Abstract
Hepatitis B viral (HBV) infection, which is one of the global public health concerns, is among the most common causes of cirrhosis and hepatocellular carcinoma. It was proposed that cytokine-mediated immunity plays a critical role in determining the outcomes of hepatitis B virus infection. Interleukin 12 (IL-12) is a heterodimeric cytokine that shows a broad range of immunoregulatory properties during immune responses and combats host invading pathogens. The main purpose of this study was to investigate the possible association between expression levels of IL-12 gene with HBV infection in patients with HBV infection. This clinical study was performed on 30 HBV patients and 30 healthy controls. SYBR Green Real-time PCR was performed to examine the expression level of IL-12 gene in HBV patients. Then, the rate of expression was calculated using the Livak ( ) method. ΔCT of samples in the two groups were compared using t test method. PCR was also used for HBV-DNA evidence. The results of our study demonstrated that the difference in mean of IL-12 gene expression between healthy subjects and HBV patients is statistically significant.
Keyword: HBV infection, Interleukin-12, Gene expression
Hepatitis B viral (HBV) infection that causes acute and chronic hepatitis as well as hepatocellular carcinoma is one of the major global health concerns [5, 14, 15, 18, 19, 21, 23, 29, 32]. It is estimated that over 300 million people throughout the world are chronically HBV carriers [5, 14] and it is known as top 10 causes of death [5, 21] with around 1 million deaths every year. Furthermore, a large proportion of HBV-infected individuals do not clear the virus which may progress to persistent infection with or without liver disease [32]. The hepatitis B virus is non-cytopathic as a member of the Hepadna virus family and the pathogenic mechanisms responsible for assorted outcome are unclear [9, 15, 19, 23, 29] but the host’s defence mechanisms may be critical in the revealing the outcome of HBV infection [2, 17]. Cytokines (secreted proteins/signaling molecules) are critical players in the pathogenesis of various diseases [30]. According to some studies, cytokines have essential role in the developmental regulation of naive CD4+ T cells into T helper 1 (Th1), Th2 cells or the recently identified IL-17 producing (Th17) helper T cells, which the appropriate T helper cell development is essential for promoting different aspects of the immune response [26, 28]. Th1 cells express interferon-gamma (IFN-γ) and develop cell-mediated immunity which is critical for the response against intracellular pathogens [7, 28]. Signals from interleukins are essential for the development of these different types of T cells and for their effector functions [1]. Interleukin-12 (IL-12) is the main cytokine that regulates cell-mediated immunity, Th1 differentiation, Th1 maintenance and IFN-γ production in T and NK cells [3, 10, 11, 13, 24, 27, 28, 30]. IFN-γ, tumor necrosis factor-alpha (TNF-α) and IL-2 as inflammatory cytokines are critical molecules for modulating the intensity and duration of the host immune responses against HBV infections. As a matter of fact, they are able to inhibit HBV gene expression and replication and play pivotal roles in the clearance of viruses from the rest of infected cells by non-cytolytic mechanisms [3, 32]. IL-12 is an important pro-inflammatory cytokine that consists of p35 and p40 subunits [3, 20, 27, 30]. The genes encoding human p35 and p40 are located on chromosomes 3 and 5, respectively [28]. IL-12 is mainly secreted by antigen-presenting cells (APC): macrophages (MQ) and dendritic cells (DCs) in response to infection caused by intracellular pathogens and host immune stimuli [3, 4, 25, 27, 28, 30]. The cellular effects of IL-12 are mediated by binding to its receptor (IL-12R) which includes β1- and β2-plasma membrane-traversing proteins [10, 13, 22]. Interestingly, IL-12 signaling results in the phosphorylation of TYK2 (Tyrosine kinase 2), Jak2 (Janus kinase 2) and STAT4 (signal transducer and activator of transcription) proteins [25, 28]. Nevertheless, IL-12 may play a key role in the evolution of virulence mechanisms shaping host–pathogen interactions. Hence, based on these unique properties of IL-12, we studied the expression levels of IL-12 gene in HBV patients from the Southeast of Iran.
In the present study, to evaluate the expression level of IL-12 gene, peripheral blood samples with anticoagulant were collected from 30 HBV patients as well as 30 healthy controls from Zabol Blood Transfusion Services (Zabol, Iran), the southeastern regions of Iran. Buffy coat and plasma were isolated from each EDTA-treated blood samples using Ficoll/paque solution (Sigma Chemical Co., USA). Exclusion criteria for HBV patients were HCV or HIV co-infection and under treatment with immune-modulator drugs. Normal adults from faculty and students in Zabol University, age- and sex-matched to the patients, were selected as healthy controls. This study was approved by the ethical committee of the University of Zabol.
HBsAg positivity of samples was evaluated by enzyme-linked immunosorbent assay (ELISA) (Behring, Marburg, Germany) according to the manufacturer’s guidelines. HBV DNA was extracted from plasma using a DNP TM Kit (CinnaGen, Iran) according to the manufacturer’s protocol and then the genomic HBV DNA was identified in patient samples using specific primers to amplify a fragment of the surface gene by a qualitative hepatitis B virus PCR detection Kit (CinnaGen, Iran) according to the manufacturer’s instruction.
Total RNA was extracted by RNX-Plus solution (CinnaGen, Iran). The quantity of extracted RNA was evaluated by Nanodrop (measuring the optical density 260/280), and the quality of extracted RNA was assessed by running 3 µl in 1 % agarose gel. The quality of RNA was indicated by the lack of a smear on the lower part of the gel (a smear indicates RNA degradation) and by the presence of 28S ribosomal RNA twice as intense as that of 18S rRNA. After good-quality total RNA was obtained, cDNA was synthesized by Takara Kit (Dalian, Japan) according to the manufacturer’s guidelines.
For the quantitative analysis of IL-12 mRNA expression profile in studied groups, SYBR Green Real time PCR method was performed. The results were normalized to the expression level of GAPDH as a housekeeping gene.The primer was originally designed for transcripts of IL-12 (AF101062.1) and GAPDH (NM_002046.5) as the internal control. The primer sequences for amplification of IL-12 and GAPDH transcripts sequences were: IL-12F: 5′-CCAAGAATGAGAGTTGCCTAA-3′, IL-12R: 5′-CACCTGGTACATCTTCAAGTC-3′; GAPDH F: 5′- GGACTCATGACCACAGTCCA-3′ and GAPDH R: 5′-CCAGTAGAGGCAGGGATGAT-3′. The ΔCT (the subtraction of Ct of IL-12 gene from the Ct of GAPDH gene) in HBV patients and ΔCT (obtained by subtracting the Ct of IL-12 gene from the Ct of GAPDH gene) in the control group were calculated and then the rate of increased expression was calculated using the Livak () method.
The statistical differences in expression level of IL-12 gene and the fold changes in patients and controls were compared via independent t test and the Livak () methods, respectively. The statistical software (IBM/SPSS Statistics version 19) was used for data analysis and p values of less than 0.05 were considered significant.
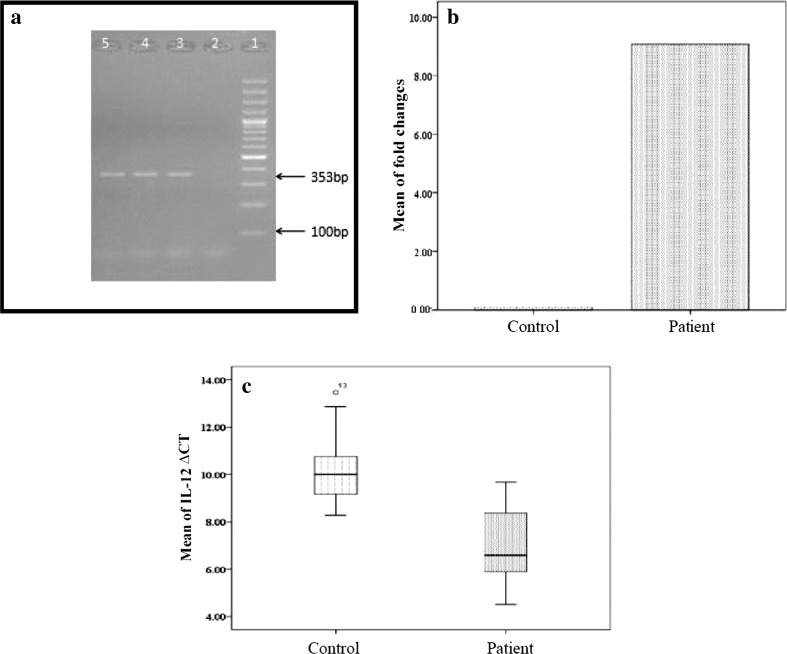
Patients and controls were matched by age and gender. The mean age of the patients and control groups was 26 ± 6 and 27 ± 6, respectively. The profile of patients with HBV infection and control group are given in Table 1. HBV-DNA was detected by PCR (Fig. 1a). Results of real-time PCR showed that, IL-12 gene relative to GAPDH gene was expressed significantly (9.06 times) higher in the PBMCs of HBV patients compared to control groups (Fig. 1b). Statistical analysis showed that the difference was significant (p < 0.001).
Table 1.
Demographic and laboratory information of hepatitis B virus-infected patient and controls
| Variant | Patient | Healthy control |
|---|---|---|
| Age | 26 ± 6 | 27 ± 6 |
| Sex | ||
| Female | 9 (30 %) | 9 (30 %) |
| Male | 21 (70 %) | 21 (70 %) |
| HBsAg positive | All of them | 0 |
| HBV-DNA | All of them | 0 |
Fig. 1.
a HBV-DNA PCR. Lane 1 DNA ladder marker, Lane 2 a negative control, Lane 3 a positive control showing the expected 353 bp product, Lanes 4 and 5 positive samples. b Comparison of expression level of IL-12 gene relative to GAPDH gene between HBV patients and control groups. c BoxPlot of mean of IL-12 ∆CT in HBV patients and control groups
Hepatitis B infection with more than 300 million HBV carriers makes it one of the most hazardous viral pathogens for worldwide public health [2, 18, 31]. The immunological features of the liver may help the maintenance of immunological tolerance present in chronic HBV infection [19]. Cytokines as potent immune modulatory molecules play crucial roles in the inflammatory and immune responses. Recent studies suggest that, Th1 and Th2 cell responses may contribute to successful treatment of hepatitis B and C and may be associated with the persistence of HBV infection, respectively. A number of investigations have indicated that HBV+ is characterized by type Th1 cytokines. In fact, IL-4 and IFN-γ are effective in the chronic progression of hepatitis [6]. IL-12 regulates innate and adaptive immune responses during infection [10], as already mentioned, this mediator is required for treating many diseases because of its Th1-inducing activity and stimulating cell-mediated immunity such as viral and bacterial infections and also cancers. Based on these studies, we assessed the expression rate of IL-12 in two different groups: HBV patients and healthy controls (Fig. 1c). Based on our results, we found that IL-12 is expressed at high levels in HBV patients, suggesting that IL-12 expression may be associated with the progression of HBV infection. T tests demonstrated that the difference of mean ΔCT for IL-12 gene between healthy subjects and patients with hepatitis B infection is statistically significant (p value < 0.001). In a transgenic mouse model study by Cavanaugh et al. [4] was shown that IL-12 can suppress replication of HBV by induction of IFN-γ. In previous researches, Rossol et al. [20] have shown that IL-12 serum levels are raised during viral clearance induced by IFN-α therapy. Milich et al. [16] reported that autoantibody production in HBeAg-transgenic mice mediated by self-reactive Th2-type cells can be suppressed by IL-12. Rizvi et al. [18] shown that no significant difference was found in serum IL-12 level in CHB patients, while, they showed both IFN-γ and IL-12 were higher in hepatitis B e-antigen (HBeAg)-negative patients than HBeAg-positive patients. Also, Researches show that IL-12 is involved in the control of various intracellular pathogens [8, 12]. IL-12-induced Th1 responses could be detrimental in some autoimmune disorders, such as MS, rheumatoid arthritis, inflammatory bowel, and graft versus-host disease [8]. Following and confirming the work of these researchers, for the first time, the results of our study indicate that IL-12 may have an essential role for viral clearance in HBV infection. Therefore, the host immunity through interaction of different cytokine expression profiles with HBV may affect in the disease progression of hepatitis. In Conclusion, HBV infection can lead to overexpression of IL-12 gene in patients with HBV infection. Thus, by knowing this fact, it may be possible to have prevention programs for preventing progression of this virus by using antiviral drugs for these patients and also achieving a better understanding of the role of IL-12 in regulation of gene activation and mechanism of the antiviral effect may aid in the development of a novel immunotherapeutic strategy for HBV infectious diseases. Therefore, our findings may lead to improvements in the clinical management of patients with HBV infection. However, other aspects of the immune response in this disease need to be investigated.
Acknowledgments
The authors gratefully acknowledge the contribution of the patients, healthy controls for their blood donations and also the Research Council of University of Zabol for their financial support of this work.
Contributor Information
Abdolhossein Zare, Email: zareabh@yahoo.com.
Ahmad Rashki, Email: ah_rashki@usal.es.
References
- 1.Batten M, Ghilardi N. The biology and therapeutic potential of interleukin 27. J Mol Med. 2007;85(7):661–672. doi: 10.1007/s00109-007-0164-7. [DOI] [PubMed] [Google Scholar]
- 2.Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87(6):1439–1449. doi: 10.1099/vir.0.81920-0. [DOI] [PubMed] [Google Scholar]
- 3.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24(4):207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavanaugh VJ, Guidotti LG, Chisari FV. Interleukin-12 inhibits hepatitis B virus replication in transgenic mice. J Virol. 1997;71(4):3236–3243. doi: 10.1128/jvi.71.4.3236-3243.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datta S, Chatterjee S, Veer V, Chakravarty R. Molecular biology of the hepatitis B virus for clinicians. J Clin Exp Hepatol. 2012;2(4):353–365. doi: 10.1016/j.jceh.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falasca K, Ucciferri C, Dalessandro M, Zingariello P, Mancino P, Petrarca C, Pizzigallo E, Conti P, Vecchiet J. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann Clin Lab Sci. 2006;36(2):144–150. [PubMed] [Google Scholar]
- 7.Ganem D, Prince AM. Hepatitis B virus infection-natural history and clinical consequences. N Engl J Med. 2004;350(11):1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 8.Gee K, Guzzo C, CheMat N, Ma W, Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8(1):40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Annu Rev Pathol Mech Dis. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 10.Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol Sci. 2010;11(3):789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones LL, Vignali DAA. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol Res. 2011;51(1):5–14. doi: 10.1007/s12026-011-8209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Komastu T, Ireland DD, Reiss CS. IL-12 and viral infections. Cytokine Growth Factor Rev. 1998;9(3):277–285. doi: 10.1016/S1359-6101(98)00017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levi B, Benish M, Goldfarb Y, Sorski L, Melamed R, Rosenne E, Ben-Eliyahu S. Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behav Immun. 2011;25(4):727–735. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lisotti A, Grenci C, Caponi A, Roda E. Chronic hepatitis B in 2008. Digest Liver Dis Suppl. 2008;2(2):3–6. doi: 10.1016/S1594-5804(09)60003-6. [DOI] [Google Scholar]
- 15.Lok ASF. Hepatitis B infection: pathogenesis and management. J Hepatol. 2000;32:89–97. doi: 10.1016/S0168-8278(00)80418-3. [DOI] [PubMed] [Google Scholar]
- 16.Milich DR, Wolf SF, Hughes JL, Jones JE. Interleukin 12 suppresses autoantibody production by reversing helper T-cell phenotype in hepatitis B e antigen transgenic mice. Proc Natl Acad Sci. 1995;92(15):6847–6851. doi: 10.1073/pnas.92.15.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondelli MU. Immunopathogenesis of viral hepatitis. Clin Rev Allergy Immunol. 2000;18(2):141–166. doi: 10.1385/CRIAI:18:2:141. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi M, Azam M, Ajmal MR, Malik A, Shukla I, Afroz N. Role of Interferon-gamma and interleukin-12 in the immunopathogenesis of hepatitis B virus infection. Euroasian J Hepato Gastroenterol. 2012;2(1):5–9. doi: 10.5005/jp-journals-10018-1022. [DOI] [Google Scholar]
- 19.Rizvi M, Azam M, Sami H, Shukla I, Malik A, Ajmal MR, Khan F, Sultan A. Role of IL-8, IL-10, IL-12, IFN-γ and TNF-α in the immunopathogenesis of acute hepatitis B virus infection. J Gastroenterol Hepatol Res. 2013;2(6):646. [Google Scholar]
- 20.Rossol S, Marinos G, Carucci P, Singer MV, Williams R, Naoumov NV. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J Clin Investig. 1997;99(12):3025. doi: 10.1172/JCI119498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shafritz DA. The molecular biology of hepatitis B virus. Mol Basis Viral Replication. 1987;136:415–435. doi: 10.1007/978-1-4684-5350-8_17. [DOI] [Google Scholar]
- 22.Taylor-Fishwick DA, Weaver JR, Grzesik W, Chakrabarti S, Green-Mitchell S, Imai Y, Kuhn N, Nadler JL. Production and function of IL-12 in islets and beta cells. Diabetologia. 2013;56(1):126–135. doi: 10.1007/s00125-012-2732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trautwein C. Mechanisms of hepatitis B virus graft reinfection and graft damage after liver transplantation. J Hepatol. 2004;41(3):362–369. doi: 10.1016/j.jhep.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 2003;19(5):641–644. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 25.Vignali DAA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S, Zhu C, Zhang R, Liu L, Wu J. Association of interleukin 27 expression and p28 gene polymorphism with chronic hepatitis B virus infection. J Toxicol Environ Health Sci. 2009;1(2):028–033. [Google Scholar]
- 27.Wang T, Husain M, Hong S, Holland JW. Differential expression, modulation and bioactivity of distinct fish IL-12 isoforms: implication towards the evolution of Th1-like immune responses. Eur J Immunol. 2014;44(5):1541–1551. doi: 10.1002/eji.201344273. [DOI] [PubMed] [Google Scholar]
- 28.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14(5):361–368. doi: 10.1016/S1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 29.Xia L, Tian D, Huang W, Zhu H, Wang J, Zhang Y, Hu H, Wu K. Upregulation of IL-23 expression in patients with chronic hepatitis B is mediated by the HBx/ERK/NF-κB pathway. J Immunol. 2012;188(2):753–764. doi: 10.4049/jimmunol.1101652. [DOI] [PubMed] [Google Scholar]
- 30.Xiong SQ, Lin BL, Gao X, Tang H, Wu CY. IL-12 promotes HBV-specific central memory CD8+ T cell responses by PBMCs from chronic hepatitis B virus carriers. Int Immunopharmacol. 2007;7(5):578–587. doi: 10.1016/j.intimp.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Yokosuka O, Arai M. Molecular biology of hepatitis B virus: effect of nucleotide substitutions on the clinical features of chronic hepatitis B. Med Mol Morphol. 2006;39(3):113–120. doi: 10.1007/s00795-006-0328-5. [DOI] [PubMed] [Google Scholar]
- 32.Zoulim F. Therapy of chronic hepatitis B virus infection: inhibition of the viral polymerase and other antiviral strategies. Antivir Res. 1999;44(1):1–30. doi: 10.1016/S0166-3542(99)00056-X. [DOI] [PubMed] [Google Scholar]