Abstract
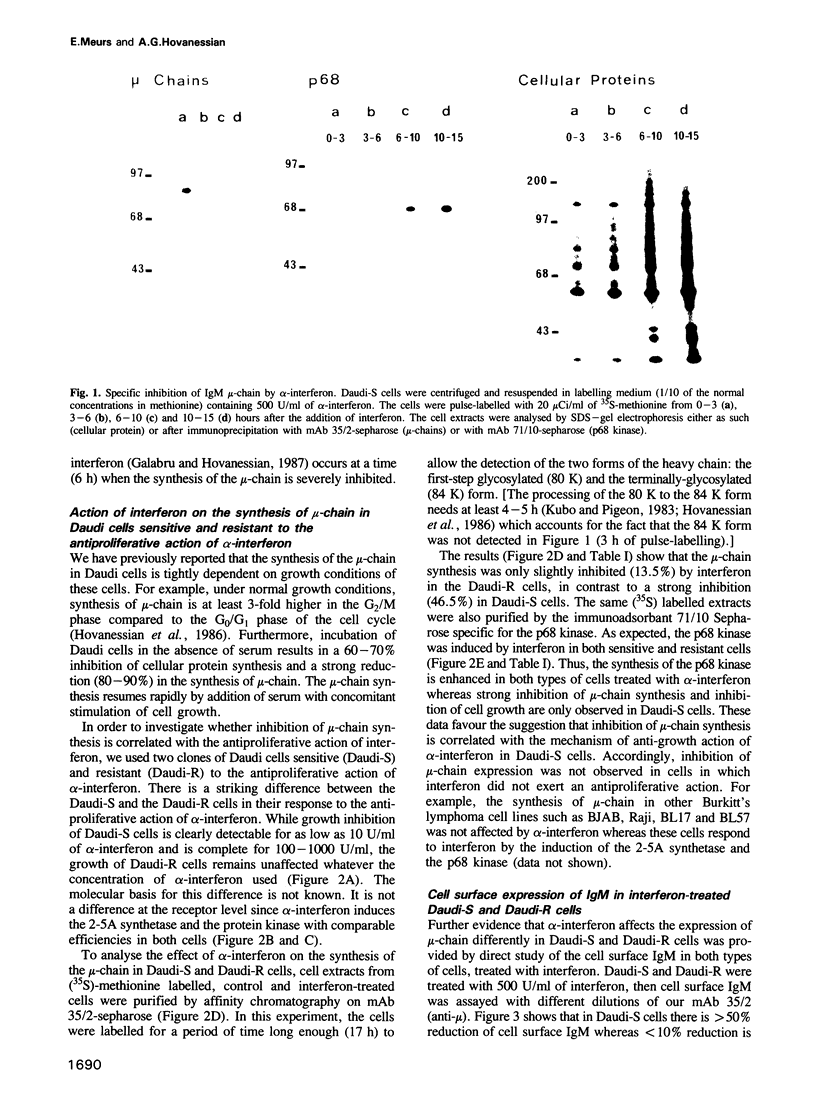
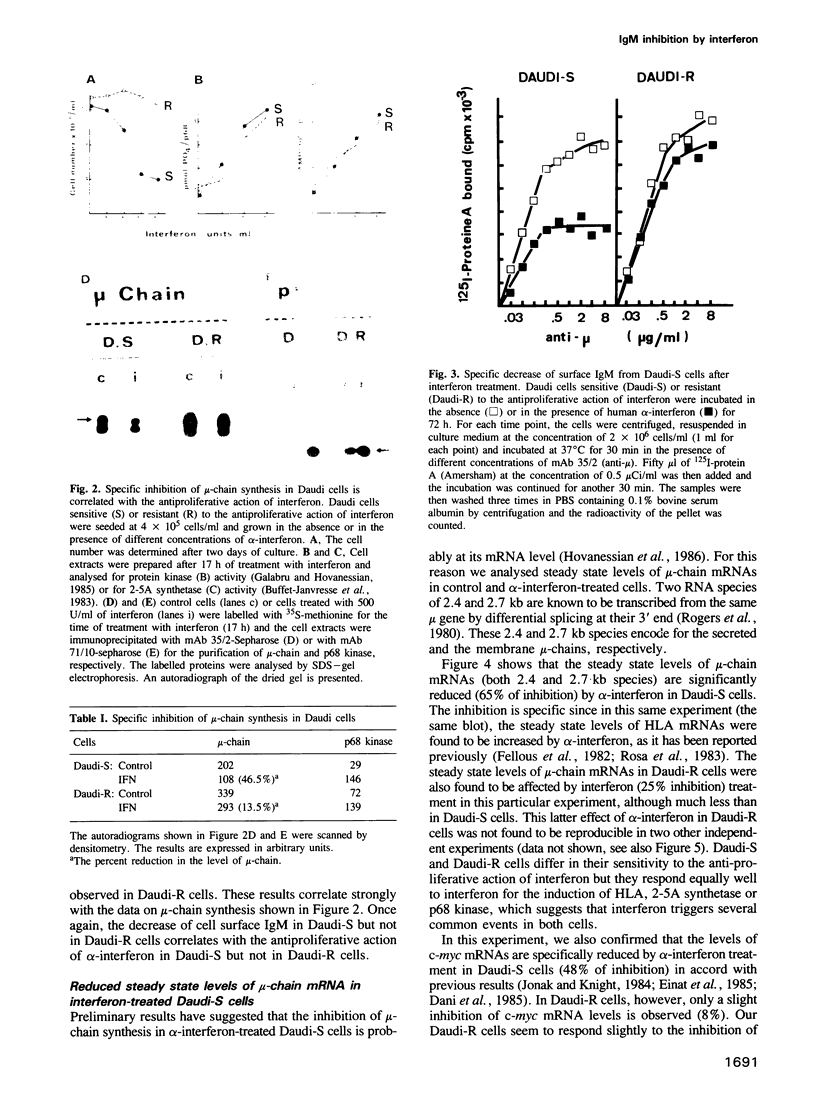
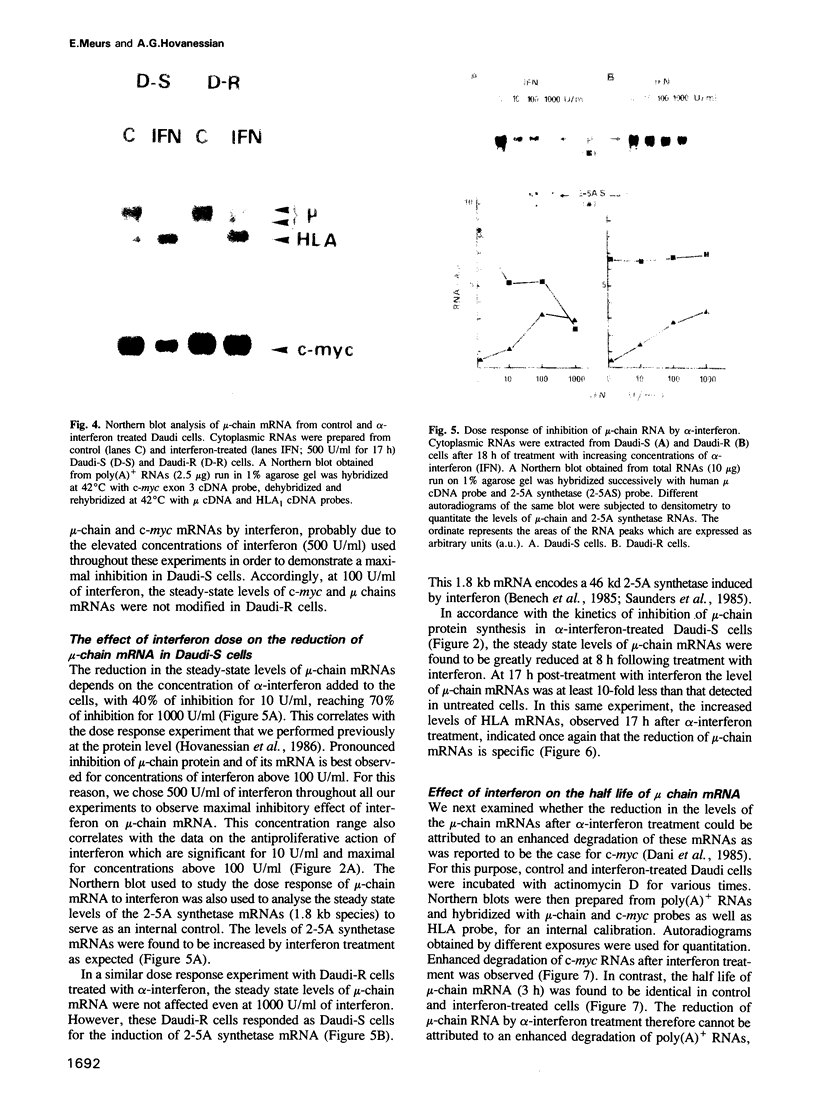
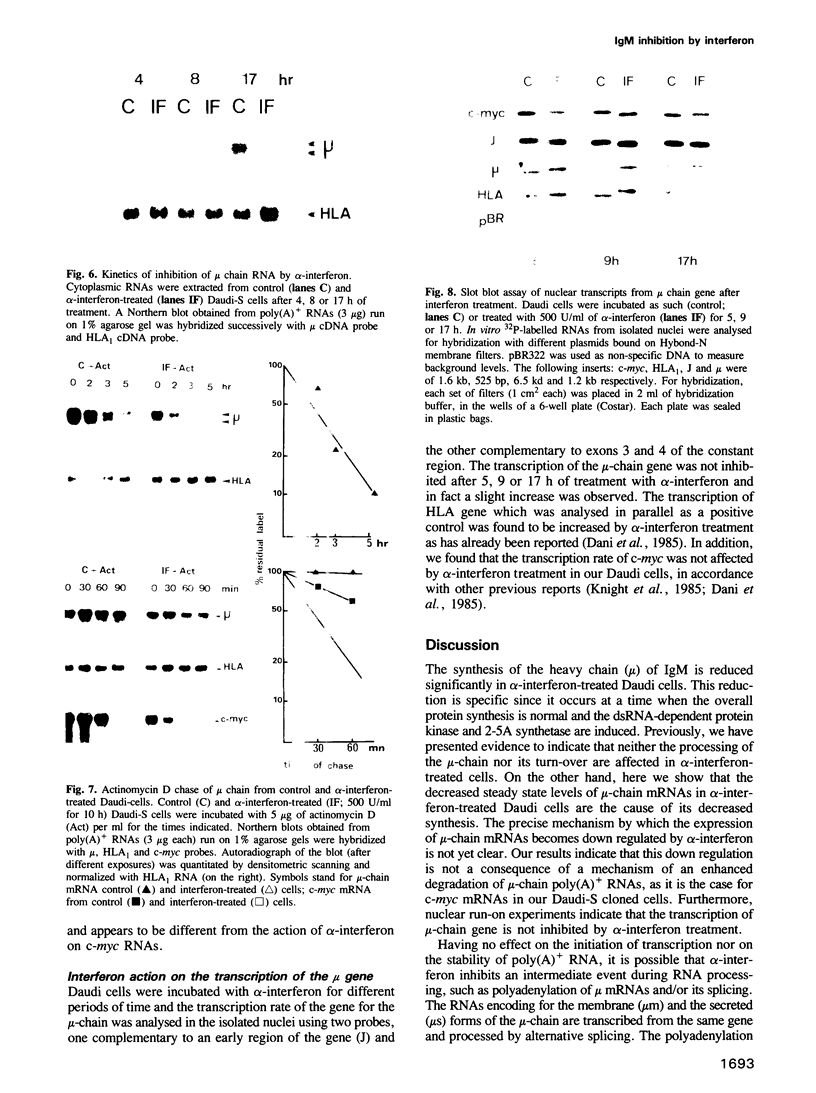
A clone of Daudi cells (Daudi-S) synthesizes the heavy chain of IgM (mu-chain) under routine conditions of cell culture. In the presence of alpha-interferon, however, synthesis of mu-chain is decreased rapidly at a time when the overall protein synthesis is not modified and the dsRNA-dependent protein kinase and the 2-5A synthetase are induced. This inhibition of mu-chain synthesis seems to be correlated with the antiproliferative action of interferon since it occurs only slightly in another clone of Daudi cells resistant (Daudi-R) to the antiproliferative action of interferon. In these resistant cells, however, the protein kinase and the 2-5A synthetase are induced by interferon. Specific inhibition of mu-chain synthesis in interferon-treated Daudi-S cells is a consequence of decreased steady-state levels of mu-chain mRNA. This effect occurs 4-8 h after addition of interferon in parallel with decreased levels of c-myc mRNA and enhanced levels of HLA mRNA. Reduced levels of mu-chain mRNA in interferon-treated Daudi-S cells is not a consequence of its enhanced degradation as shown by actinomycin D chase experiments. Furthermore, nuclear run on experiments rule out an effect on the transcription of mu-chain mRNA. Therefore, the inhibitory mechanism mediated by interferon might be at the level of termination and/or post-transcriptional processing of mu-chain RNA. In contrast, in these same interferon-treated Daudi-S cells, the inhibition of c-myc gene expression is due to an enhanced degradation of its mRNA (in accord with other reports). These data indicate that interferon can inhibit gene expression by different mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benech P., Mory Y., Revel M., Chebath J. Structure of two forms of the interferon-induced (2'-5') oligo A synthetase of human cells based on cDNAs and gene sequences. EMBO J. 1985 Sep;4(9):2249–2256. doi: 10.1002/j.1460-2075.1985.tb03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffet-Janvresse C., Magard H., Robert N., Hovanessian A. G. Assay and the levels of 2-5A-synthetase in lymphocytes of patients with viral, bacterial and autoimmune diseases. Ann Immunol (Paris) 1983 Sep-Oct;134D(2):247–258. doi: 10.1016/s0771-050x(83)80090-4. [DOI] [PubMed] [Google Scholar]
- Dani C., Mechti N., Piechaczyk M., Lebleu B., Jeanteur P., Blanchard J. M. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4896–4899. doi: 10.1073/pnas.82.15.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois M. F., Vignal M., Le Cunff M., Chany C. Interferon inhibits transformation of mouse cells by exogenous cellular or viral genes. Nature. 1983 Jun 2;303(5916):433–435. doi: 10.1038/303433a0. [DOI] [PubMed] [Google Scholar]
- Einat M., Resnitzky D., Kimchi A. Close link between reduction of c-myc expression by interferon and, G0/G1 arrest. Nature. 1985 Feb 14;313(6003):597–600. doi: 10.1038/313597a0. [DOI] [PubMed] [Google Scholar]
- Exley R., Gordon J., Clemens M. J. Induction of B-cell differentiation antigens in interferon- or phorbol ester-treated Daudi cells is impaired by inhibitors of ADP-ribosyltransferase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6467–6470. doi: 10.1073/pnas.84.18.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fellous M., Nir U., Wallach D., Merlin G., Rubinstein M., Revel M. Interferon-dependent induction of mRNA for the major histocompatibility antigens in human fibroblasts and lymphoblastoid cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3082–3086. doi: 10.1073/pnas.79.10.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. G. Two interferon-induced proteins are involved in the protein kinase complex dependent on double-stranded RNA. Cell. 1985 Dec;43(3 Pt 2):685–694. doi: 10.1016/0092-8674(85)90241-7. [DOI] [PubMed] [Google Scholar]
- Galabru J., Hovanessian A. Autophosphorylation of the protein kinase dependent on double-stranded RNA. J Biol Chem. 1987 Nov 15;262(32):15538–15544. [PubMed] [Google Scholar]
- Galli G., Guise J. W., McDevitt M. A., Tucker P. W., Nevins J. R. Relative position and strengths of poly(A) sites as well as transcription termination are critical to membrane versus secreted mu-chain expression during B-cell development. Genes Dev. 1987 Jul;1(5):471–481. doi: 10.1101/gad.1.5.471. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Robbins P. W. Yeast mutants deficient in protein glycosylation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7466–7470. doi: 10.1073/pnas.80.24.7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härfast B., Huddlestone J. R., Casali P., Merigan T. C., Oldstone M. B. Interferon acts directly on human B lymphocytes to modulate immunoglobulin synthesis. J Immunol. 1981 Nov;127(5):2146–2150. [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Itani T., Kiji Y., Ariga H. Possible function of the c-myc product: promotion of cellular DNA replication. EMBO J. 1987 Aug;6(8):2365–2371. doi: 10.1002/j.1460-2075.1987.tb02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak G. J., Knight E., Jr Selective reduction of c-myc mRNA in Daudi cells by human beta interferon. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1747–1750. doi: 10.1073/pnas.81.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Gilbert C. S., Stark G. R., Kerr I. M. Differential regulation of interferon-induced mRNAs and c-myc mRNA by alpha- and gamma-interferons. Eur J Biochem. 1985 Dec 2;153(2):367–371. doi: 10.1111/j.1432-1033.1985.tb09312.x. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Anton E. D., Fahey D., Friedland B. K., Jonak G. J. Interferon regulates c-myc gene expression in Daudi cells at the post-transcriptional level. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1151–1154. doi: 10.1073/pnas.82.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel P. Biochemistry of interferons and their actions. Annu Rev Biochem. 1982;51:251–282. doi: 10.1146/annurev.bi.51.070182.001343. [DOI] [PubMed] [Google Scholar]
- Lin S. L., Garber E. A., Wang E., Caliguiri L. A., Schellekens H., Goldberg A. R., Tamm I. Reduced synthesis of pp60src and expression of the transformation-related phenotype in interferon-treated Rous sarcoma virus-transformed rat cells. Mol Cell Biol. 1983 Sep;3(9):1656–1664. doi: 10.1128/mcb.3.9.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Montagnier L., Gruest J. Cell-density-dependence for growth in agarose of two human lymphoma lines and its decrease after Epstein-Barr virus conversion. Int J Cancer. 1979 Jan 15;23(1):71–75. doi: 10.1002/ijc.2910230113. [DOI] [PubMed] [Google Scholar]
- O'Gorman M. R., Oger J., Kastrukoff L. F. Reduction of immunoglobulin G secretion in vitro following long term lymphoblastoid interferon (Wellferon) treatment in multiple sclerosis patients. Clin Exp Immunol. 1987 Jan;67(1):66–75. [PMC free article] [PubMed] [Google Scholar]
- Peters M., Ambrus J. L., Zheleznyak A., Walling D., Hoofnagle J. H. Effect of interferon-alpha on immunoglobulin synthesis by human B cells. J Immunol. 1986 Nov 15;137(10):3153–3157. [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Molecular cloning of a human histocompatibility antigen cDNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6081–6085. doi: 10.1073/pnas.77.10.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts T. H., Forster A., Milstein C. P. Human immunoglobulin heavy chain genes: evolutionary comparisons of C mu, C delta and C gamma genes and associated switch sequences. Nucleic Acids Res. 1981 Sep 25;9(18):4509–4524. doi: 10.1093/nar/9.18.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J., Early P., Carter C., Calame K., Bond M., Hood L., Wall R. Two mRNAs with different 3' ends encode membrane-bound and secreted forms of immunoglobulin mu chain. Cell. 1980 Jun;20(2):303–312. doi: 10.1016/0092-8674(80)90616-9. [DOI] [PubMed] [Google Scholar]
- Rosa F., Hatat D., Abadie A., Wallach D., Revel M., Fellous M. Differential regulation of HLA-DR mRNAs and cell surface antigens by interferon. EMBO J. 1983;2(9):1585–1589. doi: 10.1002/j.1460-2075.1983.tb01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samid D., Chang E. H., Friedman R. M. Biochemical correlates of phenotypic reversion in interferon-treated mouse cells transformed by a human oncogene. Biochem Biophys Res Commun. 1984 Feb 29;119(1):21–28. doi: 10.1016/0006-291x(84)91612-7. [DOI] [PubMed] [Google Scholar]
- Saunders M. E., Gewert D. R., Tugwell M. E., McMahon M., Williams B. R. Human 2-5A synthetase: characterization of a novel cDNA and corresponding gene structure. EMBO J. 1985 Jul;4(7):1761–1768. doi: 10.1002/j.1460-2075.1985.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soslau G., Bogucki A. R., Gillespie D., Hubbell H. R. Phosphoproteins altered by antiproliferative doses of human interferon- beta in a human bladder carcinoma cell line. Biochem Biophys Res Commun. 1984 Mar 30;119(3):941–948. doi: 10.1016/0006-291x(84)90864-7. [DOI] [PubMed] [Google Scholar]
- Strunk R. C., Cole F. S., Perlmutter D. H., Colten H. R. gamma-Interferon increases expression of class III complement genes C2 and factor B in human monocytes and in murine fibroblasts transfected with human C2 and factor B genes. J Biol Chem. 1985 Dec 5;260(28):15280–15285. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Wreschner D. H., James T. C., Silverman R. H., Kerr I. M. Ribosomal RNA cleavage, nuclease activation and 2-5A(ppp(A2'p)nA) in interferon-treated cells. Nucleic Acids Res. 1981 Apr 10;9(7):1571–1581. doi: 10.1093/nar/9.7.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]