Abstract
Objective. To compare the 24-month course of health-related quality of life (HRQoL) in patients with long-standing RA treated with a conventional synthetic (cs) or a first, second or third biologic (b) DMARD in daily rheumatological care.
Methods. Patients enrolled in the German biologics register RABBIT who were observed over at least 12 months were stratified according to the nth bDMARD started at enrolment. HRQoL was captured by the SF36 health survey. Within strata of sequential bDMARD therapy, we examined patients’ HRQoL at baseline and at follow-ups in comparison with the general population, the 24-month course of HRQoL of different bDMARDs and the proportion of patients exceeding the minimal detectable improvement of physical and mental health sum scores.
Results. All patients reported remarkably lower scores of physical and mental health than the general population at baseline and month 12. In each stratum of sequential bDMARD therapy, patients improved significantly by month 12 and remained stable until month 24. The improvement of HRQoL was not attributable to a particular bDMARD. The following proportions of patients exceeded the minimal detectable improvement of at least 17.85 Physical Component Scale scores or 22.18 Mental Component Scale score points: csDMARD (n = 1113) 31.1%/22.3%, first bDMARD (n = 1352) 39.9%/29.7%, second bDMARD (n = 730) 37.3%/26.2% and third bDMARD (n = 680) 34.2%/30.9%.
Conclusion. Lasting improvement of both physical and mental health is achievable even for severely affected RA patients with a history of more than one bDMARD failure. Nevertheless, impairment of HRQoL in RA patients is enormous compared with the general population.
Keywords: health-related quality of life, rheumatoid arthritis, biologics register, minimal detectable change
Rheumatology key messages
Compared with the general population, health-related quality of life in RA patients is still enormously impaired.
DMARD therapy significantly improves health-related quality of life, even in RA patients with multiple treatment failures.
RA patients’ clinical characteristics are more relevant predictors of response to treatment than the particular DMARD.
Introduction
RA, which affects ∼1% of the adult population worldwide [1], is characterized by painful joint inflammation and potential joint damage in the advanced stage of disease; extra-articular manifestations, drug-induced adverse events, and distinct co-morbidities, such as cardiovascular disease, frequently complicate the disease. Consequently, health-related quality of life (HRQoL) as indicated by physical, mental and social functioning was shown to be significantly reduced in RA patients compared with the respective general populations [2–5].
Based on clinical signs and symptoms, standardized measures of RA disease activity and treatment response have been, for example, the composite DAS in 28 joints (DAS28) or the ACR response. Additionally, HRQoL was recommended to be used as an important patient-reported outcome measure of RA in clinical trials [6], and has been increasingly requested by regulating agencies, such as the National Institute of Health and Care Excellence (NICE) (UK) and Institute for Quality and Efficiency in Healthcare (IQWiG) (Germany), for health technology assessments, which may have great impact on national health-care expenditure. The preferred instrument has been the Medical Outcomes Study 36-Item Short-Form Health Survey (SF36), because it is validated for RA [7] and cross-culturally translated [8, 9]. It measures four subscales of physical and mental health, respectively; each subscale scores from 0 to 100, with higher scores indicating better HRQoL [10].
In randomized placebo-controlled trials (RCTs), notable improvement of HRQoL was found in patients with active RA treated with biologic (b) DMARDs [11–23]. RCTs comparing the different agents regarding their impact on HRQoL are lacking. Moreover, a large portion of RA patients treated in daily care are not eligible for enrolment in RCTs [24]. Patients who are not eligible for traditional RCTs have more co-morbid conditions and poorer functional status, both of which have a significant impact on HRQoL. Furthermore, considering the characteristics of patients with no, one or at least two bDMARD failures, we hypothesized that these patient/treatment groups also have different HRQoL outcomes. We aimed to describe the impairment of patients with RA compared with the general population in eight dimensions of quality of life (SF36) and, furthermore, to compare four different treatment groups regarding HRQoL, with RA patients stratified by the number of bDMARD failures. Maintaining this stratification into treatment groups, we examined the effects of individual biologic agents on HRQoL and the portions of patients who achieved a clinically relevant minimal detectable improvement (MDI).
Methods
Study design and patients
The German biologics register RABBIT is an ongoing prospective, observational cohort study on long-term safety and effectiveness of bDMARDs and conventional synthetic (cs) DMARD treatment in RA patients in daily rheumatological care. Details of the RABBIT study design are published elsewhere [25]. Briefly, patients of at least 18 years of age meeting the 1987 ACR criteria for RA are eligible for enrolment at the start of treatment with one of the licensed bDMARDs or a csDMARD (control group) after failure of at least one DMARD. They are observed for up to 10 years. Prior to enrolment, all patients have to give their informed consent. The study protocol of RABBIT was approved by the ethics committee of the Charité-Universitätsmedizin Berlin.
The SF36 health survey on patient-reported HRQoL was introduced in RABBIT in 2007 and also approved by the ethics committee of the Charité-Universitätsmedizin Berlin. Patients were eligible for the present analysis if they were enrolled from 1 January 2007 onwards and had a minimal observation time of 12 months by 30 July 2012. Patients enrolled with a csDMARD who had been treated with a bDMARD prior to enrolment were excluded from this analysis. All publications from the RABBIT register are approved by the ethics committee of the Charité-Universitätsmedizin Berlin.
Assessments
After baseline assessment, follow-up visits were at months 3 and 6, and then every 6 months; the first 24 months of observation were used for the present analysis. Patients were extensively monitored, particularly covering all treatment changes; at each visit, physicians assessed the clinical status and reported treatment details, including start and stop dates of DMARDs. The composite DAS28 was calculated using ESRs. Co-morbid conditions were documented at baseline.
Limitations in activities of daily life were assessed by the Hannover Functional Status Questionnaire, which measures functional capacity as a percentage of full function and is comparable to the HAQ [26]. At baseline and at months 12 and 24, HRQoL was captured by the SF36 health survey. This survey measures physical and mental health on eight subscales, which are summed up in two scales, the physical and mental component scale (PCS and MCS). Each scale comprises four dimensions scored from 0 to 100, with higher scores indicating better HRQoL [27]. We calculated summary scores of the PCS and MCS, as well as single dimension scores following the manual instructions (SF-36v2 Health Survey, Version 2 [10, 27]).
Strata of sequential bDMARD therapy
We stratified patients according to the number of bDMARD failures. The groups consist of patients who were enrolled at the start of a first bDMARD, a second bDMARD or at least a third bDMARD. Patients in the control cohort (csDMARD) started a new csDMARD after at least one csDMARD failure at enrolment and were biologic naive.
Definition of the main outcomes
To contrast the HRQoL of our RA patients with that of the general population, we used recently published SF36 data of a representative sample of the German population collected between 2008 and 2011 [28, 29]. We compared the subscales of physical and mental health reported for the German population with those of our age- and sex-adjusted patients at baseline and month 12. Data are presented as web charts, separately for each stratum of sequential DMARD therapy. The courses of the SF36 PCS and MCS summary scores from baseline to months 12 and 24, respectively, were assessed for each stratum of sequential bDMARD therapy as well as for each particular bDMARD group within the respective therapy strata.
We used the PCS and MCS summary scores to determine the proportion of patients of each stratum who improved beyond the MDI at months 12 and 24 of observation, respectively. The MDI is defined to indicate an improvement from baseline that is not caused by random variation or explainable by measurement errors of the instrument [30, 31]. Patients who stated at baseline a PCS or MCS summary score in a range of 100% minus the respective MDI value were per se not able to achieve the MDI; these patients were not included in this analysis.
Statistical analysis
The course of the SF36 PCS and MCS summary scores were examined by linear mixed effects models. We applied this class of model to yield unbiased estimates in the presence of missing data in the longitudinal response variable [32]. A preferable attribute of these models is that observations with partly missing data in the response are not discarded from analysis; the observations do still contribute to the estimation of the overall mean response. To examine the influence of the respective therapy stratum on the mean course of the PCS summary score over time, the model was adjusted for sex, age at baseline, the therapy stratum, the observation time and an interaction term of time and the therapy stratum. In subanalyses, we evaluated the effect of the particular bDMARDs on the summary scores, stratified for each stratum of first, second or third bDMARD therapy. In this model, we adjusted for age and sex, for the baseline status of the PCS score and the particular bDMARD. The stratified analysis ensured that stratum-specific differences in the baseline values of the summary scores were retained. We performed an analogous approach for the MCS summary scores. Respective mean PCS and MCS values for the 24-month observation period are presented as least-squares means with 95% CIs.
Published data on test–retest correlations (ρ) [10] were used to calculate the MDI for the physical and mental health scales according to the formula MDI = [33]. The s.d. was empirically estimated from data of our RABBIT patients. Statistical Analysis System (SAS) software (version 9.3; SAS Institute GmbH, Heidelberg, Germany) was used for computations. P < 0.05 were considered statistically significant.
Results
Patients’ characteristics at baseline
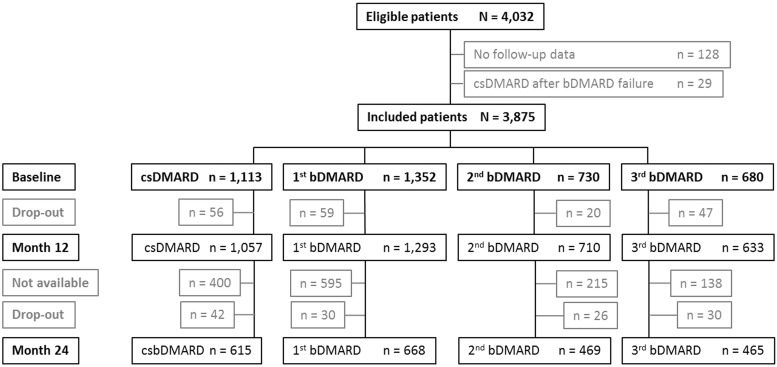
Data relating to 3875 patients with long-standing RA were included in the present analysis; about a third of all patients were enrolled after at least one bDMARD failure (Fig. 1). At baseline, strata of sequential bDMARD therapy reflected patients’ disease severity at study entry (Table 1). Taking patients treated with a first bDMARD at enrolment as reference, the strongest differences were found against biologic-naive patients or against patients receiving a third bDMARD at enrolment. Significant differences were observed for disease characteristics such as disease duration, DAS28, functional status Hannover Functional Status Questionnaire, pain, fatigue and the SF36 PCS and MCS sum scores. Patients receiving a third bDMARD at enrolment were younger and had suffered longest from RA; in any of the considered disease characteristics, their status was most impaired.
Fig. 1.
RABBIT patients included in the present analysis
bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; not available: patients with a regular observation time of <24 months.
Table 1.
Baseline characteristics of RA patients stratified by sequential DMARD therapy
| Characteristic | csDMARD | First bDMARD | Second bDMARD | Third bDMARD |
|---|---|---|---|---|
| n | 1113 | 1352 | 730 | 680 |
| Age, mean (s.d.), years | 58.5 (12.8) | 57.2 (12.5) | 57.0 (12.6) ns | 56.0 (12.7) |
| Female, n (%) | 831 (74.7) | 1,010 (74.7) | 571 (78.2) ns | 543 (79.9) |
| Disease duration, mean (s.d.), years | 6.2 (7.1) | 9.6 (8.7) | 12.6 (9.4) | 14.7 (9.3) |
| RF, positive, n (%) | 620 (55.7) | 985 (73.7) | 574 (79.4) | 524 (77.3) ns |
| Co-morbidities, n (%) | ||||
| 0 co-morbidity | 360 (32.3) | 414 (30.6) | 186 (25.5) | 165 (24.3) |
| 1 co-morbidity | 313 (28.1) | 359 (26.6) | 160 (21.9) | 179 (26.3) |
| 2 co-morbidities | 233 (20.9) | 267 (19.7) | 160 (21.9) | 127 (18.7) |
| ≥3 co-morbidities | 207 (18.6) | 312 (23.1) | 224 (30.7) | 209 (30.7) |
| Depression | 70 (6.3) | 77 (5.7) | 40 (5.5) | 45 (6.6) |
| Disease activity, mean (s.d.), DAS28 | 4.5 (1.3) | 5.2 (1.3) | 5.2 (1.4) ns | 5.4 (1.3) |
| Percentage of full functional status, mean (s.d.), FFbH | 72.3 (21.5) | 65.0 (23.3) | 59.9 (23.2) | 54.5 (23.1) |
| Patient-reported NRS, mean (s.d.), 0–10, 0 best | ||||
| Pain severity | 5.4 (2.4) | 5.9 (2.3) | 6.0 (2.2) ns | 6.4 (2.2) |
| Fatigue severity | 4.6 (2.7) | 5.2 (2.7) | 5.3 (2.6) | 5.7 (2.6) |
| Global health state | 5.3 (2.1) | 5.9 (2.0) | 5.9 (2.1) ns | 6.3 (2.0) |
| SF36 summary scores | ||||
| PCS, mean (s.d.) | 43.3 (22.1) | 35.2 (19.5) | 33.7 (19) ns | 30.5 (18.2) |
| MCS, mean (s.d.) | 57.3 (23.6) | 51.6 (22.8) | 51.6 (22.6) ns | 49.4 (22.5) |
| Number of previous DMARDs, mean (s.d.) | 1.4 (0.7) | 2.6 (1.0) | 2.8 (1.2) | 3.1 (1.3) |
csDMARD: conventional synthetic DMARD; bDMARD: biologic DMARD; FFbH: Hannover Functional Status Questionnaire; NRS: numeric rating scale; PCS: physical component scale; MCS: mental component scale; ns: not significant.
In the stratum of the first bDMARD, 83.0% of the patients received TNF inhibitors, 8.1% tocilizumab and 7.7% rituximab, respectively. The most frequent second bDMARD was a second TNF inhibitor (38.1%), followed by rituximab (34.8%) and tocilizumab (19.3%). Rituximab (49.4%), tocilizumab (21.8%) and abatacept (18.1%) were the most frequently used substances in the third bDMARD stratum.
HRQoL of RA patients in the context of the German general population
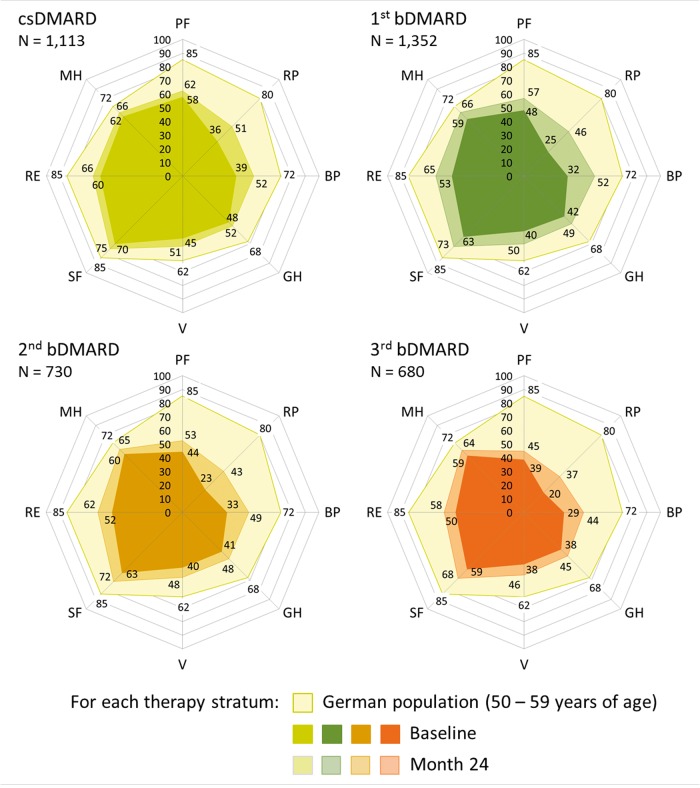
At baseline, all RA patients showed a severely impaired status of HRQoL, especially in dimensions of physical but also of mental health compared with the general population; furthermore, the degree of impairment increased with each step towards a further bDMARD started at enrolment (Fig. 2). At month 12, an improvement was found in all therapy strata throughout all subscales of HRQoL, with the greatest extent in role physical and bodily pain of the physical component scale. However, scores of both physical and mental health remained significantly diminished in all treatment groups compared with the general population, even after 12 months of treatment, irrespective of the history of DMARD therapy (Fig. 2).
Fig. 2.
Health-related quality of life of RA patients in context with the German general population
bDMARD: biologic DMARD; BP: bodily pain; csDMARD: conventional synthetic DMARD; GH: global health; MH: mental health; PF: physical functioning; RE: role emotional; RP: role physical; SF: social functioning; V: vitality.
The 24-month course of HRQoL and the impact of particular bDMARDs on this course
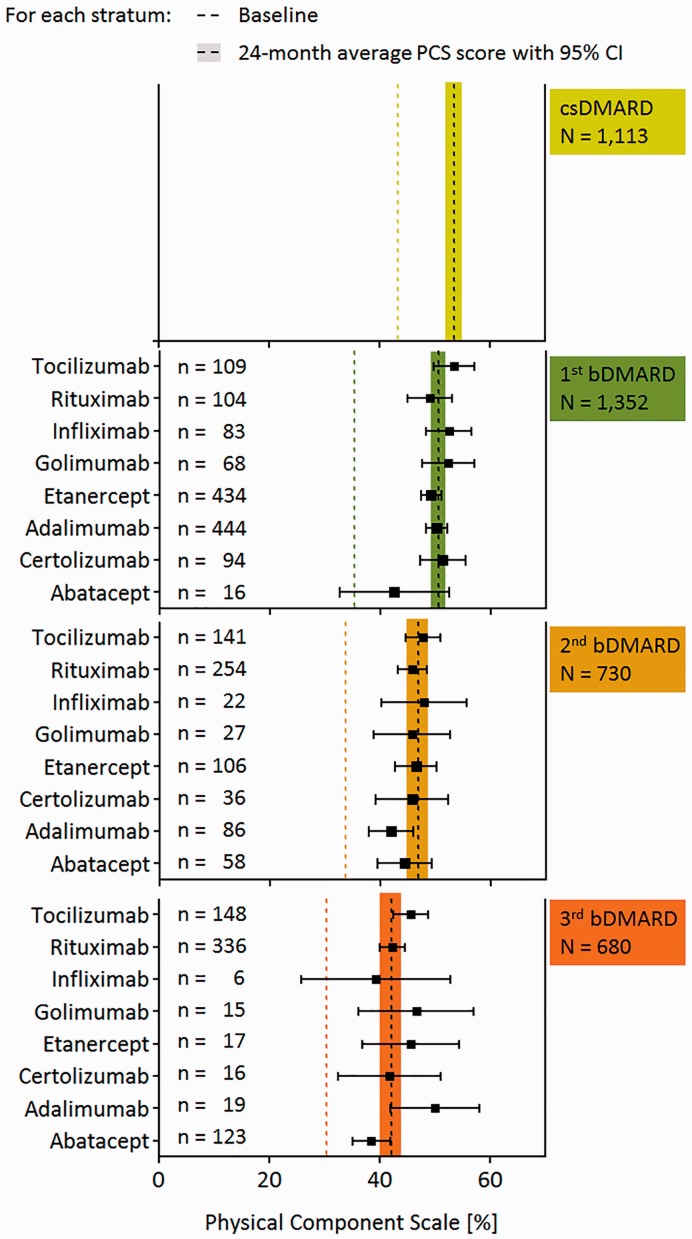
The 24-month course of HRQoL was examined using the summary scores of the PCS and MCS. In all therapy strata, improvement of both the mean PCS and MCS summary scores between baseline and month 12 was statistically significant (P < 0.05). We found a positive but insignificant time trend between months 12 and 24, indicating that the improved scores at month 12 were maintained during the second year of observation (data not shown); therefore, we present the mean scores of the PCS scales of months 12 and 24 for each therapy stratum as an average score (average 24-month score; Fig. 3). Within each therapy stratum, we did not find any significant differences between the average 24-month PCS score and the 24-month PCS scores of the particular bDMARDs, demonstrating that there is no significant effect of any particular bDMARD on the course of physical health.
Fig. 3.
Average 24-month scores of the physical component scale of each stratum of sequential DMARD therapy
bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; PCS: physical component scale.
Respective average 24-month scores of the MCS were [least square means (95% CI)] 63.8 (62.4, 65.2) for the csDMARD group, 63.0 (61.8, 64.3) for the first bDMARD group, 60.3 (58.6, 62.0) for the second bDMARD group and 59.4 (57.6, 61.2) for the third bDMARD group. As shown for physical health, there was no significant effect of any particular bDMARD on the course of mental health within each therapy stratum.
Minimal detectable improvements
The MDIs for the present study population were 17.95 PCS score points and 22.18 MCS score points. As expected, the frequency of long-standing RA patients who stated good physical health and were not able to exceed the MDI per se was low; this frequency decreased further with proceeding sequential bDMARD therapies (nceiling; Table 2).
Table 2.
Proportion of RA patients of each stratum of sequential DMARD therapy exceeding the minimal detectable improvement of the SF36 physical and mental component scales
| csDMARD | First bDMARD | Second bDMARD | Third bDMARD | ||
|---|---|---|---|---|---|
| nmonth12 | 1113 | 1352 | 730 | 680 | |
| nmonth24 | 713 | 757 | 515 | 542 | |
| Physical component scale | |||||
| Month 12 | nceiling (%) | 62 (5.6) | 33 (2.4) | 14 (1.9) | 9 (1.3) |
| nMDI (%) | 330 (31.4) | 509 (38.6) | 247 (34.5) | 207 (30.9) | |
| Month 24 | nceiling (%) | 41 (5.8) | 18 (2.4) | 8 (1.6) | 7 (1.3) |
| nMDI (%) | 209 (31.1) | 295 (39.9) | 189 (37.3) | 183 (34.2) | |
| Mental component scale | |||||
| Month 12 | nceiling (%) | 267 (24) | 226 (16.7) | 116 (15.9) | 88 (12.9) |
| nMDI (%) | 201 (23.8) | 349 (31) | 163 (26.6) | 168 (28.4) | |
| Month 24 | nceiling (%) | 166 (23.3) | 137 (18.1) | 76 (14.8) | 70 (12.9) |
| nMDI (%) | 122 (22.3) | 184 (29.7) | 115 (26.2) | 146 (30.9) | |
bDMARD: biologic DMARD; csDMARD: conventional synthetic DMARD; MDI: minimal detectable improvement; nceiling: patients excluded from the analysis because they had a physical component scale > 82.05 or a mental component scale > 77.82, respectively, and were for that reason not able to exceed the MDI; nMDI (%): percentages refer only to the number of patients included in this analysis (n − nceiling).
In each stratum of sequential DMARD therapy, >30% of patients exceeded the MDI of the PCS and >20% exceeded the MDI of the MCS. There were no significant differences between proportions of patients exceeding the MDIs after 12 and 24 months of observation within each therapy stratum. At month 12, the MDI of the PCS was exceeded by significantly more patients treated with the first bDMARD than by patients treated with csDMARDs or at least the third bDMARD; with the first bDMARD stratum as referent, the respective odds ratios were as follows: csDMARD 0.73 (95% CI 0.61, 0.86), second bDMARD 0.84 (95% CI 0.69, 1.01), third bDMARD 0.71 (95% CI 0.58, 0.87). Likewise, the MDI of the MCS was exceeded by significantly more patients treated with a first bDMARD than by patients treated with csDMARDs [odds ratio (OR) 0.65; 95% CI 0.54, 0.80) or with a second bDMARD (OR 0.79; 95% CI 0.64, 0.98). At month 24, ORs of the PCS and MCS of the csDMARD stratum remained significant (odds ratio: 0.67; 95% CI 0.52, 0.87). After adjustment for baseline differences, the proportion of patients of the csDMARD and first bDMARD stratum exceeding the MDI of the PCS were not significantly different any more.
Discussion
The present analysis provides novel data concerning the effectiveness of today’s DMARD therapy on HRQoL in an unselected population of patients with long-standing RA. We found for all of our therapy strata significant improvement of HRQoL at month 12, lasting throughout the second year of observation. Despite this improvement, RA patients were still markedly impaired in their HRQoL in comparison with the general population.
Data from RCTs demonstrated better improvement of HRQoL in patients treated with one of the licensed bDMARDs compared with the respective placebo group [11–23]. However, patients with more than one bDMARD failure have usually been excluded from these strictly designed studies even though they represent a key population in daily rheumatological care [24]. Here, we showed that RA severity characterized by, for example, higher disease activity, lower functional status and more co-morbid conditions, was associated with single or multiple bDMARD treatment failures. Both this tendency and the preference for particular bDMARDs at each step of sequential bDMARD therapy make head-to-head comparisons of bDMARD treatment difficult. The attainable change for the better of HRQoL was most probably determined by the particular step on the ladder of sequential DMARD therapy, that is, the respective stratum of sequential bDMARD therapy, rather than by the particular biologic agent.
Up to now, direct comparisons of data on HRQoL between patients treated with biologic agents in daily rheumatological care and the respective general population are sparse. Similar to data from a Norwegian survey among RA patients [5], we found that our patients with long-standing RA were markedly impaired in comparison with the German general population, not only in all dimensions of physical health scores, but also in those of the mental health scores; minimal scores were found for the dimensions role emotional and vitality at baseline. At months 12 and 24, physical as well as mental health scores were significantly and clinically relevantly improved in our patients, including those patients with more than one bDMARD failure. However, even in biologic-naive patients treated with at least a second csDMARD, HRQoL remained remarkably reduced in comparison with the general population. In this context, concerns may emerge that the impaired status of HRQoL in csDMARD patients marks the upper bound of a maximal treatment effect in RA patients; this is even more understandable when co-morbid conditions of these patients are considered. Compared with a representative subsample of the German population aged between 50 and 60 years [34], we found that our RABBIT patients aged 50–60 years suffered about twice as often from diabetes mellitus (4.7 vs 9.4%), eight times as often from hypertension (4.7 vs 39.5%) and almost four times as often from osteoporosis (4.0 vs 15.4%) [34]. Qualifying the impairment contributed by co-morbidities, compared with patients of our cohort without any co-morbid condition, RABBIT patients suffering from one of these co-morbidities had on average a 5.0 points (95% CI 3.7, 6.3) lower PCS during the first 12 months of observation, while patients suffering from hypertension, osteoporosis and diabetes had on average a 14.5 points (95% CI 8.8, 20.2) lower PCS (data not shown). Furthermore, the number of patients not achieving MDI increases with the number of prevalent co-morbidities (0/1/≥2 co-morbidities: 61.9%/65.8%/67.7%; trend test: P < 0.01). In particular, patients in the second and third bDMARD therapy stratum were affected by more than two co-morbid conditions. This strengthens the idea of HRQoL as an integral measure of disease burden, and its impairment in RA patients is most likely to result from the orchestration of a multi-morbid health state of patients in daily rheumatological care. The treatment target of DMARDs is the disease activity of the RA, but they are not able to improve clinical signs and symptoms of diabetes, hypertension or other co-morbid conditions if already present. In this view, patients with early RA who are obviously at high risk of developing cardiovascular and other co-morbid conditions associated with disease activity should become a particular target population for means of prevention. Concerning limitations, our approach of stratifying patients according to the nth bDMARD used at enrolment did not differentiate between patients receiving a third TNF-α inhibitor and patients who started on B cell depletion after failures of one TNF-α inhibitor and an inhibitor of either IL-6 or T cell co-stimulation.
At the cohort level, improvement of absolute scores of HRQoL after 12 or 24 months of observation was better in patients of the RABBIT cohort enrolled with a first bDMARD than in RCT patients treated with a specific bDMARD [13, 17]. We attribute this difference partly to the method of last-observation-carried-forward used in these RCTs; dependent on the proportion of missing data, absolute levels of PCS scores may be reduced considerably. In addition, patients enrolled in RCTs were affected by a notably higher disease activity, with baseline DAS28 values of at least six, and accordingly, lower baseline PCS scores of about 30 [13, 17]. It is conceivable that remarkably high disease activity scores as well as lowest PCS scores are more difficult to improve than those representing a somewhat moderate impairment, respectively.
In our cohort, the proportions of patients exceeding the MDI of the PCS and MCS, respectively, were lower than the proportion of patients achieving a clinically relevant improvement in RCTs [13, 14, 17]. This difference is most likely due to the definition of the threshold to identify patients with improvement of HRQoL; we used the MDI, which is statistically defined and based on the measurement precision of the instrument (here the SF36); therefore, the MDI is considerably higher than respective thresholds of clinically relevant improvement used by other authors [31, 33]. Furthermore, achieving MDI in PCS (MCS) is associated with clinical improvement; RABBIT patients obtaining good or moderate EULAR response at month 3 had 77% (34%) higher chance of exceeding MDI at month 12 (PCS: odds ratio: 1.77; 95% CI 1.53, 2.05]; and MCS: OR 1.34; 95% CI 1.11, 1.62). However, the overall rate of patients achieving a good or moderate EULAR response is considerable lower than in RCTs; 77.6% of patients in the RAPID trial [35] had good or moderate EULAR response at week 12 compared with 41.9–52.2% in RABBIT, depending on the therapy stratum. This is in line with previously reported differences between response rates of patients in RCTs and those in observational studies [36].
To conclude, considering treatment history including more than one bDMARD failure, we showed an improvement of HRQoL in all patients with severe, long-standing RA in response to both non-biologic and biologic DMARDs. Nevertheless, as of the middle of 2012, impairment of HRQoL in RA patients has remained enormous compared with the general population.
Acknowledgements
The RABBIT register is supported by a joint, unconditional grant from AbbVie, Bristol Myers Squibb, Merck-Sharp & Dohme, Pfizer, Roche and UCB.The principal investigators and their team had full academic freedom in study design and conduct, data analysis and publication of results. These stipulations were delineated in their contract with the sponsors. For the purpose of information, all six funding companies received the manuscript 30 days prior to submission. Publication of this article was not contingent on their approval. The data interpretation, drafting, critical revision and approval of the final manuscript were performed solely by the authors. K.G., A.R., J.L., A.Z. and A.S. had full access to all data of this study and take responsibility for data integrity and accuracy of the analysis. Study concept and design: J.L., A.Z. and A.S. Acquisition of the data: H.J.B., W.D., A.L. and M.S. Analysis and interpretation of the data: K.G., A.R., J.L., A.Z. and A.S. Drafting the manuscript: K.G. and A.R. Critical revision of the manuscript for important intellectual content: K.G., A.R., A.Z., A.S., J.L., M.S., H.J.B., W.D. and A.L. Obtained funding: J.L., A.Z. and A.S. Study supervision: J.L., A.Z., A.S. and M.S.
Funding: No specific funding was received from any funding bodies in the public, commercial or not-for-profit sectors to carry out the work described in this manuscript.
Disclosure statement: A.Z. has received lecture honoraria from BMS, MSD, Pfizer, Roche and UCB. A.S. has received lecture honoraria from AbbVie, BMS, MSD, Pfizer, Roche, Sanofi-Aventis and UCB. K.G. was supported by a joint, unconditional grant from Abbott/AbbVie, Amgen/Swedish Orphan Biovitrum, Bristol-Myers Squibb, Merck Sharp & Dohme, Pfizer, Roche and UCB and has received lecture honoraria from Bristol-Myers Squibb. H.J.B. has received lecture honoraria from UCB. M.S. has received grant/research support from Abbvie, Actelion, Merck Serono, Pfizer and Roche, is a consultant for Abbvie, Roche and UCB and is a member of the speakers’ bureau with Abbvie, Chugai, Roche, Pfizer and UCB. All other authors have declared no conflicts of interest.
References
- 1.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am 2001;27:269–81. [DOI] [PubMed] [Google Scholar]
- 2.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358:903–11. [DOI] [PubMed] [Google Scholar]
- 3.Husted JA, Gladman DD, Farewell VT, Cook RJ. Health-related quality of life of patients with psoriatic arthritis: a comparison with patients with rheumatoid arthritis. Arthritis Rheum 2001;45:151–8. [DOI] [PubMed] [Google Scholar]
- 4.Kosinski M, Kujawski SC, Martin R, et al. Health-related quality of life in early rheumatoid arthritis: impact of disease and treatment response. Am J Manag Care 2002;8:231–40. [PubMed] [Google Scholar]
- 5.Uhlig T, Loge JH, Kristiansen IS, Kvien TK. Quantification of reduced health-related quality of life in patients with rheumatoid arthritis compared to the general population. J Rheumatol 2007;34:1241–7. [PubMed] [Google Scholar]
- 6.Newman SP. Psychosocial measures in musculoskeletal trials. J Rheumatol 1997;24:979–84. [PubMed] [Google Scholar]
- 7.Linde L, Sørensen J, Ostergaard M, Hørslev-Petersen K, Hetland ML. Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol 2008;35:1528–37. [PubMed] [Google Scholar]
- 8.Keller SD, Ware JE, Jr, Gandek B, et al. Testing the equivalence of translations of widely used response choice labels: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol 1998;51:933–44. [DOI] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Keller SD, Gandek B, Brazier JE, Sullivan M. Evaluating translations of health status questionnaires. Methods from the IQOLA project. International Quality of Life Assessment. Int J Technol Assess Health Care 1995;11:525–51. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF-36 health survey (standard & acute forms). Lincoln: QualityMetric Incorporated, 2000. [Google Scholar]
- 11.Schiff M, Bessette L. Evaluation of abatacept in biologic-naive patients with active rheumatoid arthritis. Clin Rheumatol 2010;29:583–91. [DOI] [PubMed] [Google Scholar]
- 12.Torrance GW, Tugwell P, Amorosi S, Chartash E, Sengupta N. Improvement in health utility among patients with rheumatoid arthritis treated with adalimumab (a human anti-TNF monoclonal antibody) plus methotrexate. Rheumatology 2004;43:712–8. [DOI] [PubMed] [Google Scholar]
- 13.Strand V, Mease P, Burmester GR, et al. Rapid and sustained improvements in health-related quality of life, fatigue, and other patient-reported outcomes in rheumatoid arthritis patients treated with certolizumab pegol plus methotrexate over 1 year: results from the RAPID 1 randomized controlled trial. Arthritis Res Ther 2009;11:R170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strand V, Smolen JS, van Vollenhoven RF, et al. Certolizumab pegol plus methotrexate provides broad relief from the burden of rheumatoid arthritis: analysis of patient-reported outcomes from the RAPID 2 trial. Ann Rheum Dis 2011;70:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathias SD, Colwell HH, Miller DP, et al. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin Ther 2000;22:128–39. [DOI] [PubMed] [Google Scholar]
- 16.van der Heijde D, Klareskog L, Singh A, et al. Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis 2006;65:328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genovese MC, Han C, Keystone EC, Hsia EC, et al. Effect of golimumab on patient-reported outcomes in rheumatoid arthritis: results from the GO-FORWARD study. J Rheumatol 2012;39:1185–91. [DOI] [PubMed] [Google Scholar]
- 18.Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med 2000;343:1594–602. [DOI] [PubMed] [Google Scholar]
- 19.Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum 2004;50:1051–65. [DOI] [PubMed] [Google Scholar]
- 20.Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968–80. [DOI] [PubMed] [Google Scholar]
- 21.Strand V, Burmester GR, Ogale S, et al. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatology 2012;51:1860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006;54:2793–806. [DOI] [PubMed] [Google Scholar]
- 23.Mease PJ, Revicki DA, Szechinski J, et al. Improved health-related quality of life for patients with active rheumatoid arthritis receiving rituximab: Results of the Dose-Ranging Assessment: International Clinical Evaluation of Rituximab in Rheumatoid Arthritis (DANCER) Trial. J Rheumatol 2008;35:20–30. [PubMed] [Google Scholar]
- 24.Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum 2006;54:3399–407. [DOI] [PubMed] [Google Scholar]
- 25.Zink A, Listing J, Kary S, Ramlau P, et al. Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis 2005;64:1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lautenschlager J, Mau W, Kohlmann T, et al. [Comparative evaluation of a German version of the Health Assessment Questionnaire and the Hannover Functional Capacity Questionnaire]. Z Rheumatol 1997;56:144–55. [DOI] [PubMed] [Google Scholar]
- 27.Ware JE. How to score version two of the SF-36 health survey. Kosinski M, ed.
- 28.Ellert U, Kurth BM. [Health related quality of life in adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:643–9. [DOI] [PubMed] [Google Scholar]
- 29.Scheidt-Nave C, Kamtsiuris P, Gößwald A, et al. German health interview and examination survey for adults (DEGS) - design, objectives and implementation of the first data collection wave. BMC Public Health 2012;12:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther 2008;88:733–46. [DOI] [PubMed] [Google Scholar]
- 31.Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999;52:861–73. [DOI] [PubMed] [Google Scholar]
- 32.Molenberghs G, Kenward MG. Missing data in clinical studies. Chichester: John Wiley & Sons, 2007. [Google Scholar]
- 33.Harvill LM. Standard error of measurement. Educ Meas 1991;10:33–41. [Google Scholar]
- 34.Engstler H, Motel-Klingebiel A. Altern im Wandel. Befunde des deutschen Alterssurveys (DEAS). Stuttgart: Kohlhammer, 2010. [Google Scholar]
- 35.Keystone EC, Curtis JR, Fleischmann RM, et al. Rapid improvement in the signs and symptoms of rheumatoid arthritis following certolizumab pegol treatment predicts better longterm outcomes: post-hoc analysis of a randomized controlled trial. J Rheumatol 2011;38:990–6. [DOI] [PubMed] [Google Scholar]
- 36.Zink A, Strangfeld A, Schneider M, et al. Effectiveness of tumor necrosis factor inhibitors in rheumatoid arthritis in an observational cohort study: comparison of patients according to their eligibility for major randomized clinical trials. Arthritis Rheum 2006;54:3399–407. [DOI] [PubMed] [Google Scholar]