Abstract
Respiratory viruses are a major public health problem because of their prevalence and high morbidity rate leading to considerable social and economic implications. Cranberry has therapeutic potential attributed to a comprehensive list of phytochemicals including anthocyanins, flavonols, and unique A-type proanthocyanidins. Soy flavonoids, including isoflavones, have demonstrated anti-viral effects in vitro and in vivo. Recently, it was demonstrated that edible proteins can efficiently sorb and concentrate cranberry polyphenols, including anthocyanins and proanthocyanins, providing greatly stabilized matrices suitable for food products. The combination of cranberry and soy phytoactives may be an effective dietary anti-viral resource. Anti-viral properties of both cranberry juice-enriched and cranberry pomace polyphenol-enriched soy protein isolate (CB-SPI and CBP-SPI) were tested against influenza viruses (H7N1, H5N3, H3N2), Newcastle disease virus and Sendai virus in vitro and in ovo. In our experiments, preincubation with CB-SPI or CBP-SPI resulted in inhibition of virus adsorption to chicken red blood cells and reduction in virus nucleic acid content up to 16-fold, however, CB-SPI and CBP-SPI did not affect hemagglutination. Additionally, CB-SPI and CBP-SPI inhibited viral replication and infectivity more effectively than the commercially available anti-viral drug Amizon. Results suggest CB-SPI and CBP-SPI may have preventative and therapeutic potential against viral infections that cause diseases of the respiratory and gastro-intestinal tract.
Keywords: Cranberry, Polyphenols, Flavonoids, Anti-viral
Introduction
Food deficiencies occur worldwide every year, increasing urgency for nutritional interventions with functional foods. In all countries of the world, nutritional deficiencies are prevalent for major food substances: proteins, unsaturated fats, complex carbohydrates, vitamins and minerals. Functional food ingredients combining basic nutrients and phytoactive plant extracts can alleviate these deficiencies in safe and practical food products.
By definition, functional food is a food given an additional function (often one related to health-promotion or disease prevention) by adding new ingredients or more of existing ingredients. Therefore, functional food development is of great interest to consumers, industries, governments and universities [2]. There is a need for the development of shelf stable functional food products enriched with essential micronutrients and bioactive compounds.
Cranberry is an attractive candidate for the creation of new types of functional foods. North American cranberry (Vaccinium macrocarpon Ait) was cultivated since the early 1800’s and mostly sold as fresh fruits and sauces until 1950. Today cranberries are mostly consumed as processed products including juices, juice cocktails, sauces and sweetened-dried fruits [41]. Cranberry contains a wide array of polyphenolic compounds [38] including up to 17 different anthocyanins, as well as proanthocyanidins [13], which are an excellent source of antioxidants, and are associated with multiple human health benefits, including prevention of urinary tract infections [35, 40], and lowering the risk of cardiovascular disease and cancer [29]. Additionally, anti-viral properties of cranberry constituents have been recently demonstrated [20, 23, 42].
Cranberry fruits are recognized for their tart, astringent flavor, and unsweetened cranberry juice is unpalatable to the majority of consumers. Furthermore, it has been demonstrated that processing of cranberry into dietary supplements impacts proanthocyanidins, that are vulnerable to heat or oxidation [14]. Delivering intact and biologically-active cranberry phytochemicals in a palatable food matrix may provide health benefits to the general population and especially children. It has been recently reported that defatted soybean flour is an efficient matrix for sorption and concentration of anthocyanins and other polyphenols, but not sugars, from cranberry and blueberry juice [31]. Anthocyanins, proanthocyanidins, and flavonols were stabilized and preserved in cranberry polyphenol-enriched matrices of soy, pea, and hemp proteins, as well as peanut and soy flour [15, 31, 32]. Complexation with protein matrices did not alter the chemical composition of cranberry phytochemicals. Additionally, cranberry polyphenol enriched matrices showed both gram positive and gram negative anti-bacterial activities [15, 31]. Soy isoflavones, compounds in the flavonoid class of polyphenols, have also demonstrated anti-viral effects in vitro and in vivo [1].
Respiratory viruses are a major public health problem because of their prevalence and high morbidity rate leading to considerable social and economic implications. Substantial influenza burden remains despite expanded recommendations for vaccine [26] and anti-viral agent use [27]. There is recognized urgency in developing anti-viral agents with new mechanisms of action, and in continuing the work on new inhibitors directed against influenza polymerase, hemagglutinin, M gene, and other targets [3]. The combined application of natural viral inhibitors may be used successfully to potentiate anti-viral efficacy and may enable medication dose reduction. The aim of our research was to evaluate the anti-viral properties of a novel functional food ingredient—a cranberry-polyphenol enriched soy protein powder.
Materials and methods
Materials
Phosphate buffered saline (PBS, pH 7.4) was purchased from Amresco (Solon, OH, USA). 10-day-old chicken eggs and 50 % chicken red blood cell (cRBC) suspensions were obtained from Almaty chicken factory farm (Almaty, Kazakhstan). All water used in the experiments was purified using an E-pure water purification system with the minimum resistivity of 17.6 MΩ cm (Barnstead, Dubuque, IA). Anti-viral medication Amizon (Amizonum®, Farmak Ltd, Ukraine) served as a positive control. Amizon or l-methyl-4-(benzylaminocarbonyl) pyridinium iodide [4], CAS Registry Number: 201349-37-3 is a low toxicity compound with well established anti-viral properties [22] that has been used for influenza prophylaxis and treatment. Tamiflu (F. Hoffmann-La Roshe Ltd., Switzerland) and Rimantadine (OlainFarm, Latvia) were used in study of orthomyxoviruses only.
Cranberry polyphenol enriched soy protein matrices
Cranberry polyphenol enriched soy protein matrices were produced as described in detail previously [31] with minor modifications. Total polyphenols were determined by Folin-Ciocalteu method [36] and reported as gallic acid equivalents. Briefly, cranberry juice concentrate (Fruit Smart, Grandview, WA) was diluted 4 to 5-fold depending on total polyphenolic content of starting material. The diluted juice was mixed with soy protein isolate (ADM, Decatur, IL) at 10:1 (v/w) ratio. The mixture was adjusted to pH 2 and centrifuged for 10 min at 4000 rpm to separate solid from liquid. The solid matrix was lyophilized to produce a dry powder, CB-SPI, containing 3 % total polyphenols sorbed onto the protein matrix. Total polyphenols were determined by quantifying the difference between the total polyphenol content before and after sorption divided by the dry weight of CB-SPI to obtain mg polyphenols per gram SPI. Proanthocyanidin composition of CB-SPI was previously described [15]. CB-SPI was dispersed in PBS to concentrations indicated in experiments.
To produce the cranberry pomace extract, frozen depectinized cranberry pomace (BNK, Wisconsin Rapids, WI) was blended with 50 % ethanol at a 5:1 ratio (v/w) and pH adjusted to 2. The mixture was incubated in a rotating flask immersed in an 80 °C water bath for 2 h, centrifuged at 4000 rpm for 10 min and supernatant was filtered through Miracloth (Calbiochem, EMD Millipore, Darmstadt, Germany). Total polyphenols were quantified as described above. Extracts were combined with a calculated amount of SPI and dried to produce CBP-SPI containing 10 % total polyphenols sorbed onto the protein matrix during the co-drying process. Lyophilized CBP-SPI was dispersed in PBS to desired concentrations and used for anti-viral experiments. Phytochemical-enriched protein CBP-SPI has been characterized in detail, previously [32].
Viruses
A group of viruses commonly used in medical and veterinary research was selected to cover different aspects of viral pathogenesis. Sendai virus is frequently used as a model for entry of enveloped viruses into animal cells [6, 21]. Newcastle Disease Virus has well researched anti-neoplastic and pleiotropic immune responses [17]. Influenza virus can cause intestine diseases. Orthomyxoviruses: highly pathogenic avian influenza virus, A/FPV/Rostock/34 (H7N1) and avian influenza virus A/Tern/South Africa/1/61 (H5N3), and paramyxoviruses, Sendai virus (strain 960) and Newcastle disease virus (strain Beadetta) were obtained from the state collection of viruses at Ivanovsky Institute of Virology (Moscow, Russia). Orthomyxovirus A/Almaty/8/98 (H3N2) was obtained from the collection of microorganisms at the Research Institute for Biological Safety Problems (Kazakhstan).
Macrophages and MDCK cells
Bone marrow cell suspensions recovered from tibias and femurs of mice were suspended in DMEM medium (Gibco, Life Technologies) containing 4 g/L glucose, 1 mM pyruvate and 3.97 mM L-alanyl-l-Glutamine, 10 % heat-inactivated fetal calf serum (FCS, Dominique Dutscher SAS), streptomycin (50 µg/mL) and penicillin (50 IU/mL) (Biochrom AG, IBS International) and 50 ng/mL recombinant mouse CSF-1 (rmCSF-1) (ImmunoTools, Germany). Cells were distributed in bacteriologic plastic flasks (Corning Life Science, 7 × 105 cells/ml) and were incubated at 37 °C in a 7.5 % CO2 atmosphere for one day.
Madin–Darby canine kidney (MDCK) cells were obtained from The Research Institute for Biological Safety Problems (Kazakhstan). MDCK cells were cultured as monolayers in the MEM medium (PAA Labaratories GmbH, Germany) supplemented with 10 % heat-inactivated fetal bovine serum (PAA Labaratories).
Cytotoxicity evaluation
The cytotoxicity evaluation was performed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] method. 33–35 Briefly, 100 µL of each extract/fraction dilutions (1:2—ranging from 15.6 to 2000 µg/mL prepared in cell culture medium) was added to each well with macrophages (106 cells/well). As a control 100 µL of medium was added to cells. Plates were incubated under the same conditions for 4 h. After 4 h, the medium was removed by suction from all wells and 50 µL of MTT (Sigma, 1 mg/mL) solution prepared in cell culture medium were added to each well and the plates were incubated once more for 2 h. After the MTT solution was removed without disturbing the cells, 50 µL of DMSO was added to each well to dissolve the formazan crystals. After gently shaking the plates, the crystals were completely dissolved, and absorbances were read on a multi-well spectrophotometer (Sunrise™, Tecan) at 540 nm. The CC50 was defined as the cytotoxic concentration of each sample that reduced the absorbance of treated cells to 50 % when compared with that of the control.
Chicken embryo lethality assay
The CB-SPI and CBP-SPI preparations were each diluted to the following concentrations: 50, 25, 12, 6, 3, 1.5 and 0.75 mg/ml in sterile PBS. A single dose of 100 μL of each dilution was inoculated into the allantoic cavity of 10-day-old embryonated chicken eggs of seven serial groups according to their concentrations; a control group was inoculated with sterile PBS. The eggs were incubated in a humidified incubator at 37 °C for 5 days, and embryo death was recorded.
Virus yield reduction assay
CB-SPI and CBP-SPI were tested for in ovo anti-viral activity. A mixture containing 100 μl of influenza virus, corresponding to 100 EID50/ml, and 100 μl of PBS (control) or CBP-SPI or CB-SPI, with final doses of 0.05, 0.5 and 5.0 mg per chicken embryo, were incubated for 30 min at 37 °C and used to inoculate 10-day-old fertilized chicken eggs by the allantoic route. Five replicates of each assay were performed in three independent experiments. Inoculated eggs were incubated at 37 °C in a humidified incubator. After incubation for 24–72 h, eggs were chilled for 12 h at 4 °C, the allantoic fluid was harvested and used for HA titer. Five dilutions were tested, and the effective anti-viral concentration determined by regression analysis.
Virucidal assay
Equal volumes of viral inocula and 50 mg/ml CB-SPI or CBP-SPI (5 % w/v) were mixed and incubated for 30 min at room temperature. The mixture was titrated by tenfold dilutions and used to inoculate 10-day-old fertilized chicken eggs by the allantoic route. After incubation for 24–72 h at 37 °C in a humidified incubator, EID50 was determined as described by Reed and Muench [30]. A viral inoculum, treated with medium without cranberry polyphenol-enriched matrices, was used as control for each virus. Infectivity suppression was expressed as the difference between log10 (EID50 treatment) and log10 (EID50 control). Three independent experiments with triplicate treatments were performed.
Hemagglutination assay
Hemagglutination assay is a commonly used method of influenza virus titration [18]. Two-fold serial dilutions of virus were made in 50 μl PBS using U-shaped 96-well microtiter plates. 50 μl of a 0.75 % suspension of cRBCs were added to each well and mixed by gentle agitation. The plate was incubated at room temperature until control wells showed complete settling of cRBCs. Wells with complete HA were recorded as positive for HA and wells with a distinct button formation were recorded as negative for HA. The HA titer was read as the reciprocal of the dilution of virus in the last well showing complete HA [11, 19].
Hemagglutination inhibition assay
The assay was carried out using influenza viruses (H7N1, H3N2, H5N3), Newcastle disease virus, and Sendai virus according to a method described by Pedersen [25] with some modifications. Hemagglutination inhibition assay was performed to evaluate the effects of CB-SPI and CBP-SPI preparations on viral adsorption to target cells, demonstrating interference on hemagglutination. Standardized cRBC solutions were prepared according to Pedersen [25]. The influenza virus solution, 50 μl, was mixed with an equal volume of CB-SPI or CBP-SPI (50 μL) in two-fold serial dilutions in PBS and incubated for 1 h at 4 °C. Next, the solution was mixed with an equal volume of a 0.75 % cRBC suspension (50 μl) and incubated for 1 h at room temperature. HA titer was calculated as the reciprocal of the highest dilution that produced complete HA. Assays were done in triplicate and three independent experiments were performed.
Inhibition of virus adsorption
The inhibition of virus adsorption assay was performed to evaluate the effects of both CB-SPI and CBP-SPI on viral adsorption to target cells. Standardized 5 % cRBC solutions were prepared according to Pedersen [25]. The influenza virus solution, 50 μl, was mixed with an equal volume of CB-SPI or CBP-SPI in a two-fold serial dilution in PBS. After incubation for 30 min at 4 °C, 20× volume of 5 % cRBC solution was added to each tube. After incubation for an additional 30 min at 4 °C, the mixture was centrifuged at 3000 rpm for 5 min and supernatant was used for the hemagglutination assay titer. Positive controls contained virus solutions and cells and negative controls contained saline and cells [34, 37]. Assays were done in triplicate and three independent experiments were performed.
Interaction of CB-SPI and CBP-SPI with virus
Equal volumes (300 μl) of allantoic virus, 108 EID50, and 2 % of preparations were mixed and incubated for 30 min at 4 °C. The mixture was centrifuged at 6000 rpm for 5 min and supernatant was used for real time-PCR quantification of viral RNA [5].
RNA was extracted from supernatant liquid using RNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. The RNA was converted to full-length cDNA in the following reaction: 2.5 μl of DMPC (di-methyl pyrocarbonate) water, 5 μl of 5× First Strand buffer (Invitrogen), 0.5 μl of 10 mMdNTP mix (Amersham Biosciences), 2 μl of 50 mM UNi12 primer, 32 U of RNAguard (Amersham Biosciences), 200 U of MMLV reverse transcriptase (Invitrogen) and 5 μl RNA solution in total volume of 25 μl. Reactions were incubated at 42 °C for 60 min followed by inactivation of the enzyme at 95 °C for 5 min. PCR amplification with HA gene specific primers (Forward primer: 5′-AGCAAAAGCAGGGGA-3′, Reverse primer: 5′-AGTAGAAACAAGGGTGTT-3′) was performed on the PikoReal 96 Real Time PCR System (Thermo Scientific, USA) to amplify the product containing the fragment HA gene. The PCR mix contained: SybrGreen mix 10 μl (Thermo Scientific), 240 nM each of forward primer and reverse primer, ROX 0.04 μl and 5 μl cDNA for a 20 μl reaction volume. Reactions were placed in a thermal cycler at 94 °C for 2 min, then cycled 35 times between 94 °C 60 s, annealing at 48 °C for 60 s, and elongation at 72 °C for 180 s and finally kept at 8 °C until later use.
Statistical analysis
Results were expressed as mean ± SD of three independent experiments, unless otherwise noted. One-way ANOVA was used to evaluate the difference between the test samples. Student’s unpaired t test was used to evaluate the difference between the test sample and untreated control. A p value <0.05 was considered statistically significant. GraphPad PRISM software (La Jolla, CA) was used for statistical analysis.
Results
Cytotoxicity and chicken embryo lethality of CB-SPI and CBP-SPI
Cytotoxic concentrations (CC50) of cranberry juice concentrate, SPI, CB-SPI and SBP-SPI are presented in Table 1. For both CB-SPI and CBP-SPI CC50 exceeded 500 µg/mL in macrophages and CC50 exceeded 400 µg/mL in MDCK cells. These cytotoxicity levels are significantly lower that the cytotoxicity of cranberry juice concentrate as well as many plant extracts evaluated for antiviral properties. For SPI, the CC50 was lower than for the polyphenol enriched matrices: 500 and 390 µg/mL for macrophage and MDCK cells, respectively. For cranberry juice concentrate, the CC50 were 150 and 130 µg/mL for macrophage and MDCK cells, respectively.
Table 1.
Cytotoxicity of the tested preparations
| Preparation | CC50 (µg/mL) for macrophages | CC50 (µg/mL) for MDCK |
|---|---|---|
| CB | 150 ± 32 | 130 ± 32 |
| SPI | 500 ± 34 | 390 ± 34 |
| CB-SPI | 520 ± 46 | 425 ± 46 |
| CBP-SPI | 525 ± 34 | 438 ± 34 |
CB cranberry juice concentrate; SPI soy protein isolate; CB-SPI cranberry juice polyphenols sorbed onto soy protein matrix; CBP-SPI cranberry pomace extract polyphenols sorbed onto soy protein matrix
Chicken embryo lethality assay showed that cranberry juice or cranberry pomace polyphenol-enriched soy protein isolate (CB-SPI or CBP-SPI) had no toxicity in the concentration range investigated. LD50 for tested preparations exceed 100 mg per chicken embryo.
Thus, the combination of SPI with cranberry anthocyanins reduced the potential cytotoxic effects of the initial ingredients.
Effect of CB-SPI and CBP-SPI on virus yield reduction
The commercial anti-viral agents Rimantadine and Tamiflu were not used in experiments with paramyxoviruses, since they are affecting only the influenza virus. Amizon is an anti-viral agent usually used for the treatment of DNA and RNA containing viruses (HSV, hepatitis and mumps) therefore in our experiments we used this drug.
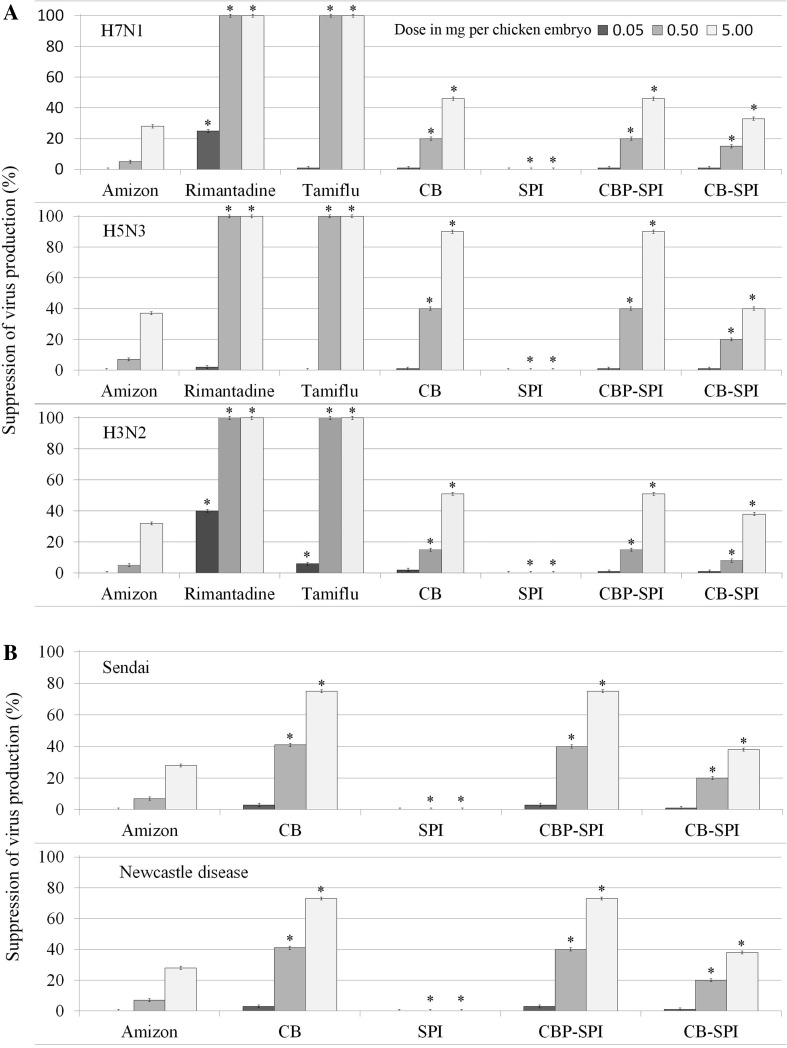
Preincubation with CB-SPI or CBP-SPI suppressed the reproduction of 100 EID50 (fifty percent embryo infectious dose) of ortho- and paramyxoviruses [19]. The investigated doses of CB-SPI and CBP-SPI ranged from 0 to 5 mg per chicken embryo (or from 0 to 100 mg per kg of embryo weight). A 5 mg dose of CB-SPI or CBP-SPI per embryo led to the suppression of reproduction of 100 EID50 of the virus from 24 - 90 %, depending on the virus (Fig. 1). For orthomyxoviruses, suppression of virus reproduction was greatest for H5N3, followed by H3N2, and H7N1. Both paramyxoviruses were affected in a similar manner. Anti-viral properties of CB-SPI and CBP-SPI were compared to activity of the commercial anti-viral agents Amizon, Rimantadine and Tamiflu. Anti-viral activity of CB-SPI and CBP-SPI against orthomyxoviruses was lower compared to Tamiflu and Rimantadine (Fig. 1 A). It may be due to the different mechanisms of action of these substances on the influenza virus. Amizon inhibits virus reproduction by changing the homeostasis of an organism which is a possible mechanism of action of investigated enterosorbents (CB-SPI and CBP-SPI). Anti-viral activity of CB-SPI compared to Amizon was stronger against orthomyxoviruses (Fig. 1a) but weaker against paramyxoviruses (Fig. 1b). Suppression of all viruses was significantly greater with CBP-SPI compared with Amizon (Fig. 1).
Fig. 1.
Suppression of virus production by CB-SPI and CBP-SPI. Doses of 0,05; 0,5 and 5,0 mg per chicken embryo are presented. a orthomyxoviruses; b paramyxoviruses. Error bars represent SD for n = 15. * Statistically significant difference (p < 0.05) compared to Amizon
It was shown that the anti-viral activity of CB was slightly higher than CB-SPI against orthomyxoviruses and paramyxoviruses. At the same time CB-SPI was much more stable than CB, indicating that it could retain anti-viral efficacy after a long storage (up to 24 months). Total polyphenols sorbed onto the protein matrix were 3 % into CB-SPI and 10 % into CBP-SPI, consequently anti-viral activity of CBP-SPI was higher because of the higher content of chemically active substances.
Virucidal activity of CB-SPI and CBP-SPI
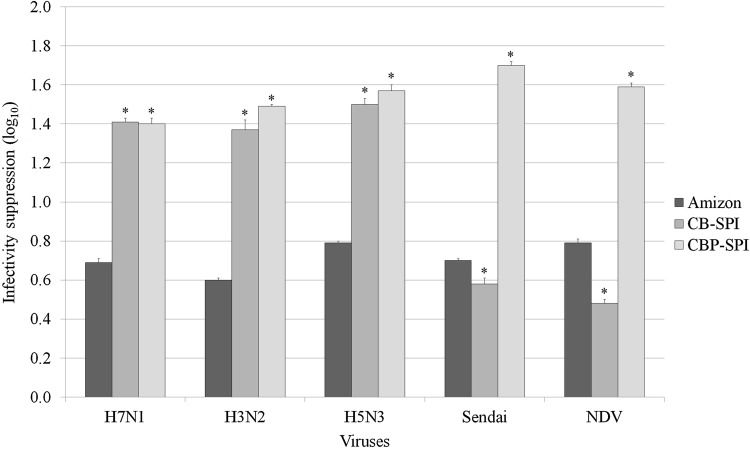
Cranberry polyphenol enriched soy protein matrices suppressed virus infectivity ranging from 0.48 to 1.59 lg which corresponds from 50 to 90 % of infectious viral particles (Fig. 2). Results show pronounced anti-viral properties of CB-SPI and CBP-SPI which exceeded the virucidal activity of Amizon against orthomyxoviruses, H7N1, H3N2, and H5N3. CBP-SPI, but not CB-SPI, also demonstrated significantly greater suppression of infectivity of paramyxoviruses, Newcastle disease virus and Sendai virus, compared to Amizon.
Fig. 2.
Suppression of virus infectivity by CB-SPI and CBP-SPI A dose of 5 mg per chicken embryo is presented. Error bars represent SD for n = 9. * Statistically significant difference (p < 0.05) compared to Amizon
Effect of CB-SPI and CBP-SPI on hemagglutination of viruses
Influenza virus titration using the hemagglutination assay confirmed viability of all strains used in the experiments (data not shown). The effect on hemagglutination by preincubation with CB-SPI and CBP-SPI concentrations ranging from 0 to 2.5 % was evaluated for influenza viruses (H7N1, H3N2, H5N3), Newcastle disease virus and Sendai virus. We detected no significant differences in hemagglutination (data not shown). These results indicated that CB-SPI and CBP-SPI did not directly interfere with binding of viruses to cell surface receptors.
Inhibition of virus adsorption by CB-SPI and CBP-SPI
The effect of CB-SPI and CBP-SPI on virus adsorption was evaluated in cRBCs. Preincubation of ortho- and paramyxoviruses with CB-SPI and CBP-SPI reduced adsorption of viruses to target cells in a concentration dependent manner (data not shown). At a high concentration of 2.5 % we observed 40–70 % inhibition of virus adsorption.
Effect of CB-SPI and CBP-SPI on viral RNA abundance
Real-time qPCR was used to quantify residual viral RNA in suspension after preincubation with CB-SPI or CBP-SPI. It was demonstrated that preincubation with CB-SPI and CBP-SPI reduced the number of virus nucleic acid copies by eightfold (average of 4 replicates Ct ± SD; 18.15 ± 0.13) and 16-fold (19.42 ± 0.42), respectively, compared to control (15.28 ± 0.24) (Fig. 3).
Fig. 3.
Inhibition of level of viral RNA in suspension after preincubation with CB-SPI and CBP-SPI
Discussion
Health effects of cranberry, cranberry products, and isolated cranberry components in humans and animals are debated. Recently, a number of studies have pointed out that cranberry A-type proanthocyanidins exhibit unique microbial anti-adhesion properties [16]. It was reported that cranberry metabolites inhibit adhesive abilities rather than kill bacteria. Evidence of protection from cancer, cardiovascular disease, and inflammation by cranberry phytochemicals is growing, while neuroprotection and anti-viral activity also have begun to draw new consideration. Soy protein isolate phytochemicals include fatty acids, saponins and isoflavones [8]. Isoflavones are the most studied bioactives of soybeans and have demonstrated anti-viral effects, including inhibition of infectivity, on a wide range of human viruses [1].
Our data suggest that cranberry compounds, most likely polyphenols, stabilized in an edible protein matrix may interact with cellular receptors, making it possible to use cranberry preparations to suppress virus reproduction. Two glycoproteins of the influenza virus envelope, hemagglutinin and neuraminidase recognize sialic acid. Initiation of virus infection involves multiple hemagglutinins binding to sialic acids on carbohydrate side chains of target cell surface glycoproteins and glycolipids [12]. Considerable progress has been made toward understanding the structural basis of interaction of the two major surface glycoproteins of influenza A virus with their common ligand/substrate. Carbohydrate chains terminating in sialic acid that bind hemagglutinins are attractive targets for anti-viral agent development. Cranberry phytochemicals may also bind hemagglutinins and prevent initiation of infection.
Cranberry phytochemical components include phenolic acids, benzoates, hydroxycinnamic acids, terpenes and organic acids. Flavonoids, especially anthocyanins, flavonols, and proanthocyanidins, have attracted research attention [24]. Cranberry proanthocyanidins have unusual A-type linkages [9, 10] compared to the more common B-type linkages found in other tannin-rich foods. Phytochemical analyses indicated that cranberry proanthocyanidins are composed primarily of oligomers containing a minimum one A-type interflavan bond [3, 24].
Anti-viral properties of high molecular weight cranberry compounds were recently discovered [20, 23, 42]. It was reported that cranberry high molecular weight non-dialyzable material (more than 15,000 MW) was significantly more potent than cranberry proanthocyanidins in inhibiting hemagglutination [42]. Non-dialyzable material showed potent activity against neuraminidase in influenza A and B strains, suggesting therapeutic potential against viral infections [23]. Su et al. [39] used standardized plaque assays to demonstrate that cranberry juice and proanthocyanidins have significant anti-viral effects on human enteric viral surrogates murine norovirus (MNV-1), feline calici virus (FCV-F9), MS2 (ssRNA) bacteriophage, and phiX-174 (ssDNA). In general, presence of ortho-trihydroxyl groups in the B-ring of anthocyanidin was important in compounds exhibiting anti-HSV. Double interflavan linkages gave rise to interesting antiviral effects (HSV and HIV) [7].
In our research, preincubation with CB-SPI or CBP-SPI in concentrations up to 2.5 % (w/v; 25 mg/mL) did not affect hemagglutination of ortho- and paramyxoviruses, hence the anti-viral mode of action of CB-SPI or CBP-SPI may be other than interference with binding of viruses to target cell surface receptors.
In this study we demonstrated that CB-SPI and CBP-SPI inhibited adsorption of viruses to target cells and decreased reproduction of ortho- and paramyxoviruses in ovo. Additionally, we demonstrated that virus nucleic acid content was reduced by eightfold with CB-SPI preincubation and 16-fold with CBP-SPI preincubation compared to control. Both matrices are formulated with SPI, thus differences in activity are attributed to cranberry polyphenols. These differences in the activity may be attributed to lower total polyphenol concentration of CB-SPI (3 % total polyphenols) compared to CBP-SPI (10 % total polyphenols). This is also applicable to the inhibition of virus adsorption to cRBCs where CBP-SPI was more effective than CB-SPI. Adsorption of all tested strains was inhibited by CBP-SPI or CB-SPI in a dose dependent manner (Fig. 3).
Suppression of viral reproduction by CBP-SPI significantly exceeded that of Amizon whereas suppression of viral reproduction by CB-SPI was comparable to Amizon (Fig. 1). At the dose of 5 mg (0.5 mg of total polyphenols) per chicken embryo CBP-SPI suppressed virus reproduction in the range of 45–90 % depending on the virus strain. CBP-SPI suppression of viral reproduction was more pronounced for H5N3 and Sendai virus and Newcastle disease virus, and lower for H7N1 and H3N2.
Suppression of infectivity of orthomyxoviruses in virucidal experiments was significantly higher for CB-SPI and CBP-SPI compared to Amizon (Fig. 2). In the case of paramyxoviruses, virucidal activity of CBP-SPI was significantly higher than CB-SPI and Amizon and virucidal activity of CB-SPI was lower than Amizon. CBP-SPI virucidal activity was similar in all tested strains. Overall, our results indicate that CB-SPI and CBP-SPI exhibit moderate anti-viral properties through prevention of virus adsorption as well as removal and destruction of virions from an in vitro host environment.
Our data suggest that soy protein/cranberry polyphenol complex effectively inhibits adsorption, replication, and infectivity of ortho- and paramyxoviruses that cause diseases of the respiratory and gastro-intestinal tract [28, 33]. New functionally-enhanced soy proteins may be the basis for development of novel, food-based therapeutic products.
Acknowledgments
This work was supported by Grants 0113PK00473 and 0114PK00303 from the Ministry of Education and Science of the Republic of Kazakhstan, Grant PSC-CUNY 66638-00 44 and an SBIR Phase 1 grant from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Grant 1R43DK092104-01A1. Diana M. Cheng was supported by NIH training grant T32: 5T32AT004094-04.
References
- 1.Andres A, Donovan SM, Kuhlenschmidt MS. Soy isoflavones and virus infections. J Nutr Biochem. 2009;20:563–569. doi: 10.1016/j.jnutbio.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betoret E, Betoret N, Vidal D, Fito P. Functional foods development: trends and technologies. Trends Food Sci Technol. 2011;22:498–508. doi: 10.1016/j.tifs.2011.05.004. [DOI] [Google Scholar]
- 3.Bresee J, Hayden FG. Epidemic influenza—responding to the expected but unpredictable. N Engl J Med. 2013;368:589–592. doi: 10.1056/NEJMp1300375. [DOI] [PubMed] [Google Scholar]
- 4.Bukhtiarova TA, Trinus FP, Danilenko VF, Danilenko GI, Ovrutskii VM, Sharykina NI. Structure and antiinflammatory activity of isonicotinic and nicotinic amides. Pharm Chem J. 1997;31:597–599. doi: 10.1007/BF02464277. [DOI] [Google Scholar]
- 5.Chan C, Lin K, Chan Y, Wang Y, Chi Y, Tu H, Shieh H, Liu W. Amplification of the entire genome of influenza A virus H1N1 and H3N2 subtypes by reverse-transcription polymerase chain reaction. J Virol Methods. 2006;136:38–43. doi: 10.1016/j.jviromet.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Chang A, Dutch RE. Paramyxovirus fusion and entry: multiple paths to a common end. Viruses. 2012;4:613–636. doi: 10.3390/v4040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Bruyne T, Pieters L, Witvrouw M, De Clercq E, Berge V, Vlietinck A. Biological evaluation of proanthocyanidin dimers and related polyphenols. J Nat Prod. 1999;62(7):954–958. doi: 10.1021/np980481o. [DOI] [PubMed] [Google Scholar]
- 8.Fang N, Yu S, Badger TM. Comprehensive phytochemical profile of soy protein isolate. J Agric Food Chem. 2004;52:4012–4020. doi: 10.1021/jf049842y. [DOI] [PubMed] [Google Scholar]
- 9.Foo LY, Lu Y, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry. 2000;54:173. doi: 10.1016/S0031-9422(99)00573-7. [DOI] [PubMed] [Google Scholar]
- 10.Foo LY, Lu Y, Howell AB, Vorsa N. A-type proanthocyanidin trimers from cranberry that Inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 11.Francis T, Pearson HE, Salk JE, Brown PN. Immunity in human subjects artificially infected with influenza virus, Type B. Am J Public Health Nations Health. 1944;34:317–334. doi: 10.2105/AJPH.34.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem. 2010;285:28403–28409. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Georgi L, Johnson-Cicalese J, Honig J, Das S, Rajah V, Bhattacharya D, Bassil N, Rowland L, Polashock J, Vorsa N. The first genetic map of the American cranberry: exploration of synteny conservation and quantitative trait loci. Theor Appl Genet. 2013;126:673–692. doi: 10.1007/s00122-012-2010-8. [DOI] [PubMed] [Google Scholar]
- 14.Grace MH, Massey AR, Mbeunkui F, Yousef GG, Lila MA. Comparison of health-relevant flavonoids in commonly consumed cranberry products. J Food Sci. 2012;77:H176–H183. doi: 10.1111/j.1750-3841.2012.02788.x. [DOI] [PubMed] [Google Scholar]
- 15.Grace M, Guzman I, Roopchand ED, Moskal K, Cheng MD, Pogrebnyak N, Raskin I, Howell A, Lila MA. Stable binding of alternative protein-enriched food matrices with concentrated cranberry bioflavonoids for functional food applications. J Agric Food Chem. 2013;61:6856–6864. doi: 10.1021/jf401627m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell AB. Cranberry proanthocyanidins and the maintenance of urinary tract health. Crit Rev Food Sci Nutr. 2002;42:273–278. doi: 10.1080/10408390209351915. [DOI] [PubMed] [Google Scholar]
- 17.Kapczynski DR, Afonso CL, Miller PJ. Immune responses of poultry to newcastle disease virus. Dev Comp Immunol. 2013 doi: 10.1016/j.dci.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Killian ML. Hemagglutination assay for the avian Influenza virus. Methods Mol Biol. 2008;436:47–52. doi: 10.1007/978-1-59745-279-3_7. [DOI] [PubMed] [Google Scholar]
- 19.Klimov A, Balish A, Veguilla V, Sun H, Schiffer J, Lu X, Katz JM, Hancock K. Influenza virus titration, antigenic characterization, and serological methods for antibody detection. Influenza Virus. 2012;865:25–51. doi: 10.1007/978-1-61779-621-0_3. [DOI] [PubMed] [Google Scholar]
- 20.Lipson S, Sethi L, Cohen P, Gordon R, Tan I, Burdowski A, Stotzky G. Antiviral effects on bacteriophages and rotavirus by cranberry juice. Phytomedicine. 2007;14:23–30. doi: 10.1016/j.phymed.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morodomi Y, Inoue M, Hasegawa M, Okamoto T, Maehara Y, Yonemitsu Y. Sendai virus-based oncolytic gene therapy. In: Wei M, Good D, editors. Novel gene therapy approaches. Rijeka: InTech Publisher; 2013. pp. 183–194. [Google Scholar]
- 22.Nesterova N, Zagorodnya S, Danilenko V, Baranova G, Golovan A. Studying of anti-epstein–barr virus activity of amizon and their derivative. Antiviral Res. 2008;78:A61. doi: 10.1016/j.antiviral.2008.01.130. [DOI] [Google Scholar]
- 23.Oiknine-Djian E, Houri-Haddad Y, Weiss EI, Ofek I, Greenbaum E, Hartshorn K, Zakay-Rones Z. High molecular weight constituents of cranberry interfere with influenza virus neuraminidase activity. Planta Med. 2012;78:962–967. doi: 10.1055/s-0031-1298579. [DOI] [PubMed] [Google Scholar]
- 24.Pappas E, Schaich K. Phytochemicals of cranberries and cranberry products: characterization, potential health effects, and processing stability. Crit Rev Food Sci Nutr. 2009;49:741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen J. Hemagglutination-Inhibition test for avian influenza virus subtype identification and the detection and quantitation of serum antibodies to the avian influenza virus. In: Spackman E, editor. avian influenza virus. Totowa: Humana Press; 2008. pp. 53–66. [DOI] [PubMed] [Google Scholar]
- 26.Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013;64:189–202. doi: 10.1146/annurev-med-120611-145115. [DOI] [PubMed] [Google Scholar]
- 27.Poehling KA, Edwards KM, Griffin MR, Szilagyi PG, Staat MA, Iwane MK, Snively BM, Suerken CK, Hall CB, Weinberg GA. The burden of influenza in young children, 2004–2009. Pediatrics. 2013;131:207–216. doi: 10.1542/peds.2012-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poulakou G, Pérez M, Rello J. Severe acute respiratory infections in the postpandemic era of H1N1. Curr Opin Crit Care. 2012;18:441–450. doi: 10.1097/MCC.0b013e32835605f2. [DOI] [PubMed] [Google Scholar]
- 29.Radulovic NS, Blagojevic PD, Stojanovic-Radic ZZ, Stojanovic NM. Antimicrobial plant metabolites: structural diversity and mechanism of action. Curr Med Chem. 2013;20:932–952. [PubMed] [Google Scholar]
- 30.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. [Google Scholar]
- 31.Roopchand DE, Grace M, Kuhn P, Cheng DM, Plundrich N, Poulev A, Howell A, Fridlender B, Lila MA, Raskin I. Efficient sorption of polyphenols to soybean flour enables natural fortification of foods. Food Chem. 2012;131:1193–1200. doi: 10.1016/j.foodchem.2011.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roopchand DE, Krueger CG, Moskal K, Fridlender B, Lila MA, Raskin I. Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem. 2013;141:3664–3669. doi: 10.1016/j.foodchem.2013.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy A, Saraf S. Limonoids: overview of significant bioactive triterpenes distributed in plants kingdom. Biol Pharm Bull. 2006;29:191–201. doi: 10.1248/bpb.29.191. [DOI] [PubMed] [Google Scholar]
- 34.Serkedjieva J, Toshkova R, Antonova-Nikolova S, Stefanova T, Teodosieva A, Ivanova I. Effect of a plant polyphenol-rich extract on the lung protease activities of influenza-virus-infected mice. Antiviral Chem Chemother. 2007;18:75–82. doi: 10.1177/095632020701800203. [DOI] [PubMed] [Google Scholar]
- 35.Shmuely H, Ofek I, Weiss EI, Rones Z, Houri-Haddad Y. Cranberry components for the therapy of infectious disease. Curr Opin Biotechnol. 2012;23:148–152. doi: 10.1016/j.copbio.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Lester P, editor. Methods in enzymology. New York: Academic Press; 1999. pp. 152–178. [Google Scholar]
- 37.Spalatin J, Hanson RP, Beard PD. The hemagglutination-elution pattern as a marker in characterizing newcastle disease virus. Avian Dis. 1970;14:542–549. doi: 10.2307/1588616. [DOI] [PubMed] [Google Scholar]
- 38.Su Z. Anthocyanins and flavonoids of Vaccinium L. Pharm Crops. 2012;3:7–37. doi: 10.2174/2210290601203010007. [DOI] [Google Scholar]
- 39.Su X, Howell AB, D’Souza DH. The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiol. 2010;27:535–540. doi: 10.1016/j.fm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Tao Y, Pinzón-Arango PA, Howell AB, Camesano TA. Oral consumption of cranberry juice cocktail inhibits molecular-scale adhesion of clinical uropathogenic Escherichia Coli. J Med Food. 2011;14:739–745. doi: 10.1089/jmf.2010.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vorsa N, Johnson-Cicalese J. American cranberry. In: Badenes ML, Byrne DH, editors. Fruit breeding. New York: Springer; 2012. pp. 191–223. [Google Scholar]
- 42.Weiss EI, Houri-Haddad Y, Greenbaum E, Hochman N, Ofek I, Zakay-Rones Z. Cranberry juice constituents affect influenza virus adhesion and infectivity. Antiviral Res. 2005;66:9–12. doi: 10.1016/j.antiviral.2004.12.011. [DOI] [PubMed] [Google Scholar]