Summary
In cultured cells, an increase in cellular levels of reactive oxygen species (ROS) can be detected using multiple techniques including colorimetric assays, immunoblotting, and immunofluorescence. These methods can also be applied for ROS measurement in tissue samples, but often require tissue homogenization, and therefore do not distinguish between the different cell types within a tissue. Here, we describe a detailed protocol for determination of altered oxidative stress levels in different cell types in tissues, by detecting ROS-caused alteration of macromolecules using immunohistochemistry (IHC). This method is demonstrated by using 4HNE as a marker for lipid peroxidation in mouse pancreas tissue that contains precancerous lesions high in cellular oxidative stress.
Keywords: reactive oxygen species, immunohistochemistry, lipid peroxidation, 4HNE
1. Introduction
Aberrant, net accumulation of reactive oxygen species (ROS) in cells and tissues has been implicated in numerous diseases such as diabetes, neurodegenerative disorders and cancer, but also shortening of lifespan and organismal aging. Reactive oxygen species (ROS) are highly reactive molecules containing oxygen with unpaired electrons. Generation of ROS can be induced by environmental and other extracellular sources or internally in cellular organelles during biological processes. Inside a cell, ROS are generated as byproducts in many organelles including mitochondria, endoplasmic reticulum and peroxisomes. These highly reactive molecules not only attack DNA to cause DNA damage and adduct formation (i.e. DNA double strand breaks, 8-hydroxy-2-deoxyguanosine/8-oxo-dG), but also lead to protein oxidation (i.e. nitro-tyrosine) and lipid peroxidation (i.e. 4 hydroxy-2-noneal/4HNE, malondialdehyde). Elimination of excess cellular ROS is mediated by scavenging systems including superoxide dismutase, catalase, glutathione peroxidase and peroxiredoxins (1). Since long-term imbalance between cellular ROS production and elimination has been implicated in organismal aging and onset and progression of numerous disorders including neurodegenerative diseases and cancer, it is important to be able to evaluate cellular ROS levels in clinical patient tissue samples or in animal models recapitulating disease (1, 2). In this chapter, we provide an immunohistochemistry protocol to assess cellular oxidative stress levels, using mouse pancreatic precancerous lesions and the lipid peroxidation product 4HNE as an indicator of ROS. With minor modifications (i.e. first antibody, and adjustment of dilution) this protocol can be applied to also detect DNA adducts, protein oxidation and other lipid peroxidation products (Table 1) by immunohistochemistry in any tissues of interest.
Table 1.
Antigens that can be targeted to evalaute cellular ROS levels in tissue immunohistochemistry
| Antibody directed against | Readout for ROS | Tissue Fixation | Reference |
|---|---|---|---|
| 8-hydroxy-2-deoxyguanosine (8-oxo-dG) | DNA damage | formalin ethanol | 3 4 |
| 8-nitroguanine | DNA damage | formalin | 5, 6 |
| thymidine glycol (TG) | DNA damage | formalin | 7 |
| dinitrophenyl (DNP) | protein oxidation | methacam | 8, 9 |
| nitrotyrosine | protein oxidation | formalin | 10, 11 |
| 4-hydroxy-2-noneal (4HNE) | lipid peroxidation | formalin | 3, 12 |
| malondialdehyde (MDA) | lipid peroxidation | formalin | 13, 14 |
| acrolein (ACR) | lipid peroxidation | paraformaldehyde | 15, 16 |
| methyglyoxal (MG) | lipid peroxidation | formalin | 17 |
| hexanoyl-lysine (HEL) | lipid peroxidation | formalin | 18, 19 |
| crotonaldhyde (CRA) | lipid peroxidation | paraformaldehyde formalin | 20 21 |
| 7-ketocholesterol (7-KC) | lipid peroxidation | N/A* | 22, 23 |
forzen tissue section
2. Materials
2.1. Buffers
Sodium citrate buffer pH 6.0: 10 mM sodium citrate, 0.05 % Tween 20, in distilled H2O. Adjust pH to 6.0.
Phosphate-buffered saline (PBS): Dissolve 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4 and 0.24 g of KH2PO4 in 800 ml of distilled H2O; then adjust pH to 7.4; and then adjust volume to 1 L with additional distilled H2O.
TBST buffer pH 7.6: 50 mM Tris, 150 mM NaCl, 0.05 % Tween 20, in distilled H2O. Adjust pH to 7.6.
Blocking buffer: 5 % goat serum in 1× TBST buffer.
95% ethanol solution: 95 mL 100% ethanol plus 5 mL distilled H2O.
80% ethanol solution: 80 mL 100% ethanol plus 20 mL distilled H2O.
3% H2O2 solution: 5 mL 30% H2O2 (commercially-available) plus 45 mL distilled H2O.
2.2. Immunohistochemistry
Rabbit anti-4HNE sera from Alpha Diagnostic International Inc. (San Antonio, TX, USA); or other antibodies directed against antigens that can serve as readout for increased oxidative stress (Table 1).
HRP-conjugated goat-anti-rabbit antibody (or other HRP-conjugated secondary antibody directed against the species in which the primary antibody was raised).
3,3’-diaminobenzidine (DAB) peroxidase substrate kit (available from multiple vendors).
2.3. Other Materials
Tissue slides with tissue of interest.
Pressure cooker or steamer.
Staining jar or holder (use polyethylene instead of glass).
Pap pen (optional).
Sharp-end forcep tweezers.
Standard IHC mounting medium (available from multiple vendors).
Coverslips.
3. Methods
Unless otherwise specified, carry out all procedures at room temperature (20 °C).
3.1. Deparaffinization of Tissue Slides
Incubate tissue slides in xylene for 5 min.
Repeat step 1 for another two times (see Note 1).
3.2. Rehydration of Tissue Slides
Perform 3 washes with 100% ethanol (see Note 2), 3 min for each wash.
Perform 2 washes with 95% ethanol, 3 min for each wash.
Perform 2 washes with 80% ethanol, 3 min for each wash.
Rinse tissue slides in distilled water for 5 min, twice.
3.3. Antigen Retrieval
Heat tissue slides (see Notes 3 and 4) in sodium citrate buffer pH 6.0 at 95 – 100 °C for 20 min.
Remove the heat and let tissue slides cool in sodium citrate buffer on the bench until the temperature reaches room temperature (see Note 5).
Wash tissue slides with phosphate buffered saline (PBS) for 5 min, three times (see Note 6).
3.4. Immunohistochemical Staining of Tissue Slides
Incubate tissue slides with 3% hydrogen peroxide for 10 min.
Wash tissue slides with PBS for 5 min; repeat this step three times.
Place tissue slides in blocking buffer for 1 h at room temperature.
Prepare 4HNE antibody solution by adding rabbit anti-4HNE antibody to blocking buffer at a dilution of 1:600 (see Note 7).
Remove tissue slides from blocking buffer.
Apply 4HNE antibody solution to tissue sample area (see Note 8) and incubate overnight at 4 °C (see Note 9).
Remove 4HNE antibody solution from tissue slides.
Wash tissue slides with TBST buffer for 5 min; repeat this step 3 times.
Prepare secondary antibody solution by adding HRP-conjugated goat-anti-rabbit antibody in blocking buffer at a dilution recommended by the manufacturer.
Remove tissue slides from TBST buffer.
Apply HRP-conjugated goat-anti-rabbit secondary antibody solution to the tissue slide (see Note 10) and incubate for 30 min at room temperature (20 °C).
Remove secondary antibody solution from tissue slides.
Wash tissue slides with TBST buffer for 5 min; repeat this step 3 times.
Prepare DAB substrate solution according to the manufacturer’s instructions.
Apply DAB substrate solution to tissue slides. Ensure that the substrate solution completely covers the tissue sample area.
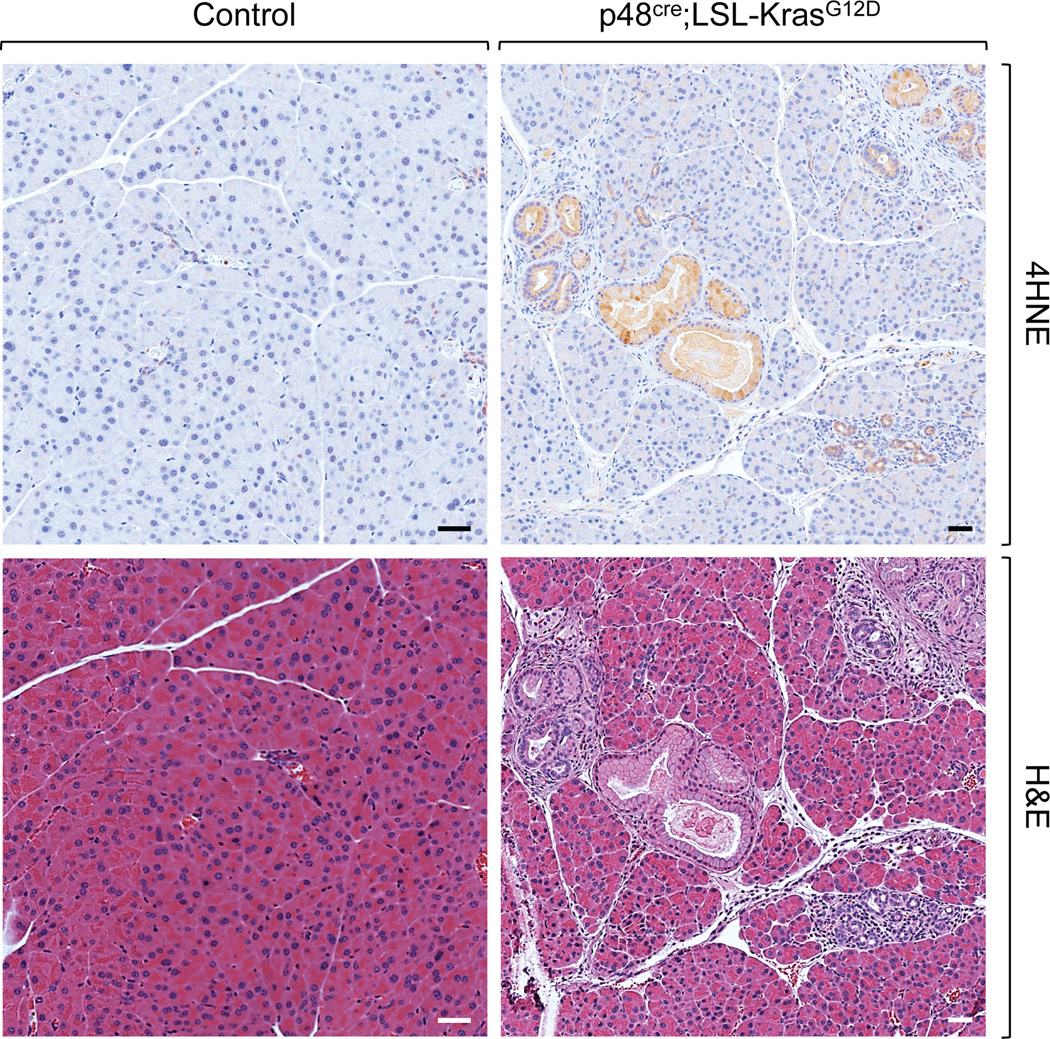
Watch closely as tissue sample color turns into brown (see Note 11). When the signal intensity reaches the ideal condition (not too light, not too dark; Fig. 1), stop the reaction by immersing slides in distilled water.
Wash tissue slides with distilled water for 5 min, twice.
Fig. 1.
Detection of elevated ROS levels in pre-cancerous lesions. Staining of pancreatic tissues from a control mouse and a p48cre;LSL-KrasG12D mouse, in which Kras with a proto-oncogenic mutation (KrasG12D) was expressed under a pancreatic cell-specific transcription factor (p48). Expression of KrasG12D leads to pre-cancerous pancreatic lesions that show high levels of oxidative stress. Tissues were fixed in formalin and then subjected to 4HNE immunohistochemistry (top panel) as described in this chapter. Additional hematoxylin & eosin (H&E) staining was performed to show pancreas morphology (bottom panel). The data shows that our protocol is effective to detect oxidative stress-mediated lipid oxidation in the abnormal pancreatic lesions (brown staining), but not in the control. Scale bar: 40 µm.
3.5. Dehydration and Mounting of Tissue Slides
Incubate tissue slides with 80% ethanol for 10 seconds, twice (see Note 12).
Incubate tissue slides with 95% ethanol for 10 seconds, twice.
Incubate tissue slides with 100% ethanol for 10 seconds, twice.
Incubate tissue slides with xylene for 10 seconds, twice.
Apply mounting media to the tissue slides and apply coverslips (see Note 13).
Acknowledgement
This work was supported by NIH grants CA140182 and GM086435 to PS.
Footnotes
Xylene is flammable and a health hazard. This step should be carried out in a chemical fume hood with outdoor exhaust ventilation.
Ethanol is flammable and a hazardous material. This step should be carried out in a chemical fume hood with outdoor exhaust ventilation.
A pressure cooker or steamer is ideal for this key step because it can maintain a fairly constant temperature nearing boiling point.
Do not use a glass staining jar or holder in this step because they will crack at high temperature. A polyethylene staining jar which can endure high temperature is best suitable for this process. In addition, using a polyethylene staining jar inside the streamer reduces the amount of sodium citrate buffer to be used.
Make sure that the surface of the tissue sample area is always moist and kept in buffer or solution.
Immediately proceed to the immunostaining procedure is highly recommended after antigen retrieval.
This dilution (1:600) for the 4HNE antibody is optimized for mouse pancreas tissues. For other tissue types, either mouse or human, optimal dilution can vary and needs to be determined.
If you want to decrease the amount of 4HNE antibody solution that will be used in the next step, create a water-proof barrier by circling the tissue sample area on the glass slide using a pap pen.
To avoid the 4HNE antibody solution from drying out during overnight incubation, the tissues slide can be placed on top of a wet paper towel in a sealable plastic container. This preserves the moisture inside the container.
Ensure that the goat-anti-rabbit antibody solution completely covers the tissue sample area.
The time period of incubation to develop signals varies and depends on the (DAB) peroxidase substrate kit used (see manufacturer’s instructions). To test the incubation time for optimal signal intensity, it is recommended using tissue samples that have high levels of cellular ROS and control tissue slides (see Fig. 1). At ideal development time, a clear difference of 4HNE signal intensity between positive and negative tissue samples should be observed.
Steps 1 – 4 should be performed in a chemical fume hood with outdoor exhaust ventilation.
To avoid generating air bubbles between tissue sample area and coverslip, drop mounting media directly on top of the tissue area. Then use sharp-end forcep tweezers to hold the coverslip at one end and let the other end of the coverslip stand on the tissue slide. Slowly close the gap between tissue slide and coverslip by putting down the coverslip entirely.
References
- 1.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quintanilla RA, Orellana JA, von Bernhardi R. Understanding risk factors for Alzheimer’s disease: interplay of neuroinflammation, connexin-based communication and oxidative stress. Arch Med Res. 2012;43:632–644. doi: 10.1016/j.arcmed.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Young O, Crotty T, O’Connell R, et al. Levels of oxidative damage and lipid oxidation in thyroid neoplasia. Head Neck. 2010;32:750–756. doi: 10.1002/hed.21247. [DOI] [PubMed] [Google Scholar]
- 4.De Luca G, Russo MT, Degan P, et al. A role for oxidized DNA precursors in Huntington’s disease-like striatal neurodegeneration. PLoS Genet. 2008;4:e1000266. doi: 10.1371/journal.pgen.1000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraku Y, Kawanishi S. Immunohistochemical analysis of 8-nitroguanine, a nitrative DNA lesion, in relation to inflammation-associated carcinogenesis. Methods Mol Biol. 2009;512:3–13. doi: 10.1007/978-1-60327-530-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Horiike S, Kawanishi S, Kaito M, et al. Accumulation of 8-nitroguanine in the liver of patients with chronic hepatitis C. J Hepatol. 2005;43:403–410. doi: 10.1016/j.jhep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Ito K, Yano T, Morodomi Y, et al. Serum antioxidant capacity and oxidative injury to pulmonary DNA in never-smokers with primary lung cancer. Anticancer Res. 2012;32:1063–1067. [PubMed] [Google Scholar]
- 8.Aksenov MY, Aksenova MV, Butterfield DA, et al. Protein oxidation in the brain in Alzheimer’s disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 9.Smith MA, Sayre LM, Anderson VE, et al. Cytochemical demonstration of oxidative damage in Alzheimer disease by immunochemical enhancement of carbonyl reaction with 2,4-dinitrophenylhydrazine. J Histochem Cytochem. 1998;46:731–735. doi: 10.1177/002215549804600605. [DOI] [PubMed] [Google Scholar]
- 10.Win S, Than TA, Han D, et al. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J Biol Chem. 2011;286:35071–35078. doi: 10.1074/jbc.M111.276089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vlahos R, Stambas J, Bozinovski S, et al. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog. 2011;7:e1001271. doi: 10.1371/journal.ppat.1001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kweider N, Huppertz B, Wruck CJ, et al. A role for Nrf2 in redox signaling if the invasive extravillous trophoblast in severe early onset IUGR associated with preeclampsia. PLoS One. 2012;7:e47055. doi: 10.1371/journal.pone.0047055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neria F, Castilla MA, Sanchez RF, et al. Inhibition of JAK2 protects renal endothelial and epithelial cells from oxidative stress and cyclosporine A toxicity. Kidney Int. 2009;75:227–234. doi: 10.1038/ki.2008.487. [DOI] [PubMed] [Google Scholar]
- 14.Kong X, Zhang Y, Wu HB, et al. Combination therapy with losartan and pioglitazone additively reduces renal oxidative and nitrative stress induced by chronic high fat, sucrose, and sodium intake. Oxid Med Cell Longev. 2012;2012:856085. doi: 10.1155/2012/856085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gregg SQ, Gutierrez V, Robinson A, et al. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55:609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okuno Y, Nakamura-Ishizu A, Otsu K, et al. Pathological neoangiogenesis depends on oxidative stress regulation by ATM. Nat Med. 2012;18:1208–1216. doi: 10.1038/nm.2846. [DOI] [PubMed] [Google Scholar]
- 17.Oya T, Hattori N, Mizuno Y, et al. Methylglyoxal modification of protein. Chemical and immunochemical characterization of methylglyoxal-arginine adducts. J Biol Chem. 1999;274:18492–18502. doi: 10.1074/jbc.274.26.18492. [DOI] [PubMed] [Google Scholar]
- 18.Fukuchi Y, Miura Y, Nabeno Y, et al. Immunohistochemical detection of oxidative stress biomarkers, dityrosine and N(epsilon)-(hexanoyl)lysine, and C-reactive protein in rabbit atherosclerotic lesions. J Atheroscler Thromb. 2008;15:185–192. doi: 10.5551/jat.e543. [DOI] [PubMed] [Google Scholar]
- 19.Tomaru M, Takano H, Inoue K, et al. Pulmonary exposure to diesel exhaust particles enhances fatty change of the liver in obese diabetic mice. Int J Mol Med. 2007;19:17–22. [PubMed] [Google Scholar]
- 20.Shibata N, Kawaquchi M, Uchida K, et al. Protein-bound crotonaldehyde accumulates in the spinal cord of superoxide dismutase-1 mutation-associated familial amyotrophic lateral sclerosis and its transgenic mouse model. Neuropathology. 2007;27:49–61. doi: 10.1111/j.1440-1789.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi-Niida M, Shibata N, Morikawa S, et al. Crotonaldehyde accumulates in glial cells of Alzheimer’s disease brain. Acta Neuropathol. 2006;111:422–429. doi: 10.1007/s00401-006-0044-1. [DOI] [PubMed] [Google Scholar]
- 22.Myoishi M, Hao H, Minamino T, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 23.Moreira EF, Larrayoz IM, Lee JW, et al. 7-Ketocholesterol is present in lipid deposits in the primate retina: potential implications in the induction of VEGF and CNV formation. Invest Ophthalmol Vis Sci. 2009;50:523–532. doi: 10.1167/iovs.08-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]