Highlights
-
•
Changes of gravitational forces affect oscillations of circadian clock genes.
-
•
The effects of microgravity on the circadian clock persist during the recovery process from microgravity.
-
•
Linking of mechanotransduction to circadian rhythms reveals changes in physiology.
Abbreviations: FAK, focal adhesion kinases; GPCR, G-protein-coupled receptors; hg, hypergravity; mAbs, monoclonal antibodies; RPM, Random Positioning Machine
Keywords: Microgravity, Circadian clock, Mechanotransduction, Bmal1, Rev-erbα, Keratinocytes
Abstract
Microgravity and sudden changes of gravitational forces exert numerous effects on tissues, organs and apparatus. Responses to these forces variably applied to cells indicate the existence of mechanotransduction pathways able to modulate transcription. Oscillation of circadian clocks similarly influences many cellular and metabolic processes. Here we hypothesized that signals derived from changes of gravitational forces applied to epidermal cells might influence their physiology in harmony with the oscillation of the molecular clock. In this study, we describe amplified oscillations of Bmal1 circadian clock gene in human keratinocytes exposed to short simulated microgravity and to rapid variation of gravitational forces. We found that exposure to microgravity enhances the amplitude of the Bmal1 feedback loop sustained by an apparently lower variability of Rev-erbα transcription, while recovery from microgravity is characterized by increased amplitude of Bmal1 expression and elongation of the oscillatory periods of Bmal1 and Rev-erbα. These data highlight the existence of integrated signaling network connecting mechanosensitive pathways to circadian gene regulation.
1. Introduction
Mechanical stimuli are crucial to many biological processes that take place in cells, tissue and organs. Almost any cell is able to respond to mechanical cues that are registered by specific sensor molecules and commuted into biochemical signals that regulate cell shape and morphology, but also participate to the execution of numerous programs, including cell proliferation, differentiation and migration. The most common and typical forces applied to living cells in vivo are shear flow, tensile stretch, compressive forces, and a variety of mechanical stimuli sometimes different for magnitude, frequency and duration.
The molecular sensors responsible to trace forces applied to living cells, are numerous and include extracellular-matrix proteins, receptors and transmembrane proteins, components of the cytoskeleton, voltage or ligand sensitive ion channels [1–3]. These structures, organized to contain or function themselves as “mechanoreceptors” respond to mechanical stress by generating signals that become finely integrated with the signaling network with the intent to override, synergize or simply tune biological responses.
In this context, gravity and sudden variations of gravitational forces have significant impact on living cells, with reflection on tissue and organ physiology. This is evident in astronauts that, exposed to micro-gravitational status for a long period of time, suffer of loss bone, immunosuppression [4], cardiovascular problems [5], changes in metabolism and body temperature activity [6,7], sleep defects [8,9], blood pressure alteration, hormone and neurotransmitter impaired secretion [10]. Some of these effects (e.g. loss bone or heart rate changes) can be variably ascribed to the altered equilibrium of signaling events, in cells anymore exposed to compressional mechanical forces [11], whereas others might be related to circadian desynchronization with the environment [7].
As a yet unexplored hypothesis, we wanted to verify whether microgravity would impact on circadian gene regulation at individual cell compartment. To test this, we exposed HaCaT human keratinocytes to microgravity in the Random Positioning Machine (RPM) device for different periods of time, monitoring variation in the expression of the core clock genes Bmal1 and Rev-erbα. Selection of this cellular model was based on previous studies demonstrating the presence in human keratinocytes and melanoma cells of functional clock genes subjected to circadian oscillation for gene expression [12–14]. Using this model we recently described the expression and nucleolar localization of a novel splicing variant of Period 2 gene (Per2S) [15], suggesting a critical role for the nucleolus in the regulation of circadian rhythms. On the other hand, keratinocytes are also known to respond to mechanical stretches by increasing, in particular conditions, proliferation and migration rates [16].
In a simple experimental setting we found that microgravity amplifies Bmal1/Rev-erbα-sustained circadian oscillations, indicating the existence of integrated gene networks connecting the machinery of mechanotransduction to the cellular timing system of the circadian clock.
2. Materials and methods
2.1. Cells and treatments
Human spontaneously transformed keratinocyte HaCaT cells were cultured in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) plus antibiotics. Cells were seeded in Opticell units (Biocrystal Ltd, Westerville, OH, USA) and allowed to attach on both the two inner membrane surfaces of the chamber by repetitive turning upside down every 15 min during 4 h. All the experiments were performed after 24 h from cell plating.
Microgravity conditions were obtained by positioning the Opticell chambers containing HaCaT cells in the Random Positioning Machine (RPM) device (Dutch Space, Leiden, Netherlands) inside a humidified incubator (5% CO2 at 37 °C), setting the angular velocity of rotation at 90°/sec as maximum and 30°/sec as minimum, in a random mode [17]. Under this experimental condition, the cells were exposed to simulated microgravity conditions ranging from 0 to 0.01g, and experiments performed for different periods of time (48 h or 60 h at 37 °C).
Synchronization of the molecular clock machinery was obtained by incubating HaCaT cells with either 1 μM Dexamethasone (Dex) (Sigma–Aldrich Inc., Saint Louis, MO, USA) [12], 10 μM Forskolin (Forsk) (Sigma–Aldrich) [18].
2.2. RNA extraction and cDNA synthesis
Total RNAs were extracted from HaCaT cells using the Quick-RNA MiniPrep (Zymo Research, Irvine, CA, USA) and concentrations were determined using NaNoDrop spectrophotometer (Thermo scientific, Huntsville, TX, USA). All the cDNAs were synthesized by the reverse transcription of 1 μg of total RNA using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Richmond, CA, USA). Reactions were performed according to manufacturer’s instructions.
2.3. PCR amplification and quantitation of RNAs by real-time PCR
The oligonucleotide primers necessary for the amplification of the cDNA fragments of Bmal1, Rev-erbα expression and for the ribosomal 18s RNA housekeeping gene were chosen utilizing the online tool Primer-BLAST [19] and purchased from Invitrogen (Carlsbad, CA, USA). The following primers were used: for Bmal1 target gene: 5′-AGCCACGGTGGTGCTGGCTA-3 (sense), 5′-AACCAATGAAGGCCCAGGATTCCAC-3′ (anti-sense); for Rev-erbα target gene: 5′-CGCAACCTCTAGTTTGAGTCAAGGTC-3′ (sense), 5′-ACGCCACCTGTGTTGTTGTTGGA-3′ (anti-sense); for GAPDH target gene: 5′-CATCAGCAATGCCTCCTGCAC-3′ (sense), 5′-GTCATGAGTCCTTCCACGATACCAA-3′ (anti-sense); for the 18s rRNA housekeeping gene: 5′-CGAGCCGCCTGGATACC-3′ (sense), 5′-CATGGCCTCAGTTCCGAAAA-3′ (anti-sense).
For each primer pair, we performed no-template control and no-reverse-transcriptase control (RT negative) assays, which produced negligible signals. Real-time PCR were carried out in 96-well plate using iQ SYBR Green Supermix (Bio-Rad) and the iCycler Real-Time Detection System (iQ5; Bio-Rad). The relative quantification of gene expression was assessed by comparative threshold cycle method (2-ΔΔCT), normalizing the gene expression to ribosomal 18S RNA and expressing the data as fold increase. All the experiments have been done in triplicates and are reported as mean ± SD.
2.4. Immunofluorescence
HaCaT cells exposed to gravitational forces and pharmacological synchronization of circadian genes were fixed with 4% paraformaldehyde in PBS for 30 min at 25 °C. Fixed cells were permeabilized using 0.1 M glycine for 20 min at 25 °C followed by additional 5 min of incubation with 0.1% Triton X-100 at 25 °C. Permeabilized cells were then incubated for 1 h at 25 °C with the appropriate primary monoclonal antibodies (mAbs): mouse mAb anti-β1 integrin, (1:1000 in PBS; ST2–16, Santa Cruz Biotechnology, CA, USA), mouse mAb anti-cytokeratins (1:100 in PBS; clone MNF116, DAKO, Carpinteria, CA, USA), rabbit polyclonal anti-Ki67 (1:50 in PBS; Zymed Laboratories Inc, San Francisco, CA, USA), mouse monoclonal FITC-conjugated anti-M30 (1:250 in PBS; clone M30, Roche, Mannheim, Germany). Primary antibody staining were visualized using either a goat anti-mouse IgG-FITC (1:50 in PBS; Cappel Research Products, Durham, NC, USA) or a goat anti-rabbit IgG-Texas Red (1:200 in PBS; Jackson Immunoresearch Laboratories, West Grove, PA, USA) for 30 min at 25 °C. Nuclei were stained with DAPI (1:1000 in PBS; Sigma–Aldrich). Sample slides were mounted with mowiol (Sigma–Aldrich) for observation and scanned in a series of 0.5 μm sequential sections with an ApoTome System (Zeiss, Oberkochen, Germany) connected with an Axiovert 200 inverted microscope (Zeiss) for the analysis of fluorescent signals; all the images were processed using the Axiovision software (Zeiss). The three dimensional reconstruction shown in each figure is a selection of three of the total number of serial optical sections evaluated under the microscope. Quantitative analysis of the percentage of Ki67 positive cells was assessed evaluating 400 cells from each sample, randomly observed in 5 optical fields from three different experiments. Results have been expressed as mean values ± standard errors (SE). p value were calculated using one-way analysis of variance – ANOVA – followed by Bonferroni post hoc test, and significant level has been defined as p < 0.05.
3. Results
3.1. Simulated microgravity modifies Bmal1 and Rev-erbα circadian oscillations
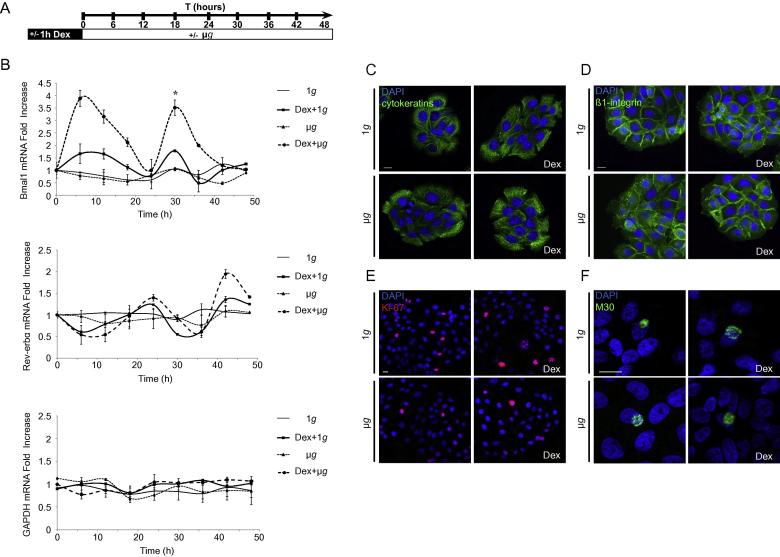
The effects of microgravity can be monitored analyzing the oscillations of the molecular clock genes taken at different end-point times in cells previously synchronized by pharmacological treatment. In our experiments, we treated HaCaT keratinocytes with dexamethasone 1 μM for 1 h. This treatment allows for the synchronization of the cell clock machinery [12] and precedes the exposure of cells to the variation of gravitational forces. We choose four different conditions for the analysis: exposure of unsynchronized and Dex-synchronized cells to normal gravitational force (1g), and exposure of unsynchronized and Dex-synchronized cells to micro-g environment (μg) (Fig. 1A). In this experimental setting we evaluated the oscillations of two circadian clock genes: Bmal1 and its repressor Rev-erbα.
Fig. 1.
Simulated microgravity modifies Bmal1 and Rev-erbα circadian oscillations in human keratinocytes. (A) Experimental scheme: untreated HaCaT cells or treated for 1 h with1 μM Dex for gene clock synchronization were exposed to gravitational forces of 1g or μg. Total RNA samples were collected every 6 h for consecutive 48 h and subjected to qRT-PCR analysis for Bmal1 and Rev-erbα mRNA expression. (B) Bmal1 mRNA transcripts show no oscillation in unsynchronized HaCaT cells exposed to either μg or 1g. Both Dex-synchronized cells cultures at 1g (Dex + 1g) or in μg (Dex + μg) show Bmal1 circadian oscillations, with higher Bmal1 transcription in cells exposed to μg (p < 0.05, one-way ANOVA). Rev-erbα mRNA show no oscillation in unsynchronized HaCaT cells exposed to either μg or 1g. Both Dex-synchronized cells cultures at 1g (Dex + 1g) or in μg (Dex + μg) show minimal increase of Rev-erbα circadian oscillations, with higher transcripts in cells exposed to μg. GAPDH mRNA expression is not subjected to circadian oscillations. All the experiments were done in triplicates and were evaluated for statistical significance (*p < 0.05) using the one-way ANOVA followed by Bonferroni post hoc test. Results are expressed as mean fold increase ± standard deviation (SD). (C–F) Unsynchronized and Dex-synchronized cells exposed to microgravity for 24 h were stained for immunofluorescence and nuclei are visualized by DAPI staining. Bar: 10 μm. Immunostaining with anti-cytokeratins antibody shows unmodified organization of cytosolic filaments in all the experimental conditions (C). Cells stained with anti-β1 integrin mAb display the expected signals localized on the cell surface and appear unmodified by the treatments (D). The proliferation rate, assessed in immunofluorescence with an anti-Ki67 polyclonal antibody, reveals positive cycling cells comparable in all the experimental conditions (E). The identification of apoptotic cells in immunofluorescence using the M30 mAb directed against the cleaved form of cytokeratin 18 shows similar numbers of positive cells for all the treatments (F).
Quantitative analysis for mRNA expression of Bmal1 in Dex-treated synchronized cells exposed to normal gravity indicates the existence of an oscillatory period of 24 h, with peak of expression in the first 6 h, and return to the basal levels within the 24 h. Importantly, changes in the mechanical forces applied to Dex-synchronized cells subjected to microgravity had a strong impact on the Bmal1 kinetics. Its expression peaks in the first 6 h (3.9-fold increase), remains constantly high at 12th hour (3.2-fold increase), tends to normalize only at the approach of the 24th hour (1.1-fold increase) and then recycles maintaining the same of oscillatory phase, amplitude and period kinetics for the next 24 h. Dex-treated cells exposed to microgravity display a significant enhancement of amplitude in comparison to Dex-synchronized cells kept in 1g (Fig. 1B), (p < 0.05, one-way analysis of variance – ANOVA – followed by Bonferroni post hoc test). As expected, the expression of Bmal1 in unsynchronized cells exposed to either 1g or microgravity did not show any oscillatory period during the 48 h of observation.
The rhythmic expression of Bmal1 is coordinately controlled by the transcriptional repressor Rev-erbα directly binding to the ROR elements in the Bmal1 promoter. This tightly regulated mechanism accounts for an anti-phasic expression of Rev-erbα to Bmal1. Therefore, we chose to evaluate the expression of the clock gene Rev-erbα mRNA to asses the specificity of Bmal1 oscillations observed in cells exposed to microgravity. As expected, to the highest peaks of Rev-erbα gene expression corresponds a minimal synthesis of Bmal1 mRNA, which occurs at the onset of the 24th hour. However, the amplified oscillations of Rev-erbα observed in cells exposed to microgravity were not significantly different from Dex-synchronized cells kept a 1g, indicating that Rev-erbα may not be subjected to variation of gravitational forces. Alternatively, exposure to microgravity produces limited but still efficient fluctuation of Rev-erbα proteins that might be sufficient to negatively regulate transcription of Bmal1. As an internal control for all the experimental conditions, we monitored glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression, which is not subjected to circadian rhythm oscillations (Fig. 1B).
Since the exposure of cells to decreased gravitational forces is known to induce alterations of the cytoskeleton dynamics [20,21] with changes of the adhesion properties, proliferation rate and apoptosis [17,22–24], we wanted to verify these potential effects on cells exposed for 24 h to microgravity. We choose cytokeratin and β1 integrin as markers for the evaluation of cytoskeleton dynamics, Ki67 to detect cycling cells, and the cleaved form of cytokeratin 18 for the evaluation of the early events of apoptosis. We could not appreciate any morphological change or variation in the organization of the cytokeratin filaments and β1 integrin adhesion molecules in cells cultured in microgravity environment (Fig. 1C and D). Also, comparative evaluation of proliferative and apoptotic rates in cells either exposed to microgravity or normal gravity did not show differences in the experimental condition tested (Fig. 1E and F, Table 1).
Table 1.
Quantitation of positive cells for Ki67 after μg exposure and/or Dex treatment.
| % of ki67 Positive cells |
||||
|---|---|---|---|---|
| 1g | Dex | μg | Dex + μg | |
| 24 h μg | 13.52 (±4.9) | 11.88 (±5.8) | 12.79 (±4.8) | 10.34 (±4.2) |
| 60 h μg | 8.66 (±4.6) | 8.71 (±4.7) | 7.97 (±4.3) | 7.40 (±4.1) |
These results demonstrate that microgravitational forces applied to cells specifically amplify the synchronized molecular clock in the absence of evident physiological changes of human keratinocytes and that the experimental conditions utilized for our experiments do not represent a major source of cell stress.
3.2. The cycle periods and amplitudes of Bmal1 and Rev-erbα are enhanced during the recovery phase
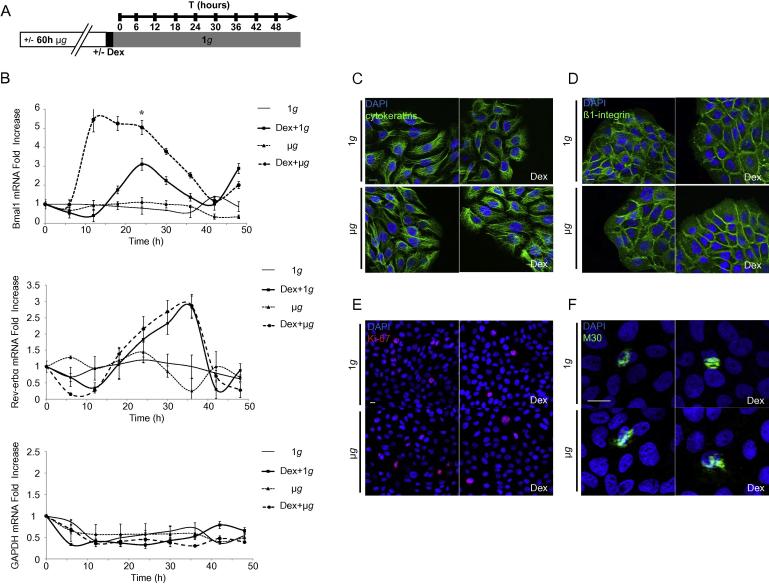
It has been recently shown that μg influences gene expression in human keratinocytes and that these effects are evident for a period time after recovery to 1g [25]. We reasoned that the oscillations of the clock genes expression Rev-erbα and Bmal1 would also be present after the exposure to microgravity, for a period of time during recovery at 1g. To verify this hypothesis, HaCaT cells were exposed to μg or 1g for consecutive 60 h and only then synchronized with Dex in 1g conditions to evaluate the expression of Rev-erbα and Bmal1 measured by qRT-PCR at 6 h, 12 h, 24 h, 30 h, 36 h, 42 h and 48 h post synchronization (Fig. 2A).
Fig. 2.
Bmal1 and Rev-erbα circadian oscillations during the recovery process from simulated microgravity. (A) Experimental scheme: HaCaT cells were exposed to μg or 1g for 60 h, then treated with 1 μM Dex for 1 h and recovered in 1g for consecutive 48 h. Total RNA samples were collected at 6 h, 12 h, 24 h, 30 h, 36 h, 42 h and 48 h during the recovery phase in 1g. Untreated cells exposed to 1g were used as control. (B) Comparative analysis of Bmal1 and Rev-erbα mRNA expression in Dex-synchronized HaCaT cells cultured either in 1g (1g + Dex) or in microgravity (μg + Dex). The kinetics of Bmal1 mRNA transcripts indicates that cells exposed to μg display atypical oscillation recovery as compared to cells recovered from 1g, different for intensity, period and time of peaking (*p < 0.05, one-way ANOVA), with a 5.5-fold increase of Bmal1 mRNA. Results are expressed as mean values ± standard deviation (SD). The Rev-erbα kinetics in synchronized cells either cultured at 1g or in the recovery phase is anti-phasic to Bmal1, with a period of 30 h and with amplitudes and peaks of equal intensity. GAPDH expression is used as an internal control. All the experiments were done in triplicates and evaluated for statistically significance using the one-way ANOVA test. Results are expressed as mean fold increase ± standard deviation (SD). (C–F) Cells were stained for immunofluorescence after 60 h of μg or 1g. Immunofluorescence was performed using specific antibodies and nuclei visualized by DAPI staining. Bar: 10 μm. Immunofluorescence with anti-cytokeratins mAb shows unchanged patterns of cytokeratins organization in HaCaT cells differently treated (C). Cells stained with anti-β1 integrins mAb do not show changes of β1-integrins dynamics in all the experimental conditions (D). Proliferative cells assessed in immunofluorescence using anti-Ki67 polyclonal antibody. There were no significant changes in the percentage of Ki67 positive cells among the various treatments (E). Number of apoptotic events evaluated by an anti-M30 staining shows no differences for all the conditions tested (F).
We evaluated the circadian oscillations of Rev-erbα and Bmal1 during the recovery phase from microgravity for consecutive 48 h. The results indicate that Rev-erbα and Bmal1 oscillations are variably affected for intensity of expression, time of peaking and period length during recovery. In particular, Bmal1 oscillations in Dex-synchronized cells previously exposed to microgravity were significantly and constantly higher as compared to cells continuously cultured at 1g conditions (Fig. 2B). Furthermore, we noticed a significant anticipation of Bmal1 expression with peak at 12 h from synchronization and a lengthened cycle period, more pronounced in Dex-treated cells in recovery (p < 0.05, one-way ANOVA followed by Bonferroni post hoc test). By contrast, the analysis of Bmal1 expression revealed no oscillations in asynchronous cells pre-cultured at 1g or μg conditions. The parallel evaluation of Rev-erbα indicated a general anti-phasic expression as compared to Bmal1, with nadir at the 12 h from synchronization corresponding to the zenith of Bmal1, with a period of about 30 h for synchronized cells, either cultured at 1g or in the recovery phase and with amplitudes and peaks of the same intensity. Again, GAPDH mRNA did not show any circadian oscillations (Fig. 2B).
Cell integrity and functional behaviour were anyhow assessed at the end of the 60 h of culture in μg, through the evaluation of cytokeratin organization, integrin dynamics, number of apoptotic or proliferative cells (Fig. 2C and F, Table 1).
3.3. The effects of microgravity on clock genes synchronization do not depend on the pharmacologic treatment
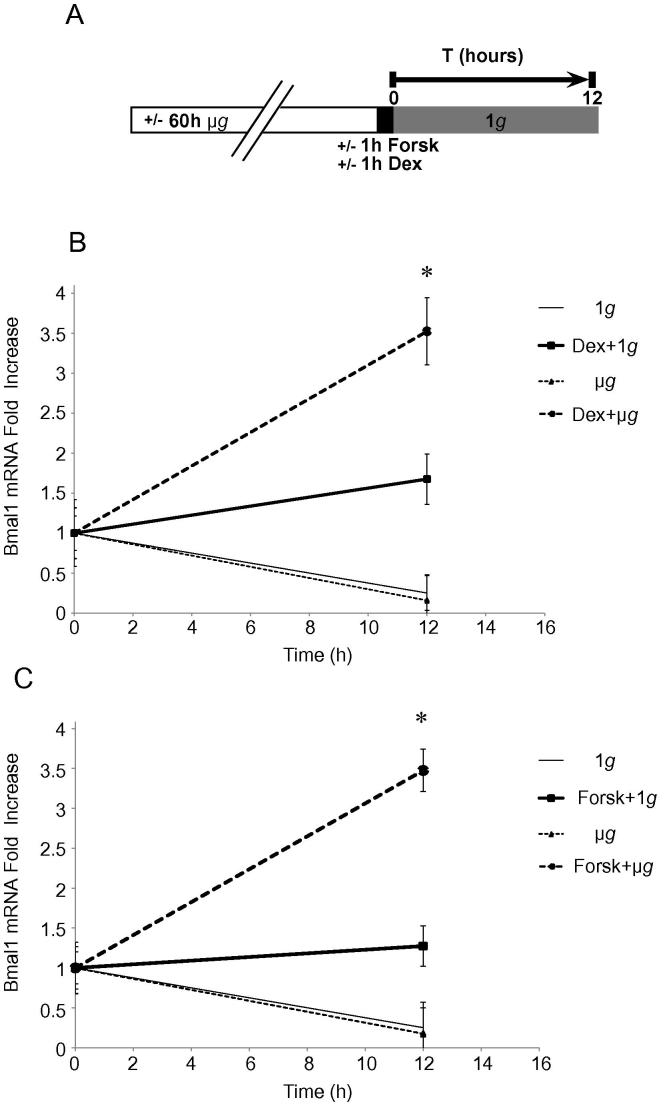
Genes of the peripheral clock can be synchronized by numerous pharmacologic treatments, each one interfering with identified signaling pathway [26]. To verify the specificity of our treatment for synchronization of the clock machinery and to ascertain the absence of interference with other pathways, we duplicate the experiment of microgravity preconditioning exposing HaCaT cells to 1 h of 1 μM forskolin after 60 h of μg. Bmal1 mRNA was quantified by qRT-PCR at 12 h, corresponding to the zenith of expression (Fig. 3A). As expected, the two synchronization protocols (Dex and Forsk) showed overlapping results with peaks of Bmal1 expression equally high in Dex- and Forsk-treated cells (Fig. 3B and C) compared to cells continuously cultured at 1g conditions.
Fig. 3.
Genes clock synchronization is not depending on the pharmacologic treatment. (A) Experimental scheme: HaCaT cells exposed to either μg or 1 g for 60 h were treated for 1 h with either Dex 1 μM or Forskolin (Forsk) 10 μM for synchronization and evaluated for Bmal1 expression after 12 h. (B) Comparative analysis of Bmal1 mRNA expression in Dex-synchronized HaCaT cells cultured either in 1 g (1g + Dex) or in microgravity (μg + Dex) reveals a 3.5-fold increase of Bmal1 mRNA in cells subjected to gravitational changes. (C) Bmal1 expression (3.5-fold increase) could be detected in μg + Forsk-treated cells as compared to 1g + Forsk-treated cells. Results are expressed as mean values ± standard deviation (SD). *p < 0.05, one-way ANOVA followed by Bonferroni post hoc test.
4. Discussion
Numerous and substantiated evidence indicates that light is the primary environmental cue that entrains circadian clocks. However, gravity and change of gravitational forces affect circadian patterns with significant changes of general behavior, hormone synthesis, body temperature and also metabolism [6–8,10,27–29]. These observations highlight the importance of the biological responses that cells display to counteract mechanical cues and, among these, changes of gravitational forces applied to cells.
It has been previously shown that rats exposed to hypergravity (hg) from mid-gestation though early lactation manifest severe alterations of multiple organs regulating metabolic activity of mammary, liver and adipose tissues with dramatic effects on maternal behavior and pup survival. These changes of the homeorhetic responses leading also to an unsuccessful lactation, are sustained by defective hormones incretion and alteration of circadian gene expression [30]. However, there is no direct evidence that microgravity and sudden changes of gravitational forces may influence the circadian oscillations of clock genes in cells not subjected to hormonal or metabolic control.
Through mechanoreceptors, epithelial cells initiate bidirectional inside-outside mechanotransduction, coupling ECM molecules and cell–cell adhesion structures, e-cadherin/α-catenin, integrins-focal adhesion kinases (FAK) complexes and G-protein-coupled receptors (GPCR) to intracellular signaling pathways through the recruitment and activation of PKC, PI3K, MAP and Rho kinases. In skin, convergence of these signals seems to be mediated by mechanosensitive transcription factors such as β-catenin, YAP and AP-1 [1]. Skin is a suitable model to study these effects: in fact, circadian rhythm in HaCaT cells is sustained by high-amplitude oscillation of the clock genes, Per2, Per3, Bmal1 and Rev-Erbα [12]. However, increased amplitudes of circadian genes expression by microgravity have not been reported before. We report that microgravitational forces applied to HaCaT cells specifically amplify the molecular clock gene Bmal1, whose amplitude, time of peaking and period, parallel the expression of the Rev-erbα clock genes. We also describe amplitude changes and significant lengthened of Bmal1 and Rev-erbα cycle periods in the recovery phase.
In fact, quantitative analysis of Bmal1 mRNA in Dex-treated synchronized HaCaT cells exposed to microgravity indicates a general increase of amplitudes with peak of a 3.9-fold increase at 6 h from synchronization. Furthermore, we observed similar effects on Bmal1 oscillation in cells exposed to μg for 60 h, then suddenly exposed to normal gravitational force. In this case, Bmal1 mRNA expression is 5.5-fold higher after 6 h from synchronization as compared to HaCaT cells that, maintained at the inertial exposure of 1g constantly show a sinusoidal Bmal1 oscillation of 24 h. By contrast, an increased length period and higher peaks of Bmal1 expression characterize the recovery phase. As expected, to the highest peaks of Rev-erbα gene expression correspond a minimal synthesis of Bmal1 mRNA, indicating a general maintenance of the transcriptional control of Rev-erbα over Bmal1 along the circadian cycle. Interestingly, 6 h is the time constantly observed and necessary for HaCaT cells to produce the maximal Bmal1 peak of expression in the transition from 1g to microgravity, while 12 h is the time necessary to observe Bmal1 peak in cells readjusting to 1g. However, while the mRNA expression levels of Rev-erbα faithfully recapitulate the anti-phasic expression to Bmal1, lack of fluctuation of Rev-erbα seems to indicate the existence of more complex mechanisms that govern their expression in cells exposed to variation of gravitational forces, and the molecular links sustaining the elongation of cycle periods are presently unknown. These effects can be explained in light of the existence of mechanotransduction machinery able to translate variation of mechanical forces applied to cells by gravitational changes into transcriptional regulated clock gene expression.
Taken together, our results demonstrate that microgravity and sudden changes of gravitational status specifically influence the molecular clock, without affecting the physiology of human keratinocytes, at least in the window of observation of 48 h. Importantly, the effects of μg on clock oscillation are detectable also during the recovery period in 1g conditions. In this case, enhanced Bmal1 transcription would be the consequence of additional mechanical stress on cells shifting from microgravity to 1g. This “double mechanical hit” in cells first exposed to microgravity, then back to 1g, would not only explain persistence of high Bmal1 amplitudes during recovery period, but even justify its further increase found in our experimental setting (3.9 in microgravity; 5.5-fold from microgravity to 1g), with a cycle period that appears to be significantly lengthened (36 h period for Bmal1, 30 h for Rev-erbα during the recovery phase). Translated to the experience of astronauts returning from space, these findings indicate the early phase of the recovery period as a critical moment for rehabilitation from microgravity, and would suggest the need of rapid and harmonic re-entrainment with our zeitgeber (see Table 1).
We did not observe appreciable morphological changes as measured by reorganization of cytokeratins and β1 integrins, rather than functional modification expressed by growth or cell death rates. This is not surprising, since only in a few cases are strength and amplitude of mechanical signals sufficient to induce adaptive changes in cell morphology, or modify the patterns of proliferation and differentiation. In general, a single type of mechanical load is unlikely to be sufficient to generate competent mechanotransductive signals to specify for defined phenotypes. Nevertheless, these signals are generated and utilized to refine transductional signals and implement transcription. To date, no mechanosensors have been identified that specifically transduce variation of gravitational forces and the pathways involved in the transcriptional control of clock genes. It will be important to pursue studies that will shed light on mechanisms of skin physiology and the interrelationships between circadian clock, mechanotransduction and cancer.
Acknowledgments
This work was partially supported by Grants from ASI – Italian Space Agency (I/003/11/0 and 2014-010-R.0), from MIUR and from AIRC – Associazione Italiana per la Ricerca sul Cancro (IG 15858), Italy.
Contributor Information
Maurizio Alimandi, Email: maurizio.alimandi@uniroma1.it.
Maria Rosaria Torrisi, Email: mara.torrisi@uniroma1.it.
References
- 1.Wang J., Zhang Y., Zhang N., Wang C., Herrler T., Li Q. An updated review of mechanotransduction in skin disorders: transcriptional regulators, ion channels, and microRNAs. Cell. Mol. Life Sci. 2015;72:2091–2106. doi: 10.1007/s00018-015-1853-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey J.D., Dufresne E.R., Schwartz M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janmey P.A., McCulloch C.A. Cell mechanics: integrating cell responses to mechanical stimuli. Annu. Rev. Biomed. Eng. 2007;9:1–34. doi: 10.1146/annurev.bioeng.9.060906.151927. [DOI] [PubMed] [Google Scholar]
- 4.Zayzafoon M., Meyers V.E., McDonald J.M. Microgravity: the immune response and bone. Immunol. Rev. 2005;208:267–280. doi: 10.1111/j.0105-2896.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 5.Hargens A.R., Richardson S. Cardiovascular adaptations, fluid shifts, and countermeasures related to space flight. Respir. Physiol. Neurobiol. 2009;169:S30–S33. doi: 10.1016/j.resp.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Robinson E.L., Fuller C.A. Gravity and thermoregulation: metabolic changes and circadian rhythms. Pflugers Arch. 2000;441:R32–R38. doi: 10.1007/s004240000329. [DOI] [PubMed] [Google Scholar]
- 7.Murakami D.M., Fuller C.A. The effect of 2G on mouse circadian rhythms. J. Gravit. Physiol. 2000;7:79–85. [PubMed] [Google Scholar]
- 8.Czeisler C.A., Chiasera A.J., Duffy J.F. Research on sleep, circadian rhythms and aging: applications to manned spaceflight. Exp. Gerontol. 1991;26:217–232. doi: 10.1016/0531-5565(91)90014-d. [DOI] [PubMed] [Google Scholar]
- 9.Mallis M.M., Deroshia C.W. Circadian rhythms, sleep, and performance. Aviat. Space Environ. Med. 2005;76:94–107. [PubMed] [Google Scholar]
- 10.Whitson P.A., Charles J.B., Williams W.J., Cintrón N.M. Changes in sympathoadrenal response to standing in humans after spaceflight. J. Appl. Physiol. 1995;79:428–433. doi: 10.1152/jappl.1995.79.2.428. [DOI] [PubMed] [Google Scholar]
- 11.Nagaraja M.P., Risin D. The current state of bone loss research: data from spaceflight and microgravity simulators. J. Cell. Biochem. 2013;114:1001–1008. doi: 10.1002/jcb.24454. [DOI] [PubMed] [Google Scholar]
- 12.Spörl F., Schellenberg K., Blatt T., Wenck H., Wittern K.P., Schrader A., Kramer A. A circadian clock in HaCaT keratinocytes. J. Invest. Dermatol. 2011;131:338–348. doi: 10.1038/jid.2010.315. [DOI] [PubMed] [Google Scholar]
- 13.Zanello S.B., Jackson D.M., Holick M.F. Expression of the circadian clock genes clock and period1 in human skin. J. Invest. Dermatol. 2000;115:757–760. doi: 10.1046/j.1523-1747.2000.00121.x. [DOI] [PubMed] [Google Scholar]
- 14.Bjarnason G.A., Jordan R.C., Wood P.A., Li Q., Lincoln D.W., Sothern R.B., Hrushesky W.J., Ben-David Y. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am. J. Pathol. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avitabile D., Genovese L., Ponti D., Ranieri D., Raffa S., Calogero A., Torrisi M.R. Nucleolar localization and circadian regulation of Per2S, a novel splicing variant of the Period 2 gene. Cell. Mol. Life Sci. 2013;71:2547–2559. doi: 10.1007/s00018-013-1503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong V.W., Longaker M.T., Gurtner G.C. Soft tissue mechanotransduction in wound healing and fibrosis. Semin. Cell Dev. Biol. 2012;23:981–986. doi: 10.1016/j.semcdb.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Pardo S.J., Patel M.J., Sykes M.C., Platt M.O., Boyd N.L., Sorescu G.P., Xu M., van Loon J.J., Wang M.D., Jo H. Simulated microgravity using the Random Positioning Machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am. J. Physiol. Cell Physiol. 2005;288 doi: 10.1152/ajpcell.00222.2004. C1211–21. [DOI] [PubMed] [Google Scholar]
- 18.Balsalobre A., Marcacci L., Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- 19.Ye J., Coulouris G., Zaretskaya I., Cutcutache I., Rozen S., Madden T.L. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 2012;13:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grosse J., Wehland M., Pietsch J., Ma X., Ulbrich C., Schulz H., Saar K., Hübner N., Hauslage J., Hemmersbach R., Braun M., van Loon J., Vagt N., Infanger M., Eilles C., Egli M., Richter P., Baltz T., Einspanier R., Sharbati S.G.D. Short-term weightlessness produced by parabolic flight maneuvers altered gene expression patterns in human endothelial cells. FASEB J. 2012;26:639–655. doi: 10.1096/fj.11-194886. [DOI] [PubMed] [Google Scholar]
- 21.Nabavi N., Khandani A., Camirand A., Harrison R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone. 2011;49:965–974. doi: 10.1016/j.bone.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 22.Grimm D., Bauer J., Kossmehl P., Shakibaei M., Schöberger J., Pickenhahn H., Schulze-Tanzil G., Vetter R., Eilles C., Paul M., Cogoli A. Simulated microgravity alters differentiation and increases apoptosis in human follicular thyroid carcinoma cells. FASEB J. 2002;16:604–606. doi: 10.1096/fj.01-0673fje. [DOI] [PubMed] [Google Scholar]
- 23.Pietsch J., Bauer J., Egli M., Infanger M., Wise P., Ulbrich C., Grimm D. The effects of weightlessness on the human organism and mammalian cells. Curr. Mol. Med. 2011;11:350–364. doi: 10.2174/156652411795976600. [DOI] [PubMed] [Google Scholar]
- 24.Shi F., Wang Y.C., Zhao T.Z., Zhang S., Du T.Y., Yang C.B., Li Y.H., Sun X.Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PLoS ONE. 2012;7:e40365. doi: 10.1371/journal.pone.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clement J.Q., Lacy S.M., Wilson B.L. Gene expression profiling of human epidermal keratinocytes in simulated microgravity and recovery cultures results morphology of HEK001 cells cultured. Geno. Prot. Bioinfo. 2008;6:14–17. doi: 10.1016/S1672-0229(08)60017-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izumo M., Sato T.R., Straume M., Johnson C.H. Quantitative analyses of circadian gene expression in mammalian cell cultures. PLoS Comput. Biol. 2006;2:e136. doi: 10.1371/journal.pcbi.0020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dijk D.J., Neri D.F., Wyatt J.K., Ronda J.M., Riel E., Ritz-De Cecco A., Hughes R.J., Elliott A.R., Prisk G.K., West J.B., Czeisler C.A. Sleep, performance, circadian rhythms, and light-dark cycles during two space shuttle flights. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R1647–R1664. doi: 10.1152/ajpregu.2001.281.5.R1647. [DOI] [PubMed] [Google Scholar]
- 28.Holley D.C., DeRoshia C.W., Moran M.M., Wade C.E. Chronic centrifugation (hypergravity) disrupts the circadian system of the rat. J. Appl. Physiol. 2003;95:1266–1278. doi: 10.1152/japplphysiol.00707.2002. [DOI] [PubMed] [Google Scholar]
- 29.Fuller P.M., Warden C.H., Barry S.J., Fuller C.A. Effects of 2-G exposure on temperature regulation, circadian rhythms, and adiposity in UCP2/3 transgenic mice. J. Appl. Physiol. 2000;89:1491–1498. doi: 10.1152/jappl.2000.89.4.1491. [DOI] [PubMed] [Google Scholar]
- 30.Casey T., Zakrzewska E.I., Maple R.L., Lintault L., Wade C.E., Baer L.A., Ronca A.E., Plaut K. Hypergravity disruption of homeorhetic adaptations to lactation in rat dams include changes in circadian clocks. Biol. Open. 2012;1:570–581. doi: 10.1242/bio.2012687. [DOI] [PMC free article] [PubMed] [Google Scholar]