Abstract
Neurogliaform (RELN+) and bipolar (VIP+) GABAergic interneurons of the mammalian cerebral cortex provide critical inhibition locally within the superficial layers. While these subtypes are known to originate from the embryonic caudal ganglionic eminence (CGE), the specific genetic programs that direct their positioning, maturation, and integration into the cortical network have not been elucidated. Here, we report that in mice expression of the transcription factor Prox1 is selectively maintained in postmitotic CGE-derived cortical interneuron precursors and that loss of Prox1 impairs the integration of these cells into superficial layers. Moreover, Prox1 differentially regulates the postnatal maturation of each specific subtype originating from the CGE (RELN, Calb2/VIP, and VIP). Interestingly, Prox1 promotes the maturation of CGE-derived interneuron subtypes through intrinsic differentiation programs that operate in tandem with extrinsically driven neuronal activity-dependent pathways. Thus Prox1 represents the first identified transcription factor specifically required for the embryonic and postnatal acquisition of CGE-derived cortical interneuron properties.
SIGNIFICANCE STATEMENT Despite the recognition that 30% of GABAergic cortical interneurons originate from the caudal ganglionic eminence (CGE), to date, a specific transcriptional program that selectively regulates the development of these populations has not yet been identified. Moreover, while CGE-derived interneurons display unique patterns of tangential and radial migration and preferentially populate the superficial layers of the cortex, identification of a molecular program that controls these events is lacking.
Here, we demonstrate that the homeodomain transcription factor Prox1 is expressed in postmitotic CGE-derived cortical interneuron precursors and is maintained into adulthood. We found that Prox1 function is differentially required during both embryonic and postnatal stages of development to direct the migration, differentiation, circuit integration, and maintenance programs within distinct subtypes of CGE-derived interneurons.
Keywords: bipolar, mouse genetics, neurogliaform, RELN, transcription, VIP
Introduction
Mammalian neocortical circuits are primarily comprised of two interconnected neuronal cell types: excitatory glutamatergic projection neurons with pyramidal morphologies and a smaller population of inhibitory GABAergic interneurons (∼20%), the majority of which project axons locally. GABAergic interneurons can be categorized into numerous diverse subtypes based on their morphology, connectivity, laminar position, molecular expression profile, and intrinsic firing properties (Kubota et al., 1994; Markram et al., 2004; Kubota et al., 2011; DeFelipe et al., 2013; Fino et al., 2013). It is increasingly becoming evident that this diversity reflects specialized roles for each cortical interneuron subtype in shaping activity within the mature neuronal circuit (Buzsáki et al., 2007; Thomson and Lamy, 2007; Klausberger and Somogyi, 2008; Sohal et al., 2009; Isaacson and Scanziani, 2011; Krook-Magnuson et al., 2012; Kvitsiani et al., 2013; Larkum, 2013). How then does this diversity develop, and what are the mechanisms by which different subtypes of interneurons become integrated into the cortical network?
Work from several research groups has determined the spatiotemporal embryonic origins of GABAergic interneuron diversity (Marín and Rubenstein, 2003; Wonders and Anderson, 2006; Batista-Brito and Fishell, 2009; Gelman and Marín, 2010; Hernández-Miranda et al., 2010; Marín et al., 2010; Le Magueresse and Monyer, 2013; Miyoshi et al., 2013; Kessaris et al., 2014). GABAergic cortical interneurons are not locally generated within the cortical germinal zones; instead they arise from the ventral telencephalon (Anderson et al., 1997), in particular the medial and caudal ganglionic eminences (MGE and CGE; Wichterle et al., 2001; Nery et al., 2002; Xu et al., 2004; Butt et al., 2005; Flames et al., 2007; Fogarty et al., 2007; Miyoshi et al., 2007, 2010; Wonders et al., 2008; Rubin et al., 2010; Inan et al., 2012) as well as the preoptic area (POA; Gelman et al., 2009; Gelman et al., 2011). Cortical interneurons arising from the MGE and CGE give rise to nonoverlapping subtypes that occupy distinct layers of the developing neocortex (Nadarajah et al., 2002; Kriegstein and Noctor, 2004; Tanaka et al., 2006; Fogarty et al., 2007; Métin et al., 2007; Miyoshi et al., 2007, Miyoshi et al., 2010; Li et al., 2008; López-Bendito et al., 2008; Rubin et al., 2010; Sessa et al., 2010; Miyoshi and Fishell, 2011; Marín, 2013). The MGE produces ∼70% of the total GABAergic cortical interneuron population, specifically the PV (Pvalb, Parvalbumin) and SST (Sst, Somatostatin)-expressing classes, which, mirroring pyramidal neurons (Molyneaux et al., 2007; Leone et al., 2008; Rakic, 2009; Franco et al., 2011; Lui et al., 2011; Kwan et al., 2012; Franco and Müller, 2013; Gao et al., 2013), integrate into the cortex in an inside-out pattern (Miller, 1985; Fairén et al., 1986; Miyoshi et al., 2007; Rymar and Sadikot, 2007). In contrast, CGE-derived cortical interneurons comprise ∼30% of the total interneuron pool, the vast majority of which express either RELN (Reln, Reelin) or VIP (Vip, vasoactive intestinal polypeptide), including a subclass that coexpresses CR (Calb2, Calretinin; Miyoshi et al., 2010). Unlike MGE lineages, CGE-derived interneurons specifically populate the superficial layers regardless of their birthdate (Miyoshi et al., 2010; Miyoshi and Fishell, 2011). Interestingly, the serotonin receptor Htr3A is selectively expressed in CGE-derived interneurons (Lee et al., 2010; Vucurovic et al., 2010) and regulates their embryonic migration (Murthy et al., 2014). Thus there must exist distinct genetic programs that control both the migration and subtype specification of CGE-derived versus MGE-derived cortical interneurons (Kriegstein and Noctor, 2004; Hernández-Miranda et al., 2010; Tanaka and Nakajima, 2012; Marín, 2013; Kessaris et al., 2014).
Here we report that expression of the homeodomain transcription factor Prox1 is selectively maintained in postmitotic CGE-derived GABAergic cortical interneurons during embryonic and postnatal time points and directs the migration and maturation programs of each CGE-derived cortical interneuron subtype.
Materials and Methods
In vivo mouse genetics.
All animal handling and experiments were performed in accordance with protocols approved by local Institutional Animal Care and Use Committee of the NYU School of Medicine. The following genetic crosses were generated for our experiments. Total cortical GABAergic populations were visualized with Dlx5/6-Flpe; RCE:FRT (Miyoshi et al., 2010). MGE-derived GABAergic populations were visualized with Lhx6BAC-Cre; Dlx5/6-Flpe; RCE:dual (Fogarty et al., 2007; Miyoshi et al., 2010). In this cross, to exclude the labeling of blood vessels by the Lhx6BAC-Cre driver (repository stock #026555; The Jackson Laboratory), an intersectional strategy was used (Miyoshi and Fishell, 2006; Dymecki and Kim, 2007). The Dlx6a-Cre (Monory et al., 2006; repository stock number #008199; The Jackson Laboratory) and Vip-Cre (Taniguchi et al., 2011; repository stock number #010908; The Jackson Laboratory) drivers were used in our Prox1 loss-of-function studies. The Wnt3a-Cre driver (Yoshida et al., 2006; Gil-Sanz et al., 2013) was used to verify the Prox1 expression that occurs transiently within the Cajal–Retzius cells during embryogenesis (data not shown). Ai9 was used as a red-fluorescent protein (tdTomato) reporter line (repository stock number #007909; The Jackson Laboratory). The Htr3ABAC-Cre driver line was generated in a manner similar to the previously generated Htr3ABAC-EGFP line (Gong et al., 2003; Lee et al., 2010) and deposited into the GENSAT program. Briefly, the coding region of Cre was inserted into ∼200 kb of the BAC fragment including the regulatory elements of Htr3A. Compound male Dlx6a-Cre; Prox1-F/+ (Harvey et al., 2005) mice were crossed with female Prox1-C:EGFP/C:EGFP mice (Iwano et al., 2012) to generate control (Dlx6a-Cre; Prox1-C:EGFP/+) and Prox1 loss-of-function (Dlx6a-Cre; Prox1-C:EGFP/F) experimental animals. This breeding strategy was followed after we observed that the Dlx6a-Cre driver caused somatic recombination in the floxed Prox1 loss-of-function allele (Harvey et al., 2005), most likely during the phase of germline transmission, when these two alleles existed together in females. Genotyping of the two conditional Prox1 alleles was performed as previously described (Harvey et al., 2005; Iwano et al., 2012). In all of our Prox1 conditional loss-of-function experiments (Dlx6a-Cre, Htr3ABAC-Cre and Vip-Cre-mediated removal of Prox1), we did not observe any obvious difference between the controls and mutants regarding the size of the forebrain as well as the thickness of the cortical layers.
Microarray expression analysis.
For the comparison of genes selectively expressed within CGE-derived versus MGE-derived interneuron precursors, we selectively labeled each population with EGFP in vivo through the use of Mash1BAC-CreER; RCE:loxP (Battiste et al., 2007; Miyoshi et al., 2010) and Nkx2-1BAC-Cre; RCE:loxP (Xu et al., 2008; Sousa et al., 2009) compound transgenic animals, respectively. To generate labeled CGE-derived interneuron precursors within the cortex at E18.5, 4 mg of tamoxifen (in corn oil) was administered by oral gavage to the pregnant dam at E16.5 (Miyoshi et al., 2010). Note that in both genetic labeling strategies, some oligodendrocyte precursors are labeled with EGFP in addition to GABAergic neuronal precursors (Kessaris et al., 2006). By using the fluorescent dissection microscope (Olympus, MVX 10), embryos harboring green brains were selected, brains were dissected out in HBSS solution one by one, meninges were removed, and each pair of cortices was dissected out and stored in a tube with 200 μl of HBSS solution and kept on ice until the dissection was complete for the entire litter. During this step, a small piece of the embryonic brainstem was also collected in numbered tubes for genotyping. Later, HBSS was removed and 20 μl of DNase I (2000 U/ml in HBSS; Worthington) and 200 μl of Papain (20 U/ml in HBSS; Worthington) solution were immediately added. By gentle pipetting (typically approximately 10–15 times) with a 200 μl filter tip pipette, cortices were broken into small pieces. After 20 min of incubation at 37°C, 750 μl of 1% (v/v) normal horse serum (Gibco) in ice-cold HBSS and 75 μl of DNase I (2000 U/ml in HBSS) were added. After gently inverting the tubes a couple of times, tubes were centrifuged for 3 min at 0.3 relative centrifugal force (rcf), the supernatant was discarded, 500 μl of ice-cold HBSS (containing 5 μl of DNase I) was added, and the cell pellet was resuspended by gentle pipetting using a 1000 μl filter tip pipette. These cells were subjected to FACS (MoFlo; Beckman Coulter), and EGFP-positive cells were collected into numbered tubes containing 500 μl of ice-cold HBSS. Cell numbers were typically between 10,000∼20,000 and 20,000∼30,000 cells per brain for CGE-derived and MGE-derived populations, respectively. These tubes were then centrifuged for 5 min at 0.3 rcf, supernatant was discarded, and tubes were snap frozen in liquid nitrogen and stored at −80°C until mRNA extraction and subsequent labeling. RNA extraction, labeling, and subsequent microarray expression analyses using Affymetrix Gene Chips (Mouse 430A.2, which contains 45,000 probe sets covering over 39,000 transcripts) were performed at the Genomic Core Laboratory at the Memorial Sloan Kettering Center. Analyses of the changes in gene expression profiles in the conditional Prox1 loss-of-function experiments were performed in a very similar manner by comparing the mRNA transcriptomes of the EGFP-labeled cells purified from the E18.5 cortices of control heterozygous and Prox1 loss-of-function animals (Dlx6a-Cre; Prox1-C:EGFP/+ and /F).
The raw probe-level expression measurements were obtained from the Affymetrix CEL files. Analysis of microarray data was performed using Ingenuity iReport. Probe set intensities were summarized and normalized using RMA, and significant differential expression was determined by a moderated t test (LIMMA) using an FDR-adjusted p value cutoff (Q value) of 0.05 and a fold change cutoff of 1.5. For the gene expression comparison of CGE-derived versus MGE-derived interneurons, we acquired 723 significantly differentially expressed probe sets resulting in 567 genes with 382 CGE-enriched and 185 MGE-enriched genes (Tables 1 and 2). For the analysis of Prox1 loss-of-function, we acquired 93 probe sets resulting in 46 upregulated and 33 downregulated genes upon Prox1 loss-of-function. Since we analyzed three male and three female samples by chance for the comparison of control and Prox1 loss-of-function, respectively, we found several gender-specific genes with significant and large changes (Table 3).
Table 1.
Genes enriched in CGE-derived versus MGE-derived interneurons at E18.5
| Symbol: Name | Fold change |
|---|---|
| CCND2: cyclin D2 | 107.68 |
| MKI67: antigen identified by monoclonal antibody Ki-67 | 67.082 |
| TOP2A: topoisomerase (DNA) II α170 kDa | 64.226 |
| MEIS2: Meis homeobox 2 | 60.951 |
| RRM2: ribonucleotide reductase M2 | 54.829 |
| SYNPR: synaptoporin | 53.649 |
| WNT5A: wingless-type MMTV integration site family, member 5A | 43.522 |
| CENPF: centromere protein F, 350/400 kDa | 42.989 |
| CEP55: centrosomal protein 55 kDa | 39.435 |
| Ccnb1/Gm5593: cyclin B1 | 37.666 |
| CDCA5: cell division cycle associated 5 | 35.871 |
| PBK: PDZ binding kinase | 35.556 |
| EZR: ezrin | 33.941 |
| KIF2C: kinesin family member 2C | 31.704 |
| VIM: vimentin | 31.315 |
| NUSAP1: nucleolar and spindle associated protein 1 | 28.928 |
| MELK: maternal embryonic leucine zipper kinase | 27.681 |
| EMP1: epithelial membrane protein 1 | 23.414 |
| CENPA: centromere protein A | 23.144 |
| KIF22: kinesin family member 22 | 23.11 |
| PAX6: paired box 6 | 23.073 |
| CENPE: centromere protein E, 312 kDa | 22.218 |
| NR2F2: nuclear receptor subfamily 2, group F, member 2 | 21.87 |
| CKS2: CDC28 protein kinase regulatory subunit 2 | 21.21 |
| SPAG5: sperm associated antigen 5 | 21.161 |
| ECT2: epithelial cell transforming sequence 2 oncogene | 21.113 |
| RWDD3: RWD domain containing 3 | 20.515 |
| CDK1: cyclin-dependent kinase 1 | 20.515 |
| TMEM123: transmembrane protein 123 | 20.48 |
| ASPM: asp (abnormal spindle) homolog, microcephaly associated (Drosophila) | 20.245 |
| BIRC5: baculoviral IAP repeat containing 5 | 19.555 |
| DTL: denticleless E3 ubiquitin protein ligase homolog (Drosophila) | 19.304 |
| BRCA1: breast cancer 1, early onset | 17.891 |
| ANLN: anillin, actin binding protein | 16.842 |
| SHCBP1: SHC SH2-domain binding protein 1 | 16.766 |
| MIS18BP1: MIS18 binding protein 1 | 16.608 |
| EGLN3: egl nine homolog 3 (Caenorhabditis elegans) | 15.965 |
| CCNA2: cyclin A2 | 15.743 |
| TPX2: TPX2, microtubule-associated, homolog (Xenopus laevis) | 15.409 |
| CDC20: cell division cycle 20 | 15.237 |
| DEPDC1: DEP domain containing 1 | 15.082 |
| CXCL14: chemokine (C-X-C motif) ligand 14 | 14.875 |
| AURKB: aurora kinase B | 14.806 |
| MCM2: minichromosome maintenance complex component 2 | 14.643 |
| KIF18A: kinesin family member 18A | 14.561 |
| KIF20A: kinesin family member 20A | 14.086 |
| GRB10: growth factor receptor-bound protein 10 | 13.737 |
| MCM3: minichromosome maintenance complex component 3 | 13.63 |
| LRR1: leucine-rich repeat protein 1 | 13.588 |
| BUB1: BUB1 mitotic checkpoint serine/threonine kinase | 13.53 |
| RACGAP1: Rac GTPase activating protein 1 | 13.465 |
| HBA1/HBA2: hemoglobin, α-1 | 13.359 |
| DNA2: DNA replication helicase 2 homolog (yeast) | 13.219 |
| KIF11: kinesin family member 11 | 13.138 |
| RLBP1: retinaldehyde binding protein 1 | 13.003 |
| PLK4: polo-like kinase 4 | 12.999 |
| NCAPG: non-SMC condensin I complex, subunit G | 12.724 |
| PSRC1: proline/serine-rich coiled-coil 1 | 12.714 |
| CCNB2: cyclin B2 | 12.388 |
| PROX1: prospero homeobox 1 | 12.378 |
| STIL: SCL/TAL1 interrupting locus | 12.191 |
| PRC1: protein regulator of cytokinesis 1 | 12.157 |
| HTR3A: 5-hydroxytryptamine (serotonin) receptor 3A, ionotropic | 11.839 |
| CCDC109B: coiled-coil domain containing 109B | 11.835 |
| CRYAB: crystallin, α-B | 11.75 |
| GMNN: geminin, DNA replication inhibitor | 11.692 |
| TTK: TTK protein kinase | 11.145 |
| SPC25: SPC25, NDC80 kinetochore complex component, homolog | 11.138 |
| TUBA1C: tubulin, α-1c | 11.057 |
| TCF19: transcription factor 19 | 10.957 |
| CDC6: cell division cycle 6 | 10.9 |
| NEDD9: neural precursor cell expressed, developmentally downregulated 9 | 10.727 |
| UHRF1: ubiquitin-like with PHD and ring finger domains 1 | 10.651 |
| SORCS3: sortilin-related VPS10 domain containing receptor 3 | 10.229 |
| SPON1: spondin 1, extracellular matrix protein | 10.21 |
| HMGB2: high mobility group box 2 | 10.171 |
| RAD51AP1: RAD51 associated protein 1 | 10.081 |
| RAI14: retinoic acid induced 14 | 10.036 |
Table 2.
Genes enriched in MGE-derived versus CGE-derived interneurons at E18.5
| Symbol: Name | Fold change |
|---|---|
| PALD1: phosphatase domain containing, paladin 1 | 56.966 |
| GRIA1: glutamate receptor, ionotropic, AMPA 1 | 27.832 |
| LHX6: LIM homeobox 6 | 22.68 |
| C8orf4: chromosome 8 open reading frame 4 | 18.462 |
| NXPH1: neurexophilin 1 | 14.768 |
| ID3: inhibitor of DNA binding 3, dominant negative helix-loop-helix protein | 11.804 |
| CXCR7: chemokine (C-X-C motif) receptor 7 | 11.699 |
| MEF2C: myocyte enhancer factor 2C | 11.461 |
| MAFB: v-maf musculoaponeurotic fibrosarcoma oncogene homolog B (avian) | 11.068 |
| GUCY1A3: guanylate cyclase 1, soluble, α-3 | 10.147 |
| CUX2: cut-like homeobox 2 | 9.613 |
| RPH3A: rabphilin 3A homolog (mouse) | 8.384 |
| NPY: neuropeptide Y | 8.362 |
| MAN1A1: mannosidase, α, class 1A, member 1 | 8.053 |
| THBS1: thrombospondin 1 | 8.04 |
| TGFB2: transforming growth factor, β2 | 7.935 |
| KLF5: Kruppel-like factor 5 (intestinal) | 7.882 |
| HNMT: histamine N-methyltransferase | 7.848 |
| IGFBP4: insulin-like growth factor binding protein 4 | 7.575 |
| RPP25: ribonuclease P/MRP 25 kDa subunit | 7.443 |
| PPARGC1A: peroxisome proliferator-activated receptor γ, coactivator 1 α | 7.198 |
| NID2: nidogen 2 (osteonidogen) | 7.115 |
| CBFA2T3: core-binding factor, runt domain,α-subunit 2; translocated to, 3 | 7.053 |
| DMD: dystrophin | 6.861 |
| PLXDC2: plexin domain containing 2 | 6.792 |
| C1QTNF4: C1q and tumor necrosis factor-related protein 4 | 6.643 |
| SCN1A: sodium channel, voltage-gated, type I, α-subunit | 6.414 |
| RBFOX1: RNA binding protein, fox-1 homolog (C. elegans) 1 | 6.172 |
| GALC: galactosylceramidase | 6.013 |
| APC: adenomatous polyposis coli | 5.977 |
| DGKG: diacylglycerol kinase, γ 90 kDa | 5.965 |
| KCNK2: potassium channel, subfamily K, member 2 | 5.918 |
| SST: somatostatin | 5.719 |
| Gm16489: predicted gene 16489 | 5.705 |
| RUFY2: RUN and FYVE domain containing 2 | 5.469 |
| TGFB3: transforming growth factor, β3 | 5.267 |
| SEMA3A: sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3A | 5.218 |
| SMARCA2: SWI/SNF-related, matrix-associated, actin-dependent regulator of chromatin, subfamily a, member 2 | 5.136 |
| PHLDA1: pleckstrin homology-like domain, family A, member 1 | 5.095 |
| NOX4: NADPH oxidase 4 | 5.046 |
| MMP2: matrix metallopeptidase 2 (gelatinase A, 72 kDa gelatinase, 72 kDa type IV collagenase) | 5.013 |
| EPB41L4A: erythrocyte membrane protein band 4.1 like 4A | 4.869 |
| SLA: Src-like-adaptor | 4.596 |
| CDH9: cadherin 9, type 2 (T1-cadherin) | 4.587 |
| CACNA2D2: calcium channel, voltage-dependent, α2/δ-subunit 2 | 4.552 |
| DPF1: D4, zinc and double PHD fingers family 1 | 4.485 |
| PKP4: plakophilin 4 | 4.453 |
| Ptprd: protein tyrosine phosphatase, receptor type, D | 4.411 |
| Pisd-ps3: phosphatidylserine decarboxylase, pseudogene 3 | 4.404 |
| RUNX1T1: runt-related transcription factor 1; translocated to, 1 (cyclin D-related) | 4.39 |
| NETO1: neuropilin (NRP) and tolloid (TLL)-like 1 | 4.39 |
| PCMTD1: protein-l-isoaspartate (d-aspartate) O-methyltransferase domain containing 1 | 4.323 |
| NPNT: nephronectin | 4.183 |
| MYH7: myosin, heavy chain 7, cardiac muscle, β | 4.141 |
| SCUBE1: signal peptide, CUB domain, EGF-like 1 | 4.083 |
| ARHGAP20: Rho GTPase activating protein 20 | 4.016 |
| DCX: doublecortin | 4 |
| KCNMB2: potassium large conductance calcium-activated channel, subfamily M, β member 2 | 3.909 |
| SOX6: SRY (sex determining region Y)-box 6 | 3.891 |
| SATB1: SATB homeobox 1 | 3.882 |
| CDK20: cyclin-dependent kinase 20 | 3.818 |
| SPATS2L: spermatogenesis associated, serine-rich 2-like | 3.802 |
| KIF2A: kinesin heavy chain member 2A | 3.753 |
| PDE2A: phosphodiesterase 2A, cGMP-stimulated | 3.641 |
| Fhod3: formin homology 2 domain containing 3 | 3.514 |
| KCTD12: potassium channel tetramerization domain containing 12 | 3.479 |
| SERPINI1: serpin peptidase inhibitor, clade I (neuroserpin), member 1 | 3.476 |
| FAM19A5: family with sequence similarity 19 (chemokine (C-C motif)-like), member A5 | 3.476 |
| ALK: anaplastic lymphoma receptor tyrosine kinase | 3.385 |
| FURIN: furin (paired basic amino acid cleaving enzyme) | 3.371 |
| ARL4D: ADP-ribosylation factor-like 4D | 3.32 |
| FUT9: fucosyltransferase 9 (α (1,3) fucosyltransferase) | 3.304 |
| PTPRT: protein tyrosine phosphatase, receptor type, T | 3.295 |
| RYR2: ryanodine receptor 2 (cardiac) | 3.28 |
| MTM1: myotubularin 1 | 3.259 |
| SKI: v-ski sarcoma viral oncogene homolog (avian) | 3.232 |
| CNTNAP2: contactin associated protein-like 2 | 3.223 |
| DUSP26: dual specificity phosphatase 26 (putative) | 3.206 |
| TENM2: teneurin transmembrane protein 2 | 3.185 |
| MAP4K5: mitogen-activated protein kinase kinase kinase kinase 5 | 3.178 |
| ATP1B1: ATPase, Na+/K + transporting, β1 polypeptide | 3.15 |
| CDH13: cadherin 13, H-cadherin (heart) | 3.139 |
| VSTM2B: V-set and transmembrane domain containing 2B | 3.098 |
| SPTBN1: spectrin, β, nonerythrocytic 1 | 3.082 |
| EPHA5: EPH receptor A5 | 3.056 |
| Pcdhb21: protocadherin β21 | 3.042 |
| SLC22A3: solute carrier family 22 (extraneuronal monoamine transporter), member 3 | 3.041 |
| CNTNAP4: contactin associated protein-like 4 | 3.04 |
| ATP1A3: ATPase, Na+/K + transporting, α3 polypeptide | 3.034 |
Table 3.
Gene expression comparison between Prox1 heterozygous versus null in CGE-derived interneuron precursors within the E18.5 cortex
| Symbol: Name | Fold change |
|---|---|
| Genes upregulated in CGE-derived Prox1 loss-of-function cells in the E18.5 cortex | |
| XIST: X inactive-specific transcript (nonprotein coding) | 376.517 |
| SLN: sarcolipin | 5.321 |
| SFRP2: secreted frizzled-related protein 2 | 4.251 |
| SCN1A: sodium channel, voltage-gated, type I, α-subunit | 3.998 |
| C1QTNF1: C1q and tumor necrosis factor-related protein 1 | 3.558 |
| NTNG1: netrin G1 | 3.009 |
| PROX1: prospero homeobox 1 | 2.717 |
| DLK1: δ-like 1 homolog (Drosophila) | 2.709 |
| MSRB2: methionine sulfoxide reductase B2 | 2.384 |
| SVIL: supervillin | 2.377 |
| DUSP26: dual specificity phosphatase 26 (putative) | 2.287 |
| TGFBI: transforming growth factor, β-induced, 68 kDa | 2.15 |
| Etl4: enhancer trap locus 4/KIAA1217 | 2.142 |
| GABRA2: GABA A receptor, α2 | 2.14 |
| DBC1: deleted in bladder cancer 1 | 2.083 |
| FGA: fibrinogen α chain | 2.034 |
| Rnd3: Rho family GTPase 3 | 1.991 |
| SEZ6L: seizure-related 6 homolog (mouse)-like | 1.958 |
| HCN1: hyperpolarization activated cyclic nucleotide-gated potassium channel 1 | 1.926 |
| PAG1: phosphoprotein associated with glycosphingolipid microdomains 1 | 1.915 |
| Iigp1: interferon inducible GTPase 1 | 1.896 |
| MPDZ: multiple PDZ domain protein | 1.893 |
| DNAH8: dynein, axonemal, heavy chain 8 | 1.886 |
| SLC4A4: solute carrier family 4, sodium bicarbonate cotransporter, member 4 | 1.86 |
| NRP2: neuropilin 2 | 1.783 |
| RNGTT: RNA guanylyltransferase and 5′-phosphatase | 1.782 |
| APCDD1: adenomatosis polyposis coli downregulated 1 | 1.756 |
| TMEM176B: transmembrane protein 176B | 1.745 |
| SHISA2: shisa homolog 2 (Xenopus laevis) | 1.706 |
| PLAGL1: pleomorphic adenoma gene-like 1 | 1.694 |
| CUX2: cut-like homeobox 2 | 1.689 |
| CALB1: calbindin 1, 28 kDa | 1.676 |
| CACHD1: cache domain containing 1 | 1.657 |
| SPC25: SPC25, NDC80 kinetochore complex component, homolog (S. cerevisiae) | 1.653 |
| ENPP3: ectonucleotide pyrophosphatase/phosphodiesterase 3 | 1.65 |
| DTNA: dystrobrevin, α | 1.634 |
| HNMT: histamine N-methyltransferase | 1.628 |
| FGF12: fibroblast growth factor 12 | 1.625 |
| SCHIP1: schwannomin interacting protein 1 | 1.586 |
| NPPA: natriuretic peptide A | 1.576 |
| EPB41L4A: erythrocyte membrane protein band 4.1 like 4A | 1.565 |
| CST3: cystatin C | 1.546 |
| TRIM25: tripartite motif containing 25 | 1.544 |
| ZFHX3: zinc finger homeobox 3 | 1.541 |
| NPTX2: neuronal pentraxin II | 1.537 |
| SEMA4A: semaphorin 4A | 1.532 |
| SLC6A15: solute carrier family 6 (neutral amino acid transporter), member 15 | 1.529 |
| Genes downregulated in CGE-derived Prox1 loss-of-function cells in the E18.5 cortex | |
| Ddx3y: DEAD (Asp-Glu-Ala-Asp) box polypeptide 3, Y-linked | 67.225 |
| EIF2S3: eukaryotic translation initiation factor 2, subunit 3 γ, 52 kDa | 51.606 |
| KDM5D: lysine (K)-specific demethylase 5D | 20.671 |
| VIP: vasoactive intestinal peptide | 14.152 |
| Uty: ubiquitously transcribed tetratricopeptide repeat gene, Y chromosome | 5.435 |
| TRIL: TLR4 interactor with leucine-rich repeats | 4.79 |
| CCK: cholecystokinin | 3.224 |
| RSPO2: R-spondin 2 | 2.076 |
| FGFR1: fibroblast growth factor receptor 1 | 1.908 |
| CRABP1: cellular retinoic acid binding protein 1 | 1.858 |
| TMEFF2: transmembrane protein with EGF-like and two follistatin-like domains 2 | 1.814 |
| CALB2: calbindin 2 | 1.787 |
| SPEG: SPEG complex locus | 1.776 |
| ID4: inhibitor of DNA binding 4, dominant negative helix-loop-helix protein | 1.762 |
| ROBO1: roundabout, axon guidance receptor, homolog 1 (Drosophila) | 1.746 |
| CRIM1: cysteine-rich transmembrane BMP regulator 1 (chordin-like) | 1.745 |
| MYO1B: myosin IB | 1.686 |
| NAV2: neuron navigator 2 | 1.671 |
| CPNE2: copine II | 1.671 |
| PTPRE: protein tyrosine phosphatase, receptor type, E | 1.665 |
| CACNA1G: calcium channel, voltage-dependent, T type, α-1G-subunit | 1.618 |
| GNAI1: guanine nucleotide binding protein (G-protein), α-inhibiting activity polypeptide 1 | 1.592 |
| CHST15: carbohydrate (N-acetylgalactosamine 4-sulfate 6-O) sulfotransferase 15 | 1.585 |
| FAM134B: family with sequence similarity 134, member B | 1.579 |
| BCKDHB: branched chain keto acid dehydrogenase E1, β-polypeptide | 1.567 |
| Rcan2: regulator of calcineurin 2 | 1.555 |
| NRIP3: nuclear receptor interacting protein 3 | 1.545 |
| ZDHHC2: zinc finger, DHHC-type containing 2 | 1.542 |
| IL-18: interleukin 18 (interferon-γ-inducing factor) | 1.536 |
| CCRN4L: CCR4 carbon catabolite repression 4-like (S. cerevisiae) | 1.535 |
| WWC1: WW and C2 domain containing 1 | 1.528 |
| PRKCE: protein kinase C, ε | 1.519 |
| HSD11B1: hydroxysteroid (11-β) dehydrogenase 1 | 1.51 |
We FACS purified EGFP-labeled cells from the E18.5 cortices of control and Prox1 loss-of-function animals (Dlx6a-Cre; Prox1-C:EGFP/+ and /F) and subsequently performed a microarray expression analysis. Genes are shown upregulated and downregulated in cells lacking Prox1, respectively. Genes are shown for FDR-adjusted p -value (<0.05) and fold change (>1.5). Note that the values of gender-linked genes are indicated with boldface as male–female split happened by chance across control and Prox1 loss-of-function brains.
CGE cell transplantation into the postnatal cortex.
CGE tissues were dissected out from control and Prox1 loss-of-function (Dlx6a-Cre; Prox1-C:EGFP/+ and /F) mutants at E14.5 and cells were dissociated and stored in individual tubes on ice. These cell suspensions were prepared in a manner identical to that described above for FACS purification of cells in our microarray analysis. However, the procedure before the cell-sorting step was changed: the cell pellets were resuspended into 200 μl of ice-cold NB/B27 tissue culture medium containing 5 μl of DNase I, instead of HBSS solution. The dissociated CGE including EGFP-labeled cells was typically between 1500 and 3000 cells/μl in a total of 200 μl. Cell concentration was adjusted to ∼5000 cells/μl by removing the supernatant after centrifuging the tubes, and cells were kept on ice typically ∼1–4 h before the subsequent transplantation step.
Cell transplantation into P3 pup cortices was performed following the protocol of the Anderson lab as previously described (Inan et al., 2012). Briefly, P3 pups were anesthetized on ice for 5 min and stabilized on a Styrofoam platform. Approximately 5 μl of CGE cells was loaded into a capillary electrode prefilled with mineral oil and loaded into an oocyte nanoinjector (Drummond). Each pup received two injections bilaterally into the somatosensory cortex of each hemisphere at a location of 1 mm lateral to the midline and 1 and 2 mm rostral of the interaural line, at a depth of 1 mm. Each injection site consisted of ∼60 mini injections of 69 nl of cell suspension (total of ∼4 μl). After injections, the pup was labeled and placed back in the nest, and analyses were performed at the age of P21.
Tissue preparation and immunohistochemistry.
Embryos were dissected out, and transcardiac perfusion was performed with cold 4% formaldehyde/PBS solution (w/v). Brain dissection was performed in PBS in a Petri dish, and brains were subsequently placed into 4% formaldehyde/PBS solution on ice. Brains were postfixed according to the developmental stages (E14.5: 20 min, E16.5: 40 min, E18.5 onward: 60 min) and, following a brief rinse in PBS, were placed into cold 25% sucrose/PBS (w/v) solution for cryoprotection until they sunk to the bottom. Exceptions were E10.5 embryos: after peeling the thin skin layer of the head, the entire bodies were placed into 4% formaldehyde/PBS solution on ice for 20 min. Later brains or embryos were placed into OCT compound (Sakura Finetek) and kept at −80°C. Cryosections were prepared at 12 μm thickness and collected on glass slides (Fisher), and, after ∼1 h of drying, the sections were stored at −80°C. For the morphological analysis of Prox1 loss-of-function cells, 100 μm cryosections were immediately placed into PBS solution and stored at 4°C until further analysis.
Immunohistochemistry was performed as previously described (Miyoshi and Fishell, 2012). All incubations were performed in PBS containing 1.5% normal donkey serum (Jackson ImmunoResearch; v/v) and 0.1% Triton X-100 (v/v; DST solution). Sections were rinsed three times in PBS to remove the residual OCT, followed by nonspecific antibody blocking, which was performed in DST solution at room temperature for 30–60 min. Primary antibody incubation was subsequently performed overnight at 4°C. Primary antibody was added to the DST solution at the following concentrations: rabbit anti-GFP (1:2000; Invitrogen, #A11122), rat anti-GFP #GF090R (1:2000; Nacalai Tesque, #04404-84), chicken anti-GFP (1:2000; Abcam, #ab13970), rat anti-RFP #5F8 (1:2000; Allele Biotechnology, #ACT-CM-MRRFP10), rabbit anti-RFP (1:2000; Clontech, #632496), goat anti-Prox1 N terminus (1:1000; R&D Systems, #AF2727), rabbit anti-Prox1 C terminus (1:1000; Abcam, #ab11941-100), mouse anti-Nkx2-1 #8G7G3/1 (1:1000; Progen, #16108), rabbit anti-Nkx2-1 #EP1584Y (1:2000; Abcam, #ab76013), goat anti-Sp8 (Santa Cruz Biotechnology, #sc-104661), mouse anti-CoupTFII #7147 (Perseus Proteomics, #PP-H7147-00), mouse anti-RELN #CR-50 (1:1000; MBL, #D223-3), goat anti-RELN (1:1000; R&D Systems, #AF3820), mouse anti-PV #PARV-19 (1:2000; Sigma, #P3088), rat anti-SST #YC7 (1:1000; Millipore Bioscience Research Reagents, #MAB354), rabbit anti-SST (1:1000; Millipore Bioscience Research Reagents, #AB5494), rabbit anti-VIP (1:1000; ImmunoStar, #20077), mouse anti-Calretinin (1:1500; Millipore Bioscience Research Reagents, #MAB1568), and rabbit anti-Calretinin (1:1500; Millipore Bioscience Research Reagents, #AB5054). Secondary antibodies conjugated with Alexa Fluor dyes 488 and 594 (Invitrogen; 1:2000 dilutions) or DyLight 649 (Jackson ImmunoResearch; 1:1000 dilutions) raised in donkey were chosen for visualizing the primary antibody staining. To visualize the cell nuclei and the laminar structure of the developing neocortex, DAPI (Sigma; 1 pg/μl in PBS and filtered) was added on slides at the end of the immunohistochemistry procedure for 10–15 min. Slides were rinsed a couple of times in PBS and then mounted in Fluoromount G (Southern Biotech). Fluorescent images were captured using a cooled-CCD camera (Princeton Scientific Instruments) using MetaMorph software (Universal Imaging).
To better visualize the morphologies of EGFP-labeled interneurons with immunohistochemistry, 100 μm cryosections were generated and, by using the confocal microscope LSM 510 (Carl Zeiss), images were captured every 4 μm of optical distance and a stacked view was made typically by combining three to four images.
After the electrophysiological recordings, post hoc immunohistochemistry was performed on 250-μm-thick vibratome sections. Sections were fixed for 2 h on ice with 4% formaldehyde containing PBS, and after 30 min of multiple PBS rinses, incubated for two or three overnights at 4°C with selected antibodies. We found that the fixation time is critical, especially for VIP immunohistochemistry. Sections were washed in PBS typically from the morning to the late afternoon and incubated at 4°C overnight with donkey secondary antibodies (The Jackson Laboratory) together with Alexa 488-conjugated streptavidin (1:1000; Invitrogen, #S-11223). Later, sections were rinsed with PBS and submerged in DAPI solution for 30 min and after multiple washes with PBS mounted and coverslipped on a slide glass with a lid with two layers of electrical tape. See details for the general immunohistochemistry procedure in the above section.
Marker and layer analysis of cortical interneuron distribution.
For the P21 analysis of Dlx6a-Cre control and Prox1 loss-of-function interneurons, pictures were taken from six representative hemispheric fields per brain for SST/RELN/EGFP/DAPI and CR/VIP/EGFP/DAPI-labeled sections. Image acquisition was performed in the somatosensory barrel field with a 10× lens and two pictures were combined to cover the cortical layers from I to VI. By using Adobe Photoshop, DAPI was placed in a different picture layer, layer borders of the cortex were drawn using the pen tool based on the DAPI signal, and then the DAPI picture layer was made invisible. The cell numbers were determined for each specific labeling (e.g., SST−/RELN+/EGFP+, SST+/RELN+/EGFP−) in cortical layers I, II/III, IV, V, and VI. At the end of the cell counting, two lines were drawn following the superficial edge of the cortex and the bottom of layer VI and total pixel numbers between these two lines were analyzed and then converted to the surface area for 2.4 m pixels = 1 mm2. Marker profiles (cell numbers for each layer) were converted to cell density (cells/1 mm2) for each section, and the values from the six fields from each brain sample were averaged per brain and final results were shown as the average ± SEM from three brains for each marker analysis. For the analysis of PV expression, since we found no overlap between PV and EGFP in both control and Prox1 loss-of-function brains, we simply counted the total numbers of PV-positive cells within the six fields, calculated the cell density, and averaged for three brains. For the P7 analysis, the profile for CR/VIP/EGFP/DAPI was analyzed. Similar analyses were performed for the images obtained from the experiments using the Htr3ABAC-Cre and Vip-Cre driver lines to remove Prox1.
Electrophysiology and data analysis.
Whole-cell patch-clamp electrophysiological recordings were performed on randomly selected cells located in neocortical layers I–III labeled with EGFP in acute brain slices prepared from control and Prox1 loss-of-function (Dlx6a-Cre; Prox1-C:EGFP/+ and /F) P16–P20 animals. Briefly, animals were decapitated and the brain was dissected out and transferred to physiological Ringer's solution (ACSF) in 4°C with the following composition (mm): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 20 glucose. The brain was then glue fixed to a stage and 250 μm slices were cut using a vibratome (Vibratome 3000 EP). The slices were allowed to recover in recording ACSF at room temperature for at least 45 min before recording. Acute slices were then placed in a recording chamber mounted on the stage of an upright microscope (Axio Scope; Zeiss) equipped with immersion differential interference contrast objectives (5× and 40×) coupled to an infrared camera system (Zeiss), perfused at a rate of 1–2 ml/min with oxygenated recording ACSF, and maintained at a temperature of 31°C. An EGFP filter was used to visualize the interneurons in epifluorescence.
Patch electrodes were made from borosilicate glass (Harvard Apparatus) and had a resistance of 4–8 MΩ. For both intrinsic electrophysiological properties and sEPSC recordings the patch pipettes were filled with a solution containing the following (in mm): 128 K-gluconate, 4 NaCl, 0.3 Na-GTP, 5 Mg-ATP, 0.0001 CaCl2, and 10 HEPES.
Experiments were performed in current-clamp and voltage-clamp mode using the Axopatch 200B amplifier (Molecular Devices). sEPSCs were recorded at Vh = −70 mV with a sampling rate of 10 kHz and were filtered on-line at 3 kHz. The recorded sEPSCs were analyzed using Mini Analysis software (Synaptosoft). The synaptic values were obtained for the average trace after visual inspection of individual events. The decay time was calculated by fitting the average trace with a single exponential. Access resistance was always monitored to ensure the stability of recording conditions. Cells were only accepted for analysis if the initial series resistance was <40 MΩ and did not change by >20% throughout the recording period. No compensation was made for access resistance and no correction was made for the junction potential between the pipette and the ACSF.
Passive and active membrane properties were recorded in current-clamp mode by applying a series of subthreshold and suprathreshold current steps and the analysis was done in Clampfit. The resting membrane potential (Vrest) was ascertained in current-clamp right after rupturing the patch by applying zero current. All values presented are average ± SEM.
Results
Within cortical interneuron lineages, Prox1 expression is selectively maintained in CGE-derived subtypes
During the development of GABAergic cortical interneuron populations, a sequential transcriptional cascade of Nkx2-1–Lhx6–Sox6/SatB1 expression has been established as critical for the specification, migration, and maturation of MGE-derived lineages (Sussel et al., 1999; Liodis et al., 2007; Butt et al., 2008; Du et al., 2008; Zhao et al., 2008; Azim et al., 2009; Batista-Brito et al., 2009; Balamotis et al., 2012; Close et al., 2012; Denaxa et al., 2012; Narboux-Nême et al., 2012). However, aside from transcription factors, Dlx, Arx, and CoupTF, which participate in the early development of both MGE and CGE cortical interneuron subtypes (Cobos et al., 2005b; Colasante et al., 2008; Friocourt et al., 2008; Kanatani et al., 2008; Lodato et al., 2011), a distinct genetic cascade that regulates CGE-derived cortical interneuron development has not yet been characterized.
To identify genes specifically enriched in CGE-derived interneurons, we performed a comparative gene expression analysis using oligonucleotide microarrays on RNA extracted from EGFP-labeled interneuron precursors derived from the CGE or the MGE that were FACS purified from the E18.5 cortex (Tables 1 and 2). Selective labeling of CGE-derived or MGE-derived cortical interneuron precursors was achieved through use of the Mash1BAC-CreER (Battiste et al., 2007; Miyoshi et al., 2010) or Nkx2-1BAC-Cre (Xu et al., 2008) driver lines, respectively. This analysis revealed that the expression of Prox1 is highly enriched (×12.4; Table 1) within CGE-derived versus MGE-derived cortical interneurons at E18.5. Prox1 is a mammalian homolog of Prospero, which determines cell fate through its asymmetric localization in dividing neuroblasts within the fruit fly larva (Choksi et al., 2006; Doe, 2008) as well as in photoreceptor progenitors (Cook et al., 2003). Similarly, in the hippocampus, Prox1 has been shown to play important roles in cell proliferation (Kaltezioti et al., 2010; Lavado et al., 2010) and fate determination (Iwano et al., 2012). Since Prox1 is further demonstrated to be essential for the development of horizontal cells, which are the interneurons of the retinal circuit (Dyer et al., 2003), we hypothesized that it plays important roles in the GABAergic local interneurons within the cerebral cortex.
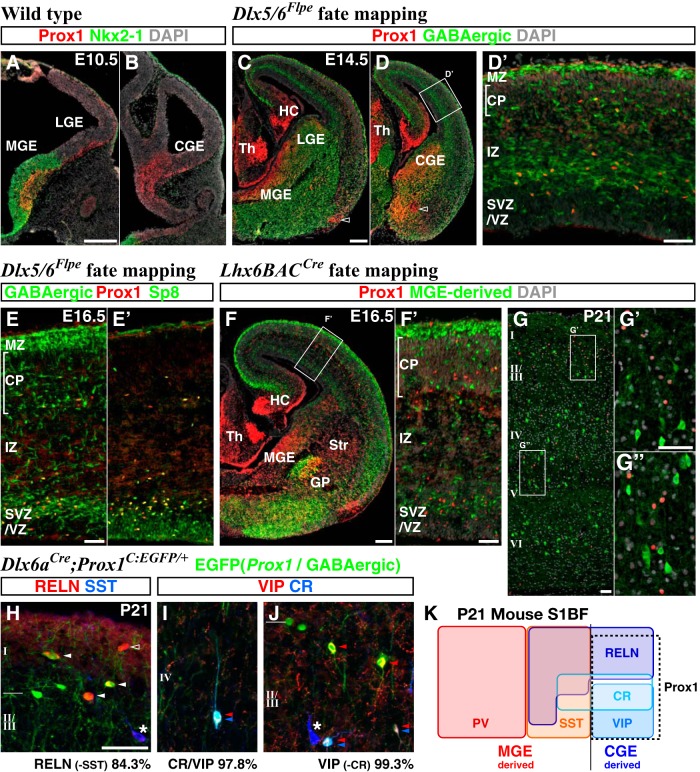
We next characterized the expression of Prox1 within the developing telencephalon using immunohistochemistry. At E10.5, Prox1 is expressed in the subventricular zones of the MGE (defined by Nkx2-1 expression), lateral GE (LGE), and CGE (Fig. 1A,B; Lavado and Oliver, 2007). This is consistent with the previous finding that Prox1 transcription is induced by Ascl1 (Mash1), a proneural factor expressed within all three ganglionic eminences (Torii et al., 1999). By E14.5, Prox1 expression is increased in the subventricular zone of the CGE (Fig. 1C,D) and in addition, is observed within the cortex and is restricted primarily to GABAergic interneuron populations, as indicated by Dlx5/6 lineage fate mapping (Miyoshi et al., 2010; Fig. 1D′; Prox1 is transiently expressed in Cajal–Retzius cells but shuts off by birth; data not shown). This timing coincides with the arrival of the earliest cohort of CGE-derived interneurons into the cortex (Miyoshi et al., 2010), several days after MGE-derived interneurons begin to populate this structure (Anderson et al., 2001; Fogarty et al., 2007; Miyoshi et al., 2007; Xu et al., 2008). At E16.5, we observed that Prox1-positive GABAergic cells (Fig. 1E) coexpressed Sp8 (Fig. 1E′), a transcription factor selectively expressed in CGE-derived but not MGE-derived or POA-derived lineages (Ma et al., 2012). To further demonstrate that Prox1 is selectively expressed within CGE-derived lineages, we generated a BAC transgenic Cre driver by using cis-regulatory elements of Htr3A gene (Htr3ABAC-Cre, GENSAT, also ×11.8 enriched in CGE lineages; Table 1) and combined this with Dlx5/6-Flpe and a dual EGFP reporter (RCE:dual) to label CGE-derived interneuron precursors. We found that Prox1 is expressed in the CGE-derived interneuron precursors labeled with the Htr3ABAC-Cre driver within the E16.5 cortex (data not shown). Consistent with these results, we did not observe Prox1 expression in immature MGE-derived interneurons labeled with EGFP by using an intersectional genetic approach (Dymecki and Kim, 2007) that combined Lhx6BAC-Cre (Fogarty et al., 2007) and Dlx5/6-Flpe drivers with the RCE:dual reporter at E16.5 (Fig. 1F,F′). Interestingly, Prox1 regulation within MGE-derived cells is lineage specific in that it entirely shuts off in cortical interneuron and globus pallidus populations (Fig. 1F; Flandin et al., 2010; Nóbrega-Pereira et al., 2010), but remains expressed in some striatal interneurons (Fig. 1F; Rubin and Kessaris, 2013).
Figure 1.
CGE-derived but not MGE-derived GABAergic cortical interneurons maintain expression of the transcription factor Prox1 during their development. A, B, Immunohistochemistry for Prox1 reveals expression within the SVZs of the ventral eminences (MGE, LGE, and CGE) as early as E10.5. Expression of Nkx2-1 largely demarcates the MGE domain. DAPI nuclear counterstain is shown in white. C, D, GABAergic neuronal precursors in the forebrain are labeled by EGFP through combinatorial use of a Dlx5/6-Flpe driver and an RCE:FRT reporter at E14.5. High levels of Prox1 expression are found in the SVZs of the MGE and CGE. Some non-GABAergic populations in the ventral forebrain also express Prox1 (open arrowheads). D′, A higher magnification picture of the neocortex in D is shown. Most Prox1-expressing cells are found to be GABAergic in the cortical plate (CP), intermediate zone (IZ), and subventricular/ventricular zones (SVZ/VZ). Non-GABAergic Prox1-expressing cells (red without green) within the marginal zone (MZ) are Cajal–Retzius cells and Prox1 expression within this population shuts off by E18.5 (confirmed by Wnt3a-Cre driver fate mapping; data not shown). E, E′, At E16.5, Prox1 expression is largely confined to GABAergic neuronal precursors within the cortex (E) and overlaps with Sp8 (E′). F, G, MGE-derived GABAergic populations are labeled with EGFP in Lhx6BAC-Cre; Dlx5/6-Flpe; RCE:dual animals. MGE-derived cortical interneuron precursors at E16.5 do not express Prox1 (F, F′). At P21, Prox1 remains absent from MGE-derived interneurons (G–G″). H–J, Analysis of Prox1 expression in specific CGE-derived cortical interneuron subtypes in Dlx6a-Cre; Prox1-C:EGFP/+ P21 brains. H, RELN-expressing interneurons that are negative for SST originate from the embryonic CGE and Prox1 expression (EGFP) are observed in 84.3% of this population albeit at variable levels (arrowheads; open arrowhead indicates Prox1-negative RELN-positive cell). SST-positive cells do not express Prox1 (asterisk). I, J, The majority of CR(Calb2)/VIP (double arrowheads) and VIP-single (single arrowhead) populations express Prox1. Most CR-positive cells lacking VIP are MGE derived (K) and do not express Prox1 (asterisk). K, A schematic correlating the molecular expression and the embryonic origin of GABAergic interneurons within the P21 mouse somatosensory barrel cortex (S1BF; adapted from Miyoshi et al., 2010). Prox1 is expressed within CGE-derived but not MGE-derived cortical interneuron subtypes. GP, globus paliidus; HC, hippocampus; Str, striatum; Th, thalamus. Scale bars: A–D, F, 200 μm; E–J, 50 μm.
We then analyzed the expression profile of Prox1 in the P21 neocortex. Using immunohistochemistry, we observed that while a substantial number of cells were found to express Prox1, it was still completely excluded from MGE-derived interneurons (Fig. 1G–G″). To further analyze which specific subtypes of CGE-derived interneurons express Prox1, we took advantage of a conditional Prox1 allele (Prox1-C:EGFP), which expresses EGFP under the control of the endogenous Prox1 locus following Cre-mediated recombination (Iwano et al., 2012). We combined this line with a forebrain pan-GABAergic Dlx6a-Cre driver (Monory et al., 2006) to analyze the morphologies and molecular markers of Prox1-expressing cortical interneurons labeled with EGFP (Dlx6a-Cre; Prox1-C:EGFP/+). Within the P21 cortex, Prox1 was detectable in most (84.3%) of the RELN-expressing (without SST) cells (Fig. 1H), although its expression levels in this population were somewhat variable (Fig. 1H, solid arrowheads). Prox1 was also expressed in almost all (98.6%) of the VIP-positive cells (Fig. 1I,J) at P21 (Fig. 1K). Conversely, the large majority of Prox1-positive interneurons expressed either VIP or RELN, and only 7.7% were negative for both markers (Fig. 1K). These results are consistent with the previous finding that Prox1 is expressed in CGE-derived interneuron subtypes (Rubin and Kessaris, 2013). We conclude that, while Prox1 is uniformly expressed in the subventricular zones of the ganglionic eminences, within cortical interneuron lineages it is selectively maintained within CGE-derived subtypes into adulthood (Fig. 1K).
Prox1 promotes the migration of CGE-derived interneuron precursors into superficial cortical layers
To test the role of Prox1 in CGE-derived interneurons, we used an additional conditional Prox1 loss-of-function allele to generate Prox1-null cells at distinct developmental stages. In this conditional loss-of-function allele (Prox1-F), exons 2 and 3 that encode the homeodomain of Prox1 are flanked by loxP sites (Harvey et al., 2005; Lavado et al., 2010), such that Prox1 expression is ablated following Cre-mediated recombination. We combined this allele to the conditional Prox1-C:EGFP line (Fig. 1H–J), in which the entire coding region of Prox1 is flanked by loxP sites, such that recombination results in a null allele in addition to EGFP expression in Prox1-expressing cells. We combined both Prox1 conditional alleles with a Dlx6a-Cre driver line (Monory et al., 2006), which allowed us to remove Prox1 from the entire telencephalic GABAergic population as these cells transit through the subventricular zone. Using EGFP as a marker, we identified the Prox1-expressing population and compared the behavior of control (heterozygous: Prox1-C:EGFP/+) versus Prox1 loss-of-function (null: Prox1-C:EGFP/F) cells (Fig. 2).
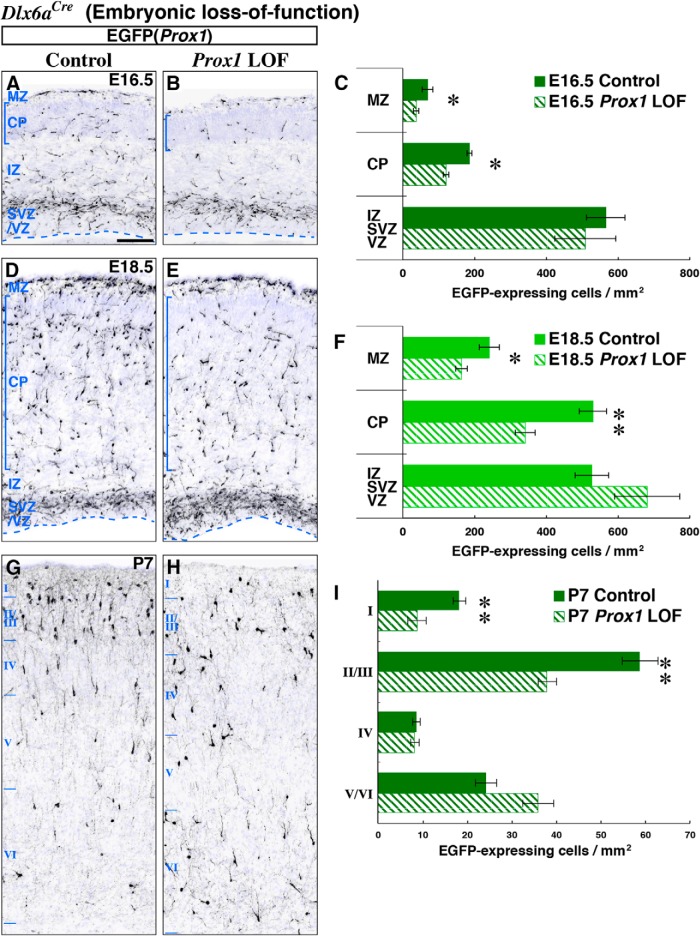
Figure 2.
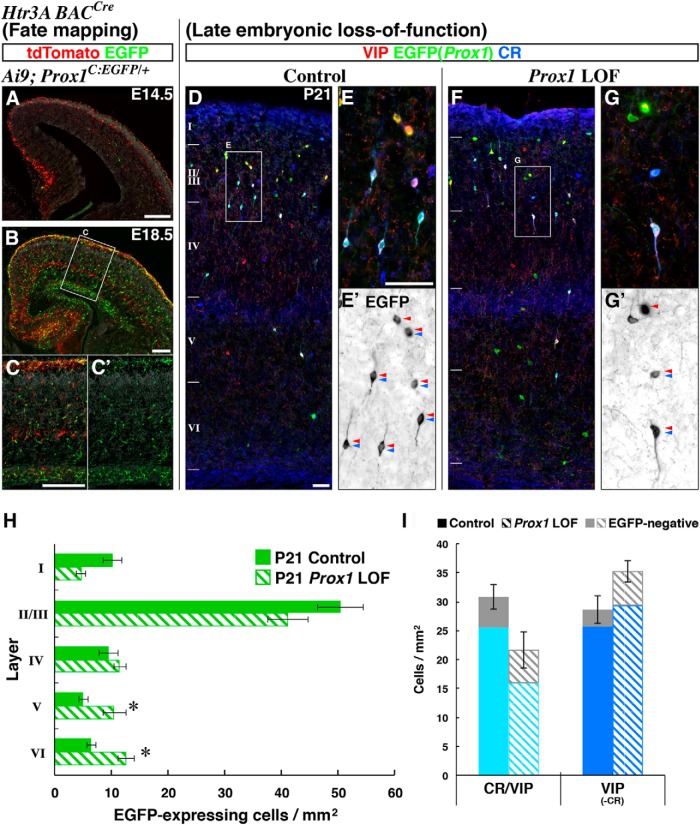
Prox1 promotes the superficial layer positioning of CGE-derived interneurons by regulating embryonic tangential migration. Localization of EGFP(Prox1)-labeled control (Dlx6a-Cre; Prox1-C:EGFP/+) and Prox1 loss-of-function (Dlx6a-Cre; Prox1-C:EGFP/F) GABAergic cells were analyzed within the cortex (shown in black). Nuclear counter staining by DAPI is shown in blue. A, B, While we found no delay in the initiation of tangential migration from the CGE into the cortex of Prox1 loss-of-function at E14.5 (Fig. 3A,B), by E16.5, we observed a decrease in Prox1-null EGFP-labeled cells reaching the marginal zone (MZ) and cortical plate (CP). Comparable numbers of EGFP-labeled cells are found in the intermediate and subventricular/ventricular zones (IZ and SVZ/VZ). C, Bar graphs comparing the cortical area normalized distribution of EGFP-labeled (GABAergic Prox1) cells at E16.5 in the control (filled) and Prox1 loss-of-function (LOF, shaded) experiments. Two-tailed t test: p = 0.0255*(MZ), p = 0.0172*(CP), p = 0.425(IZ/SVZ/VZ). D–F, By E18.5, in addition to the loss in marginal zone and cortical plate, more EGFP-labeled cells were observed within the intermediate zone and SVZ/VZ of the conditional Prox1 loss-of-function cortices. For the sake of clarity, hippocampal areas are cropped from the figures (bottom). Two-tailed t test: p = 0.0156*(MZ), p = 0.00893**(CP), p = 0.132(IZ/SVZ/VZ). G–I, At P7, mutant cells were decreased in superficial (I–III) layers, but increased in deep (V, VI) layers compared with the control cortex. Two-tailed t test: p = 0.00693**(I), p = 0.00960**(II/III), = 0.837(IV), p = 0.0842(V/VI). In addition, the population of cells with vertically oriented processes that is normally found in the superficial layers (G) was less obvious in the Prox1 loss-of-function cortex (H). All error bars indicate SEM. Scale bar, 50 μm.
As we have previously shown, CGE-derived interneuron precursors are first observed migrating tangentially through the intermediate zone of the cortex at ∼E14.5 (Miyoshi et al., 2010). At this stage, both control and Prox1 loss-of-function CGE-derived interneuron precursors exhibited comparable migration patterns within the cortex (Fig. 3A,B). This suggests that Prox1 loss-of-function does not delay the initiation of tangential migration into the cortex. By E16.5, in the control cortex, we observed an increase in the number of cells in the cortical marginal zone and within the developing cortical plate (Fig. 2A), consistent with previous analyses (Miyoshi et al., 2010; Rubin et al., 2010). In contrast, fewer Prox1-null cells had migrated into the marginal zone and cortical plate, although the intermediate and subventricular zone populations were similar to controls (Fig. 2B,C). Two days later, at E18.5, in addition to the reduction of labeled cells located in the marginal zone and cortical plate, we observed an accumulation of Prox1-null cells in the intermediate and subventricular zones (Fig. 2D–F). This indicates that Prox1 is required for the transition of CGE-derived interneuron precursors from the tangential migration stream into the cortical plate.
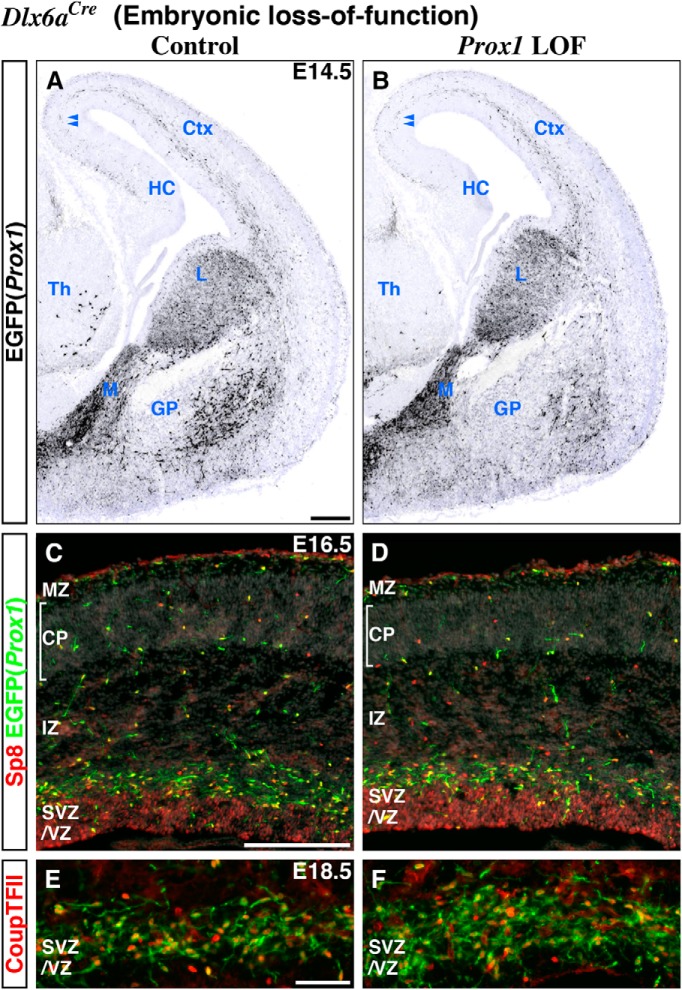
Figure 3.
Embryonic (Dlx6a-Cre) Prox1 loss-of-function does not affect the interneuron precursors for their tangential migration from the CGE into the developing cortex as well as the expression of Sp8 and CoupTFII transcription factors. We performed embryonic (Dlx6a-Cre) control (A, C, E) and Prox1 loss-of-function (Prox1-C:EGFP/+ and /F) experiments (B, D, F). A, B, At E14.5, about the time the earliest cohort of CGE-derived interneuron precursors is first observed tangentially migrating through the intermediate zone (IZ) of the cortex (Miyoshi et al., 2010), we found no obvious differences between control and Prox1-null cells labeled with EGFP (shown in black) with regard to their migration pattern or distance from the CGE. Nuclear counter staining by DAPI is shown in blue. Double arrowheads indicate the CGE-derived interneuron precursors that have reached the border area between the hippocampus (HC) and cortex (Ctx). While control EGFP(Prox1)-labeled cells are positioned laterally to the developing globus pallidus (GP) and do not express CoupTFII (Nr2f2), in the Prox1 loss-of-function ventral telencephalon, very few EGFP(Prox1)-labeled cells are observed in the comparable domain. C, D, In addition to its expression within cortical progenitors, at E16.5, the Sp8 expression pattern within EGFP(Prox1)-labeled cells is comparable between the control and Prox1 loss-of-function cortex. E, F, Higher magnification pictures of the cortical subventricular/ventricular zones (SVZ/VZ) in Figure 2D and E. While there are significantly more EGFP-labeled cells found in the SVZ/VZ of the Prox1 loss-of-function cortex at E18.5 (see also Fig. 2F), the proportion of CoupTFII expression within EGFP(Prox1)-labeled cells is not changed. M, MGE; L, LGE; Th, thalamus; MZ, marginal zone; CP, cortical plate. Scale bars: A–D, 200 μm; E, F, 50 μm.
Next, we analyzed how the abnormal migration pattern we observed in Prox1-null cells during embryonic stages ultimately impacts their localization within the postnatal cortex. We compared the laminar distribution of EGFP(Prox1)-labeled control and Prox1-null cells at P7 (Fig. 2G,H), a stage at which most GABAergic interneurons have completed their migration within the cortex (Miyoshi and Fishell, 2011; Inamura et al., 2012). In the Prox1 loss-of-function animals, we observed a marked reduction in EGFP-labeled cell numbers in layers I (47.9%) and II/III (64.5%) with a corresponding increase in layers V/VI (148.7%) compared with controls (Fig. 2I). This indicates that CGE-derived interneurons lacking Prox1 function were displaced ectopically within deeper layers. Furthermore, while many EGFP-labeled control cells exhibited vertically oriented processes in the superficial layers (Fig. 2G), these morphologies were not similarly evident in Prox1 loss-of-function cells (Fig. 2H).
Loss of Prox1 results in dysregulation of relatively few genes within CGE-derived interneuron precursors
We next examined whether Prox1 was required for the expression of the candidate CGE transcription factors CoupTFII (Tripodi et al., 2004; Kanatani et al., 2008; Miyoshi et al., 2010; Cai et al., 2013) and Sp8 (Waclaw et al., 2006; Ma et al., 2012) but found no differences between control and loss-of-function cortices (Fig. 3C–F). Thus to uncover the molecular mechanisms underlying the impaired transition of Prox1 loss-of-function cells from the intermediate to marginal zone, we performed an unbiased microarray expression analysis comparing EGFP-labeled control versus Prox1 loss-of-function interneurons that were FACS purified from the E18.5 cortex. Somewhat surprisingly, we observed significant changes in only a small number of genes (79 genes including 46 upregulated and 33 downregulated genes, fold change > 1.5, p value <0.05; data not shown), including downregulation of Vip (×14.15), Cck (×3.22), and Calb2 (Calretinin, ×1.79), markers that are expressed within mature CGE-derived interneuron subtypes (Table 3).
Previous work has shown that Dlx1/2 activity induces the expression of the transcription factor Arx (Cobos et al., 2005a; Colasante et al., 2008), promoting migration by suppressing neurite growth mediated by Pak3, a p21-activated serine/threonine kinase that acts as a downstream effector of the Rho family of GTPases (Cobos et al., 2007). However, in our microarray analysis comparing gene expression levels in control versus Prox1 loss-of-function cells, we did not reveal any measurable change in Pak3, Arx, or any of the Dlx-gene family members (data not shown). These data strongly suggest that the migration of CGE-derived interneuron precursors is independently regulated by the Dlx1/2 and Prox1 transcription factors.
Subtype-specific requirement for Prox1 in the differentiation of CGE-derived cortical interneurons
To further characterize the later effects of Prox1 loss-of-function on CGE-derived interneuron subtypes following their differentiation, we analyzed control and conditional (Dlx6a-Cre) Prox1 loss-of-function cells at P21, when interneurons have acquired their characteristic molecular markers and intrinsic electrophysiological properties. Consistent with what we have observed at P7, we found that the abnormalities in the layer distribution of EGFP-labeled cells persisted at P21, with a decrease in layers I–IV and increase in layer VI in the mutant (Fig. 4I). The total number of EGFP-expressing Prox1 cells in mutants compared with controls was reduced at P7 (82.8% of control numbers) and further decreased by P21 (74.6% of control numbers; Fig. 4J), suggesting that Prox1 is important for the maintenance of CGE-derived cortical interneurons even after they have reached their final laminar locations. Because the Dlx6a-Cre driver removes Prox1 broadly throughout the ventral telencephalic subventricular zones, we tested the specific requirement for Prox1 in CGE-derived interneurons. To do so, we used an Htr3ABAC-Cre driver to remove Prox1 only in the migrating postmitotic interneuron precursors derived from the CGE (Fig. 5). By combining with a Cre-dependent tdTomato (Ai9: R26R-CAG-loxPstop-tdTomato-WPRE polyA; Madisen et al., 2010) and Prox1-C:EGFP conditional reporter lines, we observed that the Htr3ABAC-Cre driver targets a subpopulation of migrating CGE-derived interneurons expressing Prox1 (Fig. 5A–C; data not shown). Even though this Htr3ABAC-Cre driver exhibited less efficient recombination compared with the Dlx6a-Cre driver (Figs., 2A,D, 3A) resulting in a milder phenotype, we still observed a similar displacement of EGFP-labeled cells into the deeper cortical layers (Fig. 5D–H).
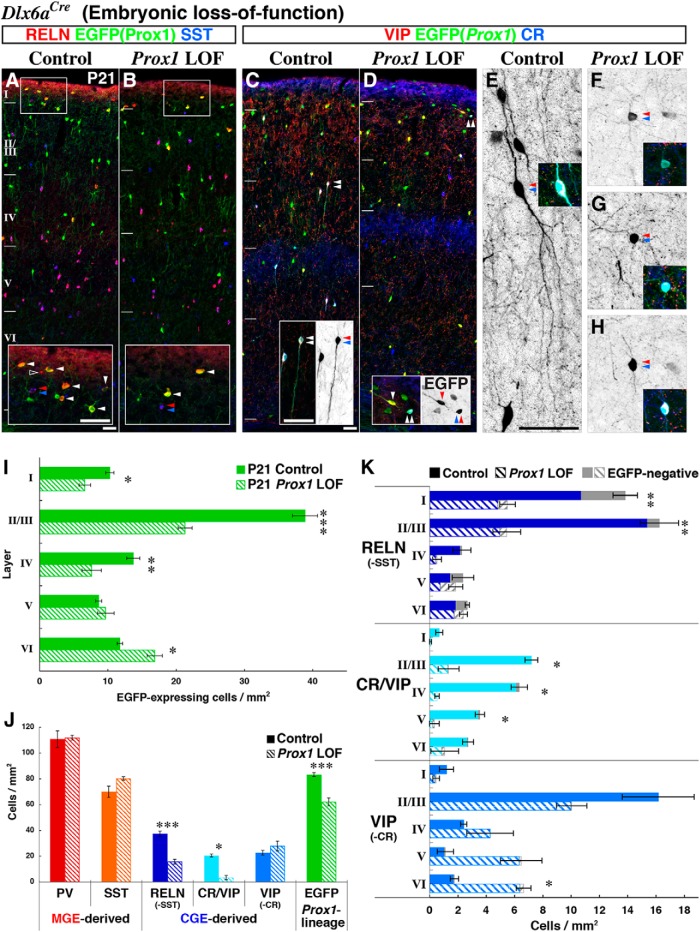
Figure 4.
Subtype-specific requirement for Prox1 in CGE-derived GABAergic cortical interneuron development. A, B, Immunohistochemistry for RELN/SST on EGFP(Prox1)-labeled cells in control and Prox1 loss-of-function (Dlx6a-Cre; Prox1-C:EGFP/+ and /F) cortices at P21. While RELN-expressing cells (negative for SST) expressed EGFP at variable levels (arrowheads), they were generally reduced in the superficial layers of conditional Prox1 loss-of-function cortices (B). Open arrowhead indicates the EGFP(Prox1)-negative RELN cells and double arrowheads indicate MGE-derived SST/RELN-expressing interneurons. C, D, Characterization of CR, VIP, and EGFP-positive cell profiles at P21. Double and single arrowheads indicate CR/VIP or VIP-single interneurons, respectively. While CR/VIP cells were severely reduced in mutants, the total numbers of VIP-single cells was unchanged. Furthermore, the remaining CR/VIP cells in mutants (D, double arrowheads) did not exhibit the characteristic bipolar morphology observed in controls (C). E–H, To better visualize the morphologies in Prox1-null CR/VIP interneurons, stacked views from confocal microscopy images were generated. CR/VIP-expressing cells (double arrowhead and inset) are found with bipolar morphology (E). In contrast, none of the CR/VIP-expressing cells remaining in the Prox1 loss-of-function cortex exhibit typical bipolar morphology (F–H). The best example of a mutant cell that we found to retain bipolar morphology to some extent is shown in H, but in most cases, obvious processes protruding from the soma could not be found (F, G). I, Layer distribution of EGFP(Prox1)-labeled control (filled bars) and Prox1 loss-of-function (LOF) cells (shaded bars) at P21. At P21, EGFP-labeled Prox1-null cells were displaced within deeper layers of the cortex, similar to the phenotype observed at P7 (Fig. 2I). Two-tailed t test: p = 0.0133*(I), p = 0.000380***(II/III), p = 0.00376**(IV), p = 0.463(V), p = 0.0148*(VI). J, Molecular expression profiles of interneurons in the P21 somatosensory barrel cortex for control and Prox1 loss-of-function experiments. The total numbers of PV(Pvalb)-expressing (red) and SST-expressing (orange) interneurons were not altered in the mutant. RELN (SST-negative) cell numbers were reduced to ∼40%, CR/VIP-interneurons were severely reduced, and the VIP-single population was unaltered. Overall, EGFP(Prox1)-expressing cells were reduced to 75% in the Prox1 loss-of-function mutant at P21. Two-tailed t test: p = 0.880(PV), p = 0.0905(SST), p = 0.000334***(RELN), p = 0.0236*(CR/VIP), p = 0.403(VIP), p = 0.00169***(EGFP). K, The laminar distribution of each RELN, CR/VIP, and VIP-single interneuron subtype is shown. In addition to control (filled bars) and Prox1 loss-of-function (shaded bars), gray bars indicate the EGFP-negative population of each subtype. While the RELN-positive population was primarily reduced in the superficial (I–IV) layers, CR/VIP-expressing cells were reduced in all layers. The VIP-single population was reduced in superficial (I–III) and increased in deep (IV–VI) layers and thus was ectopically displaced into deeper layers. Statistics are shown for the marker-positive profiles including both EGFP-positive and EGF-negative cells. Two-tailed t test: p = 0.00437**, p = 0.00127**, p = 0.1841, p = 0.6411, p = 0.551 (I–VI, RELN), p = 0.188, p = 0.0369*, p = 0.0136*, p = 0.0371*, p = 0.316 (I–VI, CR/VIP), p = 0.216, p = 0.148, p = 0.414, p = 0.0507, p = 0.0164* (I–VI, VIP). All error bars are SEM. Scale bars, 50 μm.
Figure 5.
Late embryonic (Htr3ABAC-Cre) Prox1 loss-of-function (LOF) in CGE-derived interneurons shows similar but milder phenotypes compared with pan-GABAergic early embryonic (Dlx6a-Cre) removal. We used the Htr3ABAC-Cre driver to restrict recombination to CGE-derived lineages. This driver recombines the Prox1-C:EGFP allele at somewhat later stages of embryonic cell migration than Dlx6a-Cre (Figs. 2–4) in a subpopulation of CGE-derived cells. A–C, Recombination efficiency of the Htr3ABAC-Cre driver was addressed by analyzing crosses with Prox1-C:EGFP/+ and Ai9 reporter (R26R-CAG-loxPstop-tdTomato-WPRE polyA) lines in the same animal at E14.5 (A) and E18.5 (B, C). At E14.5, the Htr3ABAC-Cre driver (A) resulted in fewer EGFP-expressing cells within the cortex compared with the Dlx6a-Cre driver (Fig. 3A). At E18.5, substantial numbers of EGFP-labeled cells were evident in the cortex, although to a lesser extent when compared with the Dlx6a-Cre driver (Fig. 2D). C, C′, A higher magnification view of B also shown for EGFP signals only (C′). The Htr3ABAC-Cre driver also targets non-GABAergic populations, most likely the Cajal–Retzius and subplate cells (red without green), in addition to CGE-derived interneuron precursors. D–G, Comparison of control and Prox1 loss-of-function (Htr3ABAC-Cre; Prox1-C:EGFP/+ and /F) cells at P21. E, G, Higher magnification of the areas in D and F are presented and EGFP signals are shown in black (E′, G′). Double arrowheads indicate CR/VIP-expressing cells and single arrowheads indicate VIP-single (CR-negative) cells. In contrast to what we observed in the early embryonic (Dlx6a-Cre) Prox1 loss-of-function study, in the Htr3ABAC-Cre mutant, not all of the remaining CR/VIP cells lost their characteristic bipolar morphologies (G′). While the CR/VIP Prox1-null cell in the middle has a round morphology with no obvious processes, the cell at the bottom exhibits a bipolar morphology (G′). H, A graph indicating the layering of late embryonic (Htr3ABAC-Cre) Prox1 loss-of-function cells in P21 cortex. Note that the decrease (Layers I–III) and increase (Layers V/VI) found in the EGFP-expressing cells of Prox1 loss-of-function cortex are consistent but milder compared with the results observed from the early embryonic (Dlx6a-Cre) loss-of-function (Fig. 4I). Two-tailed t test: p = 0.0636(I), p = 0.212(II/III), p = 0.359(IV), p = 0.0412*(V), p = 0.0205*(VI). I, Cell numbers of CR/VIP-expressing and VIP-single populations in the P21 cortex of control and late embryonic Prox1 loss-of-function animals (shaded bars). EGFP(Prox1)-negative populations are shown in gray bars. Consistent with the early embryonic (Dlx6a-Cre) loss-of-function, we also observed a reduction in CR/VIP-expressing cells, with no obvious change in the VIP-single population following late embryonic (Htr3ABAC-Cre) loss-of-function. Note that the recombination mediated by the Htr3ABAC-Cre driver does not take place in all CGE-derived population at P21 in either control or Prox1 loss-of-function animals (gray bars) compared with that observed when the Dlx6a-Cre driver was used (Figs. 1I–K, 4K). Two-tailed t test: p = 0.0852(CR/VIP), p = 0.0786(VIP). Error bars indicate SEM. Scale bars: A–C, 200 μm; D–G, 50 μm.
We next compared the molecular expression profiles of interneurons within the P21 cortices of control and Prox1 conditional loss-of-function animals (Fig. 4A–D). Within the P21 cortex, we did not observe any obvious changes in the distribution and numbers of PV-expressing and SST-expressing cells (Fig. 4A,B, red and orange bar graphs in J), indicating that Prox1 expression within the MGE subventricular zone is not required for the development of MGE-derived cortical interneurons.
CGE-derived interneurons can be divided into three broad classes based on their molecular expression profiles (Fig. 1K; Miyoshi et al., 2010) of RELN (SST negative), CR/VIP double, and VIP single (CR negative). In the cortices of conditional Prox1 loss-of-function animals, the total number of RELN-expressing (without SST) cells, a population that is largely comprised by neurogliaform and dense plexus morphologies (Miyoshi et al., 2010; Fig. 4A,B), was reduced to 42% of control numbers (Fig. 4J, dark blue bars). The loss of RELN-expressing (SST-negative) cells was particularly prominent in layers I–IV (Fig. 4K, top). Interestingly, loss of RELN-expressing cells was also observed in the Prox1-negative population (Fig. 4K, gray bars). This suggests the ∼15% of the RELN-positive population that does not express Prox1 in the P21 cortex (Figs. 1H, 4A, open arrowhead) likely still required this gene earlier during development.
We next examined the remaining two broad classes of CGE-derived interneurons, CR/VIP coexpressing and VIP-single (CR-negative) populations, both of which exhibit primarily bipolar/bitufted morphologies (Fig. 4C–H). Strikingly, the CR/VIP double-positive population was severely reduced across all cortical layers in the mutants (Fig. 4K, middle) to 16% of control numbers (Fig. 4J). Furthermore, the very few CR/VIP double-positive cells that remained lacked their characteristic bipolar morphology (Fig. 4C,D, insets; E–H, confocal stack images). Late embryonic removal of Prox1 with the CGE-specific driver Htr3ABAC-Cre also resulted in a decrease in CR/VIP cells (Fig. 5I), although the effect was less pronounced than that observed with the early embryonic Dlx6a-Cre driver, consistent with the milder layer displacement of Htr3ABAC-Cre Prox1-null cells (Fig. 5H). In addition, not all of the CR/VIP-expressing Prox1-null cells lost the bipolar morphologies in this late embryonic removal (Fig. 5G,G′). Regarding the VIP-single cells, although we did not observe any obvious change in the total number of cells (Fig. 4J), following the general trend of deep layer displacement observed in the Prox1 loss-of-function population (Fig. 4I), they were decreased in the superficial (I–III) and correspondingly increased in the deep (IV–VI) cortical layers (Fig. 4K, bottom).
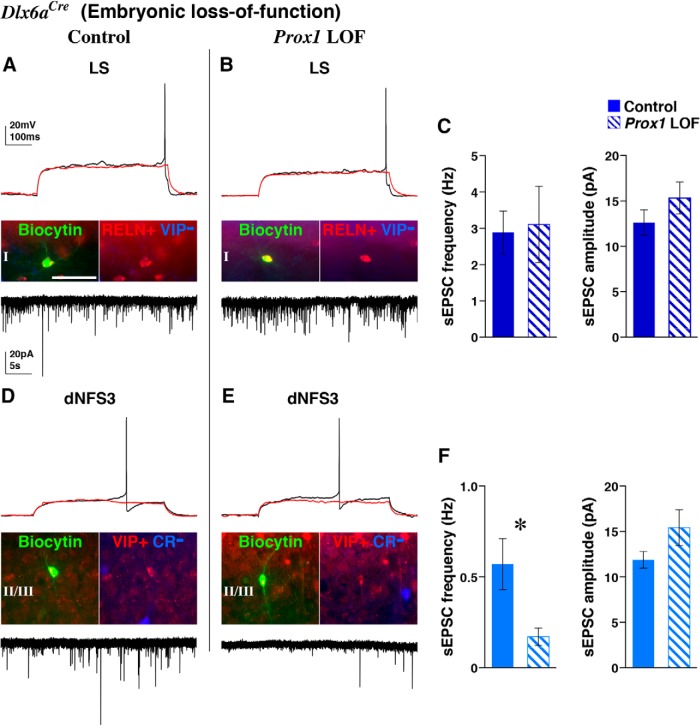
To further characterize the requirement of Prox1 in the differentiation of CGE-derived interneurons, we performed whole-cell patch-clamp recordings from EGFP-positive cells in acute brain slices (P16–P20) and analyzed their intrinsic electrophysiological properties. While we excluded the residual CR/VIP interneurons from our analysis due to severity of the null phenotype in these cells (Fig. 4F–H), all of the Prox1 loss-of-function cells we recorded in the superficial layers (I–III; 24 cells) exhibited normal resting membrane potential, displayed overshooting action potentials (APs), and could sustain high-frequency AP discharge (data not shown). Based on their membrane properties and discharge characteristics, we were able to assign the recorded cells to the previously reported CGE-derived subtype categories (Butt et al., 2005; Miyoshi et al., 2010), suggesting that their electrophysiological differentiation is largely unaffected by Prox1 loss of function. From the Prox1 loss-of-function cells recorded in layer I (17 cells), all of which were post hoc identified as RELN positive and VIP negative (Fig. 6A,B, middle), we found five late-spiking (LS; Fig. 6B), five initial-adapting, five type 1 bursting nonadapting, and one delayed intrinsic-bursting interneuron, and one cell that could not be classified. Analysis of LS neurogliaform cells (Chu et al., 2003; Támas et al., 2003; Kubota et al., 2011; DeFelipe et al., 2013; Ma et al., 2014; Pohlkamp et al., 2014) that are one of the most characteristic subtypes residing in layer I and expressing RELN did not reveal any differences across a variety of intrinsic electrophysiological measures when comparing control and Prox1-null cells (Fig. 6A,B). From the seven EGFP-positive Prox1 loss-of-function cells recorded in layers II/III, six were post hoc identified as VIP positive and negative for CR (Fig. 6D,E, middle). In these cells, we found three irregular-spiking (IS) and two type 3 delayed nonfast spiking (dNFS3) interneurons and one cell that could not be classified (Miyoshi et al., 2010). Similar to the RELN-positive classes, VIP-expressing dNFS3 (Fig. 6E) and IS (data not shown) subtypes appeared to have normal passive and active membrane properties (Fig. 6D).
Figure 6.
Reduction of excitatory events in VIP-single cortical interneurons following embryonic (Dlx6a-Cre) Prox1 removal. Control and embryonic (Dlx6a-Cre) Prox1 loss-of-function (LOF) cells labeled with EGFP were recorded in brain slices of the somatosensory cortex (P16–P20). Subsequent to electrophysiological analysis and biocytin filling, post hoc immunohistochemistry for RELN/VIP or CR/VIP was performed. A, A control cell that was identified by post hoc analysis to be positive for RELN but not VIP showed characteristic LS firing at near threshold (top, black trace; red trace is at subthreshold) and received sEPSCs at a frequency of 3 Hz (bottom trace). B, A Prox1-null RELN-positive VIP-negative cell showed a similar LS action potential discharge and received sEPSCs at a comparable frequency and amplitude to that of control cells (A). C, Analysis of the frequency (left) and amplitude (right) of sEPSCs in the control (n = 6) and Prox1 loss-of-function LS cells (n = 4, shaded bars) shows that these interneurons do not require Prox1 for the establishment of proper excitatory inputs. Mann–Whitney: p = 0.187(frequency), p = 0.442(amplitude). D, A representative example of a VIP-single (CR-negative) control cell with dNFS3 firing properties, with the sEPSCs it receives shown at the bottom. E, A trace of a dNFS3 cell (VIP-positive CR-negative) lacking Prox1. F, In contrast to RELN-positive LS cells (C), Prox1-null VIP-single interneurons (n = 4) show a dramatic reduction in the frequency of sEPSCs (left) compared with control cells (n = 4). Nevertheless, the amplitude of sEPSCs shows no change (right). Mann–Whitney: p = 0.0397*(frequency), p = 0.191(amplitude). Scale bar, 50 μm.
We next analyzed the sEPSCs received by control and Prox1-null cells as a measure of their network integration. All cells recorded received sEPSCs, but remarkably, VIP-positive Prox1 loss-of-function cells in layers II/III showed a marked reduction in the number but not the amplitude of these events (Fig. 6D,E, bottom, F). Since Prox1-null VIP-single cells that were not displaced but were properly located in layers II/III still showed a significant reduction in the sEPSCs they receive (Fig. 6F), this interneuron subtype appears to require Prox1 function to properly integrate into the cortical network. In contrast, the Prox1 loss-of-function LS cells received a comparable number of excitatory events with similar amplitude to the control cells (Fig. 6A,B, bottom, C). Thus we found a selective decrease in the excitatory drive onto VIP-single but not RELN-expressing populations after the removal of Prox1.
Our early embryonic (Dlx6a-Cre) loss-of-function experiments on forebrain GABAergic interneuron populations demonstrate a subtype-specific requirement for Prox1 within all CGE-derived interneurons. RELN-expressing cells were reduced from the superficial layers by ∼40%, and CR/VIP double-positive cells were largely eliminated from the cortex. Moreover, the CR/VIP cells that persisted failed to acquire their characteristic bipolar morphology. Finally, the VIP-single population was displaced into deeper layers, and even properly located cells exhibited a severe reduction in excitatory input.
Prox1 has a postnatal role in the maturation of CGE-derived interneurons
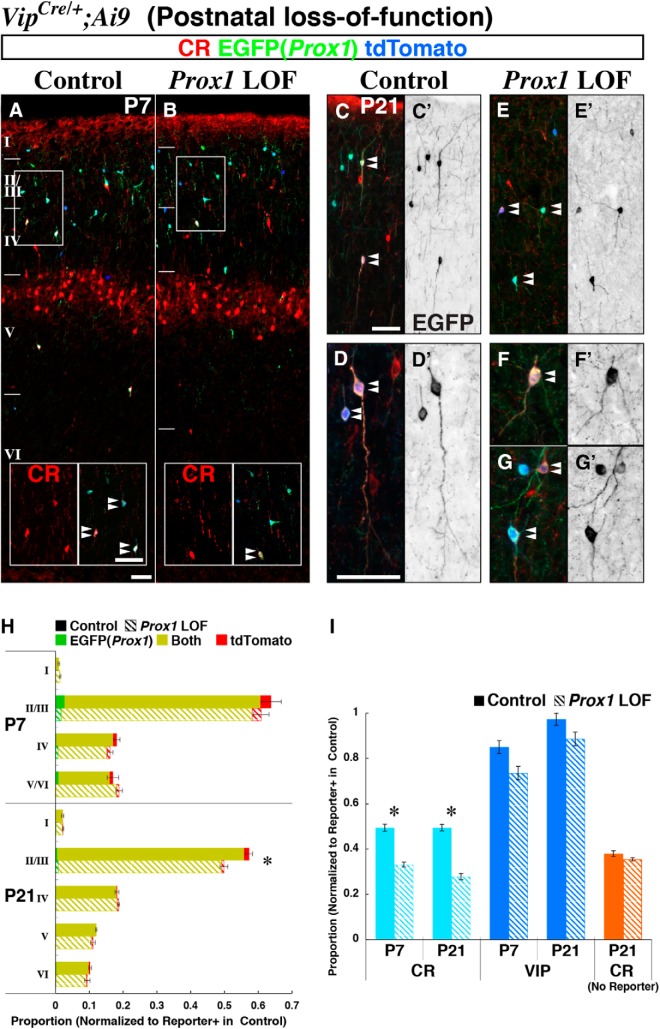
To distinguish between early and late roles for Prox1 in the development of CGE-derived interneurons (Fig. 1), we sought to examine the consequences of removing this gene during the postnatal maturation period after CGE-derived interneurons have already settled into their final laminar positions. To achieve this, we focused on the VIP-positive population, which broadly expresses Prox1 (Fig. 1I–K) and also includes the most severely affected population, the CR/VIP coexpressing subtype.
We first analyzed the timing of Prox1 removal with the Vip-Cre driver line (Taniguchi et al., 2011) by combining with a Cre-dependent tdTomato reporter line (Ai9) in control (Prox1-C:EGFP/+) and Prox1 loss-of-function (Prox1-C:EGFP/F) experiments. While at ∼E18.5 we found very few cells labeled for EGFP and tdTomato in the developing cortex (data not shown), later by P7, we observed efficient and overlapping recombination in the Prox1-C:EGFP allele and tdTomato reporter in both control (Fig. 7A) and loss-of-function (Fig. 7B) cortices. Thus the Vip-Cre driver line appeared to be well suited for us to perform postnatal Prox1 loss-of-function experiments. At P7, we observed comparable cell numbers and layer distributions of control and Prox1-null interneurons (Fig. 7H, top; total recombined cells in the Prox1 mutant was 97.4% of that in controls). This finding supports the idea that the layer displacement of VIP-single interneurons in the embryonic (Dlx6a-Cre) Prox1 loss-of-function cortex (Figs. 2I, 4I,K) results from the failure of cells to transit from the intermediate zone into the cortical plate, rather than from impaired radial migration inside the cortical plate that occurs subsequently during the first postnatal week (Miyoshi and Fishell, 2011). However, by P21 (Fig. 7C–G), in the Prox1 loss-of-function mutant we observed a progressive decrease in the number of labeled cells specifically in layers II/III (Fig. 7H, bottom). This indicates that sustained Prox1 expression enhances the survival of Vip-Cre-labeled interneurons during the postnatal period between P7 and P21.
Figure 7.
Postnatally (Vip-Cre), Prox1 is required for the differentiation and survival of CR/VIP interneurons. Postnatal conditional loss-of-function (LOF) of Prox1 was performed specifically within VIP-expressing interneurons with the Vip-Cre driver. Vip-positive cells were labeled independently of Prox1 expression by combining the tdTomato reporter (Ai9) with the control or Prox1 loss-of-function (Prox1-C:EGFP/+ or /F) alleles. This Vip-Cre driver initiates recombination in the Prox1-C:EGFP allele only after E18.5 (data not shown), thus allowing us to perform postnatal loss-of-function studies. A, B, By P7, we observed efficient recombination in both the tdTomato reporter and conditional Prox1-C:EGFP alleles in control and mutant cortices. In controls, VIP-positive interneurons exhibited a mature profile of CR (Calb2) expression by P7 (A, inset, double arrowheads), whereas in mutants, fewer cells expressed CR (B, inset). Note that layer Va pyramidal neurons have not downregulated CR at P7. C–G, Still, by P21, mutants exhibited a reduction in CR expression compared with controls, and those that remained did not show the similar characteristic bipolar morphologies observed in controls (C, D), but had kinked processes (E–G, double arrowheads). To better visualize cell morphology, EGFP is shown in black to the right of C–G (C′–G′). H, The laminar distribution of control and postnatal (Vip-Cre) Prox1 loss-of-function cells in the cortex at P7 and P21. While there was no obvious difference between the control and postnatal Prox1 conditional mutant at P7, by P21, cells were specifically reduced in layers II/III of the mutant. EGFP(Prox1) labeling and tdTomato expression (Ai9 reporter) are shown in green and red, respectively, and the overlap is indicated by yellow. Two-tailed t test: p = 0.127, p = 0.620, p = 0.577, p = 0.756(I–V/VI, P7), p = 0.953, p = 0.0301*, p = 0.735, p = 0.467, p = 0.648(I–VI, P21), p = 0.700, p = 0.121(total numbers for P7 and P21). Error bars indicate SEM for total number of recombined cells in each layer. I, CR and VIP expressions were analyzed in postnatal (Vip-Cre) Prox1 conditional mutants (labeled green and/or red) at P7 and P21. Values are shown as the proportion of labeled cells normalized to the total number of control cells at each age. While there was no change in the reporter-positive cell numbers at P7 (H), CR expression was decreased in the mutant compared with control at both ages. VIP expression was delayed in onset (P7) but recovered by P21 (the loss of VIP at P21 matches the cell loss in H, bottom). Reporter-negative CR-expressing cells (orange), most of which are derived from the MGE and coexpress SST, showed no change at P21. Two-tailed t test: p = 0.0143*(CR P7), p = 0.0120*(CR P21), p = 0.383(VIP P7), p = 0.198(VIP P21), p = 0.562(CR, no reporter). Error bars represent SEM. Scale bars: 50 μm.
Although at P7 the layer distribution of Vip-Cre-labeled cells was not different between the control and conditional Prox1 loss-of-function cells (Fig. 7H), we did observe changes in their molecular expression profiles. While approximately half (49.4%) of the Vip-Cre-labeled control population expressed CR at P7, there was a 30% decrease in CR expression in the mutant (33.2 vs 49.4% of total reporter-lableled population in control; Fig. 7I). Consistent with the cell loss found between P7 and P21, we observed a further reduction in CR expression in the mutant at P21 (Fig. 7I; control: 49.5% and mutant: 27.9% of total labeled cells in control). Furthermore, at this age, we observed morphological defects in the CR-expressing mutant cells that persisted in the adult cortex (Fig. 7E–G, double arrowheads, E′–G′). Although not as severely affected as the cells in the embryonic (Dlx6a-Cre) and late-embryonic (Htr3ABAC-Cre) Prox1 loss-of-function brains (Figs. 4F–H, 5G), CR/VIP-expressing cells in the Vip-Cre mutant still exhibited some defects in acquiring their characteristic bipolar morphologies (Fig. 7E–G,E′–G′, double arrowheads). We also analyzed the CR-expressing cells that were not labeled by Vip-Cre, the majority of which are derived from the MGE and coexpress SST (Fig. 1K), and as expected, they were unaffected in mutant animals at P21 (Fig. 7I, orange bars). Regarding the expression of VIP itself as detected by immunohistochemistry, at P7, we did observe a slight delay in the onset of its expression in conditional loss-of-function animals, but this recovered by P21 (Fig. 7I; reduction in VIP matches the cell loss at P21 shown in Fig. 7H, bottom).
From our postnatal (Vip-Cre) loss-of-function analysis, we conclude that Prox1 is necessary in the CR/VIP subclass for the proper morphogenesis, CR expression, and survival of cells after the age of P7.
Prox1 cell-autonomously specifies the CR/VIP subtype of CGE-derived interneurons independent of its role in tangential migration
Although Prox1 clearly plays important roles in the postnatal differentiation of CGE-derived interneurons, the phenotypes we observed in the CR/VIP-expressing bipolar interneuron population when Prox1 is removed embryonically (Dlx6a-Cre) versus postnatally (Vip-Cre) are quite distinct.
This raises the possibility that the bipolar subtype is particularly sensitive to its laminar positioning (Figs. 2I, 4I). Alternatively, independent of its role in cell migration, Prox1 could directly regulate the genetic program required during embryogenesis for bipolar interneurons to properly differentiate and acquire their characteristic morphologies. To distinguish between these possibilities, we dissected out the CGE from control or Prox1 loss-of-function (Dlx6a-Cre; Prox1-C:EGFP/+ or /F) embryos at E14.5. We then dissociated and transplanted these cells into wild-type postnatal cortices (P3) and analyzed later at P21 (Fig. 8A). This heterochronic transplantation approach allowed us to bypass the tangential migratory phase of CGE-derived interneuron precursors and to directly test the ability of mutant cells to differentiate within the postnatal cortex. Similar experiments using MGE-derived interneuron precursors have shown that these neurons can properly mature and integrate within the network (Martínez-Cerdeno et al., 2010) when they are heterochronically transplanted into the postnatal cortex (Wonders et al., 2008; Southwell et al., 2010; Inan et al., 2012).
Figure 8.
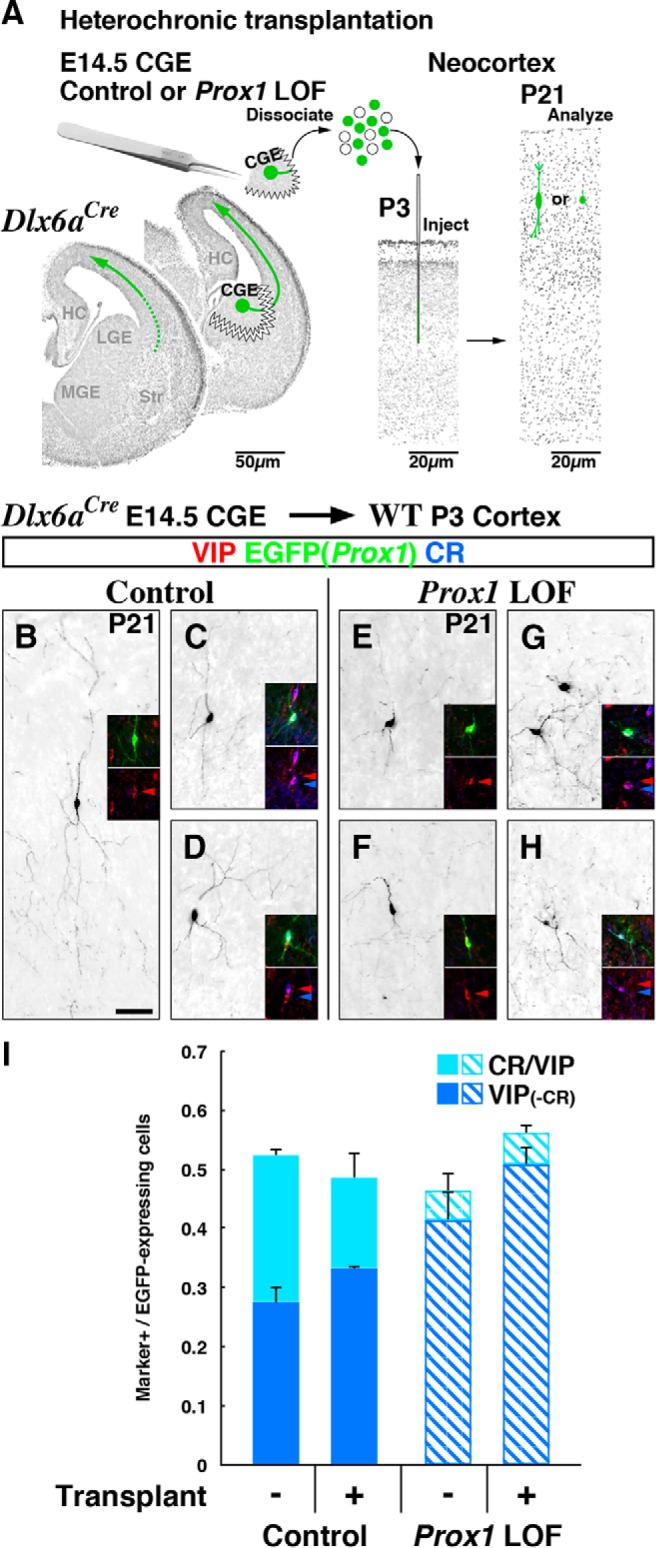
Cell migration and bipolar differentiation of CR/VIP-expressing interneurons is independently and cell-autonomously controlled by Prox1. A, A schematic of heterochronic transplantation experiments. E14.5 CGE tissue from Dlx6a-Cre control and Prox1 conditional loss-of-function (LOF) embryos (Prox1-C:EGFP/+ and /F) was dissected, dissociated, and subsequently transplanted into WT host P3 cortices, allowing CGE-derived interneuron precursors to bypass the tangential migration phase and to directly differentiate inside the cortical plate. B–D, Representative examples of transplanted control EGFP(Prox1)-labeled cells at P21 that are positive for VIP-single (B) or CR/VIP (C, D) with EGFP labeling shown in black. E–H, Prox1 loss-of-function EGFP-labeled transplanted cells positive for VIP-single (E, F) or CR/VIP (G, H) at P21. Transplantation did not rescue the embryonic (Dlx6a-Cre) Prox1 loss-of-function phenotype, as only a few EGFP(Prox1)-labeled mutant cells expressed CR/VIP and these did not possess the characteristic bipolar morphologies of this subtype (G, H). I, Bar graphs representing the proportion of CR/VIP or VIP-single-labeled populations in cells labeled by EGFP(Prox1) expression. The values obtained from the heterochronic transplantation experiments (transplant+) resembled the results without transplantation (transplant−; see also Fig. 4J) for both control and Prox1 loss-of-function. Two-tailed t test for transplant-negative versus positive: p = 0.191(VIP control), p = 0.264(CR/VIP control), p = 0.129(VIP mutant), p = 0.628(CR/VIP mutant). Error bars represent SEM. Scale bar, 50 μm.
In our control transplantation experiments, we observed EGFP-labeled cells that express VIP-single (without CR; Fig. 8B) or both CR and VIP (Fig. 8C,D) in a proportion similar to those observed in control brains where cells have tangentially migrated from the CGE into the cortex (Fig. 8I, compare the control bars; data without transplantation is shown in proportion from Fig. 4J). In addition, many of these transplanted control cells acquired bipolar morphologies by P21 (Fig. 8B–D). This demonstrates that VIP-expressing CGE-derived interneuron subtype can differentiate properly within the developing cortical plate even without following the normal migratory route from the CGE. However, when E14.5 Prox1 loss-of-function cells were transplanted, the proportion of EGFP-labeled cells coexpressing CR and VIP was reduced to one-third of that in controls (Fig. 8I, shaded bar graph, right), and their bipolar morphologies were severely attenuated (Fig. 8G,H). These results were comparable to what we observed at P21 in the embryonic Prox1 loss-of-function mutants (Dlx6a-Cre; Prox1-C:EGFP/F; Fig. 8I, compare shaded bar graphs of Prox1 loss-of-function). These results indicate that the severe phenotype in CR/VIP coexpressing bipolar interneurons that we observed in Prox1 embryonic (Dlx6a-Cre) loss-of-function animals was not due to impaired migration and displacement of cells but rather arose directly from an intrinsic requirement for Prox1 function during interneuron specification. Thus we conclude that Prox1 independently regulates both the migration and differentiation of CGE-derived cortical interneuron subtypes in a cell-autonomous manner.
Discussion
Despite the recognition that 30% of all GABAergic cortical interneurons originate from the CGE, to date, a specific transcriptional program that selectively regulates the development of these populations has not yet been identified. Moreover, while CGE-derived interneurons display unique patterns of tangential and radial migration and preferentially populate the superficial layers of the cortex, identification of a molecular program that controls these events is lacking. Here, we demonstrate that the homeodomain transcription factor Prox1, while initially broadly expressed within the ganglionic eminences, becomes restricted to postmitotic CGE-derived cortical interneuron precursors and is selectively maintained within this population into adulthood. Despite its relatively uniform expression within CGE-derived interneurons, Prox1 function is differentially required during both embryonic and postnatal stages of development and within distinct subtypes of CGE-derived interneurons (Fig. 9). During cell migration, Prox1 regulates the integration of CGE-derived interneurons into the superficial cortical layers by coordinating their transition from the subventricular/intermediate zone stream into the cortical plate. The cortical neurogliaform and dense plexus interneuron populations (RELN-positive) are reduced to 40% without Prox1 function. Furthermore, Prox1 is cell-autonomously required for the morphological development/maintenance of the CR/VIP-bipolar subtype and proper network integration of VIP-single population. As such, Prox1 is the first transcription factor to be identified that is specifically required for the differentiation, circuit integration, and maintenance of CGE-derived cortical interneurons.
Figure 9.
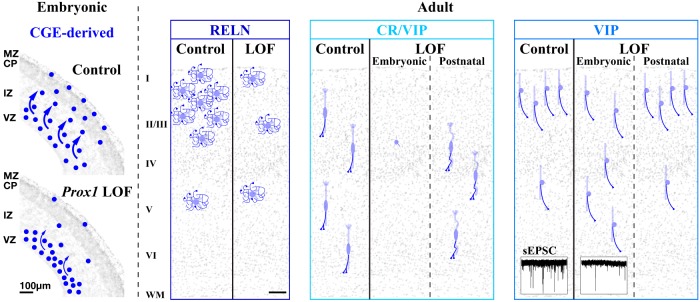
Prox1 regulates multiple developmental steps of CGE-derived interneurons in a subtype-specific manner. Left, Embryonic, Prox1 is selectively expressed within CGE-derived cortical interneuron precursors. During embryonic stages, Prox1 facilitates the transition of tangentially migrating cells from the intermediate zone (IZ) into the cortical plate (CP) and marginal zone (MZ). This step is critical for the positioning of CGE-derived interneuron precursors into the superficial layers of the neocortex. Adult, Embryonic (Dlx6a-Cre) and postnatal (Vip-Cre) loss-of-function studies reveal that Prox1 function is differentially required in a stage- and subtype-specific manner. (1) RELN-positive cells were reduced by ∼40%. (2) CR/VIP double-positive cells were mostly eliminated from the cortex and the remaining cells failed to acquire their characteristic bipolar morphology. Sustained expression of Prox1 during postnatal stages is required for the maintenance and survival of this cell class. (3) The VIP-single population was displaced into deep cortical layers. Prox1 is further required for this interneuron class to receive proper excitatory inputs and to integrate into superficial neocortical circuits. LOF, loss-of-function; VZ, ventricular zone.
Prox1 is selectively maintained in CGE-derived cortical interneuron lineages
While Prox1 is initially present in the subventricular zones of all ganglionic eminences, its expression is maintained only in CGE-derived but not MGE-derived cortical interneuron precursors during their respective migration and maturation. What mediates the selective persistence of Prox1 expression within CGE-derived cortical interneurons? It has been recently demonstrated that canonical β-catenin (Ctnnb1)-dependent Wnt signaling controls the expression of Prox1 in the pallium. Overexpression of a constitutively active form of β-catenin induces Prox1 expression uniformly within pyramidal neurons (Machon et al., 2007; Karalay et al., 2011). Conversely, perturbation of canonical Wnt signaling by misexpression of dominant-negative Lef1 (Karalay et al., 2011) increased Fgf signaling (Shimogori et al., 2004) or Zeb2 loss-of-function (Miquelajauregui et al., 2007) eliminated Prox1 expression within the dentate gyrus. Nevertheless, neither gain- nor loss-of-function manipulation of β-catenin activity in the GABAergic population targeted by the Dlx6a-Cre driver altered the pattern of Prox1 expression in MGE- and CGE-derived cortical interneuron precursors at E18.5 (data not shown). As such, to date the only demonstrated role for canonical Wnt signaling in cortical interneuron generation is in the regulation of cell proliferation within the MGE (Gulacsi and Anderson, 2008). These findings indicate that canonical Wnt signaling does not regulate Prox1 expression within CGE-derived interneurons. In the future, it will be interesting to explore how Prox1 is differentially regulated within the distinct lineages of cortical interneuron precursors.
In addition to the MGE and CGE, the POA has been shown to give rise to cortical GABAergic interneurons (Bartolini et al., 2013; Miyoshi et al., 2013). The POA harbors at least two distinct progenitor domains that can be delineated by Nkx5-1 and Dbx1 expression. POA-derived interneurons originating from an Nkx5-1 lineage have been fate mapped using a Nkx5-1BAC-Cre driver line, and while one-third of this population expresses NPY (Gelman et al., 2009), none of these interneurons were found to express PV, SST, VIP, or CR (Calb2). Interestingly, Prox1 expression was observed in this POA-derived population (Rubin and Kessaris, 2013). A second POA-derived interneuron population can be fate mapped using the Dbx1-Cre driver, and this population gives rise to interneuron classes with the molecular profiles including PV, SST, RELN, and VIP (Gelman et al., 2011). In contrast to the Nkx5-1-expressing POA lineage, Prox1 does not appear to be expressed in the majority of the mature Dbx1-Cre fate-mapped population since Prox1-positive interneurons express neither PV nor SST (Fig. 1K). To further test if Prox1 plays any roles in the differentiation and maturation of POA-derived interneurons, it may be interesting to use the Nkx5-1BAC-Cre and Dbx1-Cre lines to remove Prox1 in future studies.
Our findings demonstrate a sustained requirement for Prox1 throughout the development of CGE-derived cortical interneurons. In this regard, Prox1 is similar to Dlx1, which promotes both the embryonic migration of interneuron precursors and the postnatal maintenance of SST-expressing and VIP-expressing interneuron subtypes (Cobos et al., 2005b, 2007). However, our microarray analysis comparing gene expression levels in control versus Prox1 loss-of-function CGE-derived cells purified from the E18.5 cortex (Table 3) did not reveal any measurable change in Dlx-gene family members as well as their known target genes (Pak3 and Arx). We conclude that Dlx1 and Prox1 transcription factors independently promote the migration of CGE-derived interneurons through distinct mechanisms.
Distinct requirements for Prox1 and neuronal activity in the maturation of CGE-derived interneuron subtypes
It has been recently reported that neuronal activity is required for multiple aspects of GABAergic interneuron development (Lin et al., 2008; Bortone and Polleux, 2009; De Marco García et al., 2011; Inada et al., 2011; Inamura et al., 2012). Within the CGE-derived interneuron populations, suppression of cell activity impairs the migration and morphological development of the RELN-expressing and CR/VIP-expressing subtypes, whereas the VIP-single (CR-negative) class was not affected by this manipulation (De Marco García et al., 2011, 2015; Karayannis et al., 2012).
Here, we find that VIP-single (CR-negative) interneurons require Prox1 during embryonic stages for proper laminar positioning and for receiving proper levels of excitatory input, even though these cells exhibit relatively normal morphological development in the absence of Prox1. Since suppression of activity itself does not affect the formation of excitatory inputs onto VIP-single cells (Karayannis et al., 2012), Prox1 appears to regulate the circuit integration of this cell type in an activity-independent manner. In contrast, while excitatory inputs onto the RELN-expressing interneuron class were not affected by loss of Prox1, intrinsic activity is required for this cell type to receive inputs with the proper kinetics (Karayannis et al., 2012). Our results support the idea that each of the CGE-derived interneuron subtypes is dependent on fundamentally distinct developmental programs that are driven to different extents by a Prox1-mediated genetic cascade and intrinsic-activity driven programs (West and Greenberg, 2011). Thus it appears that Prox1 and neuronal activity independently play essential roles in mediating the connectivity of all CGE-derived interneurons in a subtype-specific manner.
In summary, we demonstrate that Prox1 function is required for the laminar positioning and development of all CGE-derived cortical interneurons (Fig. 9). Clearly, however, its actions in directing the development of CGE-derived interneurons are both stage and subtype specific. As such, it will be interesting in future studies to identify the molecular partners that work both in concert and in parallel with Prox1 to confer distinct CGE-derived interneuron properties.
Footnotes
Research in the Fishell lab is supported by National Institutes of Health grants (PPG-NS309889, RO1NS039007, RO1NS081297, and RO1MH071679), the Simons Foundation, and New York State through the NYSTEM initiative. We thank Drs. Stewart Anderson and Sonia Garel for kindly sharing their protocols for embryonic brain slice electroporation and subsequent slice culture. We greatly appreciate the following for kindly sharing their reagents: Dr. Stewart Anderson (Nkx2-1BAC-Cre), Dr. Nicoletta Kessaris (Lhx6BAC-Cre), Dr. Ulrich Müeller (Wnt3a-Cre), Dr. Marc Ekker, and Dr. John Rubenstein (Dlx6a-Cre). Finally, we thank the past and present Fishell lab members for their support and constructive comments on this project and Lihong Yin for her technical help.
The authors declare no competing financial interests.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;128:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balamotis MA, Tamberg N, Woo YJ, Li J, Davy B, Kohwi-Shigematsu T, Kohwi Y. Satb1 ablation alters temporal expression of immediate early genes and reduces dendritic spine density during postnatal brain development. Mol Cell Biol. 2012;32:333–347. doi: 10.1128/MCB.05917-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini G, Ciceri G, Marín O. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 2013;79:849–864. doi: 10.1016/j.neuron.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R, Rossignol E, Hjerling-Leffler J, Denaxa M, Wegner M, Lefebvre V, Pachnis V, Fishell G. The cell-intrinsic requirement of Sox6 for cortical interneuron development. Neuron. 2009;63:466–481. doi: 10.1016/j.neuron.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. doi: 10.1242/dev.02727. [DOI] [PubMed] [Google Scholar]
- Bortone D, Polleux F. KCC2 expression promotes the termination of cortical interneuron migration in a voltage-sensitive calcium-dependent manner. Neuron. 2009;62:53–71. doi: 10.1016/j.neuron.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S, Noctor S, Kriegstein A, Corbin JG, Fishell G. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Sousa VH, Fuccillo MV, Hjerling-Leffler J, Miyoshi G, Kimura S, Fishell G. The requirement of Nkx2-1 in the temporal specification of cortical interneuron subtypes. Neuron. 2008;59:722–732. doi: 10.1016/j.neuron.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Zhang Q, Wang C, Zhang Y, Ma T, Zhou X, Tian M, Rubenstein JL, Yang Z. Nuclear receptor COUP-TFII-expressing neocortical interneurons are derived from the medial and lateral/caudal ganglionic eminence and define specific subsets of mature interneurons. J Comp Neurol. 2013;521:479–497. doi: 10.1002/cne.23186. [DOI] [PubMed] [Google Scholar]
- Choksi SP, Southall TD, Bossing T, Edoff K, de Wit E, Fischer BE, van Steensel B, Micklem G, Brand AH. Prospero acts as a binary switch between self-renewal and differentiation in Drosophila neural stem cells. Dev Cell. 2006;11:775–789. doi: 10.1016/j.devcel.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Chu Z, Galarreta M, Hestrin S. Synaptic interactions of late-spiking neocortical neurons in layer 1. J Neurosci. 2003;23:96–102. doi: 10.1523/JNEUROSCI.23-01-00096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close J, Xu H, De Marco García N, Batista-Brito R, Rossignol E, Rudy B, Fishell G. Satb1 is an activity-modulated transcription factor required for the terminal differentiation and connectivity of medial ganglionic eminence-derived cortical interneurons. J Neurosci. 2012;32:17690–17705. doi: 10.1523/JNEUROSCI.3583-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Broccoli V, Rubenstein JL. The vertebrate ortholog of Aristaless is regulated by Dlx genes in the developing forebrain. J Comp Neurol. 2005a;483:292–303. doi: 10.1002/cne.20405. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005b;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Cobos I, Borello U, Rubenstein JL. Dlx transcription factors promote migration through repression of axon and dendrite growth. Neuron. 2007;54:873–888. doi: 10.1016/j.neuron.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasante G, Collombat P, Raimondi V, Bonanomi D, Ferrai C, Maira M, Yoshikawa K, Mansouri A, Valtorta F, Rubenstein JL, Broccoli V. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28:10674–10686. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Dev Cell. 2003;4:853–864. doi: 10.1016/S1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- De Marco García NV, Karayannis T, Fishell G. Neuronal activity is required for the development of specific cortical interneuron subtypes. Nature. 2011;472:351–355. doi: 10.1038/nature09865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco García NV, Priya R, Tuncdemir SN, Fishell G, Karayannis T. Sensory inputs control the integration of neurogliaform interneurons into cortical circuits. Nat Neurosci. 2015;18:393–401. doi: 10.1038/nn.3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, López-Cruz PL, Benavides-Piccione R, Bielza C, Larrañaga P, Anderson S, Burkhalter A, Cauli B, Fairén A, Feldmeyer D, Fishell G, Fitzpatrick D, Freund TF, González-Burgos G, Hestrin S, Hill S, Hof PR, Huang J, Jones EG, Kawaguchi Y, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denaxa M, Kalaitzidou M, Garefalaki A, Achimastou A, Lasrado R, Maes T, Pachnis V. Maturation-promoting activity of SATB1 in MGE-derived cortical interneurons. Cell Rep. 2012;2:1351–1362. doi: 10.1016/j.celrep.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Du T, Xu Q, Ocbina PJ, Anderson SA. NKX2.1 specifies cortical interneuron fate by activating Lhx6. Development. 2008;135:1559–1567. doi: 10.1242/dev.015123. [DOI] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet. 2003;34:53–58. doi: 10.1038/ng1144. [DOI] [PubMed] [Google Scholar]
- Dymecki SM, Kim JC. Molecular neuroanatomy's “Three Gs”: a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairén A, Cobas A, Fonseca M. Times of generation of glutamic acid decarboxylase immunoreactive neurons in mouse somatosensory cortex. J Comp Neurol. 1986;251:67–83. doi: 10.1002/cne.902510105. [DOI] [PubMed] [Google Scholar]
- Fino E, Packer AM, Yuste R. The logic of inhibitory connectivity in the neocortex. Neuroscientist. 2013;19:228–237. doi: 10.1177/1073858412456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flames N, Pla R, Gelman DM, Rubenstein JL, Puelles L, Marín O. Delineation of multiple subpallial progenitor domains by the combinatorial expression of transcriptional codes. J Neurosci. 2007;27:9682–9695. doi: 10.1523/JNEUROSCI.2750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flandin P, Kimura S, Rubenstein JL. The progenitor zone of the ventral medial ganglionic eminence requires Nkx2-1 to generate most of the globus pallidus but few neocortical interneurons. J Neurosci. 2010;30:2812–2823. doi: 10.1523/JNEUROSCI.4228-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty M, Grist M, Gelman D, Marín O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. J Neurosci. 2007;27:10935–10946. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Müller U. Shaping our minds: stem and progenitor cell diversity in the mammalian neocortex. Neuron. 2013;77:19–34. doi: 10.1016/j.neuron.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friocourt G, Kanatani S, Tabata H, Yozu M, Takahashi T, Antypa M, Raguénès O, Chelly J, Férec C, Nakajima K, Parnavelas JG. Cell-autonomous roles of ARX in cell proliferation and neuronal migration during corticogenesis. J Neurosci. 2008;28:5794–5805. doi: 10.1523/JNEUROSCI.1067-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao P, Sultan KT, Zhang XJ, Shi SH. Lineage-dependent circuit assembly in the neocortex. Development. 2013;140:2645–2655. doi: 10.1242/dev.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman DM, Marín O. Generation of interneuron diversity in the mouse cerebral cortex. Eur J Neurosci. 2010;31:2136–2141. doi: 10.1111/j.1460-9568.2010.07267.x. [DOI] [PubMed] [Google Scholar]
- Gelman DM, Martini FJ, Nóbrega-Pereira S, Pierani A, Kessaris N, Marín O. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9389. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman D, Griveau A, Dehorter N, Teissier A, Varela C, Pla R, Pierani A, Marín O. A wide diversity of cortical GABAergic interneurons derives from the embryonic preoptic area. J Neurosci. 2011;31:16570–16580. doi: 10.1523/JNEUROSCI.4068-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Sanz C, Franco SJ, Martinez-Garay I, Espinosa A, Harkins-Perry S, Müller U. Cajal-Retzius cells instruct neuronal migration by coincidence signaling between secreted and contact-dependent guidance cues. Neuron. 2013;79:461–477. doi: 10.1016/j.neuron.2013.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gulacsi AA, Anderson SA. Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci. 2008;11:1383–1391. doi: 10.1038/nn.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet. 2005;37:1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- Hernández-Miranda LR, Parnavelas JG, Chiara F. Molecules and mechanisms involved in the generation and migration of cortical interneurons. ASN Neuro. 2010;2:e00031. doi: 10.1042/AN20090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada H, Watanabe M, Uchida T, Ishibashi H, Wake H, Nemoto T, Yanagawa Y, Fukuda A, Nabekura J. GABA regulates the multidirectional tangential migration of GABAergic interneurons in living neonatal mice. PLoS One. 2011;6:e27048. doi: 10.1371/journal.pone.0027048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Kimura T, Tada S, Kurahashi T, Yanagida M, Yanagawa Y, Ikenaka K, Murakami F. Intrinsic and extrinsic mechanisms control the termination of cortical interneuron migration. J Neurosci. 2012;32:6032–6042. doi: 10.1523/JNEUROSCI.3446-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan M, Welagen J, Anderson SA. Spatial and temporal bias in the mitotic origins of somatostatin- and parvalbumin-expressing interneuron subgroups and the chandelier subtype in the medial ganglionic eminence. Cereb Cortex. 2012;22:820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano T, Masuda A, Kiyonari H, Enomoto H, Matsuzaki F. Prox1 postmitotically defines dentate gyrus cells by specifying granule cell identity over CA3 pyramidal cell fate in the hippocampus. Development. 2012;139:3051–3062. doi: 10.1242/dev.080002. [DOI] [PubMed] [Google Scholar]
- Kaltezioti V, Kouroupi G, Oikonomaki M, Mantouvalou E, Stergiopoulos A, Charonis A, Rohrer H, Matsas R, Politis PK. Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol. 2010;8:e1000565. doi: 10.1371/journal.pbio.1000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani S, Yozu M, Tabata H, Nakajima K. COUP-TFII is preferentially expressed in the caudal ganglionic eminence and is involved in the caudal migratory stream. J Neurosci. 2008;28:13582–13591. doi: 10.1523/JNEUROSCI.2132-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, Lie DC, Jessberger S. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2011;108:5807–5812. doi: 10.1073/pnas.1013456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayannis T, De Marco García NV, Fishell GJ. Functional adaptation of cortical interneurons to attenuated activity is subtype-specific. Front Neural Circuits. 2012;6:66. doi: 10.3389/fncir.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9:173–179. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessaris N, Magno L, Rubin AN, Oliveira MG. Genetic programs controlling cortical interneuron fate. Curr Opin Neurobiol. 2014;26:79–87. doi: 10.1016/j.conb.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Krook-Magnuson E, Varga C, Lee SH, Soltesz I. New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci. 2012;35:175–184. doi: 10.1016/j.tins.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-X. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Shigematsu N, Karube F, Sekigawa A, Kato S, Yamaguchi N, Hirai Y, Morishima M, Kawaguchi Y. Selective coexpression of multiple chemical markers defines discrete populations of neocortical GABAergic neurons. Cereb Cortex. 2011;21:1803–1817. doi: 10.1093/cercor/bhq252. [DOI] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Sestan N, Anton ES. Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development. 2012;139:1535–1546. doi: 10.1242/dev.069963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum M. A cellular mechanism for cortical associations: an organizing principle for the cerebral cortex. Trends Neurosci. 2013;36:141–151. doi: 10.1016/j.tins.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Lavado A, Oliver G. Prox1 expression patterns in the developing and adult murine brain. Dev Dyn. 2007;236:518–524. doi: 10.1002/dvdy.21024. [DOI] [PubMed] [Google Scholar]
- Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8:e1000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010;30:16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Adesnik H, Li J, Long J, Nicoll RA, Rubenstein JL, Pleasure SJ. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–1098. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Bloodgood BL, Hauser JL, Lapan AD, Koon AC, Kim TK, Hu LS, Malik AN, Greenberg ME. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–1204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liodis P, Denaxa M, Grigoriou M, Akufo-Addo C, Yanagawa Y, Pachnis V. Lhx6 activity is required for the normal migration and specification of cortical interneuron subtypes. J Neurosci. 2007;27:3078–3089. doi: 10.1523/JNEUROSCI.3055-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato S, Tomassy GS, De Leonibus E, Uzcategui YG, Andolfi G, Armentano M, Touzot A, Gaztelu JM, Arlotta P, Menendez de la Prida L, Studer M. Loss of COUP-TFI alters the balance between caudal ganglionic eminence- and medial ganglionic eminence-derived cortical interneurons and results in resistance to epilepsy. J Neurosci. 2011;31:4650–4662. doi: 10.1523/JNEUROSCI.6580-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bendito G, Sánchez-Alcaniz JA, Pla R, Borrell V, Picó E, Valdeolmillos M, Marín O. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–1624. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Yao XH, Fu Y, Yu YC. Development of layer 1 neurons in the mouse neocortex. Cereb Cortex. 2014;24:2604–2618. doi: 10.1093/cercor/bht114. [DOI] [PubMed] [Google Scholar]
- Ma T, Zhang Q, Cai Y, You Y, Rubenstein JL, Yang Z. A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb Cortex. 2012;22:2120–2130. doi: 10.1093/cercor/bhr296. [DOI] [PubMed] [Google Scholar]
- Machon O, Backman M, Machonova O, Kozmik Z, Vacik T, Andersen L, Krauss S. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev Biol. 2007;311:223–237. doi: 10.1016/j.ydbio.2007.08.038. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O. Cellular and molecular mechanisms controlling the migration of neocortical interneurons. Eur J Neurosci. 2013;38:2019–2029. doi: 10.1111/ejn.12225. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Marín O, Valiente M, Ge X, Tsai LH. Guiding neuronal cell migrations. Cold Spring Harb Perspect Biol. 2010;2:a001834. doi: 10.1101/cshperspect.a001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Métin C, Alvarez C, Moudoux D, Vitalis T, Pieau C, Molnár Z. Conserved pattern of tangential neuronal migration during forebrain development. Development. 2007;134:2815–2827. doi: 10.1242/dev.02869. [DOI] [PubMed] [Google Scholar]
- Miller MW. Cogeneration of retrogradely labeled corticocortical projection and GABA-immunoreactive local circuit neurons in cerebral cortex. Brain Res. 1985;355:187–192. doi: 10.1016/0165-3806(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Miquelajauregui A, Van de Putte T, Polyakov A, Nityanandam A, Boppana S, Seuntjens E, Karabinos A, Higashi Y, Huylebroeck D, Tarabykin V. Smad-interacting protein-1 (Zfhx1b) acts upstream of Wnt signaling in the mouse hippocampus and controls its formation. Proc Natl Acad Sci U S A. 2007;104:12919–12924. doi: 10.1073/pnas.0609863104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Directing neuron-specific transgene expression in the mouse CNS. Curr Opin Neurobiol. 2006;16:577–584. doi: 10.1016/j.conb.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. Dynamic FoxG1 expression coordinates the integration of multipolar pyramidal neuron precursors into the cortical plate. Neuron. 2012;74:1045–1058. doi: 10.1016/j.neuron.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Butt SJ, Takebayashi H, Fishell G. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–7798. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. J Neurosci. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Machold RP, Fishell G. Cortical development: neural diversity and neocortical organization. Tokyo: Springer Japan; 2013. Specification of GABAergic neocortical interneurons; pp. 89–126. [Google Scholar]
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wölfel B, Dodt HU, Zieglgänsberger W, Wotjak CT, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy S, Niquille M, Hurni N, Limoni G, Frazer S, Chameau P, van Hooft JA, Vitalis T, Dayer A. Serotonin receptor 3A controls interneuron migration into the neocortex. Nat Commun. 2014;5:5524. doi: 10.1038/ncomms6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5:218–224. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- Narboux-Nême N, Goïame R, Mattéi MG, Cohen-Tannoudji M, Wassef M. Integration of H-2Z1, a somatosensory cortex-expressed transgene, interferes with the expression of the Satb1 and Tbc1d5 flanking genes and affects the differentiation of a subset of cortical interneurons. J Neurosci. 2012;32:7287–7300. doi: 10.1523/JNEUROSCI.6068-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nat Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Nóbrega-Pereira S, Gelman D, Bartolini G, Pla R, Pierani A, Marín O. Origin and molecular specification of globus pallidus neurons. J Neurosci. 2010;30:2824–2834. doi: 10.1523/JNEUROSCI.4023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlkamp T, Dávid C, Cauli B, Gallopin T, Bouché E, Karagiannis A, May P, Herz J, Frotscher M, Staiger JF, Bock HH. Characterization and distribution of reelin-positive interneuron subtypes in the rat barrel cortex. Cereb Cortex. 2014;24:3046–3058. doi: 10.1093/cercor/bht161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin AN, Kessaris N. PROX1: a lineage tracer for cortical interneurons originating in the lateral/caudal ganglionic eminence and preoptic area. PLoS One. 2013;8:e77339. doi: 10.1371/journal.pone.0077339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin AN, Alfonsi F, Humphreys MP, Choi CK, Rocha SF, Kessaris N. The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex. J Neurosci. 2010;30:12050–12062. doi: 10.1523/JNEUROSCI.6178-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymar VV, Sadikot AF. Laminar fate of cortical GABAergic interneurons is dependent on both birthdate and phenotype. J Comp Neurol. 2007;501:369–380. doi: 10.1002/cne.21250. [DOI] [PubMed] [Google Scholar]
- Sessa A, Mao CA, Colasante G, Nini A, Klein WH, Broccoli V. Tbr2-positive intermediate (basal) neuronal progenitors safeguard cerebral cortex expansion by controlling amplification of pallial glutamatergic neurons and attraction of subpallial GABAergic interneurons. Genes Dev. 2010;24:1816–1826. doi: 10.1101/gad.575410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–i10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussel L, Marin O, Kimura S, Rubenstein JL. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–3370. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- Tamás G, Lorincz A, Simon A, Szabadics J. Identified sources and targets of slow inhibition in the neocortex. Science. 2003;299:1902–1905. doi: 10.1126/science.1082053. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Nakajima K. Migratory pathways of GABAergic interneurons when they enter the neocortex. Eur J Neurosci. 2012;35:1655–1660. doi: 10.1111/j.1460-9568.2012.08111.x. [DOI] [PubMed] [Google Scholar]
- Tanaka DH, Maekawa K, Yanagawa Y, Obata K, Murakami F. Multidirectional and multizonal tangential migration of GABAergic interneurons in the developing cerebral cortex. Development. 2006;133:2167–2176. doi: 10.1242/dev.02382. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Lamy C. Functional maps of neocortical local circuitry. Front Neurosci. 2007;1:19–42. doi: 10.3389/neuro.01.1.1.002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- Tripodi M, Filosa A, Armentano M, Studer M. The COUP-TF nuclear receptors regulate cell migration in the mammalian basal forebrain. Development. 2004;131:6119–6129. doi: 10.1242/dev.01530. [DOI] [PubMed] [Google Scholar]
- Vucurovic K, Gallopin T, Ferezou I, Rancillac A, Chameau P, van Hooft JA, Geoffroy H, Monyer H, Rossier J, Vitalis T. Serotonin 3A receptor subtype as an early and protracted marker of cortical interneuron subpopulations. Cereb Cortex. 2010;20:2333–2347. doi: 10.1093/cercor/bhp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ, 2nd, Bell SM, Erdélyi F, Szabó G, Potter SS, Campbell K. The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- West AE, Greenberg ME. Neuronal activity-regulated gene transcription in synapse development and cognitive function. Cold Spring Harb Perspect Biol. 2011;3:a005744. doi: 10.1101/cshperspect.a005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Taylor L, Welagen J, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314:127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Tam M, Anderson SA. Fate mapping Nkx2.1-lineage cells in the mouse telencephalon. J Comp Neurol. 2008;506:16–29. doi: 10.1002/cne.21529. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Flandin P, Long JE, Cuesta MD, Westphal H, Rubenstein JL. Distinct molecular pathways for development of telencephalic interneuron subtypes revealed through analysis of Lhx6 mutants. J Comp Neurol. 2008;510:79–99. doi: 10.1002/cne.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]