Highlights
-
•
Mild heat preconditioning up-regulated HSP72 expression in cultured hypothalamic cells.
-
•
siRNA-HSP72 pretreatment down-regulated HSP72 expression.
-
•
Mild heat preconditioning attenuated heat-induced cell loss.
-
•
siRNA-HSP72 pre-treatment exacerbated heat-induced cell loss.
-
•
A positive correlation between HSP72 expression and heat tolerance might exist in hypothalamic cells.
Abbreviations: HSP, heat shock protein; MHP, mild heat preconditioning; HI, heat injury; siRNA-HSP72, pSUPER plasmid expressing HSP72 small interfering RNA
Keywords: Hyperthermia, Hypothalamus, Apoptosis, Heat shock protein 72
Abstract
Heat shock protein (HSP) 72 in serum was decreased to a greater degree in patients with serious heat stroke than in those with mild heat stroke. Thus, increased levels of HSP72 appeared to correlate with a better outcome for the patient. Nevertheless, the function of HSP72 in the heat-induced hypothalamic cell death has not been assessed. In this study, we found that increasing HSP72 levels with mild heat preconditioning or decreasing HSP72 levels with pSUPER plasmid expressing HSP72 small interfering RNA significantly attenuated or exacerbated heat-induced cell death in cultured primary hypothalamic cells, respectively. Our findings suggest that HSP72 plays a pivotal role in heat-induced cell death and may be associated with heat tolerance.
1. Introduction
Evidence has accumulated to indicate that heat shock protein 72 (HSP72) promotes cell survival under various pathologic conditions by interacting with multiple components of caspase-dependent and -independent apoptotic pathways [1]. Exogenous HSP72 overexpression significantly reduced cerebral ischemia-induced neuronal loss in rats [2]. In contrast, knockout of HSP72 exacerbated infact volume in mice after focal cerebral ischemia [3]. These findings suggest that HSP72 has significant neuroprotective effects across multiple models of brain ischemia.
According to the description by Wang et al. [4], HSP27 may play a role in the response of patients to heat stroke. Many of the heatstroke patients, displayed decreased serum levels of HSP72. The decreased response was more pronounced in those with serious heatstroke than in those with mild heatstroke. Evidence has accumulated to indicate that decreased heat tolerance is associated with hypothalamic impairment [5]. Thermal injury to the hypothalamus has been hypothesized to be the primary mechanism of multiple organ failure and mortality [6,7]. To our knowledge, evidence is not available whether cellular levels of HSP in the hypothalamus play a role in heat-induced cell death.
The present study was performed to investigate whether heat-induced cell death in rat primary cultured hypothalamic cells can be attenuated or exacerbated by increasing or decreasing HSP72, respectively. The cellular levels of HSP72 were elevated by mild heat preconditioning (MHP) [8–10] or lowered by gene silencing [11,12].
2. Materials and methods
2.1. Ethics statement
All animal procedures were performed in accordance to the guideline of the Ministry of Science and Technology of the Republic of China (Taipei, Taiwan) for Animal Care and approved by the Chi Mei Medical Center Animal Care Committee (protocol no: 100/20751). Animals were housed in specific pathogen-free facilities at the Chi Mei Medical Center (Tainan, Taiwan). All surgery was performed under anesthesia using isoflurane, and all efforts were made to minimize suffering. Human euthanasia of rats was performed by carbon dioxide with a flow rate of 20% of chamber volume/min following the guidelines of the Ministry of Science and Technology of the Republic of China.
2.2. Construction of recombinant pSUPER plasmid expressing HSP72 siRNA
pSUPER vector (vector) (Oligo Engine, Seattle, WA, USA), which contains polymerase-III HI-RNA gene promoter, can direct the synthesis of siRNA-like transcripts. The target sequence for HSP72 (Gen Bank Accession No. NM_031971) was chemically synthesised (Tri-1 Biotech; Taipei, Taiwan) as complementary oligonucleotides [11]. ABLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) search of the human genome database was done to ensure that the sequence did not target other gene transcripts. The synthetic oligonucleotide siRNA-HSP72: 5′-GATCCCCGGAGATCATCGCCAACGACTTCAAGAGAGTCGTTGGCGATGATCTCCTTTTTA-3′ and 3′-GGGCCTCTAGTAGCGGTTGCTG AAGTTCTCTCAGCAACCGCTACTAGAGGAAAAATTCGA-5′ was annealed and cloned downstream of HI promoter to construct recombinant pSUPER/siRNA-HSP72 plasmid (siRNA-HSP72). The cloned HSP72 target sequence was sequence-confirmed using a DNA sequencer (ABI Prism 377; Applied Biosystems, Foster City, CA, USA).
2.3. Cell culture and stable transfection
Primary cultured hypothalamic cells were obtained from embryonic (E18d) Sprague–Dawley rat fetuses. Briefly, the brains were removed and the hypothalamus were dissected out. The tissues were cut into fragments, and incubated in 0.2% trypsin for 20 min in 37 °C. Then, DMEM medium (Hyclone, Waltham, MA, USA) containing 10% fetal bovine serum (Hyclone) was added for trypsin activation, and the tissues were dissociated by mild mechanical trituration. About 1 × 105 cells in 1 mL were plated onto poly-d-lysine-coated 6-well plates for further culture. After 4 h, the DMEM medium was changed into Neurobasal™ medium (Gibco, Carlsbad, CA, USA) supplemented with 2% B-27 serum-free supplement (Gibco) and 0.5 mM l-glutamine in a humidified incubator containing 95% air and 5% CO2 at 37 °C. Primary cell cultures were maintained for 8 days before experiments. Eight hours before hyperthermic injury, cells were transfected with pSUPER/siHSP72 plasmid and with pSUPER vector as control by using oligofectamine transfection according to the manufacturer’s instruction. The positive clones were picked and expanded to establish cell lines, and stable transfection cell lines expressing HSP72 siRNA were determined by Western blot analysis.
2.4. Western blot analysis
The cells were harvested from flasks, and lysed in a lysis buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 1 mM MgCl2, 2.5 mM VO4, 1 mM PMSF, 2.5 mM EDTA, 0.5% Triton X-100, 0.5% NP-40, 5 μg/mL of aprotonin, peptatin A, and leupeptin) for 60 min. Protein samples (500 μg) were separated on 10% or 12% SDS–PAGE gels, and transferred to Hybond-P PVDF membranes (Amersham Biosciences, Stockholm, Sweden). After blocking with 5% non-fat dry mild in TBS-T buffer (20 mM Tris, pH 7.6, 100 mM NaCl, 0.1% Twn-20) for 2 h at room temperature, the membranes were probed with a 1:1000 dilution of anti-HSP72 (DO-1, Santa Cruz Biotechnology, CA, USA) overnight at 4 °C, and a 1:5000 dilution of β-actin (AC-15, Sigma–Aldrich) to correct for differences in protein loading, followed by incubation in a 1:2000 dilution of secondary antibodies conjugated to horseradish peroxidase (Amersham Biosciences) for 1 h at room temperature. Protein bands were detected using ECL detection (Amersham Biosciences). All of the Western immunoblots were performed at least three times.
2.5. Simulated hyperthermic injury (HI) model
The primary hypothalamic cultured cells were put in a circulating 43 °C water bath for 120 min to induce HI, while the normothermic controls were kept in a 37 °C incubator for the equivalent time.
2.6. Mild heat preconditioning (MHP)
Eight hours before the start of HI, the cells were kept in a circulating 42 °C water bath for 30 min to induce HSP72 expression [8].
2.7. Cell viability
Cell viability was determined using an MTT (3-C-4.5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay (Invitrogen, Carlsbad, CA, USA) in which the yellow tetrazolium salt catalyzed by nicotinamide adenine dinucleotide (NAD)-dependent dehydrogenase (in active mitochondria) to form purple formalin salt crystals. Cells were seeded into a 96-well plate. After HI, the MTT reagent (5 mg/mL in PBS) was added to each well and then incubated for 4 h. After the assay reagent had been removed, dimethyl sulfoxide (DMSO) was added to each well to solubilize the purple crystals. Plates were then immediately analyzed using an ELISA plate reader (Biotals Instruments, Winooski, VVT, USA) at 580 nm. Cell viability was defined relative to the ratio of normothermic control cells as follow: cell viability (%) = (value of optical density of treated sample)/(value of optical density of control × 100).
2.8. Flow cytometry
Apoptosis was analyzed using a kit (Annexin V-FITC kit, Beckman Coulter, Fullerton, CA) that measures the binding of a fluorescein-labelled annexin V to phosphatidylserine residues translocated on the cell surface soon after the induction of apoptosis and before nuclear breakdown occurs. After HI, cells were collected by trypsinisation and were suspended at a concentration of 1 × 106/mL in phosphate buffered saline. All subsequent steps were done on ice. The cells were resuspended in binding buffer and incubated with annexin V-FITC for 15 min in the dark. The cells were then re-pelleted and re-suspended in binding buffer, propidium iodide was added, and a flow cytometer (Beckman Coulter, Miami, FL, USA) was used for analysis: (A) viable cells do not stain with either reagent, (B) necrotic cells stain with both reagents, and (C) apoptotic cells stain with annexin V-FITC only.
2.9. Experimental groups
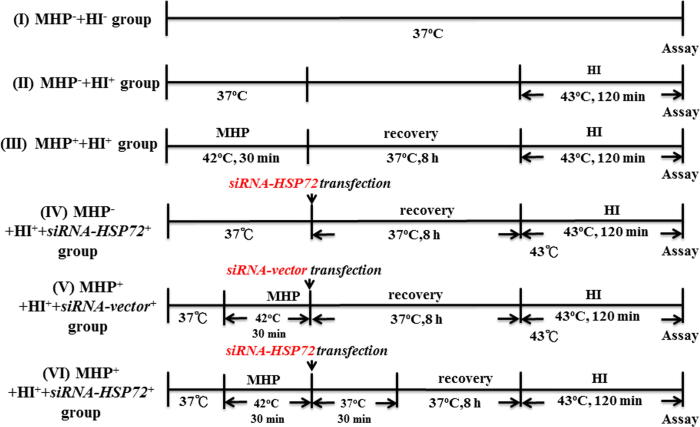
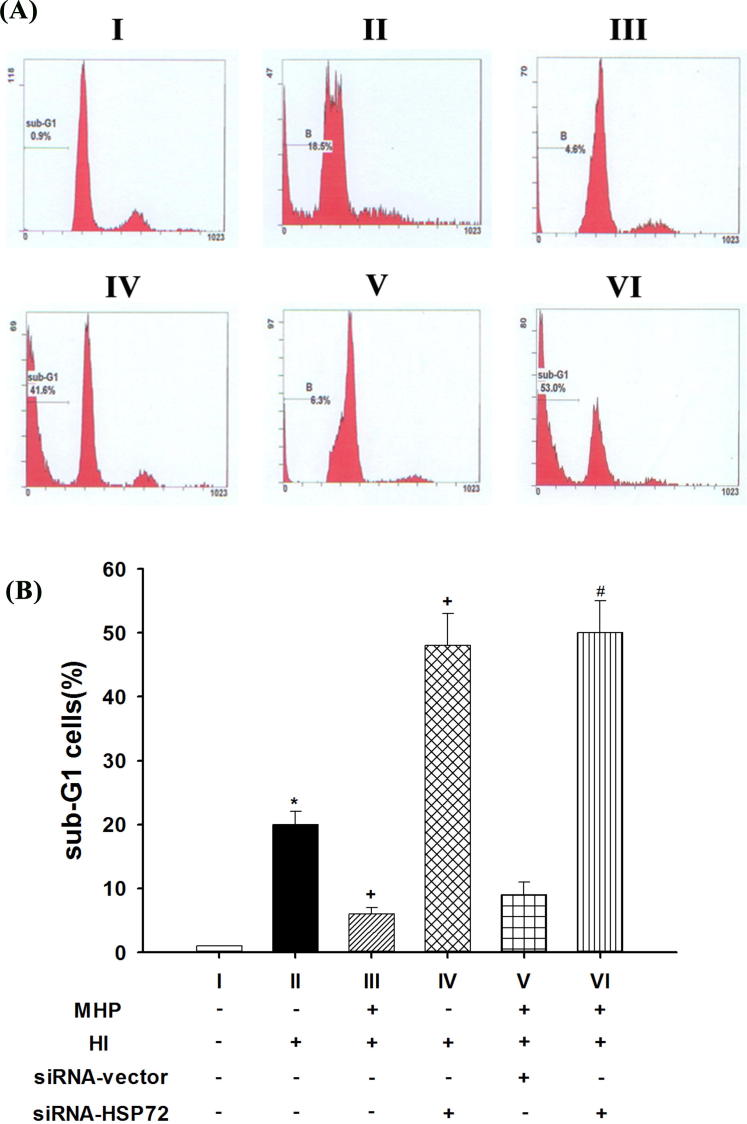
Six groups of hypothalamic cells were used in the present study (Fig. 1): (I) no mild heat preconditioning (MHP−) and no hyperthermic injury (HI) (MHP− + HI−); (II) no MHP and HI (MHP− + HI+): the cells were kept in a circulating water bath of 43 °C for 120 min; (III) MHP+ + HI+: before the start of HI, the cells were kept in a circulating 42 °C water bath for 30 min and then allowed to recover at 37 °C for 8 h; (IV) MHP− + HI+ + siRNA-HSP72+ group: the MHP− cells were transfected with pSUPER/siRNA-HSP72 8 h before the start of HI; (V) MHP+ + HI+ + siRNA-vector+ group: the MHP+ cells were transfected with siRNA-vector 8 h before the start of HI; and (VI) MHP+ + HI+ + siRNA-HSP72+ group: the MHP+ cells were transfected with siRNA-HSP72 8 h before the start of HI.
Fig. 1.
Six experimental groups: (I) no mild heat preconditioning (MHP−) and no hyperthermic injury (HI−) (MHP− + HI−); (II) no MHP and HI (MHP− + HI+); (III) MHP+ + HI+; (IV) MHP− + HI+ + siRNA-HSP72+; (V) MHP+ + HI+ + siRNA-vector+; and (VI) MHP+ + HI+ + siRNA-HSP72+. Please see Section 2 for the explanations of group abbreviations.
2.10. Statistical analysis
All assays were independently performed three times. The data are means ± standard deviation (SD). Comparisons between groups were made using analysis of variance (ANOVA) F tests. Bonferroni adjusted t-tests were used for multiple-group comparisons, and an unpaired t-test was used for single comparison. Significance was set at P < 0.05 (two-tailed).
3. Results
3.1. Cellular HSP72 was lower in siRNA-HSP72-treated cultured primary hypothalamic cells
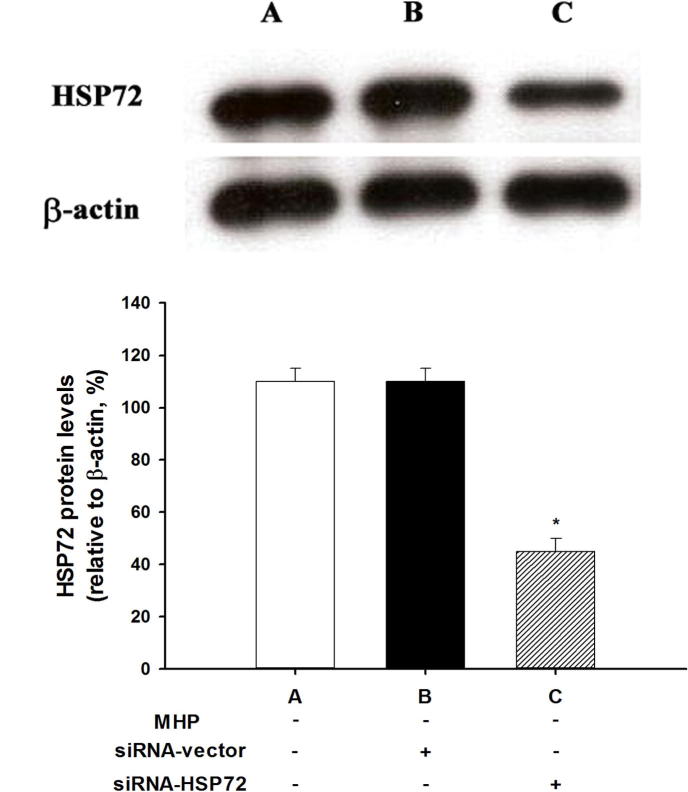
Western blotting was used to analyze cultured primary hypothalamic cells without mild heat preconditioning (MHP−). In the MHP− cells treated with siRNA-HSP72 (MHP− + siRNA-HSP72+), levels of cellular HSP72 were significantly lower than those for the MHP− controls (P < 0.05; Fig. 2C vs. Fig. 2A). In contrast, no differences were found in the cellular levels of HSP72 between the MHP− + siRNA-vector+ group and the MHP- control group (P > 0.05, Fig. 2B vs. Fig. 2A).
Fig. 2.
Effect of siRNA-HSP72 on cellular levels of HSP72 in different groups of cultured primary cells. A: MHP− control; B: MHP− + siRNA-vector; C: MHP− + siRNA-HSP72. Cellular expression of HSP72 was assessed by Western blot analysis in sham groups of rats with no MHP. The gels presented are representative of results from three separate experiments. Densitometry readings of gel bands represent mean ± SD of three separate experiment. *P < 0.05 for A vs. C.
3.2. Cellular HSP72 was higher in MHP-treated cultured primary hypothalamic cells under HI
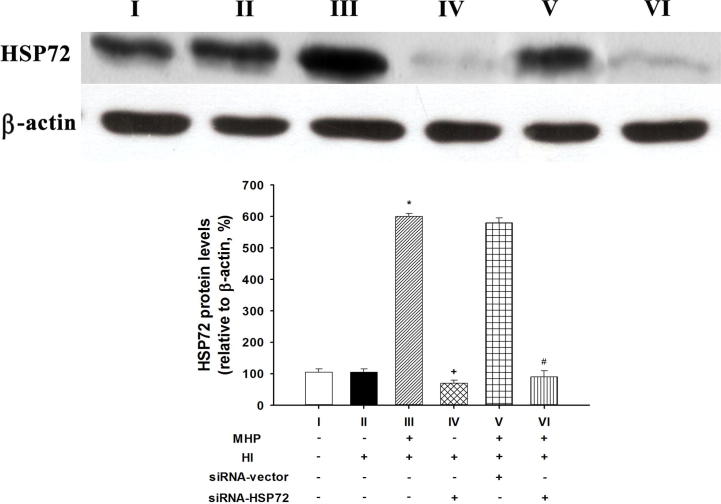
Levers of HSP72 in the cultured MHP− + HI cells were insignificantly different from MHP− + HI− cells (P > 0.05; Fig. 3II vs. Fig. 3I). In (MHP+ + HI+) cells, levels of cellular HSP72 were significantly higher than those for the (MHP− + HI) cells (P < 0.01; Fig. 3III vs. Fig. 3II). However, in (MHP− + HI+ + siRNA-HSP72) cells, levels of cellular HSP72 were significantly lower than those for the (MHP− + HI+) cells (P < 0.05; Fig. 3IV vs. Fig. 3II). Levels of HSP72 in the (MHP+ + HI+ + siRNA-HSP72), but not the (MHP+ + HI+ + siRNA-vector) cells, were significantly lower than those for the (MHP+ + HI+) cells (P < 0.01; Fig. 3VI vs. III).
Fig. 3.
Effect of mild heat preconditioning (MHP+) on cellular expression of HSP72 in different groups of cultured primary cells. I: MHP− + HI−; II: MHP− + HI+; III: MHP+ + HI+; IV: MHP− + HI+ + siRNA-72; V: MHP+ + HI+ + siRNA-vector; and VI: MHP+ + HI+ + siRNA-HSP72. Please see Section 2.9 procedures for the explanations of the abbreviation. Cellular expression of HSP72 was assessed by Western blot analysis. The gels presented are representative of results from three separate experiments. Densitometry readings of gel bands expressed as arbitrary units of relative intensities to that of MHP− + HI− control. Values represent mean ± SD of three separate experiments. *P < 0.01 for III vs. II; +P < 0.01 for IV vs. II; #P < 0.01 for VI vs. III.
3.3. HI-induced cell loss was reduced by MHP+ but exacerbated by siRNA-HSP72
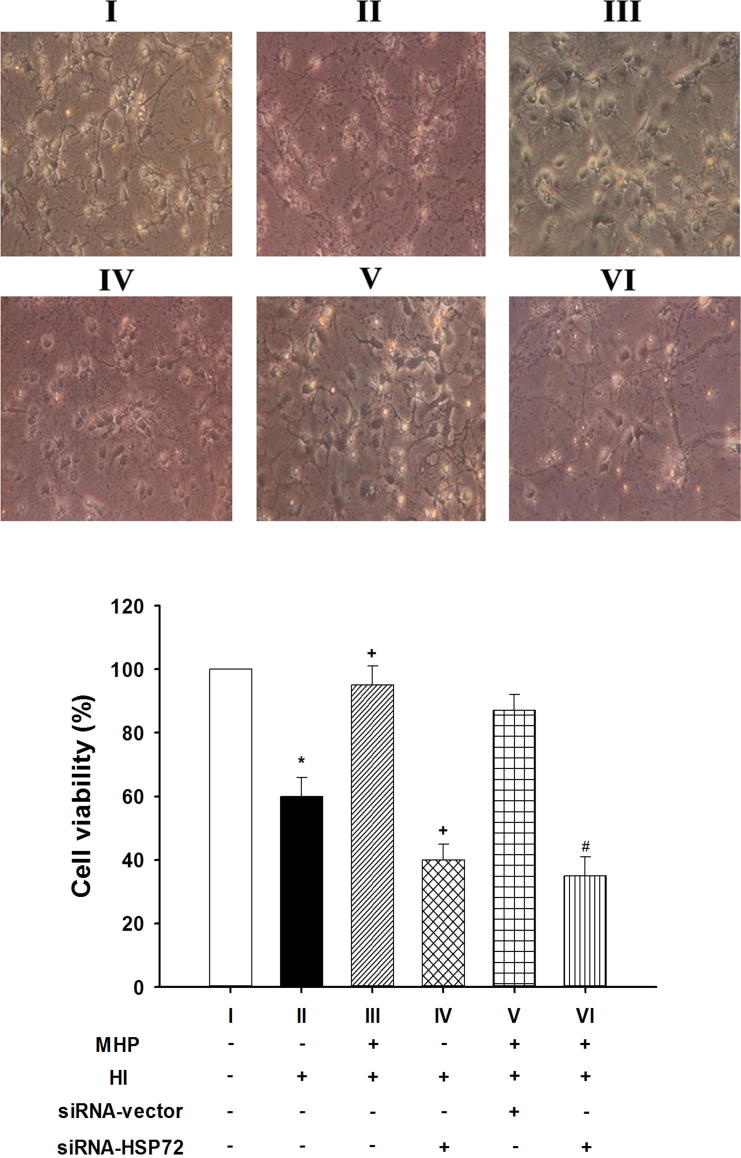
In the (MHP− + HI+) cells, percentages of cell viability were significantly lower than those for the (MHP− + HI−) cells (P < 0.05; Fig. 4II vs. Fig. 4I). The cell loss caused by HI was significantly reduced by MHP (P < 0.05; Fig. 4III vs. Fig. 4II) but significantly exacerbated by siRNA-HSP72 preconditioning (P < 0.05; Fig. 4IV vs. Fig. 4II). The beneficial effects of MHP+ in reducing HI-induced cell loss were significantly attenuated by siRNA-HSP72 (P < 0.05; Fig. 4VI vs. Fig. 4III) but not by siRNA-vector (P > 0.05; Fig. 4V vs. Fig. 4III).
Fig. 4.
The percentage of cell viability exhibited in different groups of cultured primary cells. Cells were stained with an MTT assay. Data were presented that MHP+ increased the percentage of cell viability during HI+ treatment, showed at (A) quality and (B) quantity. In contrast, siRNA-HSP72 exacerbated HI-induced cell death. The beneficial effects of MHP in reducing HI-induced cell death were attenuated by siRNA-HSP72 preconditioning but not by siRNA-vector preconditioning. *P < 0.001 for II vs. I; +P < 0.05 for II vs. III or for IV vs. II; #P < 0.05 for VI vs. III. Data are the mean ± SD of triplicate experiments. Please see Section 2.9 procedure for the explanations of group abbreviation.
3.4. HI-induced cell apoptosis was reduced by MHP+ but exacerbated by siRNA-72
In the (MHP− + HI+) cells, percentages of apoptotic cells were significantly higher than those for the (MHP− + HI−) cells (P < 0.01; Fig. 5II vs. Fig. 5I). The cellular apoptosis caused by HI was significantly attenuated by MHP (P < 0.05; Fig. 5III vs. Fig. 5II), but significantly exacerbated by siRNA-HSP72 preconditioning (P < 0.01; Fig. 5IV vs. Fig. 5II). In addition, the beneficial effects of MHP+ in reducing HI-induced cell apoptosis were significantly attenuated by siRNA-HSP72 (P < 0.05; Fig. 5VI vs. Fig. 5III) but not by siRNA-vector (P > 0.05; Fig. 5V vs. Fig. 5III).
Fig. 5.
The level or apoptosis exhibited in different groups of cultured primary cells. Apoptotic cells were detected on a propidium iodide (PI) histogram as percentage of sub G1 population (A). The percentage of apoptotic cells was determined by flow cytometry after PI labeling. (B) Data are the mean ± SD of triplicate experiments. *P < 0.01 for II vs. I; +P < 0.05 for III vs. II or for IV vs. II; #P < 0.01 for VI vs. III. In (A) panel, the data are reported as a representative experiment. Apoptotic cells are characterized by low DNA stain ability and appear below the GI peak in the distribution. Please see Section 2.9 procedures for the explanations of group abbreviation.
4. Discussion
In the central nervous system, topical application of naked small interfering RNAs (siRNA) has been used successfully to induce gene silencing [13–15]. The pSUPER RNAί system (Oligo Engine, Seattle WA, USA) efficiently and specifically downregulates gene expression [11], which functionally inactivates targeted genes. Indeed, as shown in the present results, topical application of pSUPER plasmid expressing HSP72 small interfering RNAA significantly attenuates the cellular levels of HSP72 in primary cultured hypothalamic cells.
Probably, the most striking findings are that the cellular levels of HSP72 can be elevated or lowered by mild heat preconditioning (42 °C for 30 min) or gene silencing (pSUPER plasmid expressing HSP72 small interfering RNA) in rat hypothalamic cells respectively. In addition, both hypothalamic cell loss and apoptosis caused by severe heat (43 °C for 120 min) are significantly attenuated or exacerbated by increasing or decreasing HSP72 levels in primary cultured hypothalamic cells, respectively. Thus, it appears that the extent of cellular heat tolerance depends upon the cellular levels of HSP72 in the hypothalamus. Our results are supported in part by human data showing that healthy volunteers with body temperature reached or exceeded 39 °C had elevated serum HSP72 levels whereas many of the heatstroke patients failed to show elevated serum HSP72 levels [4]. Owing to the patients with serious heatstroke had lower serum levels of HSP72 than did the patients with mild heatstroke, they promoted that the abnormally low serum levels of HSP72 was likely the “cause” of heatstroke. In addition, our results are consistent in part with transgenic mice data. Chen and colleagues [16] report that overexpression of HSP72 in the hypothalamus significantly protects against the heat-induced hypothalamic ischemia and oxidative damage, thermoregulatory dysfunction and mortality. l-arginine administration to heat stressed mice [17] or glutamine administration to heat stressed rats [18] is also shown to increase the expression of HSP72 and contributes to the protective effect of these drugs in rodents subjected to heatstroke. Geranylgeranyl-acetone, an acryclic isoprenoid, is a non-tonic inducer of HSP72, preconditioning attenuated heat-induced inflammation and multiple organ dysfunction in rats [19]. Pre-induction of HSP72 in different organs (including brain) with progressive exercise [20,21] or hypobaric hypoxia [22] preconditioning attenuates heat-induced multiple organ dysfunction in rats. A number of studies have also demonstrated that ischemic preconditioning induces neuroprotection by inducing HSP72 in neurons [23]. In addition, inducing HSP72 in brain by physical exercise is shown to protect against ischemic stroke in rats [24]. These observations indicate that the increased HSP72 levels prior to stress-induced injury may be one of the key issues. Increased or decreased HSP72 levels might attenuate or exacerbated the stress-induced injury, respectively.
In heat stressed animals, their hypothalami are fully activated, as suggested by the increase in c-fos mRNA and protein in different brain regions including the hypothalamus [25]. The hypothalamo–pituitary–adrenocortical (HPA) axis is also mobilized as suggested by the increase in fos-positive cells [26] in the hypothalamus and the increase in blood adrenocorticotropic-hormone (ACTH) and corticosterone concentrations [27,28]. Inter-individual heterogeneousness in heat tolerance could be associated with HPA axis activation states. In other words, the heat intolerant animals would display an inadequate HPA axis mobilization, whereas heat tolerant animals would benefit from an adequate HPA axis control [5]. It is not known whether hypothalamic levels of HSP72 are associated with HPA axis activity.
Although Annexin is indeed for apoptosis, PI also marks apoptotic cells [29]. This is evident in our present results in Figs. 4 and 5 (viability and apoptotic). If viability is viewed as the inverse of cell death, then Figs. 4 and 5 are virtually identical. That is, 100% viability in control conditions of Fig. 4 is basically no apoptotic cells in control condition of Fig. 5. Similarly, 50% viability for HI + HSP72 knockdown in Fig. 4 is nearly same as 50% apoptotic for same treatment in Fig.5. Based on the present data, we estimate that total dead cells (60% in Fig. 4) minus apoptotic of Fig. 5 (20%) equals 40% of necrotic cells, e.g. for treatment II.
In summary, our data show that severe heat caused hypothalamic apoptosis and injury. Mild heat preconditioning, in addition to inducing HSP72 overexpression, significantly reduces severe heat shock-induced hypothalamic cell apoptosis and injury. In contrast, pSUPER plasmid expressing HSP72 small interfering RNA reduces cellular levels of HSP72 and significantly exacerbates the heat-induced hypothalamic cell apoptosis and injury. The beneficial effects of mild heat preconditioning are significantly attenuated by silencing of selected genes and knockdown of HSP72. Our data suggest that prior expression of HSP72 by mild heat preconditioning protects against injury and apoptosis caused by severe heat in primary cultured hypothalamic cells.
Acknowledgements
This work is partially supported by grants from the Ministry of Science and Technology, Taiwan, R.O.C. (Grant No. NSC 101-2314-B-218-001-MY3, and MOST 103-2314-B-384-002) and Chi Mei Medical Center, Tainan, Taiwan (Grant No. CMFHT10401).
Kao-Chang Lin and Hung-Jung Lin conceived and designed the project and acquired the data. Kao-Chang Lin, Hung-Jung Lin, and Ching-Ping Chang analyzed and interpreted the data. Ching-Ping Chang and Mao-Tsun Lin wrote the paper.
Contributor Information
Ching-Ping Chang, Email: jessica@mail.stust.edu.tw.
Mao-Tsun Lin, Email: 891201@mail.chimei.org.tw.
References
- 1.Evans C.G., Chang L., Gestwicki J.E. Heat shock protein 70 (HSP70) as an emerging drug target. J. Med. Chem. 2010;53:4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuchiya D., Hong S., Matsumori Y., Kayama T., Swanson R.A., Dillman W.H., Liu J., Panter S.S., Weinstein P.R. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery. 2003;53:1179–1187. doi: 10.1227/01.neu.0000090341.38659.cf. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.H., Kim M., Yoon B.W., Kim Y.J., Ma S.J., Roh J.K., Lee J.S., Seo J.S. Targeted HSP70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32:2905–2912. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z.Z., Wang C.L., Wu T.C., Pan H.N., Wang S.K., Jiang J.D. Autoantibody response to heat shock protein 70 in patients with heatstroke. Am. J. Med. 2001;111:654–657. doi: 10.1016/s0002-9343(01)00974-3. [DOI] [PubMed] [Google Scholar]
- 5.Michel V., Peinnequin A., Alonso A., Buguet A., Cespuglio R., Canini F. Decreased heat tolerance is associated with hypothalamo–pituitary–adrenocortical axis impairment. Neuroscience. 2007;147:522–531. doi: 10.1016/j.neuroscience.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Mslsmud N., Haymaker W., Custer R.P. Heat stroke; a clinico-pathologic study of 125 fatal cases. Mil. Surg. 1946;99:397–449. [PubMed] [Google Scholar]
- 7.Chen S.H., Lin M.T., Chang C.P. Ischemic and oxidative damage to the hypothalamus may be responsible for heat stroke. Curr. Neuropharmacol. 2013;11:129–140. doi: 10.2174/1570159X11311020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu S.F., Chao C.M., Huang W.T., Lin M.T., Cheng B.C. Attenuating heat-induced cellular autophagy, apoptosis and damage in H9c2 cardiomyocytes by pre-inducing HSP70 with heat shock preconditioning. Int. J. Hyperther. 2013;29:239–247. doi: 10.3109/02656736.2013.777853. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y.L., Lin M.T. Heat shock protein expression protects against cerebral ischemia and monoamine overload in rat heatstroke. Am. J. Physiol. 1999;276(6 Pt 2):H1961–H1967. doi: 10.1152/ajpheart.1999.276.6.H1961. [DOI] [PubMed] [Google Scholar]
- 10.Wang J.L., Ke D.S., Lin M.T. Heat shock pretreatment may protect against heatstroke-induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock. 2005;23:161–167. doi: 10.1097/01.shk.0000150779.47107.d5. [DOI] [PubMed] [Google Scholar]
- 11.Brummelkamp T.R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 12.Chang C.K., Chou W., Lin H.J., Huang Y.C., Tang L.Y., Lin M.T., Chang C.P. Exercise preconditioning protects against spinal cord injury in rats by upregulating neuronal and astroglial heat shock protein 72. Int. J. Mol. Sci. 2014;15:19018–19036. doi: 10.3390/ijms151019018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lingor P., Koeberle P., Kügler S., Bähr M. Down-regulation of apoptosis mediators by RNAi inhibits axotomy-induced retinal ganglion cell death in vivo. Brain. 2005;128:550–558. doi: 10.1093/brain/awh382. [DOI] [PubMed] [Google Scholar]
- 14.Makimura H., Mizuno T.M., Mastaitis J.W., Agami R., Mobbs C.V. Reducing hypothalamic AGRP by RNA interference increases metabolic rate and decreases body weight without influencing food intake. BMC Neurosci. 2002;3:18. doi: 10.1186/1471-2202-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manrique C., Compan V., Rosselet C., Duflo S.G. Specific knock-down of GAD67 in the striatum using naked small interfering RNAs. J. Biotechnol. 2009;142:185–192. doi: 10.1016/j.jbiotec.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z.C., Wu W.S., Lin M.T., Hsu C.C. Protective effect of transgenic expression of porcine heat shock protein 70 on hypothalamic ischemic and oxidative damage in a mouse model of heatstroke. BMC Neurosci. 2009;10:111. doi: 10.1186/1471-2202-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee S., Premachandran S., Sharma D., Bagewadikar R.S., Poduval T.B. Therapeutic treatment with l-arginine rescues mice from heat stroke-induced death: physiological and molecular mechanisms. Shock. 2005;24:247–341. doi: 10.1097/01.shk.0000180983.55623.2b. [DOI] [PubMed] [Google Scholar]
- 18.Singleton K.D., Wischmeyer P.E. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock. 2006;25:295–299. doi: 10.1097/01.shk.0000196548.10634.02. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y.Q., Gao J.T., Liu S.H., Wu Y., Lin M.T., Fan M. Geranylgeranylacetone preconditioning may attenuate heat-induced inflammation and multiorgan dysfunction in rats. J. Pharm. Pharmacol. 2010;62:99–105. doi: 10.1211/jpp.62.01.0011. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y.W., Chen S.H., Chou W., Lo Y.M., Hung C.H., Lin M.T. Exercise pretraining protects against cerebral ischaemia induced by heat stroke in rats. Br. J. Sports Med. 2007;41:597–602. doi: 10.1136/bjsm.2006.033829. (See comment in PubMed Commons below) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung C.H., Chang N.C., Cheng B.C., Lin M.T. Progressive exercise preconditioning protects against circulatory shock during experimental heatstroke. Shock. 2005;23:426–433. doi: 10.1097/01.shk.0000159557.95285.96. [DOI] [PubMed] [Google Scholar]
- 22.Wang L.C., Chang C.P., Chio C.C., Wu M.H., Lee Y.S., Huang C.Y., Tsai K.J. Hypobaric hypoxia preconditioning attenuates experimental heatstroke syndromes via preinduction of heat shock protein 70. Am. J. Med. Sci. 2012;344:383–390. doi: 10.1097/MAJ.0b013e31824314fe. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.E., Yenari M.A., Sun G.H., Xu L., Emond M.R., Cheng D., Steinberg G.K., Giffard R.G. Differential neuroprotection from human heat shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp. Neurol. 2001;170:129–139. doi: 10.1006/exnr.2000.7614. [DOI] [PubMed] [Google Scholar]
- 24.Liebelt B., Papapetrou P., Ali A., Guo M., Ji X., Peng C., Rogers R., Curry A., Jimenez D., Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- 25.Lin M.T., Yang Y.L., Tsay H.J. c-fos expression in rat brain during heat stress. J. Therm. Biol. 1999;24:423–427. [Google Scholar]
- 26.Cham J.L., Klein R., Owens N.C., Mathai M., McKinley M., Badoer E. Activation of spinally projecting and nitrergic neurons in the PVN following heat exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R91–R101. doi: 10.1152/ajpregu.00675.2005. [DOI] [PubMed] [Google Scholar]
- 27.Curẻ M. Plasma corticosterone response in continuous versus discontinuous chronic heat exposure in rat. Physiol. Behav. 1988;45:1117–1122. doi: 10.1016/0031-9384(89)90097-8. [DOI] [PubMed] [Google Scholar]
- 28.Djordjević J., Cvijić G., Davidović V. Different activation of ACTH and corticosterone release in response to various stressors in rats. Physiol. Res. 2003;52:67–72. [PubMed] [Google Scholar]
- 29.Fiorelli T., Kirouac L., Padmanabhan J. Altered processing of amyloid precursor protein in cells undergoing apoptosis. PLoS One. 2013;8:e57979. doi: 10.1371/journal.pone.0057979. [DOI] [PMC free article] [PubMed] [Google Scholar]