokines, and cells. Accordingly, 3D collagen gels are the foundation for in vitro culture systems that model benign and malignant cellular responses in vivo (Cukierman et al. 2001; Bartling et al. 2009; Leventhal et al. 2009; Damodarasamy et al. 2010; Miron-Mendoza et al. 2010; Petrie et al. 2012).
For studies of aged collagen, tendons from the tails of young and aged mice can be extracted to yield “young” and “aged” collagenous ECM. Under physiologic conditions, the extracted collagen monomers assemble into a gel of fibrils that resembles native fibrillar collagen in vivo. As we and others have previously shown, when compared to gels made from young collagen extracts (“young collagen gels”), gels made from aged collagen extracts (“aged collagen gels”) retain many of the characteristics of native, aged tissues, which include decreased collagen density, greater disorganization and heterogeneity of collagen fibers, and increased levels of advanced glycation end-products (Jiang et al. 2000; Wu et al. 2005; Bartling et al. 2009; Damodarasamy et al. 2010). The phenomenon that aged collagen gels made in vitro retain, in some respects, the characteristics of collagenous ECM in native aged tissues in vivo makes these gels useful for in vitro studies of age-associated changes in cell-ECM interactions (Bentov et al. 2013).
In the present study, we evaluated the influence of young and aged 3D collagen gels on the behaviors of three well-characterized human prostatic epithelial cell lines in vitro. These lines were as follows: PEC, which is obtained from normal prostate tissue; BPH-1, which is derived from hyperplastic prostate and has historically represented benign proliferative prostate disease; and LNCaP, which is derived from metastatic prostate adenocarcinoma and has been a consistent example of aggressive prostate cancer (Horoszewicz et al. 1980; Hayward et al. 1995). We compared the proliferative responses of PEC, BPH-1, and LNCaP cells in aged vs. young 3D collagen gels. Differential responses of all three prostate epithelial cell lines to the young and aged gels led to studies to determine if specific differences in the composition of the young and aged collagen extracts might be responsible for the observed effects.
Materials and Methods
Cell culture
PEC cells are obtained from normal prostate tissue (Lonza, Allendale, NJ). BPH-1 cells are an SV40 T-immortalized primary human prostate epithelial cell line derived from a prostate with benign hyperplasia (Hayward et al. 1995). The LNCaP epithelial cell line is an androgen-sensitive cell line derived from prostatic adenocarcinoma that had metastasized to a lymph node (Horoszewicz et al. 1980). Cells were confirmed to be epithelial in nature by keratin immunostaining. To maintain consistency, PEC, BPH-1, and LNCaP lines were all cultured in a growth medium consisting of RPMI-1640 (Invitrogen, Grand Island, NY) with 5% fetal bovine serum (FBS) (Invitrogen) and antibiotics supplemented with 1 × 10−9 M dihydrotestosterone (Sigma-Aldrich, St. Louis, MO). All experiments were conducted in this growth medium unless otherwise specified.
Preparation of collagen extracts and gels
Young (5–6 mo) and aged (23–24 mo) C57Bl/6 male mice, an established model for the study of aging (Ershler et al. 1984), were obtained from the NIA Rodent Colony (aged mouse colony, Charles River Laboratories, Wilmington, MA). For each extraction, an equivalent wet weight of tendon tissue was isolated from each euthanized mouse. The isolated tendons from 5 young and 5–6 aged mice were pooled in respective “young” and “aged” groups, hydrated briefly in phosphate-buffered saline (PBS), rinsed in acetone and 70% isopropanol, macerated, and stirred gently overnight at 4°C in 0.05 N acetic acid. Three separate pools, representing 15 young and 16 aged mice, were utilized for the experiments. Subsequently, the “young” and “aged” collagen extracts were centrifuged for 15 min at 4,000×g to remove undissolved material. Total protein content was quantified by a bicinchoninic acid assay (Thermo Fisher Scientific Inc., Waltham, MA), and specific collagen content was quantified by the Sircol assay (Accurate Chemical and Scientific Corp., Westbury, NY). 3D gels were prepared from young and aged collagen extracts as previously described (Damodarasamy et al. 2010). For purposes of analyzing cell proliferation, cells were placed on top of the 3D gels, which have been shown to elicit changes similar to that of cells cultured within 3D gels (Haas et al. 1998).
Cell proliferation assays
Young or aged collagen gels (35 µl volume) were polymerized in each well of a 96-well plate. Subsequently, 5,000 cells were placed on each gel, and an index of the proliferative activity after 72 h of culture was measured with a Cultrex® 3DCulture Cell Proliferation Assay Kit (Trevigen Inc., Gaithersburg, MD).
Western blotting
Collagen extracts of equivalent collagen content were resolved by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) (20 µg/lane), transferred to blot membranes, and probed with 2–5 µg/ml of primary antibodies to type I collagen (Chemicon International, Temecula, CA), thrombospondin-1 (TSP-1) (Santa Cruz Biotechnology Inc., Santa Cruz, CA) and the proteoglycans decorin and biglycan (kindly provided by Dr. Larry Fisher, NIDCR/NIH). Blots were probed with appropriate horse radish peroxidase-conjugated secondary antibodies (1 µg/ml, Jackson ImmunoResearch, West Grove, PA) followed by enhanced chemiluminescence (GE Healthcare LifeSciences, Piscataway, NJ).
Measurement of hyaluronan content and molecular weight in collagen extracts
Total hyaluronan (HA) in collagen extracts was measured by a competitive enzyme-linked sorbent assay (ELSA) as previously described (Wilkinson et al. 2004; Sakr et al. 2008; Jarvelainen et al. 2009). Briefly, extracts were dialyzed, digested with proteinase K (250 µg/ml) to degrade endogenous proteins and proteoglycans. After heat-inactivation of the proteinase K, the samples were assayed for HA. HA reactivity was quantitated according to a standard curve of purified HA (Underhill 1993; Wilkinson et al. 2004; Jarvelainen et al. 2009). The molecular weight (MW) of HA in the dialyzed, proteinase K-treated preparations was evaluated by electrophoresis through 1.2% agarose as previously described (Reed et al. 2013).
HA staining of collagen gels and prostate tissues
Gels made from polymerized extracts of young and aged collagen were fixed in neutral-buffered formalin, dehydrated, embedded in paraffin, and sectioned at 5 µm. Slide-mounted, deparaffinized sections were stained for total HA using HA-binding protein (HABP) complexed with biotin (2 µg/ml), followed by Vectastain® ABC (avidin-biotin complex) and 3,3′-diaminobenzidine (DAB) (Vector Laboratories, Burlingame, CA). Images were recorded by digital light microscopy.
Slide-mounted arrays of 5 µm-thick human prostate tissue sections were obtained from de-identified specimens (courtesy of Dr. Robert L. Vessella, University of Washington) that represented normal tissue (n=10 subjects with a mean age of 61 and a range of 48–77 yr old), tissue exhibiting benign prostatic hyperplasia (BPH) (n=11 subjects with a mean age of 66 and a range of 51–76 yr old), and tissue demonstrating prostate cancer, Gleason 4 (n=9 subjects with a mean age of 59 and a range of 48–77 yr old). The sections were stained for HA in a manner similar to the collagen gels, photographed by digital microscopy, and then analyzed specifically for intensity of DAB staining in the stroma using ImageJ (National Institutes of Health freeware).
Influence of HA on cell proliferation
Cells were seeded on top of young collagen gels at subconfluence (≈5,000 cells/well). After 3 h, the culture medium was replaced with serum-free RPMI-1640 and the cells were cultured overnight. Subsequently, the cultures received either RPMI-1640/10% FBS (without added HA) or RPMI-1640/1% FBS with or without 10 µg/ml of purified 250 kDa HA (Hyalose, Oklahoma City, OK). Cell proliferation 72 h after plating was measured using the CellTiter Aqueous assay (Promega, Madison, WI).
Statistical analyses
All experiments were performed at least three separate times with a minimum of duplicate samples within each experiment. Statistical significance between young/aged groups and control/experimental groups was determined using a paired Student’s t test with unequal variance with p<0.05.
Results
Three-dimensional gels provide models of cell-ECM interactions that are relevant to cell behaviors in vivo (Lee et al. 2007; Bartling et al. 2009; Leventhal et al. 2009; Petrie et al. 2012). In this context, we and others have established that 3D gels polymerized from collagen extracted from young and aged animals induce differential cellular responses that reflect tissue aging (Jiang et al. 2000; Bartling et al. 2009; Damodarasamy et al. 2010). Moreover, aged collagenous ECM in vitro provides an environment that can simulate pathologic responses in vivo (Bentov et al. 2013).
Cell proliferation is increased on aged collagen gels compared to young collagen gels
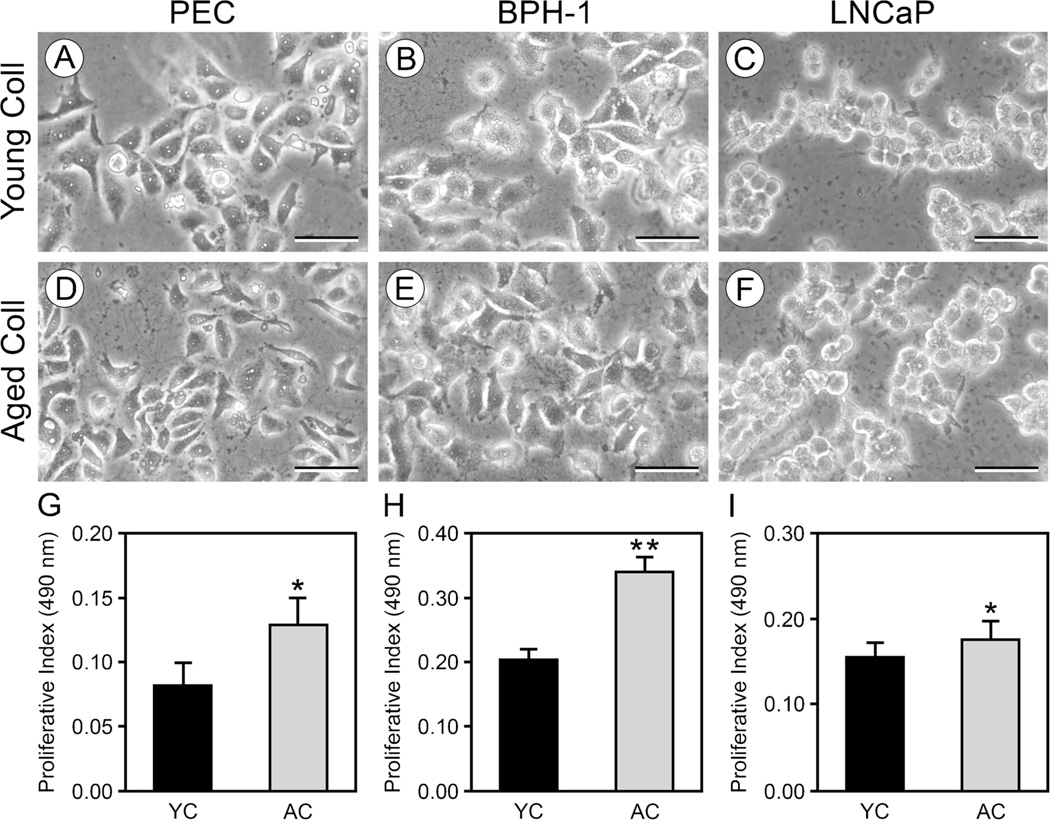
We first examined whether contact with young vs. aged collagen gels differentially influenced the proliferative responses of the PEC, BPH-1, and LNCaP cell lines. PEC and BPH-1 cells assumed a flattened, well-spread morphology on the young gels (Fig. 1A, B). In contrast, LNCaP cells maintained highly rounded shapes on young gels (Fig. 1C). On the aged gels, all three cell lines exhibited, respectively, the same spread (PEC and BPH-1 cells—Fig. 1D, E) and round (LNCaP—Fig. 1F) shapes that they had on the young gels. Although aged collagen gels did not differentially affect cell shape relative to young collagen gels, we found that the proliferation of all three lines was significantly greater on aged collagen gels relative to young collagen gels (Fig. 1G–I).
Figure 1.
Proliferation of PEC, BPH-1, and LNCaP cells is increased on aged collagen relative to young collagen. PEC and BPH-1 cells cultured for 72 h on young (A, B) or aged (D, E) collagen gels assumed a flattened morphology. In contrast, LNCaP cells cultured for 72 h on young (C) or aged (F) collagen gels had rounded contours. Proliferation measured at 72 h shows that all three cell lines: PEC (G), BPH-1 (H), and LNCaP (I) proliferated at a significantly greater rate on aged collagen (AC—gray bars) than on young collagen (YC—black bars). Values are mean± standard deviation (*p<0.05, **p<0.005). A–F scale bars=50 µm.
Aged collagen extracts have an increased content of HA relative to young collagen extracts
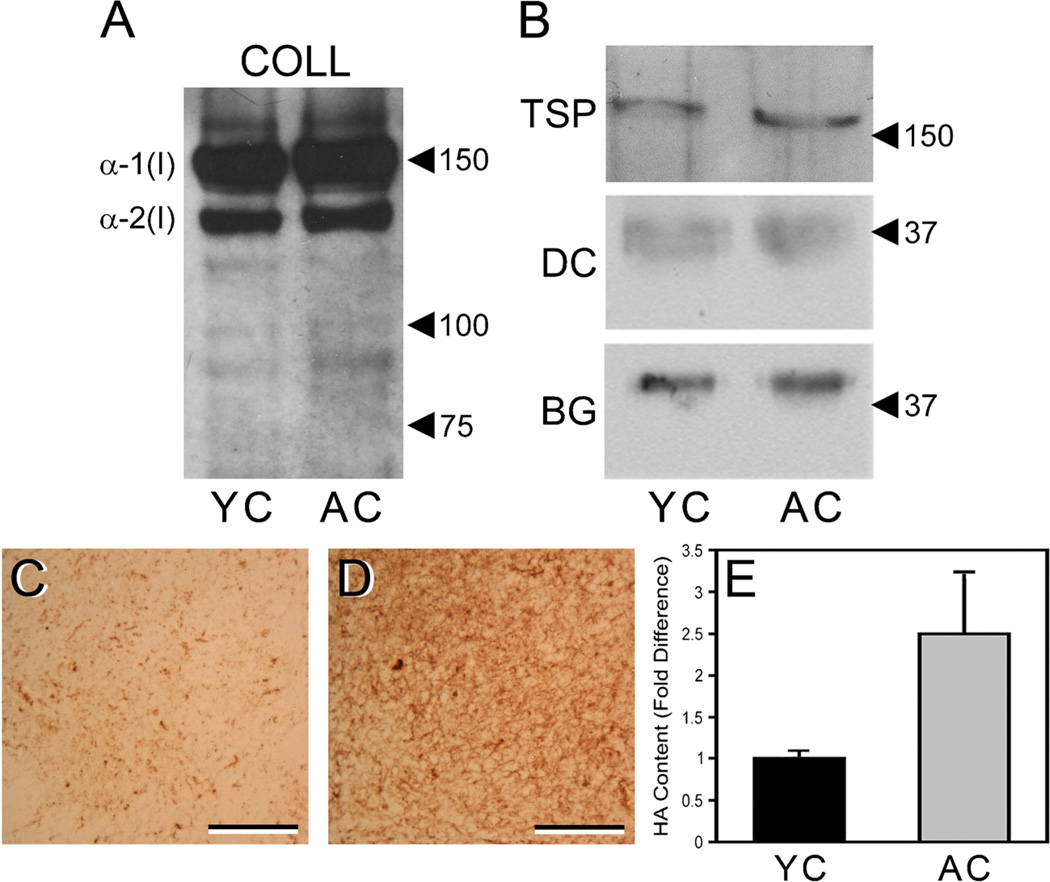
It has been shown that collagenous ECM extracted from young and aged tissues retains non-collagenous ECM molecules (Bartling et al. 2009). Age-associated differences in these non-collagenous components might contribute to observed differences in the behaviors of cells cultured on young vs. aged collagen gels. We have previously noted that extracts of tail tendon collagen from young and aged mice have a similar content of structural ECM proteins, such as laminin and fibronectin (Damodarasamy et al. 2010). In this study, we evaluated several collagen-associated molecules that can regulate cell behavior. The extracts of young and aged collagen exhibited very similar levels and patterns of type I collagen expression, as determined by SDS-PAGE-Western immunoblotting with a type I collagen antibody (Fig. 2A). As expected, both young and aged collagen extracts contained high levels of α-1(I) and α-2(I) collagen chains. Also expected was the larger quantity of α-1(I) relative to α-2(I), which reflected the 2:1 ratio of these subunits in the native collagen triple helix. A ladder of faint immunoreactive bands of lower MW was also observed. These bands likely represented collagen degradation products, which were present in substantially lower quantities compared to the intact α-1(I) and α-2(I) chains. Like type I collagen, we found that the matricellular glycoprotein TSP-1 and the proteoglycans decorin and biglycan were expressed at similar levels in the young and aged collagen extracts (Fig. 2B).
Figure 2.
Young and aged collagen extracts have similar content of type I collagen and associated non-collagenous ECM components, but there is increased content of HA in aged collagen. Extracts of young and aged collagen (YC, AC) were resolved by SDS-PAGE under reducing conditions, transferred to nitrocellulose, and stained with antibodies to specific ECM components. Both YC and AC extracts showed strong and similar staining for α-1(I) and α-2(I) chains of type I collagen (blot “COLL”) (A) and weak staining of lower MW bands that likely represent collagen degradation products. TSP-1, decorin, and biglycan were also found in similar quantities in the YC and AC extracts (B) (blots “TSP,” “DC,” and “BG,” respectively). In A and B, positions of MW markers (values are in kDa) are indicated by arrowheads. Sections of gels prepared from YC (C) and AC (D) extracts were stained with HABP to demonstrate total HA content. Note the substantially greater staining (brown color) of the aged gel vs. the young gel. In C and D, scale bars=50 µm. Correspondingly, quantitative ELSA assays of the collagen extracts (E) indicated that the AC extracts (gray bar) contained an average of 2.5-fold more HA than did the YC extracts (black bar), as determined by three separate assays of pooled extracts representing a total of 15 young and 16 aged mice. Average HA content was 8.02±1.6 ng HA per µg aged collagen vs. 3.88±2.4 ng HA per µg young collagen. Values are mean±SEM.
In subsequent experiments, we assayed the collagen extracts for the presence of the glycosaminoglycan HA, a large, unbranched polymer of the disaccharide glucuronic acid/N-acetylglucosamine that is the major non-proteinaceous ECM component of connective tissues. HA was found at substantially higher levels in gels made from aged collagen compared to corresponding gels made from young collagen, as shown by affinity-staining the gels with the HA ligand HABP (Fig. 2C, D). In quantitative ELSA assays of three separate pools (n=5 to 6 mice per pool), aged collagen extracts had a 2.5-fold greater HA content than young collagen extracts (Fig. 2E). Averaged across the three assays, the absolute content of HA in the aged collagen extracts was 8.02±1.6 ng HA per µg collagen vs. 3.88±2.4 ng HA per µg collagen for the young collagen extracts—a content difference of over 2-fold. The young and aged collagen extracts ran similarly on an agarose gel. Each contained HA covering a broad spectrum of MWs less than 750 kDa, with HA in the 250–300 kDa range predominant in the aged collagen extracts.
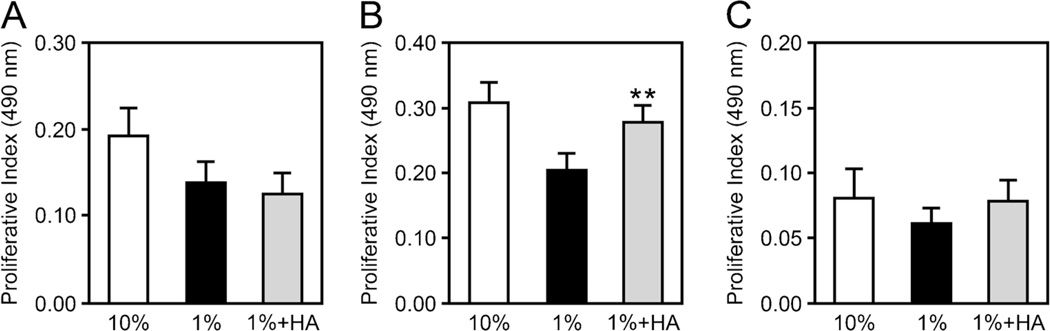
Addition of HA stimulates proliferation of BPH-1 cells cultured on young collagen gels
The specific effects of HA on cell proliferation on 3D collagen was shown by addition of purified HA to PEC, BPH-1, and LNCaP cells cultured on young collagen gels. The MW (250–300 kDa) and concentration (10 µg/ml) of HA used approximated the size and concentration of total HA we measured in the aged collagen extracts. Exposure of BPH-1 cells to the exogenous HA resulted in significantly increased proliferation relative to control cultures that did not receive HA (Fig. 3B). This increase was similar to that seen with 10% FBS, which served as the positive control. BPH-1 cells cultured on aged collagen that was pretreated with hyaluronidase (Sigma-Aldrich) (pretreatment conditions were 50 units of hyaluronidase per ml of collagen for 1 h at 37°C) exhibited decreased proliferation relative to BPH-1 cells culture on untreated aged collagen, underscoring the specific effect of HA on this cell line (data not shown). Notably, exposure of PEC and LNCaP cells to HA in an identical fashion to that of BPH-1 cells did not stimulate proliferation (Fig. 3A and C).
Figure 3.
The addition of HA stimulates proliferation of BPH-1 cells, but not PEC or LNCaP. Cells were placed on young collagen gels and exposed to culture medium/1% FBS (black bars) or medium/1% FBS with the addition of 10 µg/ml of purified 250 kDa HA (gray bars). Proliferation (measured at 72 h) of BPH-1 (B), but not PEC (A) or LNCaP (C), was significantly stimulated in the presence of HA. Medium with 10% FBS (white bars) served as a positive control. Values are mean±SEM (**p<0.005).
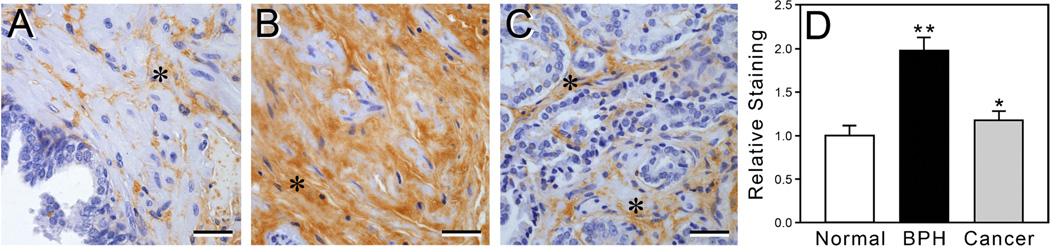
Prostate tissue exhibiting BPH has increased HA deposition in the stromal ECM
To examine the relevance of our in vitro data to prostate tissues in vivo, we used HABP to stain a series of tissue sections from normal human prostates, prostates exhibiting BPH, and prostate cancers to assess the levels of deposition of endogenous HA in the ECM of the stroma (representative images are shown in Fig. 4A–C). HA deposition in the stroma of BPH tissue was significantly greater than that found in normal prostate tissue, as measured by quantification of staining intensity in digital images (Fig. 4D). HA levels were also significantly higher in sections of prostate cancer relative to those of normal prostate tissue (but to a lesser degree than for BPH tissue vs. normal prostate) (Fig. 4D).
Figure 4.
Prostates exhibiting BPH and cancer have an elevated expression of HA in the stromal ECM relative to normal prostate. Human prostate tissue sections representing normal (n=10 subjects), BPH (n=11 subjects), and prostate cancer (n=9 subjects) were stained with HABP to visualize HA levels (brown color) in the stroma. Images of representative samples show that expression of HA (asterisks) was higher in the BPH (B) and cancerous (Gleason 4) tissues (C) relative to normal prostate tissue (A). Bar graph (D) shows quantitative analysis of the intensity of staining for HA in stromal ECM of normal, BPH, and cancerous (Gleason 4) tissue sections. Values are mean±SEM (*p<0.05, **p<0.005). A–C scale bars=50 µm.
Discussion
The microenvironment of aged tissues promotes the growth of prostate epithelial cells (Begley et al. 2008; Sprenger et al. 2010). Collagenous ECM extracted from tail tendons and polymerized into 3D gels provides an ideal model to study the effect of specific ECM components on cell behaviors (Bartling et al. 2009; Leventhal et al. 2009). Three-dimensional gels made from collagen extracted from aged animals are highly useful tools to study the effects of aged ECM on cell behaviors in vitro, which are relevant to cell-ECM interactions in vivo (Bentov et al. 2013). In this context, we found that, compared to young collagen gels, aged collagen gels elicited significantly greater proliferation of three distinct prostate epithelial cell lines: PEC, BPH-1, and LNCaP. The effect of aged collagen on the LNCaP cells was less robust than on the BPH-1 and PEC cells, a result that likely reflects the decreased responsiveness of highly malignant cells to the ECM (Sprenger et al. 2010).
Age is the greatest risk factor for benign and malignant prostatic disease (Grönberg et al. 1994; Saad et al. 2008). We focused our subsequent studies on how aged collagenous ECM might influence the proliferative responses of prostate epithelial cells. As an example, there has been much debate on the effect of collagen density and organization on cell proliferation (Provenzano et al. 2008; Leventhal et al. 2009). Some propose that a loose collagen matrix facilitates cell division, but it has also been noted that greater collagen density upregulates adhesion receptors that could ultimately stimulate pro-growth signaling pathways (Ng and Brugge 2009). Consequently, aged collagen has structural features that can both promote and inhibit epithelial proliferation.
Less is known about the presence of ECM-associated components that could differentially influence the behaviors of cells cultured in young and aged collagen gels. Collagen extracts are especially well-suited for evaluation of collagen-associated ECM molecules, as the extracts are free of cells and soluble factors, but retain posttranslationally modified collagen and associated ECM molecules (Harrison and Archer 1978; Bartling et al. 2009; Damodarasamy et al. 2010). ECM molecules associated with collagen include structural ECM proteins, such as fibronectin and laminin, and molecules that serve primarily to regulate cell behaviors, such as the matricellular proteins TSP-1 and SPARC and the proteoglycans decorin and biglycan (Bradshaw et al. 2010; Damodarasamy et al. 2010; Iozzo and Sanderson 2011; Orgel et al. 2011). Notably, we did not observe differences in the expression of TSP-1, biglycan, and decorin in young compared to aged collagen extracts. In contrast, there were marked differences in the content of the glycosaminoglycan HA, which was consistently two to threefold greater in aged collagen relative to young collagen extracts.
HA is the major non-proteinaceous component of ECM and is critical for the organization, assembly, and homeostasis of ECM, including collagen (Toole 2004; Stern and Maibach 2008; Roughley et al. 2011; Afratis et al. 2012). HA is found in prostate ECM in both benign and malignant pathology (De Klerk 1983; Pullen et al. 2001; Misra et al. 2006; Simpson and Lokeshwar 2008). HA size has differential effects on cell behavior that relate to its chain length: newly synthesized HA has a high MW(up to 2×104 kDa) and can inhibit a variety of cellular processes (Goldberg and Toole 1987; Bharadwaj et al. 2007; Tian et al. 2013). Once secreted, however, HA is rapidly cleaved by hyaluronidases into a continuum of lower MW forms (i.e., <300 kDa) that can promote the growth of prostate tissue. Consequently, there is long-standing interest in HA content, size, and mediators of HA cleavage as predictors for disease progression in the prostate (Lokeshwar et al. 1996, 2001; Kovar et al. 2006; Bharadwaj et al. 2007; Afratis et al. 2012). In prostate cancer, it is well described that increased levels of hyaluronidases (and thereby increased levels of HA degradation) correlate with aggressive disease (Lokeshwar et al. 2001; Kovar et al. 2006; Simpson and Lokeshwar 2008). Far less is known about the influence of HA on prostate hyperplasia. An increase in total HA expression and variability of HA size is a feature of benign prostate disease (De Klerk 1983; Goulas et al. 2000; Pullen et al. 2001). Given the complex nature of prostate ECM, the use of ECM extracts is particularly advantageous for studies of specific components, such as HA, that influence the behaviors of prostate epithelial cells.
In this study, aged collagen extracts contained significantly more total HA than young collagen extracts, but the MW distribution of the native HA in the aged and young extracts was relatively similar, with HA of the 250–300 kDa size predominant in the aged collagen. Of note, this size range of HA does not trigger pathways inhibitory to wound repair as noted with higher MW (>1,000 kDa) forms of HA, or induce inflammatory signals as found with very low MW (<20 kDa) forms of HA (David-Raoudi et al. 2008; Maharjan et al. 2011; Ghazi et al. 2012; Damodarasamy et al. 2014). The specific contribution of HA to promoting replication of BPH-1 cells was underscored by the significant increase in proliferation when BPH-1 cells were stimulated with purified HA in the size range (250–300 kDa) and concentration similar to that present in the aged collagen extracts. The fact that exposure to HA did not promote proliferation of PEC or LNCaP cells could indicate that other properties of aged 3D collagen were more operative in these cell types. Such properties could include a looser or more disorganized matrix, which has been shown to promote proliferative responses in many types of epithelial cell lines (Provenzano et al. 2008; Leventhal et al. 2009).
In summary, 3D gels made from collagenous extracts from aged mice significantly increased proliferative responses from benign and malignant prostate epithelial cells relative to corresponding gels made from collagenous extracts from young mice. Relative to young collagen extracts, aged collagen extracts contained substantially larger quantities of the glycosaminoglycan HA. The increased levels of HA in the aged collagen extracts might, at least in part, account for the greater proliferation of BPH-1 cells cultured on aged collagen gels. This is supported by the finding that BPH-1 cell proliferation was significantly stimulated by exposure to HA of a size range and concentration similar to that found in the aged collagen extracts. Interestingly, we found that histochemical analyses of human prostatic tissues showed significantly higher expression of HA in BPH and (to a lesser degree) prostate cancer stroma relative to stroma of normal prostate. We suggest that HA is a specific mediator in aged ECM that promotes the growth of prostate epithelial cells. In vitro systems utilizing 3D collagen gels are increasingly used to simulate cell-ECM interactions that occur in vivo. Therefore, it is important to note that the presence in collagen gels of specific non-collagenous components, such as HA, may exert a significant influence on cell behavior.
Acknowledgments
The authors wish to thank Drs. Daniella Bianchi-Frias, Cynthia Sprenger, and Amy Bradshaw for helpful discussions, and Virginia M. Green, PhD, for editorial review of the manuscript.
Contract/grant sponsor R21 AG033391 NIA/NIH (M.J.R.); R01 EB012558, NIBIB/NIH (R.B.V./T.N.W)
Footnotes
Conflict of interest The authors have no conflicts of interest to declare.
Contributor Information
Mamatha Damodarasamy, Department of Medicine, University of Washington, Harborview Medical Center, 325 9th Avenue, Box 359625, Seattle, WA 98104, USA.
Robert B. Vernon, Matrix Biology Program, Benaroya Research Institute at Virginia Mason, 1201 9th Avenue, Seattle, WA 98101, USA
Christina K. Chan, Matrix Biology Program, Benaroya Research Institute at Virginia Mason, 1201 9th Avenue, Seattle, WA 98101, USA
Stephen R. Plymate, Department of Medicine, University of Washington, Harborview Medical Center, 325 9th Avenue, Box 359625, Seattle, WA 98104, USA Veterans Affairs Puget Sound Health Care System, Seattle, Washington, USA.
Thomas N. Wight, Matrix Biology Program, Benaroya Research Institute at Virginia Mason, 1201 9th Avenue, Seattle, WA 98101, USA
May J. Reed, Email: mjr@uw.edu, Department of Medicine, University of Washington, Harborview Medical Center, 325 9th Avenue, Box 359625, Seattle, WA 98104, USA.
References
- Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- Bartling B, Desole M, Rohrbach S, Silber RE, Simm A. Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. FASEB J. 2009;23:1510–1520. doi: 10.1096/fj.08-122648. [DOI] [PubMed] [Google Scholar]
- Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine. 2008;43:194–199. doi: 10.1016/j.cyto.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentov I, Damodarasamy M, Plymate S, Reed MJ. B16/F10 tumors in aged 3D collagen in vitro simulate tumor growth and gene expression in aged mice in vivo. In Vitro Cell Dev Biol Anim. 2013;49:395–399. doi: 10.1007/s11626-013-9623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj AG, Rector K, Simpson MA. Inducible hyaluronan production reveals differential effects on prostate tumor cell growth and tumor angiogenesis. J Biol Chem. 2007;282:20561–20572. doi: 10.1074/jbc.M702964200. [DOI] [PubMed] [Google Scholar]
- Bianchi-Frias D, Vakar-Lopez F, Coleman M, Plymate SR, Reed MJ, Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment. PLoS One. 2010;1:5–10. doi: 10.1371/journal.pone.0012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–H622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Damodarasamy M, Johnson RS, Bentov I, MacCoss MJ, Vernon RB, Reed MJ. Hyaluronan enhances wound repair and increases collagen III in aged dermal wounds. Wound Repair Regen. 2014 doi: 10.1111/wrr.12192. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damodarasamy M, Vernon RB, Karres N, Chang H, Bianchi-Frias D, Nelson PS, Reed MJ. Collagen extracts derived from young and aged mice demonstrate different structural properties and cellular effects in three-dimensional gels. J Gerontol A Biol Sci Med Sci. 2010;65:209–218. doi: 10.1093/gerona/glp202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Raoudi M, Tranchepain F, Deschrevel B, Vincent JC, Bogdanowicz P, Boumediene K, Pujol JP. Differential effects of hyaluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen. 2008;16:274–287. doi: 10.1111/j.1524-475X.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- De Klerk DP. The glycosaminoglycans of normal and hyperplastic prostate. Prostate. 1983;4:73–81. doi: 10.1002/pros.2990040107. [DOI] [PubMed] [Google Scholar]
- Ershler WB, Stewart JA, Hacker P, Moore AL, Tindle BH. B16 murine melanoma and aging: slower growth and longer survival in old mice. J Natl Cancer Inst. 1984;72:161–164. doi: 10.1093/jnci/72.1.161. [DOI] [PubMed] [Google Scholar]
- Ghazi K, Deng-Pichon U, Warnet JM, Rat P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: role of molecular weight. PLoS One. 2012;7:e48351. doi: 10.1371/journal.pone.0048351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987;30:769–778. doi: 10.1002/art.1780300707. [DOI] [PubMed] [Google Scholar]
- Goulas A, Hatzichristou DG, Karakiulakis G, Mirtsou-Fidani V, Kalinderis A, Papakonstantinou E. Benign hyperplasia of the human prostate is associated with tissue enrichment in chondroitin sulphate of wide size distribution. Prostate. 2000;44:104–110. doi: 10.1002/1097-0045(20000701)44:2<104::aid-pros2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Grönberg H, Damber JE, Jonsson H, Lenner P. Patient age as a prognostic factor in prostate cancer. J Urol. 1994;152:892–895. doi: 10.1016/s0022-5347(17)32601-0. [DOI] [PubMed] [Google Scholar]
- Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J Biol Chem. 1998;273:3604–3610. doi: 10.1074/jbc.273.6.3604. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Measurement of changes in mouse tail collagen with age: temperature dependence and procedural details. Exp Gerontol. 1978;3:75–82. doi: 10.1016/0531-5565(78)90033-5. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;5:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ST, Liao KK, Liao MC, Tang MJ. Age effect of type I collagen on morphogenesis of Madin-Darby canine kidney cells. Kidney Int. 2000;57:1539–1548. doi: 10.1046/j.1523-1755.2000.00998.x. [DOI] [PubMed] [Google Scholar]
- Kovar JL, Johnson MA, Volcheck WM, Chen J, Simpson MA. Hyaluronidase expression induces prostate tumor metastasis in an orthotopic mouse model. Am J Pathol. 2006;169:1415–1426. doi: 10.2353/ajpath.2006.060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GY, Kenny PA, Lee EH, Bissell MJ. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359–365. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokeshwar VB, Lokeshwar BL, Pham HT, Block NL. Association of elevated levels of hyaluronidase, a matrix-degrading enzyme, with prostate cancer progression. Cancer Res. 1996;56:651–657. [PubMed] [Google Scholar]
- Lokeshwar VB, Rubinowicz D, Schroeder GL, Forgacs E, Minna JD, Block NL, Nadji M, Lokeshwar BL. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J Biol Chem. 2001;276:11922–11932. doi: 10.1074/jbc.M008432200. [DOI] [PubMed] [Google Scholar]
- Maharjan A, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One. 2011;6:e26078. doi: 10.1371/journal.pone.0026078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron-Mendoza M, Seemann J, Grinnell F. The differential regulation of cell motile activity through matrix stiffness and porosity in three dimensional collagen matrices. Biomaterials. 2010;31:6425–6435. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra S, Toole BP, Ghatak S. Hyaluronan constitutively regulates activation of multiple receptor tyrosine kinases in epithelial and carcinoma cells. J Biol Chem. 2006;281:34936–34941. doi: 10.1074/jbc.C600138200. [DOI] [PubMed] [Google Scholar]
- Ng MR, Brugge JS. A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell. 2009;16:455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Orgel JP, San Antonio JD, Antipova O. Molecular and structural mapping of collagen fibril interactions. Connect Tissue Res. 2011;52:2–17. doi: 10.3109/03008207.2010.511353. [DOI] [PubMed] [Google Scholar]
- Petrie RJ, Gavara N, Chadwick RS, Yamada KM. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol. 2012;197:439–455. doi: 10.1083/jcb.201201124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11–22. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen M, Thomas K, Wu H, Nambi P. Stimulation of hyaluronan synthetase by platelet-derived growth factor bb in human prostate smooth muscle cells. Pharmacology. 2001;62:103–106. doi: 10.1159/000056079. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Damodarasamy M, Chan CK, Johnson MN, Wight TN, Vernon RB. Cleavage of hyaluronan is impaired in aged dermal wounds. Matrix Biol. 2013;32:45–51. doi: 10.1016/j.matbio.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley PJ, Lamplugh L, Lee ER, Matsumoto K, Yamaguchi Y. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine. 2011;36:E914–E920. doi: 10.1097/BRS.0b013e3181f1e84f. [DOI] [PubMed] [Google Scholar]
- Saad M, Abdel-Rahim M, Abol-Enein H, Ghoneim MA. Concomitant pathology in the prostate in cystoprostatectomy specimens: a prospective study and review. BJU Int. 2008;102:1544–1550. doi: 10.1111/j.1464-410X.2008.07831.x. [DOI] [PubMed] [Google Scholar]
- Sadoun E, Reed MJ. Impaired angiogenesis in aging is associated with alterations in vessel density, matrix composition, inflammatory response, and growth factor expression. J Histochem Cytochem. 2003;51:1119–1130. doi: 10.1177/002215540305100902. [DOI] [PubMed] [Google Scholar]
- Sakr SW, Potter-Perigo S, Kinsella MG, Johnson PY, Braun KR, Goueffic Y, Rosenfeld ME, Wight TN. Hyaluronan accumulation is elevated in cultures of low density lipoprotein receptor-deficient cells and is altered by manipulation of cell cholesterol content. J Biol Chem. 2008;283:36195–36204. doi: 10.1074/jbc.M807772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi ZD, Wang H, Tarbell JM. Heparan sulfate proteoglycans mediate interstitial flow mechanotransduction regulating MMP-13 expression and cell motility via FAK-ERK in 3D collagen. PLoS One. 2011;6:15956. doi: 10.1371/journal.pone.0015956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson MA, Lokeshwar VB. Hyaluronan and hyaluronidase in genitourinary tumors. Front Biosci. 2008;13:5664–5680. doi: 10.2741/3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int J Cancer. 2010;127:2739–2748. doi: 10.1002/ijc.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008;26:106–122. doi: 10.1016/j.clindermatol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Underhill CB. Hyaluronan is inversely correlated with the expression of CD44 in the dermal condensation of the embryonic hair follicle. J Invest Dermatol. 1993;101:820–826. doi: 10.1111/1523-1747.ep12371701. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Sage EH. A novel quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvasc Res. 1999;57:118–133. doi: 10.1006/mvre.1998.2122. [DOI] [PubMed] [Google Scholar]
- Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31:92–99. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- Wu CC, Ding SJ, Wang YH, Tang MJ, Chang HC. Mechanical properties of collagen gels derived from rats of different ages. J Biomater Sci Polym Ed. 2005;16:1261–1275. doi: 10.1163/156856205774269494. [DOI] [PubMed] [Google Scholar]