Abstract
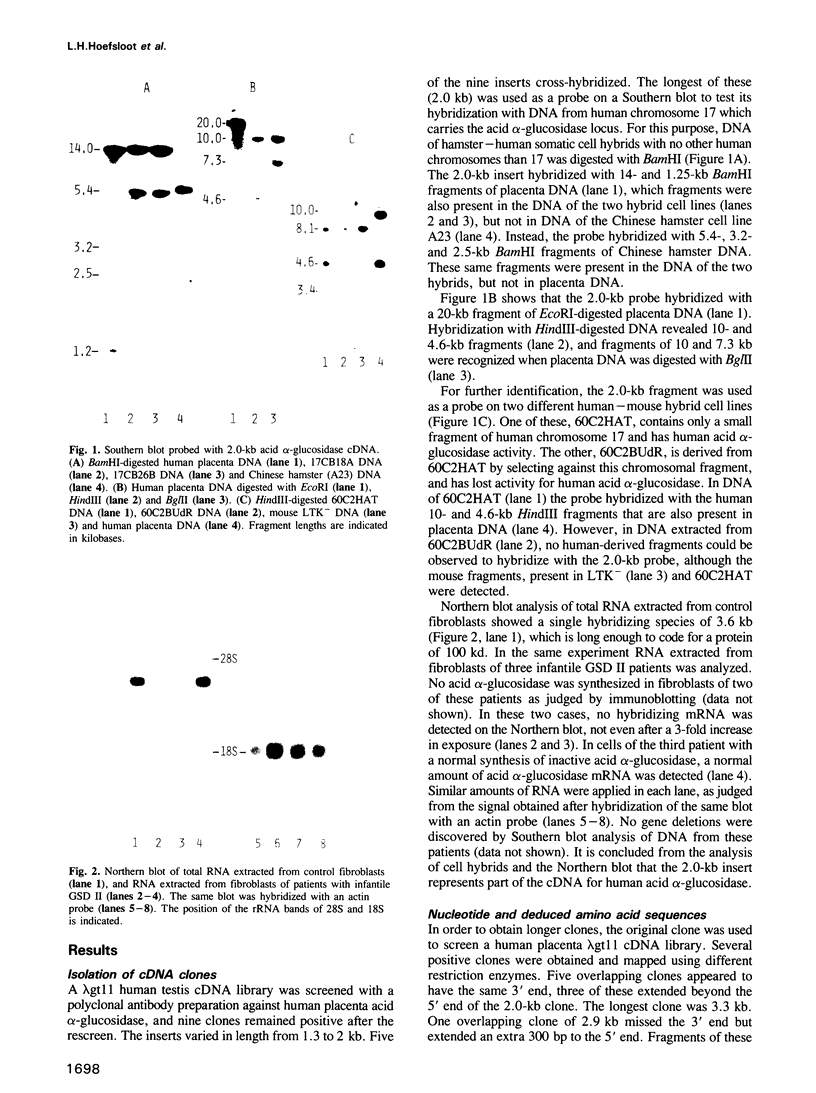
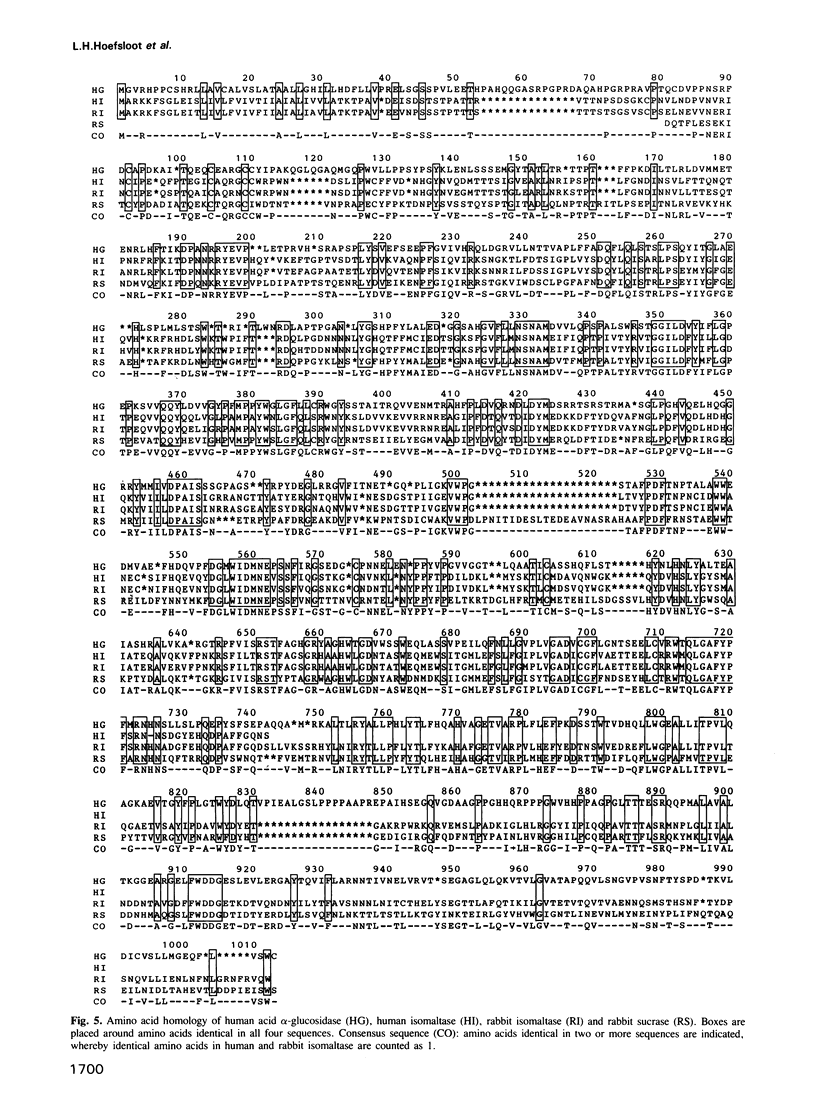
Lysosomal alpha-glucosidase (acid maltase) is essential for degradation of glycogen in lysosomes. Enzyme deficiency results in glycogenosis type II. The amino acid sequence of the entire enzyme was derived from the nucleotide sequence of cloned cDNA. The cDNA comprises 3636 nt, and hybridizes with a messenger RNA of approximately 3.6 kb, which is absent in fibroblasts of two patients with glycogenosis type II. The encoded protein has a molecular mass of 104.645 kd and starts with a signal peptide. Sites of proteolytic processing are established by identification of N-terminal amino acid sequences of the 110-kd precursor, and the 76-kd and 70-kd mature forms of the enzyme encoded by the cDNA. Interestingly, both amino-terminal and carboxy-terminal processing occurs. Sites of sugar-chain attachment are proposed. A remarkable homology is observed between this soluble lysosomal alpha-glucosidase and the membrane-bound intestinal brush border sucrase-isomaltase enzyme complex. It is proposed that these enzymes are derived from the same ancestral gene. Around the putative active site of sucrase and isomaltase, 10 out of 13 amino acids are identical to the corresponding amino acids of lysosomal alpha-glucosidase. This strongly suggests that the aspartic acid residue at this position is essential for catalytic function of lysosomal alpha-glucosidase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Belen'ky D. M., Mikhajlov V. I., Rosenfeld E. L. Carbohydrate content of acid alpha-glucosidase (gamma-amylase) from human liver. Clin Chim Acta. 1979 May 2;93(3):365–370. doi: 10.1016/0009-8981(79)90286-9. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Beratis N. G., LaBadie G. U., Hirschhorn K. Acid alpha-glucosidase: kinetic and immunologic properties of enzyme variants in health and disease. Isozymes Curr Top Biol Med Res. 1983;11:25–36. [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Green F., Edwards Y., Hauri H. P., Povey S., Ho M. W., Pinto M., Swallow D. Isolation of a cDNA probe for a human jejunal brush-border hydrolase, sucrase-isomaltase, and assignment of the gene locus to chromosome 3. Gene. 1987;57(1):101–110. doi: 10.1016/0378-1119(87)90181-8. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem. 1980 May 25;255(10):4946–4950. [PubMed] [Google Scholar]
- Hasilik A., Neufeld E. F. Biosynthesis of lysosomal enzymes in fibroblasts. Synthesis as precursors of higher molecular weight. J Biol Chem. 1980 May 25;255(10):4937–4945. [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig J., Martiniuk F., D'Eustachio P., Zamfirescu C., Desnick R., Hirschhorn K., Hirschhorn L. R., Hirschhorn R. Confirmation of the regional localization of the genes for human acid alpha-glucosidase (GAA) and adenosine deaminase (ADA) by somatic cell hybridization. Ann Hum Genet. 1984 Jan;48(Pt 1):49–56. doi: 10.1111/j.1469-1809.1984.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hunziker W., Spiess M., Semenza G., Lodish H. F. The sucrase-isomaltase complex: primary structure, membrane-orientation, and evolution of a stalked, intrinsic brush border protein. Cell. 1986 Jul 18;46(2):227–234. doi: 10.1016/0092-8674(86)90739-7. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. I. Purification and properties of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1403–1415. doi: 10.1021/bi00808a015. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. II. Kinetics of action of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1416–1422. doi: 10.1021/bi00808a016. [DOI] [PubMed] [Google Scholar]
- Konings A., Hupkes P., Versteeg R., Grosveld G., Reuser A., Galjaard H. Cloning a cDNA for the lysosomal alpha-glucosidase. Biochem Biophys Res Commun. 1984 Feb 29;119(1):252–258. doi: 10.1016/0006-291x(84)91645-0. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G. Some properties of human liver acid alpha-glucosidase. Biochim Biophys Acta. 1977 May 12;482(1):89–97. doi: 10.1016/0005-2744(77)90357-6. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martiniuk F., Mehler M., Pellicer A., Tzall S., La Badie G., Hobart C., Ellenbogen A., Hirschhorn R. Isolation of a cDNA for human acid alpha-glucosidase and detection of genetic heterogeneity for mRNA in three alpha-glucosidase-deficient patients. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9641–9644. doi: 10.1073/pnas.83.24.9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery R. K., Sybicki M. A., Forcier A. G., Grand R. J. Rat intestinal microvillus membrane sucrase-isomaltase is a single high molecular weight protein and fully active enzyme in the absence of luminal factors. Biochim Biophys Acta. 1981 Oct 13;661(2):346–349. doi: 10.1016/0005-2744(81)90024-3. [DOI] [PubMed] [Google Scholar]
- Mutsaers J. H., Van Halbeek H., Vliegenthart J. F., Tager J. M., Reuser A. J., Kroos M., Galjaard H. Determination of the structure of the carbohydrate chains of acid alpha-glucosidase from human placenta. Biochim Biophys Acta. 1987 Jan 30;911(2):244–251. doi: 10.1016/0167-4838(87)90014-8. [DOI] [PubMed] [Google Scholar]
- O'Dowd B. F., Quan F., Willard H. F., Lamhonwah A. M., Korneluk R. G., Lowden J. A., Gravel R. A., Mahuran D. J. Isolation of cDNA clones coding for the beta subunit of human beta-hexosaminidase. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1184–1188. doi: 10.1073/pnas.82.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Elferink R. P., Brouwer-Kelder E. M., Surya I., Strijland A., Kroos M., Reuser A. J., Tager J. M. Isolation and characterization of a precursor form of lysosomal alpha-glucosidase from human urine. Eur J Biochem. 1984 Mar 15;139(3):489–495. doi: 10.1111/j.1432-1033.1984.tb08032.x. [DOI] [PubMed] [Google Scholar]
- Oude Elferink R. P., Van Doorn-Van Wakeren J., Strijland A., Reuser A. J., Tager J. M. Biosynthesis and intracellular transport of alpha-glucosidase and cathepsin D in normal and mutant human fibroblasts. Eur J Biochem. 1985 Nov 15;153(1):55–63. doi: 10.1111/j.1432-1033.1985.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Palmer T. N. The maltase, glucoamylase and transglucosylase activities of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):713–724. doi: 10.1042/bj1240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. N. The substrate specificity of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):701–711. doi: 10.1042/bj1240701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaroni A., Semenza G. Partial amino acid sequences around the essential carboxylate in the active sites of the intestinal sucrase-isomaltase complex. J Biol Chem. 1976 Jun 10;251(11):3250–3253. [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Oude Elferink R. P., Tager J. M. Defects in synthesis, phosphorylation, and maturation of acid alpha-glucosidase in glycogenosis type II. J Biol Chem. 1985 Jul 15;260(14):8336–8341. [PubMed] [Google Scholar]
- Reuser A. J., Kroos M., Willemsen R., Swallow D., Tager J. M., Galjaard H. Clinical diversity in glycogenosis type II. Biosynthesis and in situ localization of acid alpha-glucosidase in mutant fibroblasts. J Clin Invest. 1987 Jun;79(6):1689–1699. doi: 10.1172/JCI113008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld E. L. Alpha-glucosidases (gamma-amylases) in human and animal organisms. Pathol Biol (Paris) 1975 Jan;23(1):71–84. [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Solomon E., Swallow D., Burgess S., Evans L. Assignment of the human acid alpha-glucosidase gene (alphaGLU) to chromosome 17 using somatic cell hybrids. Ann Hum Genet. 1979 Jan;42(3):273–281. doi: 10.1111/j.1469-1809.1979.tb00661.x. [DOI] [PubMed] [Google Scholar]
- Spiess M., Brunner J., Semenza G. Hydrophobic labeling, isolation, and partial characterization of the NH2-terminal membranous segment of sucrase-isomaltase complex. J Biol Chem. 1982 Mar 10;257(5):2370–2377. [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Bauw G., Puype M., Van Damme J., Van Montagu M. Protein-blotting on Polybrene-coated glass-fiber sheets. A basis for acid hydrolysis and gas-phase sequencing of picomole quantities of protein previously separated on sodium dodecyl sulfate/polyacrylamide gel. Eur J Biochem. 1985 Oct 1;152(1):9–19. doi: 10.1111/j.1432-1033.1985.tb09157.x. [DOI] [PubMed] [Google Scholar]
- Walvoort H. C. Glycogen storage diseases in animals and their potential value as models of human disease. J Inherit Metab Dis. 1983;6(1):3–16. doi: 10.1007/BF02391186. [DOI] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R. D., Wun T. C., Sadler J. E. cDNA cloning and expression in Escherichia coli of a plasminogen activator inhibitor from human placenta. J Biol Chem. 1987 Mar 15;262(8):3718–3725. [PubMed] [Google Scholar]
- Yuan P. M., Pande H., Clark B. R., Shively J. E. Microsequence analysis of peptides and proteins. I. Preparation of samples by reverse-phase liquid chromatography. Anal Biochem. 1982 Mar 1;120(2):289–301. doi: 10.1016/0003-2697(82)90350-5. [DOI] [PubMed] [Google Scholar]
- de Jonge A. J., de Smit S., Kroos M. A., Reuser A. J. Cotransfer of syntenic human genes into mouse cells using isolated metaphase chromosomes or cellular DNA. Hum Genet. 1985;69(1):32–38. doi: 10.1007/BF00295526. [DOI] [PubMed] [Google Scholar]
- van der Horst G. T., Hoefsloot E. H., Kroos M. A., Reuser A. J. Cell-free translation of human lysosomal alpha-glucosidase: evidence for reduced precursor synthesis in an adult patient with glycogenosis type II. Biochim Biophys Acta. 1987 Nov 20;910(2):123–129. doi: 10.1016/0167-4781(87)90064-9. [DOI] [PubMed] [Google Scholar]





