Abstract
We have screened Xenopus laevis cDNA and genomic libraries for finger motif encoding sequences by use of a synthetic oligonucleotide probe coding for a stretch of conserved amino acids, the H/C-link, which joins individual finger loops in several multi-fingered proteins. Our studies reveal that a large number of different cDNA clones encode amino acid sequences predicting multiple units of the metal-coordinating finger structure. Derived proteins are different from each other as well as from the two examples of Xenopus finger proteins reported to date, TFIIIA and X.fin. The 109 finger repeats characterized are derived from 14 different cDNA clones and have been analysed for the presence of conserved and highly variable amino acids, revealing a close structural relatedness among each other as well as with a few selected finger domains from Drosophila and mouse proteins. The results from this comparative sequence analysis are also discussed in terms of the existing models for DNA binding. All sequences are identified in an ovary cDNA library but the patterns of mRNA level for individual finger clones vary greatly during early development. The prevalence of these structures in the oocyte suggests that part of the maternal information for the realization of the developmental program utilized in Xenopus embryogenesis might be transmitted in the form of regulatory, nucleic-acid-binding proteins.
Full text
PDF

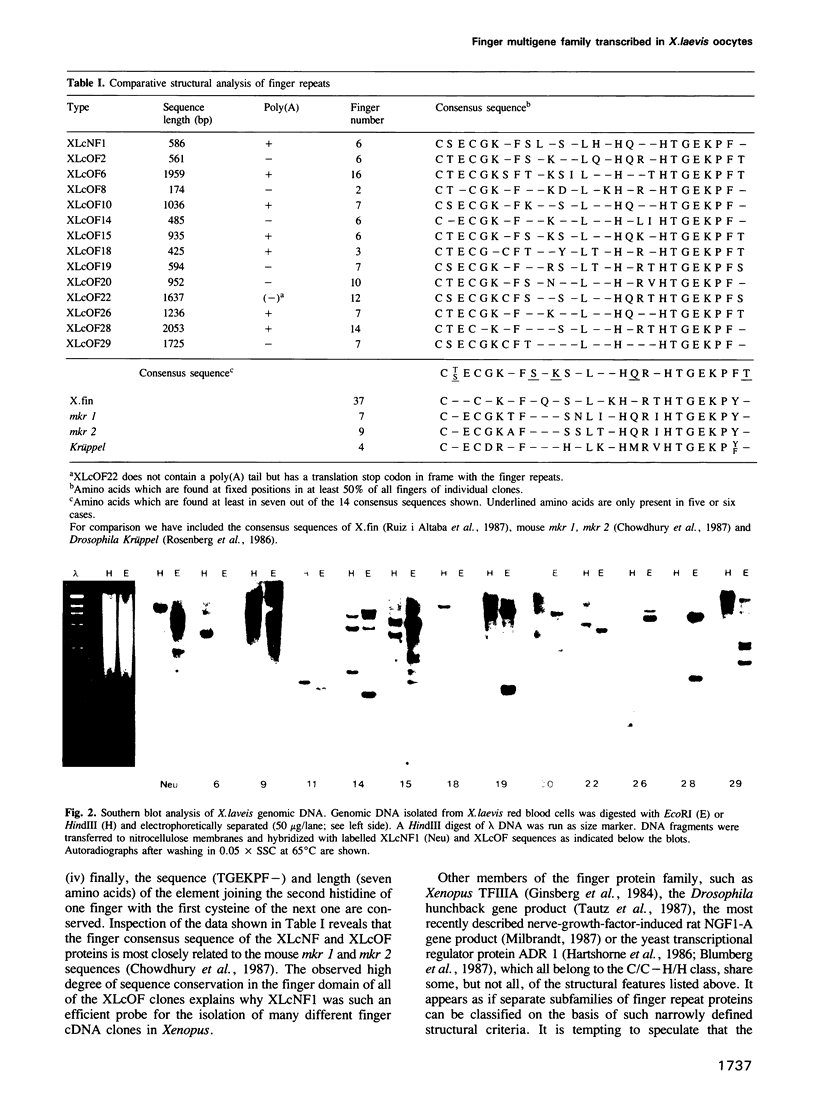

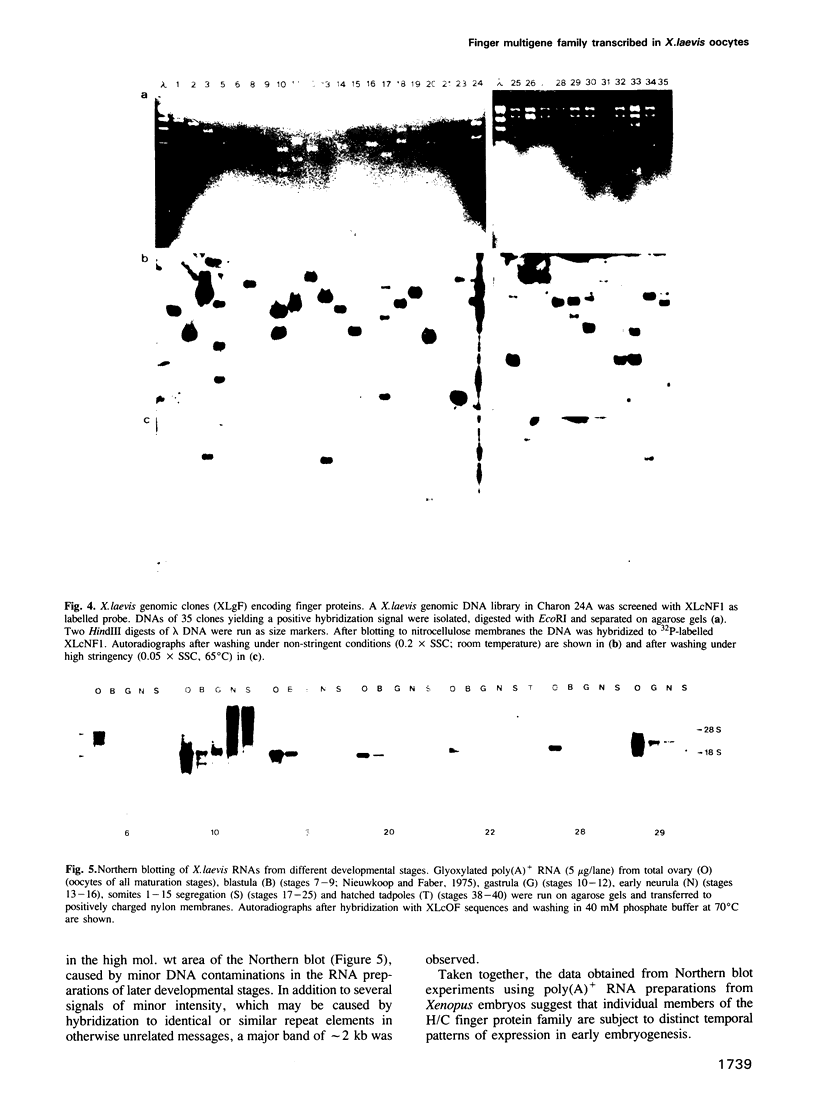
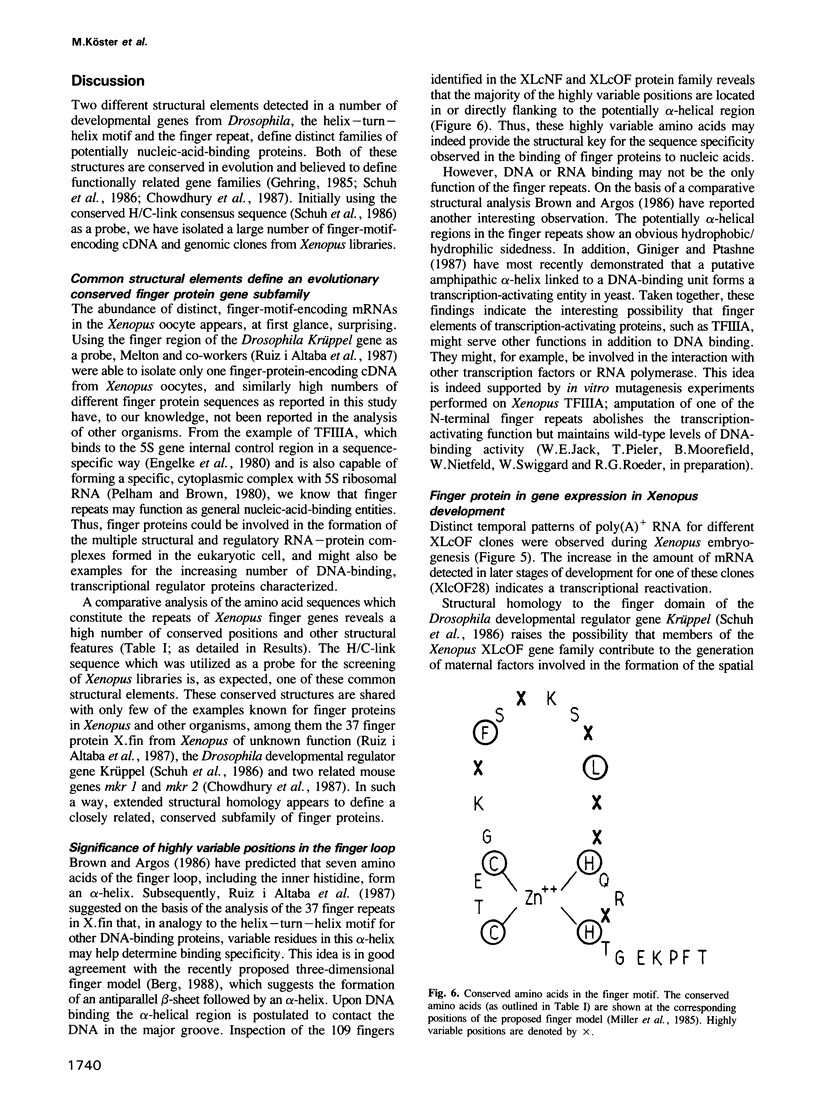

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Proposed structure for the zinc-binding domains from transcription factor IIIA and related proteins. Proc Natl Acad Sci U S A. 1988 Jan;85(1):99–102. doi: 10.1073/pnas.85.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg H., Eisen A., Sledziewski A., Bader D., Young E. T. Two zinc fingers of a yeast regulatory protein shown by genetic evidence to be essential for its function. 1987 Jul 30-Aug 5Nature. 328(6129):443–445. doi: 10.1038/328443a0. [DOI] [PubMed] [Google Scholar]
- Boulay J. L., Dennefeld C., Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. 1987 Nov 26-Dec 2Nature. 330(6146):395–398. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- Brown R. S., Argos P. Fingers and helices. Nature. 1986 Nov 20;324(6094):215–215. doi: 10.1038/324215a0. [DOI] [PubMed] [Google Scholar]
- Chowdhury K., Deutsch U., Gruss P. A multigene family encoding several "finger" structures is present and differentially active in mammalian genomes. Cell. 1987 Mar 13;48(5):771–778. doi: 10.1016/0092-8674(87)90074-2. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Engelke D. R., Ng S. Y., Shastry B. S., Roeder R. G. Specific interaction of a purified transcription factor with an internal control region of 5S RNA genes. Cell. 1980 Mar;19(3):717–728. doi: 10.1016/s0092-8674(80)80048-1. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Homeotic genes, the homeo box, and the genetic control of development. Cold Spring Harb Symp Quant Biol. 1985;50:243–251. doi: 10.1101/sqb.1985.050.01.031. [DOI] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Ginsberg A. M., King B. O., Roeder R. G. Xenopus 5S gene transcription factor, TFIIIA: characterization of a cDNA clone and measurement of RNA levels throughout development. Cell. 1984 Dec;39(3 Pt 2):479–489. doi: 10.1016/0092-8674(84)90455-0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Melton D. A. Gene transfer in amphibian eggs and oocytes. Annu Rev Genet. 1981;15:189–218. doi: 10.1146/annurev.ge.15.120181.001201. [DOI] [PubMed] [Google Scholar]
- Hartshorne T. A., Blumberg H., Young E. T. Sequence homology of the yeast regulatory protein ADR1 with Xenopus transcription factor TFIIIA. Nature. 1986 Mar 20;320(6059):283–287. doi: 10.1038/320283a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel W. J., Cole J., Lehmann A. R. Molecular analysis of ouabain-resistant mutants of the mouse lymphoma cell line L5178Y. Mutagenesis. 1987 Sep;2(5):383–389. doi: 10.1093/mutage/2.5.383. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Brown D. D. A specific transcription factor that can bind either the 5S RNA gene or 5S RNA. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4170–4174. doi: 10.1073/pnas.77.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Perry-O'Keefe H., Melton D. A. Xfin: an embryonic gene encoding a multifingered protein in Xenopus. EMBO J. 1987 Oct;6(10):3065–3070. doi: 10.1002/j.1460-2075.1987.tb02613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh R., Aicher W., Gaul U., Côté S., Preiss A., Maier D., Seifert E., Nauber U., Schröder C., Kemler R. A conserved family of nuclear proteins containing structural elements of the finger protein encoded by Krüppel, a Drosophila segmentation gene. Cell. 1986 Dec 26;47(6):1025–1032. doi: 10.1016/0092-8674(86)90817-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]