Abstract
Purpose of review
It is our opinion that there is an unmet need in Hepatology for a minimally- or noninvasive test of liver function and physiology. Quantitative liver function tests (QLFTs) define the severity and prognosis of liver disease by measuring the clearance of substrates whose uptake or metabolism is dependent upon liver perfusion or hepatocyte function. Substrates with high affinity hepatic transporters exhibit high “first-pass” hepatic extraction and their clearance measures hepatic perfusion. In contrast, substrates metabolized by the liver have low first-pass extraction and their clearance measures specific drug metabolizing pathways.
Recent Findings
We highlight one QLFT, the dual cholate test, and introduce the concept of a disease severity index (DSI) linked to clinical outcome that quantifies the simultaneous processes of hepatocyte uptake, clearance from the systemic circulation, clearance from the portal circulation, and portal-systemic shunting.
Summary
It is our opinion that dual cholate is a relevant test for defining disease severity, monitoring the natural course of disease progression, and quantifying the response to therapy.
Keywords: Chronic liver disease, Quantitative liver function tests, Cholate, Disease Severity Index (DSI), Cirrhosis, Hepatic Fibrosis
Introduction
Hepatology needs an accurate, minimally- or non-invasive test of function of the whole liver. When a patient asks, “How much liver function do I have left?”, hepatologists reply with a confusing array of stage of fibrosis, blood tests, or liver stiffness. It is time for a change.
In this review we will focus on:
principles of quantitative liver function tests (QLFTs),
function - at the crossroads between fibrosis and clinical outcome,
quantifying the portal circulation,
a disease severity index (DSI) generated from the dual cholate test, and
potential clinical applications.
Caveats
None of the quantitative liver function tests described in this review are FDA-approved. Also, this paper should be viewed as our opinion and a position paper rather than an exhaustive review of all function tests. Although manuscripts are in preparation or submitted regarding dual cholate and DSI, most of the recent information that we discuss in this paper is from presentations at meetings or abstracts and not peer-reviewed manuscripts.
Principles of Quantitative Liver Function Tests (QLFTs)
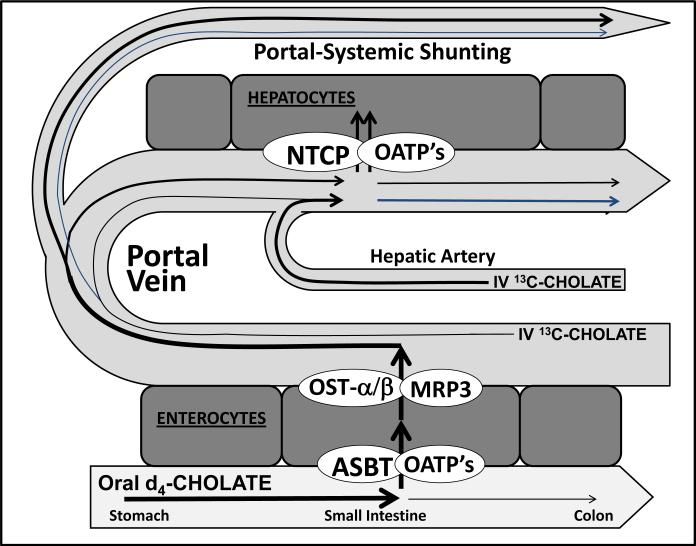
QLFTs measure liver function using liver-specific substrates that are cleared from blood by hepatic uptake, metabolism, or both1. The substrates are classified as exogenous/xenobiotic (examples include indocyanine green, propranolol, nitroglycerine, antipyrine, lidocaine, midazolam, erythromycin), natural (examples include caffeine, galactose, sorbitol), or endogenous (examples include bile acids such as cholic acid, amino acids, lipoproteins). Hepatic clearance of substrates is determined by: a) liver blood flow; b) efficiency of extraction by hepatocytes; and c) metabolism by hepatocytes. Depending on the substrate and route of administration, clearance may measure or estimate hepatic blood flow, hepatic metabolism, or both 2,3. Figure 1 displays clearance of cholate from portal and systemic circulations.
Figure 1. Delivery of intravenously and orally administered cholates to the liver for hepatic uptake.
Intravenously administered cholate is delivered to the liver via both the hepatic artery and portal vein. Orally administered cholate is absorbed via the apical membrane sodium dependent bile acid transporter (ASBT) of the enterocyte. Cholate exits the enterocyte via the organic solute transporter OST-a/OST-b, enters the portal circulation, binds to albumin, and is transported to the liver sinusoids. The sinusoidal uptake of both cholates into the hepatocytes occurs predominantly at the basolateral membrane of hepatocytes, mainly by the Na-dependent taurocholate co-transporter (NTCP) and by the Na-independent superfamily of organic anion transporting polypeptides (OATP). First-pass-clearance for cholic acid is approximately 80 to 90%.
Clearance is calculated from:
| Equation 1 |
where Dose is the amount of administered substrate and AUC (area under the curve) is the integral of the substrate's serum (plasma or blood) concentration versus time curve. Clearance and hepatic blood flow are related by:
| Equation 2 |
where HBF is hepatic blood flow and E is the first pass hepatic extraction of the substrate. When E=1, all of the substrate is cleared in a single pass through the liver and clearance equals HBF. If the substrate's volume of distribution is restricted to plasma volume, clearance must be adjusted by hematocrit to approximate HBF. An advantage of clearance methods is that they assess not only blood flow but also hepatic uptake or extraction efficiency; i.e., they measure effective hepatic perfusion and function.
Clearance of High Extraction Substrates
High extraction substrates are characterized by E>0.7, half lives of elimination measured in minutes, specific high-affinity hepatic transport systems, and ability to estimate hepatic blood flow 4. Common high-extraction substrates include galactose 5, propranolol, nitroglycerine, lidocaine 6, indocyanine green (ICG) 7, sorbitol 8, and bile acids 9.
Clearance of Low Extraction Substrates
Low extraction substrates are characterized by E <0.3, and half lives of elimination of several hours to days. The clearance of a low extraction substrate reflects hepatic metabolic capacity. Changes in liver perfusion would have little effect on the clearance of substrates with metabolism-dependent routes of elimination 10. Common substrates for measuring hepatic metabolism include phenylalanine, aminopyrine, phenacetin, methacetin, antipyrine, caffeine, diazepam, erythromycin, methionine, and α-ketoisocaproic acid 11-21.
Breath Tests
Metabolism of a substrate may also release volatile gas, such as CO2 ,which can be measured in exhaled breath. With 13C breath tests a 13C label is synthetically introduced at the substrate's site of enzyme activity, so that the rate of production of 13CO2 is related to the amount and activity of enzyme 16. Variations in the kinetics of CO2 may account for some variability in results, which may require administration of 13C-bicarbonate to quantify and adjust for variation in CO2 kinetics 17.
Dual Cholate Test
Cholate is an endogenous bile salt, synthesized in the liver from cholesterol, with a pool size of 1 to 3 g that is maintained by specific hepatic and intestinal transporters 3. Cholate has a high first-pass hepatic extraction (80 to 90%), is not metabolized, and can estimate liver blood flow and perfusion 22.
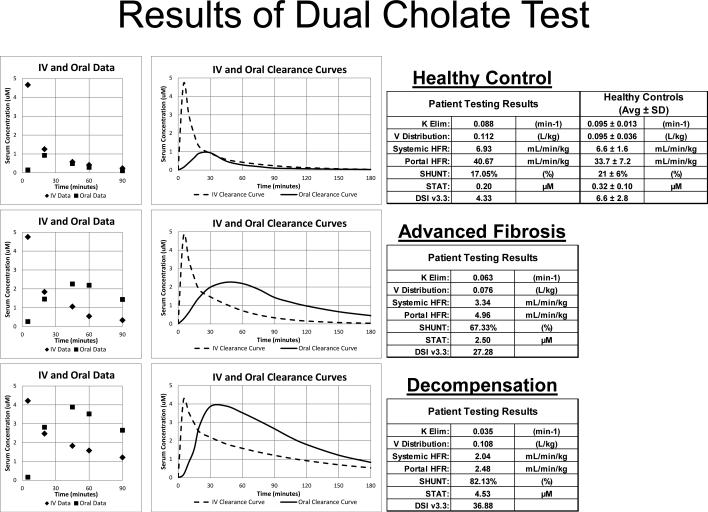
Cholates have been safely administered both orally and intravenously 1,9,23,24. In the dual test, [2,2,4,4-D]cholate (D4-cholate)is administered orally and, [24-13C]cholate (13C-cholate) is administered intravenously, simultaneously. Figure 2 gives examples of the dual cholate test in health and stages of disease. The area under the serum concentration versus time curve of D4-cholate quantifies clearance from the portal circulation (Portal hepatic filtration rate (HFR)), whereas the area under the serum concentration versus time curve of 13C-cholate quantifies clearance from the systemic circulation (Systemic HFR). The ratio of 13C (IV) to D4 (oral) cholate clearance estimates portal-systemic SHUNT 24 (Equation 3).
| Equation 3 |
This single test quantifies three key parameters of global liver function – clearance from the systemic circulation, clearance from the portal circulation, and portal-systemic shunting.
Figure 2. Individual examples of results from the Dual Cholate Test.
Three examples, a healthy control (DSI 4.33), a noncirrhotic patient by fibrosis measurement but with high DSI of 27.28 and clinical outcome, and a patient with clinical complications of cirrhosis (DSI 36.88), are shown. Serum concentrations of the administered cholates are shown in the left panels, modeled clearance curves in the middle panels, and reported results in the right panels.
Disease Severity Index (DSI)
Portal HFR, Systemic HFR, and SHUNT each bear a distinct relationship to fibrosis stage and clinical stages of cirrhosis. Modeling these test outputs for prediction of clinical outcomes produced a disease severity index (DSI) (Equation 4).
| Equation 4 |
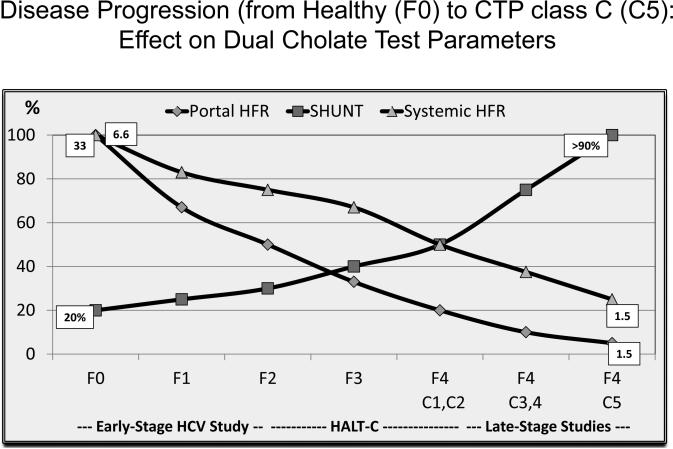
The coefficients A, B,and C and constant D were modeled from data from a long term study of patients with both early stage HCV25 and advanced fibrosis or compensated cirrhosis (Figure 3) 24,26. DSI quantifies global hepatic function and physiology and provides a direct link of DSI score to clinical outcome.
Figure 3. Model for changes in parameters of the dual cholate test from mild to end-stage liver disease.
Portal HFR, Systemic HFR, and SHUNT are plotted against the stage of fibrosis (METAVIR F0 to F4) and D'Amico clinical stages of cirrhosis (C1 to C5). This plot includes data from an early stage HCV study 25, the HALT-C trial 24-26, and a mixture of studies in clinically decompensated cirrhosis. Portal HFR declines early, SHUNT increases but more dramatically in cirrhotic stages, and Systemic HFR declines later, mainly in cirrhotic stages. The different relationships of Portal HFR, Systemic HFR, and SHUNT to stages of fibrosis and cirrhosis allowed modeling of these three parameters of the dual cholate test to generate the disease severity index (DSI).
F0 to F4 represent increasing stages of METAVIR fibrosis (Poynard et al., Lancet 1997).
C1 to C5 represent increasing stages of cirrhosis (D'Amico et al., J Hepatol 2006).
DSI range and cutoffs are similar in HCV disease, fatty liver disease, and cholestatic disease 25,27-31. DSI is linearly related to biopsy-defined fibrosis stage, and has cutoffs for predicting varices, and defining risk for future outcomes. Youden Index (J) defines a test's performance in relation to its optimal cutoff value. In predicting clinical outcomes, J score was 0.59 for DSI >19, versus 0.37 for Ishak Fibrosis score >F4. The latter J score of 0.37 is similar to the J score of 0.34 reported by Forestier and colleagues for liver stiffness measurement (LSM)32. These results suggest that function based on DSI may outperform fibrosis based on biopsy or LSM in prediction of risk for future clinical outcomes.
Function - at the Crossroads between Fibrosis and Clinical Outcomes
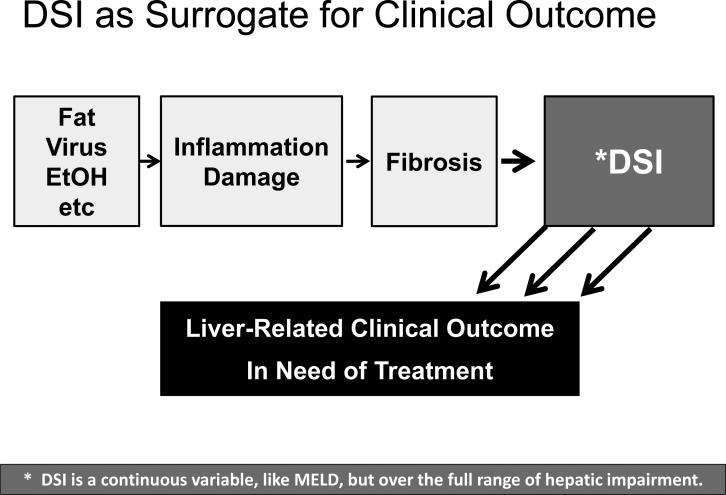
Chronic liver disease, regardless of etiology, involves injury and necrosis, inflammation, fibrosis accumulation, impairment of hepatic function, and, subsequently, clinical manifestations (Figure 4). Patients without hepatic impairment have a better prognosis compared to patients with hepatic impairment. In a long term longitudinal study of cases of chronic hepatitis C, functional impairment outperformed stage of fibrosis in predicting risk for future clinical outcomes14.
Figure 4. DSI is potentially a surrogate for clinical outcome.
A functional view of the progression of liver disease would hold that etiologic agents of disease initiate damage and injury, which is sequentially followed by inflammation, fibrosis, and hepatic impairment with concomitant alteration of the portal circulation. DSI encompasses changes in both hepatic function and the altered portal circulation and is a proximal surrogate for liver-related outcomes. Because DSI and HVPG both focus on changes to the portal circulation, DSI could be viewed as potentially a noninvasive alternative to HVPG. Unlike HVPG and MELD, which only measure impairment and track changes in advanced stages of disease, DSI measures hepatic impairment over the entire spectrum of disease from F0 to F4 and through clinical stages of cirrhosis.
In every organ functional impairment precedes the development of clinical manifestations, symptoms and complications. Nephrologists monitor creatinine clearance, cardiologists measure ejection fraction, and pulmonologists check pulmonary functions tests to define disease severity, measure progression, and assess treatments. Hepatologists need a sensitive and reliable test to measure global liver function and physiology.
Pitfalls of the Current Clinical and Laboratory Evaluation
History and physical examination, liver enzymes, standard blood tests, imaging, elastography, and liver biopsy are insensitive to early stages of liver disease 33. Blood levels of liver enzymes do not correlate with the severity of hepatic impairment – patients with very high ALT can have normal liver function, and patients with normal ALT can have clinical decompensation. Conventional liver function tests, such as bilirubin, albumin, and INR, are incorporated into clinical models (MELD and CTP) which have prognostic value in end-stage liver disease34. But MELD score and CTP score have only been validated in patients with cirrhosis, and are not useful for measuring severity or tracking progression in pre-cirrhotic stages of disease.
PROs and CONs of Measuring Fibrosis
Measuring fibrosis by liver biopsy has been a gold standard for evaluating the patient with chronic liver disease 35,36. However, liver biopsy is invasive, risky, anxiety-provoking, and subject to sampling error 37. For these reasons, noninvasive methods for estimating liver fibrosis, such as elastography or serologic fibrosis markers, or a combination of both, have begun to replace staging by liver biopsy. These noninvasive alternatives can distinguish patients with cirrhosis from those without cirrhosis, but are less able to categorize the earlier fibrotic stages or later clinical stages of disease 38-40.
PROs and CONs of Measuring HVPG
Portal pressure, as measured by hepatic venous pressure gradient (HVPG), is increasingly accepted as a surrogate for clinical outcome due to the linkage of portal hypertension to varices, ascites, and encephalopathy 41,42. However, HVPG is invasive, risky, anxiety-provoking, and can only be applied by experienced operators in specialized centers. A noninvasive alternative to HVPG is desirable. The dual cholate test is focused on the portal circulation and is potentially a noninvasive alternative to HVPG.
Quantifying the Portal Circulation
HVPG has been the traditional method for quantifying the portal circulation. But HVPG only measures pressure, is insensitive to changes occurring prior to cirrhosis, and is dampened by development of collateral vessels. Dual cholate assessed flow and hepatic uptake and can be applied over the full spectrum of alteration of the portal circulation.
Changes in the Portal Circulation at Early Stages of Disease
Despite progression of hepatic fibrosis from METAVIR F0 through F3 most patients lack signs or symptoms, standard laboratory tests are unremarkable, and HVPG is normal. Evolving pathophysiologic changes during these earlier stages of disease are not detected nor quantified! In contrast, the clearance of orally administered cholate (Portal HFR) declines by 50% or more from fibrosis stages F0 to F3 25,26. Based on the known relationships shown in Equation 5, the normal portal pressure and decreased Portal HFR likely reflect an increase in intrahepatic resistance.
| Equation 5 |
Necro-inflammation and activation of peri-sinusoidal stellate and endothelial cells in association with vasoactive cytokines account for the increased intrahepatic resistance to portal inflow 43. At this early stage of disease, cure of the underlying cause could reverse the changes in the portal circulation. This positive effect of successful treatment could be quantified by measuring Portal HFR, but not by the less sensitive measurements of fibrosis or portal pressure.
Changes in the Portal Circulation at Later Stages of Disease
As disease progresses fibrosis accumulates, hepatic resistance increases, hepatic perfusion is further compromised, and portal hypertension and portal-systemic collaterals develop 41. Nearly all complications of chronic liver disease (ascites, varices, encephalopathy) are due to these alterations of the portal circulation. At this stage HVPG correlates with risk for decompensation and mortality 42. But, HVPG is invasive and not embraced by patients. And, with emergence of portal-systemic collaterals the rise in HVPG is dampened. Surrogates for HVPG, such as imaging methods or liver stiffness, correlate with but do not directly measure or quantify the portal circulation or portal hypertension 44-50. Thus, there is need for a minimally-invasive technique to quantify the portal circulation even at late stages of disease. Unlike other tests, including other QLFTs, the dual cholate test is focused on the portal circulation and may satisfy this need.
Clinical Applications
The ability to measure functional and physiological changes over the full spectrum of disease has several advantages in characterizing disease severity, monitoring progression, or measuring therapeutic effects.
Defining and Monitoring Early Stage Disease
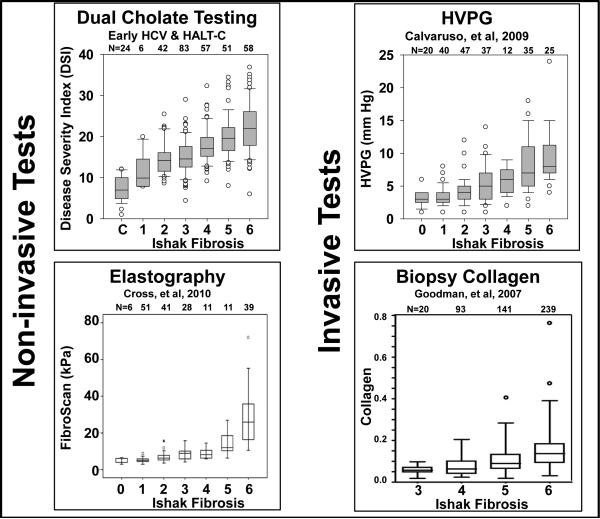
A goal of treatment is to prevent cirrhosis by treating patients at pre-cirrhotic stages of disease. However, every currently available method for measuring disease severity has inadequate accuracy in early stages of liver disease. Current methods are unable to monitor natural progression or quantify effectiveness of treatments or interventions. In contrast, DSI has correlated linearly with stage of fibrosis, from ISHAK fibrosis stages F0 to F6 27,28. A comparison of the relationships of DSI, transient elastography, HVPG, and quantitative collagen to Ishak fibrosis score is shown in Figure 5 27,28,51-54. Only DSI demonstrates stage to stage differentiation over the full range of Ishak fibrosis scores and most notably from Ishak F0 to F4. We conclude that dual cholate and DSI is uniquely able to monitor therapeutic effects in early-stage disease.
Figure 5. DSI compared to other proposed surrogates of clinical outcome.
DSI27,28, HVPG51, transient elastography52, and quantitative collagen from liver biopsy53,54 are plotted against Ishak fibrosis stage (data from 4 different studies). Sample sizes are shown at the top of each plot. DSI is linear over the full range of fibrosis and, compared to the other tests, is uniquely sensitive to earlier stages of fibrosis.
Steatosis
Chronic hepatitis C, nonalcoholic fatty liver disease, and alcoholic liver disease are associated with hepatic steatosis which can lead to inflammation and fibrosis. Unlike imaging and elastography, DSI is not influenced by steatosis (unpublished data, presented at AASLD Emerging Trends Conference on Antifibrotic Drug Trials: Strategies and Endpoints in Chicago in June 2014).
Late Stage Disease – Diagnosing Cirrhosis
Is there a need for a QLFT to diagnose cirrhosis? Transient elastography has been approved by the FDA and is the leading noninvasive alternative to biopsy for diagnosis of cirrhosis 39,47-50,55. But transient elastography (or magnetic resonance elastography) may not be accurate in patients with high BMI or hepatic steatosis. Elastography may also be influenced by hepatic inflammation or blood flow, and the equipment for test performance is concentrated in liver centers and otherwise not widely available. Other noninvasive alternatives, such as serum biomarkers or clinical and laboratory models, are less accurate.
The dual cholate test outperformed a battery of metabolic tests in predicting cirrhosis 14,24,26, and DSI>19 predicted cirrhosis and risk for future clinical outcomes 27,28. D4-cholate concentration >1μM at 60 minutes after oral dosing, is equivalent to DSI >19 27,28. A test requiring only one 60 minute serum sample could offer simplicity, wide-spread application, and cost savings over biopsy, elastography, and other noninvasive alternatives.
Late Stage Disease – Predicting Risk for Future Complications
The real issue is not whether a QLFT can diagnose cirrhosis, but whether function outperforms fibrosis in establishing prognosis. In the HALT-C cohort we found that the dual cholate test outperformed standard lab tests, Ishak fibrosis score, and other QLFTs in prediction of outcomes 14,24,26. Subsequently, we compared the performance of DSI against Ishak fibrosis score, platelet count, and MELD score first by examination of respective ROC curves. The optimum cutoffs were Ishak fibrosis score > F4, MELD > 6, and platelets <150 × 109/L. The performance of DSI in predicting risk for future outcomes was better than these other measurements27,28 .
Identifying the Patient with Varices
Esophageal varices are one of the more dangerous complications of liver disease. Detecting and treating the medium to large varices prior to their rupture and hemorrhage is desirable. In the HALT-C trial cholate shunt >35% or DSI>19 identified nearly all of the patients with medium- or large-sized varices 14,24,26, and DSI was more predictive than fibrosis stage 27,28 (Youden Index, J, was 0.51 for DSI versus 0.38 for Ishak fibrosis score).
Measuring Hepatic Improvement with HCV Treatment
Clearing HCV heals the liver. HCV RNA is undetectable within 1 to 4 weeks of treatment initiation with current multi-DAA regimens. In parallel, ALT normalizes and inflammation subsides. With sustained virologic response (SVR) hepatocyte functions improve – bilirubin drops, INR drops, and albumin increases. In contrast, resolving fibrosis and improving the portal circulation and portal hypertension are less certain effects. Fibrosis resolution, if and when it occurs, is a much slower process taking many months or years.
We measured the early effects at week 4 of HCV treatment in 31 patients who either had decompensated cirrhosis (Child-Pugh B and C) or were liver transplant recipients with and without cirrhosis 56. All patients had HCV RNA <LLOQ by Week 4 of treatment. Standard blood tests, MELD score, and CTP score did not improve. Although SHUNT did not change, HFRs and DSI improved. The significant changes in HFRs and DSI, without change in bilirubin, INR, albumin, MELD score, or CTP score, would be consistent with improvement in the hepatic microcirculation due to resolution of necroinflammation. These early responders can be identified on the basis of their baseline DSI.
The long-term impact of SVR on liver function and physiology was studied in the era of interferon-based therapy. QLFTs (caffeine, antipyrine, galactose, MEGX, dual cholate, SPECT LSS) were performed at baseline and at month 24 of followup in 24 patients after achieving SVR and in 68 nonresponders 57,58. In general, all of the functional tests improved with SVR but the most consistent and significant improvements were in DSI, SHUNT and Portal HFR. The magnitude of the improvement in the dual cholate test was similar to the magnitude of improvement in HVPG measured in a separate study 59.
Fulminant Hepatic Failure
Early recognition of prognosis in fulminant hepatic failure defines need for liver transplantation. ICG and methacetin clearance (LIMAX® test, Breath-ID ® test) are severely impaired in patients with acetaminophen or non-acetaminophen fulminant hepatic failure 60,61. However, there is not enough experience with these tests in this complex setting to currently endorse their widespread use.
Liver Transplantation
QLFTs may predict graft failure and development of complications 62-66, but it is not clear that QLFTs could improve recipient management or independently predict graft survival. QLFTs have quantified functional return in donors and recipients after living donor transplantation 67-69.
Conclusions and future perspectives
At the ‘AASLD Emerging Trends Conference on Antifibrotic Drug Trials: Strategies and Endpoints’ in Chicago in June 2014, it was suggested that the ideal test for defining liver disease severity, monitoring progression, and measuring the effectiveness of treatment should be:
Reproducible
Plausibly linked to pathogenesis of disease
Able to assess the whole organ
Minimally invasive and well tolerated
Able to measure effectively all stages of disease, especially early stages
Applicable to relevant populations
Operator independent
Applicable across centers.
In our opinion, the dual cholate test and DSI fulfill all of these criteria and deserve further consideration in the assessment of the patient with chronic liver disease.
Key Points.
Hepatology has an unmet need, a functional test that globally encompasses liver function and physiology that is also linked to clinical outcomes.
Quantitative liver function tests, particularly dual cholate and DSI, may fill this unmet need.
The dual cholate test outputs a disease severity index (DSI) that is linked to clinical outcomes.
DSI may be useful in defining disease severity, monitoring disease progression, and for measuring response to therapy.
Acknowledgements
The authors wish to acknowledge the following persons who participated in the studies of the dual cholate test and disease severity index (DSI): Shannon Lauriski, Andrea Herman RN, Jennifer DeSanto RN, Mitchell Shiffman MD, Timothy Morgan MD, John Hoefs MD, Richard Sterling MD, Teresa Curto, Ann Stoddard, Elizabeth Wright, Jacqueline O'Leary MD, James Trotter MD.
Footnotes
Financial Support and Sponsorship
None.
Conflict of Interest
Dr. Everson is the inventor of the dual cholate test and has filed patents related to this test with the University of Colorado Denver. He is founder, manager, and equity member of HepQuant LLC and the cholate tests are HepQuant-SHUNT, HepQuant-FLOW, and HepQuant-STAT. HepQuant® is a registered trademark of HepQuant LLC and its tests. Dr. Helmke and Dr Colmenero have no conflicts to report.
Contributor Information
Steve Helmke, University of Colorado Denver School of Medicine.
Jordi Colmenero, Liver Transplantation, Liver Unit Biomedical Research Institute August Pi I Sunyer (IDIBAPS) Hospital Clínic, Barcelona, Spain.
Gregory T. Everson, University of Colorado Denver School of Medicine.
Bibliography
- 1.Shrestha R, McKinley C, Showalter R, et al. Quantitative liver function tests define the functional severity of liver disease in early-stage cirrhosis. Liver Transpl Surg. 1997;3(2):166–73. doi: 10.1002/lt.500030210. [DOI] [PubMed] [Google Scholar]
- 2.Tozer MRaTN. Clinical Pharmacokinetics and Pharmacodynamics. 4th ed Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 3.Stieger B, Heger M, de Graaf W, Paumgartner G, van Gulik T. The emerging role of transport systems in liver function tests. Eur J Pharmacol. 2012;675(1-3):1–5. doi: 10.1016/j.ejphar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 4.Zeeh J, Lange H, Bosch J, et al. Steady-state extrarenal sorbitol clearance as a measure of hepatic plasma flow. Gastroenterology. 1988;95(3):749–59. doi: 10.1016/s0016-5085(88)80024-6. [DOI] [PubMed] [Google Scholar]
- 5.Henderson JM, Kutner MH, Bain RP. First-order clearance of plasma galactose: the effect of liver disease. Gastroenterology. 1982;83(5):1090–6. [PubMed] [Google Scholar]
- 6.Ercolani G, Grazi GL, Calliva R, et al. The lidocaine (MEGX) test as an index of hepatic function: its clinical usefulness in liver surgery. Surgery. 2000;127(4):464–71. doi: 10.1067/msy.2000.104743. [DOI] [PubMed] [Google Scholar]
- 7.Leevy CM, Bender J. Physiology of Dye Extraction by the Liver: Comparative Studies of Sulfobromophthalein and Indocyanine Green. Ann N Y Acad Sci. 1963;111:161–76. doi: 10.1111/j.1749-6632.1963.tb36956.x. [DOI] [PubMed] [Google Scholar]
- 8.Molino G, Cavanna A, Avagnina P, Ballare M, Torchio M. Hepatic clearance of D-sorbitol. Noninvasive test for evaluating functional liver plasma flow. Dig Dis Sci. 1987;32(7):753–8. doi: 10.1007/BF01296143. [DOI] [PubMed] [Google Scholar]
- 9.Gilmore IT, Thompson RP. Plasma clearance of oral and intravenous cholic acid in subjects with and without chronic liver disease. Gut. 1980;21(2):123–7. doi: 10.1136/gut.21.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vesell ES. Noninvasive assessment in vivo of hepatic drug metabolism in health and disease. Ann N Y Acad Sci. 1984;428:293–307. doi: 10.1111/j.1749-6632.1984.tb12305.x. [DOI] [PubMed] [Google Scholar]
- 11.Herold C, Ganslmayer M, Ocker M, et al. Inducibility of microsomal liver function may differentiate cirrhotic patients with maintained compared with severely compromised liver reserve. J Gastroenterol Hepatol. 2003;18(4):445–9. doi: 10.1046/j.1440-1746.2003.03007.x. [DOI] [PubMed] [Google Scholar]
- 12.Klinker H, Joeres R, Bomhard M, et al. [Lidocaine elimination and MEGX formation after oral lidocaine administration--a practicable test for assessment of quantitative liver function]. Z Gastroenterol. 1993;31(Suppl 2):52–5. [PubMed] [Google Scholar]
- 13.Kawasaki S, Sugiyama Y, Iga T, et al. Hepatic clearances of antipyrine, indocyanine green, and galactose in normal subjects and in patients with chronic liver diseases. Clin Pharmacol Ther. 1988;44(2):217–24. doi: 10.1038/clpt.1988.140. [DOI] [PubMed] [Google Scholar]
- 14*.Everson GT, Shiffman ML, Hoefs JC, et al. Quantitative liver function tests improve the prediction of clinical outcomes in chronic hepatitis C: results from the Hepatitis C Antiviral Long-term Treatment Against Cirrhosis Trial. Hepatology. 2012;55(4):1019–29. doi: 10.1002/hep.24752. [This study confirmed that the dual cholate test correlated with disease severity and could predict risk for future clinical outcomes. The data from this Trial was instrumental in development of the disease severity index (DSI)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalan R, Hayes PC. Review article: quantitative tests of liver function. Aliment Pharmacol Ther. 1995;9(3):263–70. doi: 10.1111/j.1365-2036.1995.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 16.Armuzzi A, Candelli M, Zocco MA, et al. Review article: breath testing for human liver function assessment. Aliment Pharmacol Ther. 2002;16(12):1977–96. doi: 10.1046/j.1365-2036.2002.01374.x. [DOI] [PubMed] [Google Scholar]
- 17.Holzhutter HG, Lock JF, Taheri P, Bulik S, Goede A, Stockmann M. Assessment of hepatic detoxification activity: proposal of an improved variant of the (13)c-methacetin breath test. PLoS One. 2013;8(8):e70780. doi: 10.1371/journal.pone.0070780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel G, Hofmann U, Heidemann H, Cosme J, Eichelbaum M. Antipyrine as a probe for human oxidative drug metabolism: identification of the cytochrome P450 enzymes catalyzing 4-hydroxyantipyrine, 3-hydroxymethylantipyrine, and norantipyrine formation. Clin Pharmacol Ther. 1996;59(6):613–23. doi: 10.1016/S0009-9236(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 19.Afolabi P, Wright M, Wootton SA, Jackson AA. Clinical utility of 13C-liver-function breath tests for assessment of hepatic function. Dig Dis Sci. 2013;58(1):33–41. doi: 10.1007/s10620-012-2340-z. [DOI] [PubMed] [Google Scholar]
- 20.Perri F, Marras RM, Ricciardi R, Quitadamo M, Andriulli A. 13C-breath tests in hepatology (cytosolic liver function). Eur Rev Med Pharmacol Sci. 2004;8(1):47–9. [PubMed] [Google Scholar]
- 21.Grattagliano I, Lauterburg BH, Palasciano G, Portincasa P. 13C-breath tests for clinical investigation of liver mitochondrial function. Eur J Clin Invest. 2010;40(9):843–50. doi: 10.1111/j.1365-2362.2010.02331.x. [DOI] [PubMed] [Google Scholar]
- 22.Dancygier H. Clinical Hepatology. Principles and practice of hepatobiliary diseases. Springer-Verlag; 2010. [Google Scholar]
- 23.Alrefai WA, Gill RK. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res. 2007;24(10):1803–23. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 24.Everson GT, Martucci MA, Shiffman ML, Sterling RK, Morgan TR, Hoefs JC. Portal-systemic shunting in patients with fibrosis or cirrhosis due to chronic hepatitis C: the minimal model for measuring cholate clearances and shunt. Aliment Pharmacol Ther. 2007;26(3):401–10. doi: 10.1111/j.1365-2036.2007.03389.x. [DOI] [PubMed] [Google Scholar]
- 25.Helmke SM, Kulig CC, Lauriski S, Herman A, Dudekula A, Everson GT. Significant alteration of the Portal Circulation in over half of the Chronic HCV Patients with Ishak Fibrosis Stage F0-F2. Hepatology. 2011;54:1328A–1329A. [Google Scholar]
- 26.Everson GT, Shiffman ML, Morgan TR, et al. The spectrum of hepatic functional impairment in compensated chronic hepatitis C: results from the Hepatitis C Anti-viral Long-term Treatment against Cirrhosis Trial. Aliment Pharmacol Ther. 2008;27(9):798–809. doi: 10.1111/j.1365-2036.2008.03639.x. [DOI] [PubMed] [Google Scholar]
- 27*.Helmke SM, DeSanto J, Herman A, Lauriski S, Everson GT. A Disease Severity Index Based on Dual Cholate Clearances and Shunt Outperforms Biopsy At Predicting Clinical Outcomes in Chronic Hepatitis C. Gastroenterology. 2013;144(5):S951–S952. [Methodology related to development of DSI and performance of DSI in patients with chronic hepatitis C.] [Google Scholar]
- 28.Helmke SM, DeSanto J, Herman A, Lauriski S, Everson GT. Alteration of the Portal Circulation across the Entire Spectrum of Fibrosis in Patients with Chronic Hepatitis C as Measured by Dual Cholate Clearances. Hepatology. 2012;56:678A–678A. [Google Scholar]
- 29.Helmke S, Wallack A, Herman A, Isberg H, Lauriski S, Everson G. A Disease Severity Index Based on Dual Cholate Clearances and Shunt Identifies Primary Sclerosing Cholangitis Waiting List Patients at Risk for Clinical Complications. American Journal of Transplantation. 2013;13:513–513. [Google Scholar]
- 30.Helmke SM, Wallack A, Herman A, Isberg H, Lauriski S, Everson GT. Slow, Moderate, and Rapid Progressors: Three Distinct Categories of Patients with Primary Sclerosing Cholangitis Detected by Functional Assessment using Cholate Testing. Hepatology. 2012;56:1133A–1133A. [Google Scholar]
- 31.Helmke SM, Wallack A, Herman A, et al. Cholate Testing Is Superior to MELD in Assessing Disease Severity in Patients with Primary Sclerosing Cholangitis. Liver Transplantation. 2013;19:S97–S98. [Google Scholar]
- 32.Forestier J, Dumortier J, Guillaud O, et al. Noninvasive diagnosis and prognosis of liver cirrhosis: a comparison of biological scores, elastometry, and metabolic liver function tests. Eur J Gastroenterol Hepatol. 2010;22(5):532–40. doi: 10.1097/MEG.0b013e3283343f58. [DOI] [PubMed] [Google Scholar]
- 33.Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48(Suppl 1):S68–92. doi: 10.1016/j.jhep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Kamath PS, Kim WR. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 35.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 37.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614–8. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 38*.Crespo G, Fernandez-Varo G, Marino Z, et al. ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57(2):281–7. doi: 10.1016/j.jhep.2012.03.016. [A comparison of elastography and biomarkers in correlating with stage of fibrosis] [DOI] [PubMed] [Google Scholar]
- 39.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–50. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 40.Banerjee R, Pavlides M, Tunnicliffe EM, et al. Multiparametric Magnetic Resonance for the noninvasive diagnosis of liver disease. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Pagan JC, Gracia-Sancho J, Bosch J. Functional aspects on the pathophysiology of portal hypertension in cirrhosis. J Hepatol. 2012;57(2):458–61. doi: 10.1016/j.jhep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Ripoll C, Groszmann R, Garcia-Tsao G, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–8. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 43*.Lee YA, Friedman SL. Reversal, maintenance, or progression: What happens to the liver after virologic cure of hepatitis C? Antiviral Research. 2014;107:23–30. doi: 10.1016/j.antiviral.2014.03.012. [A summary of what is known about fibrosis resolution after viral clearance and SVR] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berzigotti A, Reverter E, Garcia-Criado A, et al. Reliability of the estimation of total hepatic blood flow by Doppler ultrasound in patients with cirrhotic portal hypertension. J Hepatol. 2013;59(4):717–22. doi: 10.1016/j.jhep.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 45.Annet L, Materne R, Danse E, Jamart J, Horsmans Y, Van Beers BE. Hepatic flow parameters measured with MR imaging and Doppler US: correlations with degree of cirrhosis and portal hypertension. Radiology. 2003;229(2):409–14. doi: 10.1148/radiol.2292021128. [DOI] [PubMed] [Google Scholar]
- 46.Corpechot C, Gaouar F, El Naggar A, et al. Baseline values and changes in liver stiffness measured by transient elastography are associated with severity of fibrosis and outcomes of patients with primary sclerosing cholangitis. Gastroenterology. 2014;146(4):970–979. doi: 10.1053/j.gastro.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Poynard T, Vergniol J, Ngo Y, et al. Staging chronic hepatitis C in seven categories using fibrosis biomarker (FibroTest™) and transient elastography (FibroScan®). J Hepatol. 2014;60(4):706–14. doi: 10.1016/j.jhep.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Berzigotti A, Seijo S, Arena U, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology. 2013;144(1):102–111. doi: 10.1053/j.gastro.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Colecchia A, Montrone L, Scaioli E, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143(3):646–54. doi: 10.1053/j.gastro.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 50.Vizzutti F, Arena U, Romanelli RG, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45(5):1290–7. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 51.Calvaruso V, Burroughs AK, Standish R. Computer-Assisted Image Analysis of Liver Collagen: Relationship to Ishak Scoring and Hepatic Venous Pressure Gradient. Hepatology. 2009;49:1236–1244. doi: 10.1002/hep.22745. [DOI] [PubMed] [Google Scholar]
- 52.Cross TJS, Calvaruso V, Maimone S, et al. Prospective comparison of Fibroscan, King’s score and liver biopsy for the assessment of cirrhosis in chronic hepatitis C infection. Journal of Viral Hepatitis. 2010;17:546–554. doi: 10.1111/j.1365-2893.2009.01210.x. [DOI] [PubMed] [Google Scholar]
- 53.Goodman ZD, Stoddard AM, Bonkovsky HL, et al. Fibrosis progression in chronic hepatitis C: morphometric image analysis in the HALT-C trial. Hepatology. 2009;50:1738–49. doi: 10.1002/hep.23211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodman ZD, Becker RL, Jr, Pockros PJ, Afdhal NH. Progression of Fibrosis in Advanced Chronic Hepatitis C: Evaluation by Morphometric Image Analysis. Hepatology. 2007;45:886–894. doi: 10.1002/hep.21595. [DOI] [PubMed] [Google Scholar]
- 55*.Afdhal NH, Bacon BR, Patel K, et al. Accuracy of Fibroscan, Compared with Histology, in Analysis of Liver Fibrosis in Patients with Hepatitis B or C: A US Multi-center Study. Clin Gastroenterol Hepatol. 2014 Dec 17;:S1542–3565(14)01823-0. doi: 10.1016/j.cgh.2014.12.014. [doi: 10.1016/j.cgh.2014.12.014. [Epub ahead of print]. US data on Fibroscan quantification of fibrosis] [DOI] [PubMed] [Google Scholar]
- 56**.O'Leary JG, Burton JR, Helmke SM, et al. Early Improvement in the HepQuant (HQ)-SHUNT Function Test during Treatment with Ledipasvir/Sofosbuvir in Liver Transplant Recipients with Allograft Fibrosis or Cirrhosis and Patients with Decompensated Cirrhosis who have not undergone Transplantation. Hepatology. 2014;60:1134A. [Dual cholate was able to detect hepatic improvement within 4 weeks of initiation of ledipasvir/sofosbuvir in patients with advanced liver disease or post-transplant patients with wide spectrum of fibrosis and cirrhosis] [Google Scholar]
- 57.Everson GT, Shiffman ML, Hoefs JC, et al. Quantitative tests of liver function measure hepatic improvement after sustained virological response: results from the HALT-C trial. Aliment Pharmacol Ther. 2009;29(5):589–601. doi: 10.1111/j.1365-2036.2008.03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Helmke SM, Desanto JL, Herman A, Lauriski S, Everson GT. Noninvasive Cholate Testing Predicts Response to Peginterferon/Ribavirin and Measures Functional Improvement after Sustained Virological Response in Chronic Hepatitis C. J Hepatology. 2013;58:S290–291. [Dual cholate measures hepatic improvement after SVR] [Google Scholar]
- 59.Rincon D, Ripoll C, Iacono OL, et al. Antiviral Therapy Decreases Hepatic Venous Pressure Gradient in Patients with Chronic Hepatitis C and Advanced Fibrosis. Am J Gastroenterol. 2006;101:2269–74. doi: 10.1111/j.1572-0241.2006.00743.x. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt LE, Ott P, Tygstrup N. Galactose elimination capacity as a prognostic marker in patients with severe acetaminophen-induced hepatotoxicity: 10 years' experience. Clin Gastroenterol Hepatol. 2004;2(5):418–24. doi: 10.1016/s1542-3565(04)00128-4. [DOI] [PubMed] [Google Scholar]
- 61.Merle U, Sieg O, Stremmel W, Encke J, Eisenbach C. Sensitivity and specificity of plasma disappearance rate of indocyanine green as a prognostic indicator in acute liver failure. BMC Gastroenterol. 2009;9:91. doi: 10.1186/1471-230X-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potter JM, Hickman PE, Lynch SV, et al. Use of monoethylglycinexylidide as a liver function test in the liver transplant recipient. Transplantation. 1993;56(6):1385–8. doi: 10.1097/00007890-199312000-00021. [DOI] [PubMed] [Google Scholar]
- 63.Schutz E, Luy-Kaltefleiter M, Kaltefleiter M, et al. The value of serial determination of MEGX and hyaluronic acid early after orthotopic liver transplantation. Eur J Clin Invest. 1996;26(10):907–16. doi: 10.1111/j.1365-2362.1996.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt LE, Rasmussen A, Kirkegaard P, Dalhoff K. Relationship between postoperative erythromycin breath test and early morbidity in liver transplant recipients. Transplantation. 2003;76(2):358–63. doi: 10.1097/01.TP.0000076626.46866.E7. [DOI] [PubMed] [Google Scholar]
- 65.Levesque E, Saliba F, Benhamida S, et al. Plasma disappearance rate of indocyanine green: a tool to evaluate early graft outcome after liver transplantation. Liver Transpl. 2009;15(10):1358–64. doi: 10.1002/lt.21805. [DOI] [PubMed] [Google Scholar]
- 66.Olmedilla L, Pérez-Peña JM, Ripoll C, et al. Early noninvasive measurement of the indocyanine green plasma disappearance rate accurately predicts early graft dysfunction and mortality after deceased donor liver transplantation. Liver Transpl. 2009;15(10):1247–53. doi: 10.1002/lt.21841. [DOI] [PubMed] [Google Scholar]
- 67.Jochum C, Beste M, Penndorf V, et al. Quantitative liver function tests in donors and recipients of living donor liver transplantation. Liver Transpl. 2006;12(4):544–9. doi: 10.1002/lt.20627. [DOI] [PubMed] [Google Scholar]
- 68.Hori T, Iida T, Yagi S, et al. K(ICG) value, a reliable real-time estimator of graft function, accurately predicts outcomes in adult living-donor liver transplantation. Liver Transpl. 2006;12(4):605–13. doi: 10.1002/lt.20713. [DOI] [PubMed] [Google Scholar]
- 69*.Everson GT, Hoefs JC, Niemann CU, et al. Functional elements associated with hepatic regeneration in living donors after right hepatic lobectomy. Liver Transpl. 2013;19(3):292–304. doi: 10.1002/lt.23592. [Dual cholate measures return of function after right lobectomy in liver donors] [DOI] [PMC free article] [PubMed] [Google Scholar]