Abstract
This study investigated the clinicopathological characteristics and the surgical outcome in patients with non-small cell carcinoma (NSCLC) with parietal pleura invasion or chest wall invasion (p3/T3). This study clinicopathologically evaluated 760 patients who had undergone a resection for NSCLC between 1999 and 2008. There were 43 (5.7 %) patients with p3/T3 NSCLC. The patients included 37 males and 6 females. The histological types included 23 squamous cell carcinomas, 13 adenocarcinomas, 3 large cell carcinomas, 3 pleomorphic carcinomas, and 1 spindle cell carcinoma. Pneumonectomy was performed in 2 patients, bilobectomy in 1, lobectomy in 31, segmentectomy in 3, and partial resection of the lung in 6. The combined resection regions were parietal pleural in 23, ribs in 16, pericardium in 2, and diaphragm in 2 patients. Major complications included empyema in 1, chylothorax in 1, and postoperative bleeding in 1 patient. The first recurrence sites in 16 patients with recurrent disease were the lung in 5 patients, brain in 3, bone in 2, adrenal gland in 2, skin in 2, liver in 1, mesenterium in 1, mediastinal lymph node in 1, axillary lymph node in 1, and carcinomatous pleuritis in 1. The overall 5-year survival rate after surgery was 50.6 %. An en bloc resection for p3/T3 NSCLC provides a modestly favorable prognosis. Local recurrence was observed in a minority of case, and recurrence by distant metastasis was observed in most cases, suggesting a greater need for postoperative chemotherapy.
Keywords: Parietal pleural invasion, Chest wall invasion, Surgical resection, Non-small cell lung cancer, Surgical outcome
Introduction
Lung cancer is among the most prevalent and lethal cancers worldwide, and non-small cell lung cancer (NSCLC) comprises approximately 85 % of lung cancer cases [1, 2]. The 5-year survival rate for patients with NSCLC is very poor because most patients already have advanced-stage disease at diagnosis [3]. Lung cancer is also known to be a highly treatment-refractory cancer. Early detection and surgical resection remains a mainstay for improving the survival of lung cancer patients [4]. The survival of early-stage NSCLC is reported to be 50–80 % in stages I and II [5, 6]. This wide range of survival rates suggests that patients who undergo surgery have markedly different disease progression.
The T factor in the TNM classification system not only describes the size of the primary tumor but also contains factors about the invasion of adjacent structures. Tumors with parietal pleural invasion or invasion into the chest wall (soft tissue and/or bone) are classified as T3, and patients with such types of lung cancer are divided into stage IIB or stage IIIA according to the presence of lymph node metastasis in the UICC TNM staging system [7]. Chest wall invasion is not common, occurring in 2–8 % of patients with NSCLC undergoing surgical resection [8–10]. The clinical characteristics of parietal pleural invasion or chest wall invasion in NSCLC have remained unclear due to the low incidence of these conditions. For instance, the prognostic impact of the depth of invasion in the chest wall has been controversial. Furthermore, the selection of surgical procedures (e.g., extrapleural resection vs. combined resection of the ribs) is another point of controversy. This study retrospectively investigated the clinicopathological characteristics and the surgical outcome in patients with NSCLC with either parietal pleural invasion or chest wall invasion (p3/T3 disease).
Patients and Methods
The hospital records of 760 consecutive patients who underwent the resection of non-small cell lung cancer between 1999 and 2008 were reviewed. Preoperative assessments included chest roentgenography and computed tomography (CT) of the chest, upper abdomen, and brain. PET scans were used for the assessment of clinical staging since 2005. Clinical N2 status was defined by the presence of a lymph node more than 1 cm in a short-axis diameter. Bone scintigraphy was performed to detect any bone metastasis. MRI (magnetic resonance imaging) of the brain was routinely employed after 2001. Bronchoscopy was routinely performed to obtain a pathological diagnosis by a transbronchial lung biopsy, and to evaluate endobronchial staging. The patients’ records, including their clinical data, preoperative examination results, details of any surgeries, histopathological findings, and the TNM stages of all patients were also reviewed. The pulmonary function was evaluated using spirometry and arterial blood gas analysis. Predictive postoperative lung function was considered as operable if the forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1.0) were greater than 800 and 600 ml/m2, respectively. All resected specimens, including the primary tumor and resected hilar and mediastinal lymph nodes, were examined to determine both the tumor histology and the extent of lymph node metastases. Intraoperative frozen sections were performed if invasion of the tumor was suspected at the surgical margins. The histopathological findings were classified according to the World Health Organization criteria, and the UICC TNM staging system (7th edition) was employed [7, 11].
Follow-up information was obtained from all patients through office visits or telephone interviews either with the patient, with a relative, or with their primary physicians. The patients were evaluated every 3 months by chest roentgenography, and chest CT scans and bone scintigraphy were obtained every 6 months for the first 2 years after surgery and annually thereafter. The mean observation time was 3.5 years.
The survival curve was calculated by the Kaplan–Meier method and compared using the log-rank test for univariate analysis. Categorical variables were compared by Fisher’s exact test. Differences were considered to be significant if the P value was less than 0.05. The Statview V software program (Abacus Concept, Berkeley, CA) was used for all statistical analyses.
Results
A total of 760 patients underwent a resection for non-small cell lung cancer between 1999 and 2008 at the Second Department of Surgery, University of Occupational and Environmental Health. The current study evaluated 43 patients (5.7 %) with p3/T3 NSCLC. The patients included 37 males and 6 females (Table 1). The mean age of the patients was 68.7 years (range 47–81 years). All the patients had a smoking habit, and the average smoking pack-year index was 55. The tumor diameter ranged from 1.0 to 9.8 cm (average 4.5 cm). The clinical stage was diagnosed as stage I in 16 patients, stage II in 16, and stage IIIA in 11. Chief complaints were chest pain in 14 patients (32.5 %), hemosputa in 4 (9.3 %), cough in 3 (7.0 %), and no symptoms in 22 (51.1 %). The histological types included 23 squamous cell carcinomas (53 %), 13 adenocarcinomas (30 %), 3 large cell carcinomas (7 %), 3 pleomorphic carcinomas (7 %), and 1 spindle cell carcinoma (2 %). Concerning the operative procedures, pneumonectomy was performed in 2 patients, lobectomy in 31, bilobectomy in 1, segmentectomy in 3, and partial resection of the lung in 6 patients. The combined resection regions were parietal pleural in 23, ribs in 16, pericardium in 2, and diaphragm in 2 patients. When a chest wall resection with a resection of more than 2 ribs was performed, the reconstruction of the chest wall was performed using polypropylene (Marlex®) mesh in 14 patients. The defect in the pericardium was reconstructed with expanded polytetrafluoroethylene surgical membranes (ePTFE; Gore-Tex®). The lymph node metastasis was pathologically diagnosed as N0 in 32 patients, N1 in 7, and N2 in 4. Preoperative radiation therapy was performed in four patients. Regarding postoperative adjuvant treatment, 10 patients underwent radiation therapy and 13 patients received chemotherapy.
Table 1.
Characteristics of the patients with NSCLC with parietal pleura or chest wall invasion
| Average of age (range) | 68.7 (47–81) |
| Gender; male/female | 37/6 |
| Smoker/Non-smoker | 43/0 |
| Operative procedure of lung resection | |
| Pneumonectomy | 2 |
| Bilobectomy | 1 |
| Lobectomy | 31 |
| Segmentectomy | 3 |
| Partial resection | 6 |
| Combined resection | |
| Parietal pleura only | 23 |
| Rib | 16 |
| Pericadium | 2 |
| Diaphragma | 2 |
| Pathological nodal status | |
| N0 | 32 |
| N1 | 7 |
| N2 | 4 |
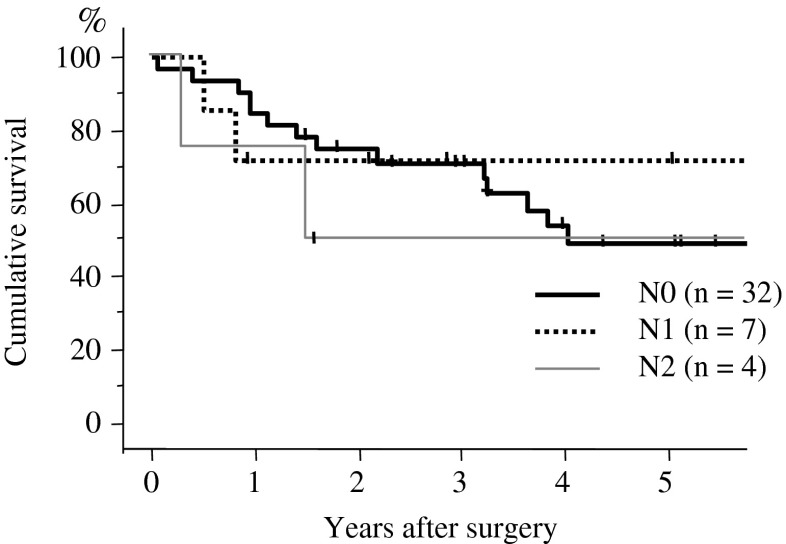
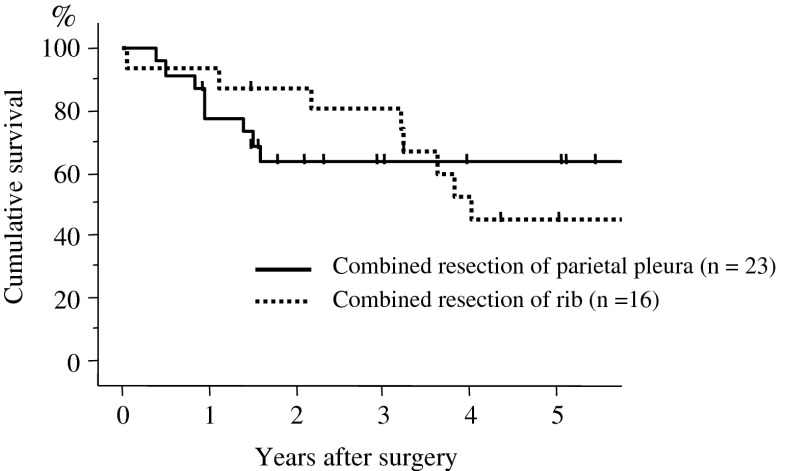
Major complications included empyema in 1, chylothorax in 1, and postoperative bleeding in 1 patient. Minor complications included arrhythmia in two patients. One patient (with empyema) died within 30 days of the operation. Recurrence was observed in 16 patients. The first recurrence sites were the lung in 5 patients, brain in 3, bone in 2, adrenal gland in 2, skin in 2, liver in 1, mesenterium in 1, mediastinal lymph node in 1, axillary lymph node in 1, and carcinomatous pleuritis in 1 (Table 2). The 5-year survival rate after surgery was 50.6 % (Fig. 1). According to the N factor, the 5-year survival rates in patients with N0, N1, and N2 disease were 50.1, 71.4, and 50.7 %, respectively (Fig. 2). When the prognosis in the patients without lymph node metastasis was compared by T factor, the 5-year survival rates in T1a, T1b, T2a, T2b, and T3 (>7 cm) were 89.1, 86.1, 67.0, 50.9, and 60.2 %, respectively (Fig. 3). The 5-year survival rate in patients who underwent combined resection with the parietal pleura was 63.4 %, and for those who underwent a combined resection of the chest wall (ribs) was 44.9 % (Fig. 4).
Table 2.
The first recurrence sites in the patients with NSCLC with parietal pleura or chest wall invasion
| Distant metastasis | |
| Brain | 3 |
| Lung | 5 |
| Adrenal gland | 2 |
| Bone | 2 |
| Skin | 2 |
| Liver | 1 |
| Mesenterium | 1 |
| Mediastinal lymph node | 1 |
| Axillary lymph node | 1 |
| Local recurrence | |
| Carcinomatous pleuritis | 1 |
Fig. 1.
Overall survival curves for the patients with NSCLC with p3/T3 disease. The overall survival in each period was analyzed by the Kaplan–Meier method. The postoperative 5-year survival rate was 50.6 %
Fig. 2.
Overall survival curves for the patients with p3/T3 disease according to lymph node metastasis. The postoperative 5-year survival rates of N0, N1, and N2 disease were 50.1, 71.4, and 50.7 %, respectively. There was no significant difference between these groups
Fig. 3.
Overall survival curves for the patients according to the T factor. The 5-year survival rates of T1a, T1b, T2a, T2b, and T3 (>7 cm) among N0 patients were 89.1, 86.1, 67.0, 50.9, and 60.2 %, respectively, and that of the patients with T3 (p3) N0 disease was 50.1 %
Fig. 4.
Overall survival curves for the patients with p3/T3 disease according to the selected surgical procedures. The postoperative 5-year survival rate was 64.3 % in patients who underwent combined resection of the parietal pleura and 44.9 % in patients who underwent combined resection of the ribs. No significant difference was observed based on the surgical procedure used
Discussion
The p3/T3 NSCLC includes cancers that have invaded the parietal pleura only, as well as those that have invaded the chest wall of the soft tissue or ribs. Coleman first reported long-term survival following pulmonary resection with en bloc excision of the chest wall in 1947 [12]. Early reports considered chest wall invasion as a contraindication to pulmonary resection due to the significant surgical mortality [13, 14]. The treatment for patients with locally advanced NSCLC has developed over the past two decades; however, the surgical approach selected for treatment still has a crucial role as a component of multimodality therapy [10, 15].
Regarding preoperative diagnosis, chest CT is an efficient modality to evaluate chest wall invasion and the extent of involvement of lung cancer. MRI is a better tool for evaluating the depth of chest wall invasion [16]. Akata et al. reported that respiratory dynamic MR improved the accuracy of conventional CT scans or MRI in the prediction of chest wall invasion of lung cancer, especially in the presence of a peripheral mass abutting the chest wall surface without obvious chest wall invasion [17]. Thoracic pain was previously reported to be present in 40–50 % of patients with chest wall invasion [8, 18]. In this study, chest pain was observed in 32 % of patients, suggesting that the absence of local pain is not always good indicator of a lack of chest wall invasion.
A histological examination revealed squamous cell carcinoma to be the most common subtype, and it constitutes 40–60 % of NSCLC cases [8, 18, 19]. In this study, squamous cell carcinoma accounted for 53 % of the cases. Several investigators have reported that more than 80 % of their patients were male [8, 9, 18, 19]. In line with these reports, in this study, 86 % of the patients were male, and all of them were smokers. The average tumor size was 4.5 cm in this study, and the average diameter of all resected lung cancers (n = 717) in our hospital during the same period was 2.9 cm. The tumor size in the patients with p3/T3 disease was significantly larger than with other types of lung cancer.
Extrapleural resection was performed only if there were flimsy adhesions to the chest wall and there was no tumor in the surgical margin macroscopically. The intraoperative frozen section was not performed routinely in the case of extrapleural resection. An en bloc chest wall resection was performed in cases where the tumor had invaded the chest wall beyond the parietal pleura on the basis of preoperative findings of CT or MRI. In cases where there was fixation beyond the parietal pleura, or any doubt about invasion of deeper structures based on intraoperative findings, the en bloc chest wall resection was also carried out. The resection margins were checked with frozen sections in the majority of cases. The present study indicated that the postoperative prognosis of extrapleural resection was similar to that of chest wall resection. Matsuoka et al. also reported that the depth of invasion and the type of resection (extrapleural or chest wall) did not affect survival [20]. Similar conclusions were drawn by other investigators [9, 21]. McCaughan showed that there was no significant difference in the 5-year survival rate between the extrapleural and chest wall resections, and noted that an extrapleural resection sufficed if the cancers involved only the parietal pleura, and negative surgical margins were confirmed by intraoperative frozen section analysis [21]. On the other hand, the depth of invasion was reported to be an unfavorable prognostic factor [8, 22]. Intercostal muscle or rib invasion indicated a poor prognosis, presumably because these tissues are richly supplied with blood, thereby promoting distant metastasis. However, cancers whose invasion was grossly limited to the parietal pleura might be successfully controlled by pre- or postoperative radiation.
Regarding the 5-year survival after surgery, reported rates ranged from 25 % to 67 % [8, 18, 20, 22]. Studies on the postoperative mortality rate have been reported to be 3.2–7.8 % [10]. In the present study, the rate of occurrence of major complications was 7.0 % (empyema, chylothorax, and postoperative bleeding), and the postoperative mortality rate was 2.3 %. According to most of previous studies, N1 or N2 disease was a significant unfavorable prognostic factor in comparison with T3N0M0 disease [8, 9, 18–23]. However, in the present study, no difference in the prognosis was observed between patients with and without lymph node metastasis, likely because there were a too few patients with lymph node metastasis. The present study showed recurrence of the lung cancer in 16 patients, and most patients had distant metastases as a first recurrence. Only one patient had a local recurrence (Table 2). Little information exists regarding the efficacy of adjuvant chemotherapy for p3/T3 disease. However, our results suggested a greater need for postoperative chemotherapy, as well as the local control of NSCLC with p3/T3 disease.
The postoperative prognosis of patients with p3/T3 NSCLC demonstrated a modestly favorable survival (excess of 50 %) with an acceptable surgical mortality. A surgical resection is also recommended as the first-line treatment for p3/T3 NSCLC. The 5-year survival rate was not significantly different between the patients with involvement of the parietal pleura only and the patients with invasion of the chest wall of the soft tissue or ribs. Further clinical studies will be necessary to evaluate the efficacy of adjuvant therapy for the patients with p3/T3 NSCLC.
Acknowledgments
Conflict of interest
None to declare
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 3.Spiro SG, Silvestri GA. One hundred years of lung cancer. Am J Respir Crit Care Med. 2005;172:523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 4.Hanagiri T, Baba T, So T, et al. Time trends of surgical outcome in patients with non-small cell lung cancer. J Thorac Oncol. 2010;5:825–829. doi: 10.1097/JTO.0b013e3181d5e47f. [DOI] [PubMed] [Google Scholar]
- 5.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352:2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 6.Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227–234. doi: 10.1016/j.lungcan.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 8.Facciolo F, Cardillo G, Lopergolo M, Pallone G, Sera F, Martilli M. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg. 2001;121:649–656. doi: 10.1067/mtc.2001.112826. [DOI] [PubMed] [Google Scholar]
- 9.Akay H, Cangir AK, Kutlay H, Kavukçu S, Okten I, Yavuzer S. Surgical treatment of peripheral lung cancer adherent to the parietal pleura. Eur J Cardiothorac Surg. 2002;22:615–620. doi: 10.1016/S1010-7940(02)00408-6. [DOI] [PubMed] [Google Scholar]
- 10.Stoelben E, Ludwig C. Chest wall resection for lung cancer: indications and techniques. Eur J Cardiothorac Surg. 2009;35:450–456. doi: 10.1016/j.ejcts.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Travis WD, Colby TV, Corrin B, Shimosato Y, Brambilla E. Histological type of lung and pleural tumors. World Health Organization international histological classification of tumors. 3. Berlin: Springer; 1999. pp. 31–47. [Google Scholar]
- 12.Coleman FP. Primary carcinoma of the lung, with invasion of the ribs pneumonectomy and simultaneous block resection of the chest wall. Ann Surg. 1947;126:156–168. doi: 10.1097/00000658-194708000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piehler JM, Pairolero PC, Weiland LH, Offord KP, Payne WS, Bernatz PE. Bronchogenic carcinoma with chest wall invasion: factors affecting survival following en bloc resection. Ann Thorac Surg. 1982;34:684–691. doi: 10.1016/S0003-4975(10)60909-5. [DOI] [PubMed] [Google Scholar]
- 14.Trastek VF, Pairolero PC, Piehler JM, et al. En bloc (non-chest wall) resection for bronchogenic carcinoma with parietal fixation. Factors affecting survival. J Thorac Cardiovasc Surg. 1984;87:352–358. [PubMed] [Google Scholar]
- 15.Allen MS, Mathisen DJ, Grillo HC, Wain JC, Moncure AC, Hilgenberg AD. Bronchogenic carcinoma with chest wall invasion. Ann Thorac Surg. 1991;51:948–951. doi: 10.1016/0003-4975(91)91011-J. [DOI] [PubMed] [Google Scholar]
- 16.Ohno Y, Sugimura K, Hatabu H. MR imaging of lung cancer. Eur J Radiol. 2002;44:172–181. doi: 10.1016/S0720-048X(02)00267-X. [DOI] [PubMed] [Google Scholar]
- 17.Akata S, Kajiwara N, Park J, et al. Evaluation of chest wall invasion by lung cancer using respiratory dynamic MRI. J Med Imaging Radiat Oncol. 2008;52:36–39. doi: 10.1111/j.1440-1673.2007.01908.x. [DOI] [PubMed] [Google Scholar]
- 18.Doddoli C, D’Journo B, Le Pimpec-Barthes F, et al. Lung cancer invading the chest wall: a plea for en-bloc resection but the need for new treatment strategies. Ann Thorac Surg. 2005;80:2032–2040. doi: 10.1016/j.athoracsur.2005.03.088. [DOI] [PubMed] [Google Scholar]
- 19.Voltolini L, Rapicetta C, Luzzi L, et al. Lung cancer with chest wall involvement: predictive factors of long-term survival after surgical resection. Lung Cancer. 2006;52:359–364. doi: 10.1016/j.lungcan.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka H, Nishio W, Okada M, Sakamoto T, Yoshimura M, Tsubota N. Resection of chest wall invasion in patients with non-small cell lung cancer. Eur J Cardiothorac Surg. 2004;26:1200–1204. doi: 10.1016/j.ejcts.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 21.McCaughan BC, Martini N, Bains MS, McCormack PM. Chest wall invasion in carcinoma of the lung. Therapeutic and prognostic implications. J Thorac Cardiovasc Surg. 1985;89:836–841. [PubMed] [Google Scholar]
- 22.Magdeleinat P, Alifano M, Benbrahem C, et al. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg. 2001;71:1094–1099. doi: 10.1016/S0003-4975(00)02666-7. [DOI] [PubMed] [Google Scholar]
- 23.Demir A, Gunluoglu MZ, Kara HV, Buyukpinarbasili N, Dincer SI. Prognostic factors in resected T3 non-small cell lung carcinoma: perineural invasion as a new prognostic factor. Thorac Cardiovasc Surg. 2008;56:93–98. doi: 10.1055/s-2007-965059. [DOI] [PubMed] [Google Scholar]