Abstract
Objective
Autologous HIV-1 infected CD4+ primary T cells (aHIV+CD4) have been shown to be largely resistant to Natural Killer (NK) cell mediated lysis due to viral strategies of immune evasion. We have previously shown that a pre-activation of NK cells with Plasmacytoid Dendritic Cells can significantly augment lysis of aHIV+CD4 through a mechanism dependent on Interferon-alpha (IFN-α).
Design
The goal of the present study is to identify the specific NK activating receptors involved in NK lysis of aHIV+CD4 following IFN-α activation.
Methods
PBMC were incubated with aHIV+CD4 to induce the secretion of endogenous levels of IFN-α and drive NK activation. We then utilized a standard chromium lysis assay to assess the degree of IFN-α activated lysis of aHIV+CD4 in the presence or absence of masking antibodies to a panel of NK activating receptors and co-receptors.
Results
Direct recognition of HIV-1 infected, but not uninfected, autologous CD4+ primary T cells by PBMC induced the secretion IFN-α (Median 2280 pg/ml, p<0.001, n=9) that, in turn, activated NK cells (p<0.001, n=12) and significantly increased their cytolytic potential against aHIV+CD4 (p<0.01, n=12). The masking of NKp46 (p<0.01, n=8) and NKG2D (p<0.05, n=8), but not 2B4, NTBA, NKp30 or NKp44, significantly reduced IFN-α activated lysis of aHIV+CD4.
Conclusions
Taken together, these results demonstrate that endogenous levels of IFN-α secreted by pDCs induce NK cells to lyse aHIV+CD4 via the engagement of NKp46 and NKG2D.
Keywords: AIDS, NK Cells, NKp46, NKG2D, Interferon-alpha, Cytotoxicity
Introduction
The ability of NK cells to discriminate between normal and abnormal cells involves complex interactions between inhibitory (iNKRs) and activating (aNKRs) NK cell receptors [1–5]. Under physiological conditions, the binding of iNKRs such as NKG2A and killer immunoglobulin-like receptors (KIRs) to autologous MHC-class-I (MHC-I) molecules induces negative regulatory signals that switch off NK cells [6–11]. Heterologous target cells expressing iNKR-mismatched MHC-I proteins exhibit a naturally increased target cell sensitivity to NK cell lysis. In contrast, normally resistant autologous target cells become susceptible to NK cell cytotoxicity during viral infection or tumor transformation when MHC-I proteins are down-regulated. Following the reduction of inhibitory signals, NK cells then require the engagement of aNKRs to induce the killing of susceptible target cells. Examples of aNKRs include: the NKG2D receptor that recognizes stress-induced ligands [12–15], the Fc-γIII receptor (CD16) which mediates antibody dependent cytotoxicity [16–18], activating KIRs lacking inhibitory motifs [19–21], and the Natural Cytotoxicity Receptor Family (NKp46, NKp30, NKp44) which directly recognize viral or cellular antigens [22–27]. NK cell effector functions are also modulated by co-stimulatory receptors such as 2B4 or NTBA that can synergize with other aNKRs to induce higher levels of cellular lysis [28, 29]. Likewise, cytokines such as IL-2, IL-12, IL-15, IL-21 or Interferon-alpha (IFN-α) can also augment lysis of susceptible targets cells by pre-activating NK cells [30–38].
The autologous HIV-1 infected CD4+ primary T cell (aHIV+CD4) NK assay system represents the most physiologically relevant in vitro model for measuring NK activity due to the complete match between MHC-I alleles on HIV+CD4 target cells and iNKRs on NK cells [39–41]. However, aHIV+CD4 have been shown to be largely resistant to lysis by NK cells in vitro due to viral strategies of immune evasion [39, 42, 43]. We have previously shown that NK cytotoxicity against aHIV+CD4 can be significantly augmented by Plasmacytoid Dendritic Cell (pDC) activation of NK cells through an IFN-α dependent-mechanism [44]. We have also observed that purified pDC alone are sufficient to recognize aHIV+CD4 and secrete high amounts of IFN-α that in turn can activate NK cells [45]. However, the specific receptors utilized by NK cells during IFN-α activated lysis of autologous HIV+CD4 remains undetermined. Using a modified version of our aHIV+CD4/pDC recognition system, we now investigated the specific aNKRs involved in lysis of aHIV+CD4 following activation of NK cells with endogenous levels of IFN-α.
Materials and Methods
HIV-1 infection
Peripheral blood mononuclear cells (PBMCs) were isolated from 20 healthy uninfected donors according to informed consent and Institutional Review Board approval from The Wistar Institute. PBMCs were stimulated for 3 days with 10 µg/ml PHA-p (Sigma Aldrich, MO) and 100 IU/ml hIL-2 (PeproTech, Rocky Hill, NJ). CD4+ primary T cells were isolated by positive selection using anti-CD4 magnetic beads as described by the manufacturer (Miltenyi Corporation, CA). 5×106 activated CD4+ T cells were spinfected with 150 ng of p24 containing supernatant of the CXCR4-tropic HIV-1 isolate TYBE as previously described [44]. After 4 days of infection, we enriched HIV-1 infected cells that downregulated the CD4 receptor during infection (X>70% infectivity per donor) by removing uninfected CD4+ T cells using anti-CD4 depletion magnetic beads (Miltenyi) as previously described [39].
Flow cytometry
The following antibodies were used at the recommended dilution of 0.25 µg antibody/million cells: CD3 (SK7), CD4 (SK3), CD16 (3GB), CD56 (B159), CD69 (FN50). Cell surface staining for CD69 activation was carried on CD56+/CD3− gated NK cells with gates set upon unstimulated control cells. For intracellular staining of the HIV-1 p24 gag protein, CD4+ T cells were permeabilized with the Cytofix/Cytoperm kit (BD Pharmingen) as described by the manufacturer and stained with the anti-p24 KC57 FITC antibody (Beckman Coulter, CA). Samples were collected on a LSRII Cytometer (BD) and were analyzed with FlowJo software (Tree Star Incorporated, Ashland OR).
NK chromium51 release cytotoxicity assay
HIV-1 infected or uninfected CD4+ primary T cells were generated over a 7 day period as described above and incubated with autologous PBMC isolated from a second blood draw at a 25:1 PBMC:CD4 ratio for 18 hours as depicted in Figure 1, panel A. Following overnight incubation, NK cells were tested for upregulation of the CD69 activation marker by flow cytometry and IFN-α secretion into the supernatant was measured by Interferon alpha-2a ELISA (PBL Biomedical Laboratories, NJ) as described previously [44]. On the day of chromium lysis assay, a second aliquot of HIV-1 infected CD4+ primary T cells was labeled with 100 µCu Na251CrO4 for 3 hours and incubated with autologous PBMC effector cells (containing activated NK cells as described above) at a 100:1 PBMC/CD4 ratio in a 4-hour chromium lysis assay as described previously [44]. Saturating concentrations of specific monoclonal antibodies (mAbs) were added to the PBMC cultures for masking experiments utilizing a one fourth total volume of antibody per well (50 µl in a 200 µl total volume). The following masking antibodies (mAbs) kindly provided by Prof. Alessandro Moretta were used for the study: BAT221 (IgG1, anti NKG2D), BAB-281 (IgG1, anti-NKp46), F252 (IgM, anti NKp30), KS38 (IgM, anti-NKp44), KL247 (IgM, anti-NKp46), CO54 (IgM, anti-2B4), ON56 (IgG2a, anti-NTBA), and FS280 (IgG2a, anti-CD56). The appropriate IgG1 or IgM blocking antibody for NKp46 was chosen for blocking experiments when compared to NKG2D (IgG1) and NKp30/NKp44 (IgM) while anti-CD56 was used as a control antibody.
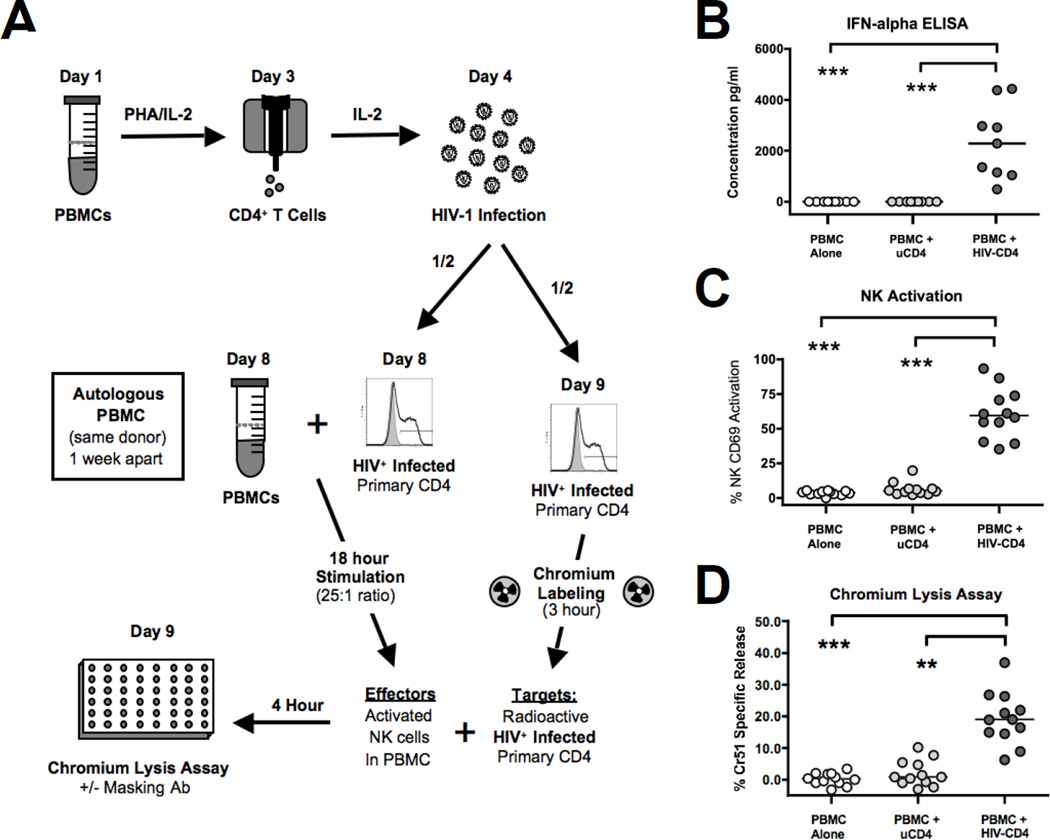
Figure 1. Endogenous IFN-α activation of NK cells stimulates lysis of HIV-1 infected autologous CD4 primary T cells (aHIV+CD4).
A) Experimental approach undertaken to determine the ability of IFN-α activated NK cells to lyse aHIV+CD4 either in the presence or in the absence of masking antibodies against different NK activating receptors. PBMCs were incubated for 18 hours with HIV-1 infected or uninfected autologous CD4+ primary T cells at a 25:1 PBMC/CD4 ratio and then re-incubated with another aliquot of the same chromium labeled aHIV+CD4 at a 100:1 E:T ratio in a standard 4 hour lytic assay. B) Summary graph showing the level of IFN-α (pg/ml) secreted by PBMCs following 18 hour incubation with either HIV-1 infected or uninfected autologous CD4+ primary T cells. Data represents 9 donors tested. C) Summary graph showing the surface expression of the activation marker CD69 on CD3−/CD56+ NK cells within total PBMCs following the incubation with either HIV-1 infected or uninfected CD4+ autologous T cells. Data represents 12 donors tested. D) Summary graph showing the degree of Cr51 specific release as indicative of target cell lysis in PBMC incubated for 18 hours with autologous HIV-1 infected or uninfected CD4+ primary T cells at a 25:1 PBMC/CD4 ratio and then re-incubated with another aliquot of the same chromium labeled aHIV+CD4 at a 100:1 E:T ratio. Data represents 12 donors tested. For graphs B-D, the statistical analyses were performed by using a paired, non-parametric Friedman ANOVA with a Dunn post-test. In all cases, significant results have two-sided p values of p<0.05, p<0.01, p<0.001 denoted with a single, double or triple asterisk in graphs, respectively.
Statistical analysis
All graphic presentations and statistical analysis were performed with Prism software (GraphPad Software, La Jolla, CA). In individual representative experiments, error bars depict the standard deviation. In composite graphs of multiple experiments, cross bars represent the median. Statistical analysis of three groups was carried out using a Friedman matched pair, non-parametric ANOVA test with a post-hoc Dunn analysis. p-values were two-sided with alphas noted in figures by asterisks as follows: p<0.05 (*), p<0.01 (**) or p<0.001 (***).
Results
Direct Recognition of aHIV+CD4 induces the secretion of IFN-α, that in turn leads to NK cell activation and triggers cytotoxicity against aHIV+CD4 through NKp46 and NKG2D
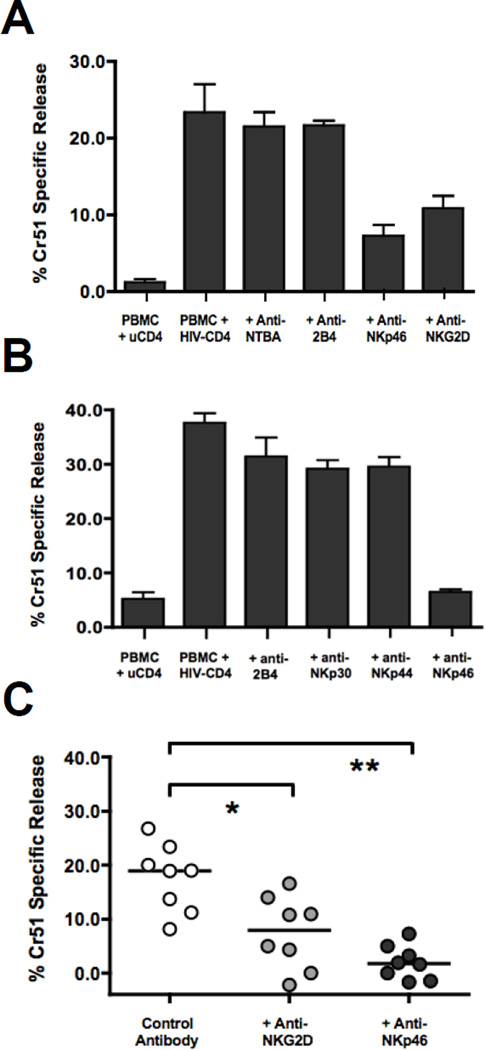
In order to investigate the specific NK activating receptors involved in IFN-α activated NK lysis of HIV-1 infected autologous CD4+ primary T cells (aHIV+CD4), we utilized a modified version of our previously published autologous experimental approach [45]. As shown in Figure 1, this model system involves the incubation of PBMC for 18 hours with aHIV+CD4 to induce the secretion of IFN-α (Median 2280 pg/ml, p<0.001, n=9) that in turn stimulates NK activation (p<0.001, n=12) and significantly increases the NK cytolytic potential against aHIV+CD4 (p<0.01, n=12). We have previously determined that depletion of pDCs from PBMC prior to incubation with aHIV+CD4 abrogated the secretion of IFN-α and NK cell activation [45]. We have also shown that depletion of NK cells from IFN-α stimulated PBMCs reduced lysis of aHIV+CD4 to undetectable levels and that lysis of aHIV+CD4 can be recapitulated with purified NK cells incubated solely with IFN-α [44]. We next performed masking experiments with a panel of monoclonal antibodies (mAbs) specific for different NK activating receptors in order to identify the NK cell receptor pathways regulating IFN-α activated lysis of aHIV+CD4. As shown in two representative experiments (Figure 2A and B) and across multiple donors (Figure 2C), the masking of either NKp46 or NKG2D significantly reduced the IFN-α activated lysis of aHIV+CD4 (p<0.01 and p<0.05, n=8). In contrast, the blocking of the co-stimulatory receptors NTBA and 2B4 (Figure 2A) or natural cytotoxicity receptors NKp30 and NKp44 (Figure 2B) did not alter IFN-α activated lysis of aHIV+CD4. Taken together, these results demonstrate that endogenous levels of IFN-α secreted by pDCs in response to HIV-1 infected target cells induce NK cells to lyse aHIV+CD4 via the engagement of NKp46 and NKG2D.
Figure 2. IFN-α activated lysis of aHIV+CD4 is blocked with masking antibodies specific for NKp46 and NKG2D.
A–B) Two representative experiments showing the degree of IFN-α activated NK lysis of HIV-1 infected CD4+ primary T cells (as measured by Cr51 specific release) in the presence or absence of masking antibodies against various NK activating receptors. Autologous PBMC were incubated for 18 hours with uninfected (first bar) or HIV-1 infected (next 5 bars) CD4+ primary T cells at a 25:1 PBMC/CD4 ratio and then re-incubated with another aliquot of chromium labeled HIV-1 infected CD4+ primary T cells at 100:1 E:T ratio in a four hour chromium lysis assay with masking antibodies against A) NTBA, 2B4, NKG2D, NKp46 or B) 2B4, NKp30, NKp44, NKp46. C) Summary graph showing in the above-mentioned experimental approach in a subset of 8 donors where IFN-α activated NK lysis of HIV-1 infected CD4+ primary T cells was measured in the presence of masking antibodies against NKG2D or NKp46 in the same experiment. The statistical analyses were performed by using a paired, non-parametric Friedman ANOVA with a Dunn post-test. In all cases, significant results have two-sided p values of p<0.05, p<0.01, p<0.001 denoted with a single, double or triple asterisk in graphs, respectively.
Discussion
Utilizing a physiologically relevant model system for measuring NK lysis, we demonstrate here that endogenous levels of IFN-α secreted by pDCs induce NK cells to lyse aHIV+CD4 via the engagement of NKp46 and NKG2D (but not 2B4, NTBA, NKp30 or NKp44). Our results showing a key role of NKG2D in triggering the NK cell-mediated clearance of aHIV+CD4 are in line with previous studies reporting the up-regulation of NKG2D ligands on the surface of HIV-1 infected cells [40, 41]. We now extend this finding by demonstrating that the NKp46 receptor is also required for lysis of aHIV+CD4 following IFN-α activation. As both cellular and viral antigens can act as ligands for the NKp46 receptor, an exploratory proteomic approach may be needed to discover the ligand induced on aHIV+CD4 in future studies.
Of note, previous studies have also shown a role for NKp44 in NK recognition of HIV-1 infected target cells from HIV-1 infected viremic subjects [27]. In our experimental approach, we could not detect any contribution of NKp44 in the clearance of aHIV+CD4 following IFN-α activation. This finding is not unexpected as NKp44 is not expressed on resting NK cells [4, 16, 23, 27, 29, 41, 46, 47]. Rather, NKp44 is up-regulated during chronic HIV-1 viral infection or following stimulation of NK cells with certain cytokines such as IL-2 (but not IFN-α) [25, 27, 29, 46, 48]. Similarly, it has been previously reported that the blocking of NKG2D, but not NKp46, reduces NK lysis of aHIV+CD4 [41]. The discrepancy between this study and our results may be related to the absence of IFN-α stimulation and testing lysis of whole NK populations versus selected NK subsets as effectors. Previously, Ward et al. utilized depleted NK cell cultures where NK cells expressing inhibitory receptors were removed to stimulate NK lysis [41]. Here, we utilized endogenous IFN-α activation of total NK cell populations to overcome the immune evasion mechanisms of HIV-1. It remains to be determined if IFN-α selectively activates lysis of aHIV+CD4 by NK cells expressing inhibitory receptors that have been licensed to kill during ontogeny. The implications of our data to HIV-1 infected subjects pertain to retained NK function during viral control, as prolonged viremia is associated with NK dysfunction. Specifically, during ART immune reconstitution we would expect a recovery of IFN-α-stimulated NK lytic activity against HIV-1 infected autologous CD4+ primary T cells by the same mechanism as described here for NKp46 and NKG2D (and potentially NKp44). Future longitudinal studies addressing ART, the degree of CD4 reconstitution and NK/pDC functional recovery will be needed to determine the timeline to NK cell responses against autologous infected targets following ART suppression.
Taken together, our results demonstrate that pDC driven NK activation through IFN-α stimulates lysis of aHIV+CD4 through a mechanism dependent on NKp46 and NKG2D. Having established the anti-HIV activity of IFN-α2 as an immunotherapy in antiretroviral therapy suppressed subjects [49], and a role for pDC frequency and control of viral replication [50, 51], our results here further highlight innate immunity and pDC/NK crosstalk in control over HIV-1. Conversely, the reduction in pDC function and NKp46 receptor expression during viremia [46, 52–54] suggests that a loss of IFN-α activated NK recognition of HIV-1 infected target cell through NKp46 may contribute to higher HIV-1 replication and disease progression.
Acknowledgements
We are grateful to Dr. Alessandro Moretta and Dr. Emanuela Marcenaro for generating and providing all NK masking reagents used in the study. The CXCR4-tropic HIV-1 isolate TYBE was expanded and tittered at the University of Pennsylvania Centers for AIDS Research Viral Core Facility. We thank Deborah Davis for her work as Phlebotomist at the Wistar Institute. Support for Shared Resources utilized in this study was provided by Cancer Center Support Grant (CCSG) P30CA010815 to The Wistar Institute. This study was supported by grants from the National Institutes of Health (AI51225, AI47760, U01AI065279, AI07632, AI068405), the Philadelphia Foundation, the funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health the Italian Ministry of Health (Ricerca Finalizzata, Bando ISS, Grants RF-ICH-2009-1299677 to D.M. and RF-ICH-2009-1304134 and to J.M). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors have no financial conflicts of interest.
Author Contributions
Costin Tomescu (Completed the in vitro assays, co-analyzed the data, co-wrote the manuscript), Domenico Mavilio (Co-coordinated the study design, edited manuscript), Luis J. Montaner (Coordinated the study, co-analyzed the data, co-wrote the manuscript.
References
- 1.Biassoni R, Ugolotti E, De Maria A. NK cell receptors and their interactions with MHC. Curr Pharm Des. 2009;15:3301–3310. doi: 10.2174/138161209789105225. [DOI] [PubMed] [Google Scholar]
- 2.Blery M, Olcese L, Vivier E. Early signaling via inhibitory and activating NK receptors. Hum Immunol. 2000;61:51–64. doi: 10.1016/s0198-8859(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 3.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 5.Tomasello E, Blery M, Vely F, Vivier E. Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin Immunol. 2000;12:139–147. doi: 10.1006/smim.2000.0216. [DOI] [PubMed] [Google Scholar]
- 6.Boyton RJ, Altmann DM. Natural killer cells, killer immunoglobulin-like receptors and human leucocyte antigen class I in disease. Clin Exp Immunol. 2007;149:1–8. doi: 10.1111/j.1365-2249.2007.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooley S, Xiao F, Pitt M, Gleason M, McCullar V, Bergemann TL, et al. A subpopulation of human peripheral blood NK cells that lacks inhibitory receptors for self-MHC is developmentally immature. Blood. 2007;110:578–586. doi: 10.1182/blood-2006-07-036228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 9.Long EO, Burshtyn DN, Clark WP, Peruzzi M, Rajagopalan S, Rojo S, et al. Killer cell inhibitory receptors: diversity, specificity, and function. Immunol Rev. 1997;155:135–144. doi: 10.1111/j.1600-065x.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 10.Vivier E, Romagne F. Good news, bad news for missing-self recognition by NK cells: autoimmune control but viral evasion. Immunity. 2007;26:549–551. doi: 10.1016/j.immuni.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 12.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pende D, Cantoni C, Rivera P, Vitale M, Castriconi R, Marcenaro S, et al. Role of NKG2D in tumor cell lysis mediated by human NK cells: cooperation with natural cytotoxicity receptors and capability of recognizing tumors of nonepithelial origin. Eur J Immunol. 2001;31:1076–1086. doi: 10.1002/1521-4141(200104)31:4<1076::aid-immu1076>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 15.Andre P, Castriconi R, Espeli M, Anfossi N, Juarez T, Hue S, et al. Comparative analysis of human NK cell activation induced by NKG2D and natural cytotoxicity receptors. Eur J Immunol. 2004;34:961–971. doi: 10.1002/eji.200324705. [DOI] [PubMed] [Google Scholar]
- 16.Biassoni R, Bottino C, Cantoni C, Moretta A. Human natural killer receptors and their ligands. Curr Protoc Immunol. 2002;Chapter 14(Unit 14):10. doi: 10.1002/0471142735.im1410s46. [DOI] [PubMed] [Google Scholar]
- 17.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 18.Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10:147–164. doi: 10.1016/s0966-3274(02)00062-x. [DOI] [PubMed] [Google Scholar]
- 19.Carr WH, Rosen DB, Arase H, Nixon DF, Michaelsson J, Lanier LL. Cutting Edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–651. doi: 10.4049/jimmunol.178.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long BR, Ndhlovu LC, Oksenberg JR, Lanier LL, Hecht FM, Nixon DF, Barbour JD. KIR3DS1 Conferral of Enhanced Natural Killer Cell Function in Early HIV-1 Infection. J Virol. 2008;82:4785–4792. doi: 10.1128/JVI.02449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Connor GM, Guinan KJ, Cunningham RT, Middleton D, Parham P, Gardiner CM. Functional polymorphism of the KIR3DL1/S1 receptor on human NK cells. J Immunol. 2007;178:235–241. doi: 10.4049/jimmunol.178.1.235. [DOI] [PubMed] [Google Scholar]
- 22.Arnon TI, Achdout H, Lieberman N, Gazit R, Gonen-Gross T, Katz G, et al. The mechanisms controlling the recognition of tumor- and virus-infected cells by NKp46. Blood. 2004;103:664–672. doi: 10.1182/blood-2003-05-1716. [DOI] [PubMed] [Google Scholar]
- 23.Arnon TI, Lev M, Katz G, Chernobrov Y, Porgador A, Mandelboim O. Recognition of viral hemagglutinins by NKp44 but not by NKp30. Eur J Immunol. 2001;31:2680–2689. doi: 10.1002/1521-4141(200109)31:9<2680::aid-immu2680>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 24.Bottino C, Biassoni R, Millo R, Moretta L, Moretta A. The human natural cytotoxicity receptors (NCR) that induce HLA class I-independent NK cell triggering. Hum Immunol. 2000;61:1–6. doi: 10.1016/s0198-8859(99)00162-7. [DOI] [PubMed] [Google Scholar]
- 25.Hudspeth K, Silva-Santos B, Mavilio D. Natural cytotoxicity receptors: broader expression patterns and functions in innate and adaptive immune cells. Front Immunol. 2013;4:69. doi: 10.3389/fimmu.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 27.Vieillard V, Strominger JL, Debre P. NK cytotoxicity against CD4+ T cells during HIV-1 infection: a gp41 peptide induces the expression of an NKp44 ligand. Proc Natl Acad Sci U S A. 2005;102:10981–10986. doi: 10.1073/pnas.0504315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M, et al. Human natural killer cell receptors and co-receptors. Immunol Rev. 2001;181:203–214. doi: 10.1034/j.1600-065x.2001.1810117.x. [DOI] [PubMed] [Google Scholar]
- 29.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, et al. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 30.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 31.Caligiuri MA, Murray C, Robertson MJ, Wang E, Cochran K, Cameron C, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chehimi J, Starr SE, Frank I, Rengaraju M, Jackson SJ, Llanes C, et al. Natural killer (NK) cell stimulatory factor increases the cytotoxic activity of NK cells from both healthy donors and human immunodeficiency virus-infected patients. J Exp Med. 1992;175:789–796. doi: 10.1084/jem.175.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldman M, Howell D, Fitzgerald-Bocarsly P. Interferon-alpha-dependent and - independent participation of accessory cells in natural killer cell-mediated lysis of HSV-1-infected fibroblasts. J Leukoc Biol. 1992;52:473–482. doi: 10.1002/jlb.52.5.473. [DOI] [PubMed] [Google Scholar]
- 34.Nutt SL, Brady J, Hayakawa Y, Smyth MJ. Interleukin 21: a key player in lymphocyte maturation. Crit Rev Immunol. 2004;24:239–250. doi: 10.1615/critrevimmunol.v24.i4.20. [DOI] [PubMed] [Google Scholar]
- 35.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 36.Trinchieri G, Wysocka M, D'Andrea A, Rengaraju M, Aste-Amezaga M, Kubin M, et al. Natural killer cell stimulatory factor (NKSF) or interleukin-12 is a key regulator of immune response and inflammation. Progress in Growth Factor Research. 1992;4:355–368. doi: 10.1016/0955-2235(92)90016-b. [DOI] [PubMed] [Google Scholar]
- 37.Varchetta S, Oliviero B, Mavilio D, Mondelli MU. Different combinations of cytokines and activating receptor stimuli are required for human natural killer cell functional diversity. Cytokine. 2013;62:58–63. doi: 10.1016/j.cyto.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Waldmann T. The contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for the immunotherapy of rheumatological diseases. Arthritis Res. 2002;4(Suppl 3):S161–S167. doi: 10.1186/ar584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonaparte MI, Barker E. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood. 2004;104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 40.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, et al. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 2008;4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood. 2007;110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonaparte MI, Barker E. Inability of natural killer cells to destroy autologous HIV-infected T lymphocytes. Aids. 2003;17:487–494. doi: 10.1097/00002030-200303070-00003. [DOI] [PubMed] [Google Scholar]
- 43.Ward JP, Bonaparte MI, Barker E. HLA-C and HLA-E reduce antibody-dependent natural killer cell-mediated cytotoxicity of HIV-infected primary T cell blasts. Aids. 2004;18:1769–1779. doi: 10.1097/00002030-200409030-00005. [DOI] [PubMed] [Google Scholar]
- 44.Tomescu C, Chehimi J, Maino VC, Montaner LJ. NK Cell Lysis of HIV-1-Infected Autologous CD4 Primary T Cells: Requirement for IFN-Mediated NK Activation by Plasmacytoid Dendritic Cells. J Immunol. 2007;179:2097–2104. doi: 10.4049/jimmunol.179.4.2097. [DOI] [PubMed] [Google Scholar]
- 45.Chehimi J, Papasavvas E, Tomescu C, Gekonge B, Abdulhaqq S, Raymond A, et al. Inability of plasmacytoid dendritic cells to directly lyse HIV-infected autologous CD4+ T cells despite induction of tumor necrosis factor-related apoptosis-inducing ligand. J Virol. 2010;84:2762–2773. doi: 10.1128/JVI.01350-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Maria A, Fogli M, Costa P, Murdaca G, Puppo F, Mavilio D, et al. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30 and NKp44) Eur J Immunol. 2003;33:2410–2418. doi: 10.1002/eji.200324141. [DOI] [PubMed] [Google Scholar]
- 47.Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, Moretta L, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol. 2004;34:2313–2321. doi: 10.1002/eji.200425251. [DOI] [PubMed] [Google Scholar]
- 48.Fausther-Bovendo H, Sol-Foulon N, Candotti D, Agut H, Schwartz O, Debre P, Vieillard V. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS. 2009;23:1077–1087. doi: 10.1097/QAD.0b013e32832cb26b. [DOI] [PubMed] [Google Scholar]
- 49.Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, et al. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207:213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Papasavvas E, Chehimi J, Azzoni L, Pistilli M, Thiel B, Mackiewicz A, et al. Retention of functional DC-NK cross-talk following up to 18 weeks therapy interruptions in chronically suppressed HIV type 1+ subjects. AIDS Res Hum Retroviruses. 2015;26:1047–1049. doi: 10.1089/aid.2010.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomescu C, Liu Q, Ross BN, Yin X, Lynn K, Mounzer KC, et al. A correlate of HIV-1 control consisting of both innate and adaptive immune parameters best predicts viral load by multivariable analysis in HIV-1 infected viremic controllers and chronically-infected non-controllers. PLoS One. 2014;9:e103209. doi: 10.1371/journal.pone.0103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chehimi J, Azzoni L, Farabaugh M, Creer SA, Tomescu C, Hancock A, et al. Baseline Viral Load and Immune Activation Determine the Extent of Reconstitution of Innate Immune Effectors in HIV-1-Infected Subjects Undergoing Antiretroviral Treatment. J Immunol. 2007;179:2642–2650. doi: 10.4049/jimmunol.179.4.2642. [DOI] [PubMed] [Google Scholar]
- 53.Kuri-Cervantes L, de Oca GS, Avila-Rios S, Hernandez-Juan R, Reyes-Teran G. Activation of NK cells is associated with HIV-1 disease progression. J Leukoc Biol. 2014;96:7–16. doi: 10.1189/jlb.0913514. [DOI] [PubMed] [Google Scholar]
- 54.Mavilio D, Benjamin J, Daucher M, Lombardo G, Kottilil S, Planta MA, et al. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc Natl Acad Sci U S A. 2003;100:15011–15016. doi: 10.1073/pnas.2336091100. [DOI] [PMC free article] [PubMed] [Google Scholar]