Abstract
Background:
The corticotropin-releasing factor is a stress-related neuropeptide that modulates locus coeruleus activity. As locus coeruleus has been involved in pain and stress-related patologies, we tested whether the pain-induced anxiety is a result of the corticotropin-releasing factor released in the locus coeruleus.
Methods:
Complete Freund’s adjuvant-induced monoarthritis was used as inflammatory chronic pain model. α-Helical corticotropin-releasing factor receptor antagonist was microinjected into the contralateral locus coeruleus of 4-week-old monoarthritic animals. The nociceptive and anxiety-like behaviors, as well as phosphorylated extracellular signal-regulated kinases 1/2 and corticotropin-releasing factor receptors expression, were quantified in the paraventricular nucleus and locus coeruleus.
Results:
Monoarthritic rats manifested anxiety and increased phosphorylated extracellular signal-regulated kinases 1/2 levels in the locus coeruleus and paraventricular nucleus, although the expression of corticotropin-releasing factor receptors was unaltered. α-Helical corticotropin-releasing factor antagonist administration reversed both the anxiogenic-like behavior and the phosphorylated extracellular signal-regulated kinases 1/2 levels in the locus coeruleus.
Conclusions:
Pain-induced anxiety is mediated by corticotropin-releasing factor neurotransmission in the locus coeruleus through extracellular signal-regulated kinases 1/2 signaling cascade.
Keywords: anxiety, corticotropin-releasing factor, locus coeruleus, pain, pERK1/2
Introduction
Corticotropin-releasing factor (CRF) is a neuropeptide released from neurons in the paraventricular nucleus (PVN) of the hypothalamus that activates stress-related hypophysial structures (Bale and Vale, 2004). Extrahypophysial CRF operates as a neurotransmitter in several brain areas, influencing different actions related to the stress response (Valentino and Wehby, 1988). Also, it may exacerbate many chronic diseases, in particular those involving severe pain like arthritis (Zautra et al., 2007), a prevalent inflammatory condition. Equally, the emergence of anxiety due to persistent pain is a negative factor commonly reported by arthritic patients (Gyurcsik et al., 2014), representing in itself a stressful situation (Hummel et al., 2010) and inducing similar effects to other stressors (Vierck et al., 2010). Indeed, it is estimated that up to 20% of patients with arthritis will develop depression and/or anxiety (Covic et al., 2012). Although inflammatory pain is a stressor that may modulate the hypothalamic-pituitary-adrenal axis (Bomholt et al., 2004), the neurobiological features and behavioral repercussion of such an association remain poorly understood.
CRF acts at different sites in important regulatory pain structures, directly implicating this molecule in pain modulation (Lariviere and Melzack, 2000). In particular, the locus coeruleus (LC), the major noradrenaline source in the brain, is one important target for CRF neurotransmission (Valentino and Van Bockstaele, 2008). Besides its important role in modulating ascending and descending pain pathways, the LC also represents a convergent nucleus that is correlated with adaptive responses to stress (Valentino and Van Bockstaele, 2008). Thus, the activity of CRF in the LC could influence chronic inflammatory pain, and it would not be unreasonable to hypothesize that the onset of emotional changes produced by pain might be the result of stress-induced CRF release.
The extracellular signal-regulated kinases 1/2 (ERK1/2) cascade is a strong candidate to mediate the effects of CRF in pain, and it is known to participate in CRF receptor signaling in neuronal cells (Hauger et al., 2006). In addition, CRF administration into the LC region promotes c-Fos and ERK1/2 activation in the prefrontal cortex (Snyder et al., 2012). Accordingly, we demonstrated that animals suffering chronic inflammation also display an anxio-depressive phenotype, with an enhancement of ERK1/2 activation in the prefrontal cortex (Borges et al., 2014).
To evaluate whether CRF neurotransmission in the LC triggers the development of anxiety in chronic inflammation (eg, a model of rheumatoid arthritis), an antagonist of the CRF receptors was microinjected into the LC, and the nociceptive and anxiety behavior as well as the activation of ERK1/2 in the LC were evaluated.
Methods
Animals
Harlan Sprague-Dawley male rats (250–300g) were provided by the Experimental Unit of the University of Cádiz (ES110120000210). Animals were housed 2 to 4 per cage with ad libitum access to food and water and kept under controlled conditions of lighting (12-h-light/-dark cycle), temperature (22ºC), and humidity (45–60%). The protocols followed the European Communities Council Directive of 22 September 2010 (2010/63/EC), Spanish Law (RD 1201/2005), and the ethical guidelines for investigation of experimental pain in animals (Zimmermann, 1983) and were reviewed and approved by the Institutional Ethical Committee for animal care and use.
Monoarthritis Model of Inflammatory Pain
Monoarthritis (MA) was induced as previously described (Butler et al., 1992), injecting the left tibiotarsal joint of rats anaesthetized with isoflurane (4% to induce and 2% to maintain: Abbott, Spain) with 50 µL of complete Freund’s adjuvant (CFA) solution containing 30mg of desiccated Mycobacterium butyricum (Difco Laboratories), paraffin oil (3mL), saline (2mL), and Tween 80 (500 µL). Animals that developed polyarthritis were excluded. The control rats were injected with the vehicle solution (paraffin oil, saline, and Tween 80). The experimental design is represented in supplementary Figure 1a.
Surgery and Intra-LC Drug Administration
Animals were anesthetized with an intraperitoneal injection of ketamine (100mg/kg) and xylazine (20mg/kg) and placed in a stereotaxic apparatus with the head tilted at an angle of 15° to the horizontal plane. A guide cannula (22 gauge, 15-mm length) was implanted into the contralateral LC (lambda: AP = -3.2mm, ML -1.1mm, and DV -6.2mm; Figure 1a; supplementary Figure S1b) and was fixed with skull screws and dental cement. A stainless-steel wire was inserted into the guide cannula to prevent occlusion. Five days after recovery, animals were immobilized and the steel wire was cut. Microinjection was performed by inserting an injector cannula (30 gauge) that was 1mm longer than the guide cannula (16mm). Animals received 28ng or 34ng of α-helical CRF(9–41) (αCRF) dissolved in sterile water (0.5 µL; Sigma Aldrich, Ref. C246), which blocks the CRF I and II receptors, inhibiting the endogenous CRF activity. Sterile water was used as a vehicle. Behavioral tests or sacrifice for Western-blot procedures were performed 10 to 25 minutes after drug/vehicle administration (see supplementary Material and supplementary Figure 1a). As no differences between doses were observed in terms of pERK1/2 expression, the behavioral effects were analyzed in animals receiving the 28ng αCRF. Behavior was assessed in groups of 6 animals, and random animals were selected for histological verification of the cannula implantation.
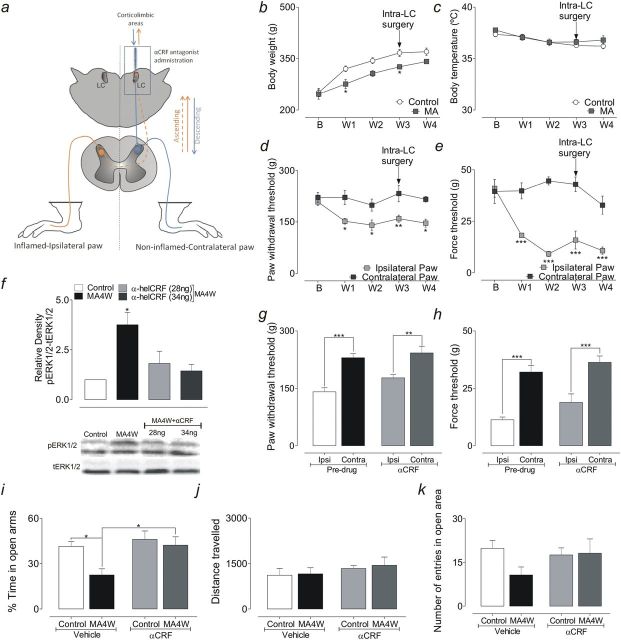
Figure 1.
(a) Schematic representation of the anatomical pathways implicated. Briefly, the contralateral locus coeruleus (LC) indirectly receives inputs from the inflamed paw (red dashed line; ascending pathways) and, subsequently, the information is sent to corticolimbic areas. Additionally, the LC sends direct projections to the spinal cord (blue straight line; descending pathways). (b) Body weight of the control and monoarthritic (MA) rats. (c) Body rectal temperature of control and MA rats. (d) Mechanical hyperalgesia represented by a significant decrease in the paw withdrawal threshold of the ipsilateral paw of MA rats. (e) Mechanical allodynia represented by a significant decrease in the force threshold of the ipsilateral paw of MA rats. *P<.05, **P<.01, ***P<.001; repeated measures followed by a Bonferroni posthoc test comparing control vs MA for the same week (b and c) or comparing the ipsilateral vs contralateral paw for the same week (d and e). (f) Graph depicting the expression of phosphorylated extracellular signal-regulated kinases 1/2 (pERK1/2) in the locus coeruleus (LC) after intra-LC administration of the α-helical CRF(9–41) (αCRF) receptor antagonist, showing that the significant increase of pERK1/2 in week 4 (MA4W) animals was no longer observed when this antagonist was administered: *P<.05 (1-way ANOVA followed by Dunnett’s posthoc test). (g) Graph showing that the local administration of the αCRF antagonist had no significant effect on mechanical hyperalgesia in MA4W rats. (h) Graph showing that local administration of the α-helical CRF antagonist had no significant effect on mechanical allodynia in the ipsilateral paw of MA rats. **P<.01, ***P<.001 (2-way ANOVA followed by Bonferroni posthoc test). (i) Graph showing that the time spent in the open arms decreased in MA4W rats receiving the vehicle alone, but this effect was successfully reversed by administration of the αCRF antagonist. *P<.05 (2-way ANOVA followed by Bonferroni posthoc test). (j) Graph showing that local administration of the α-helical CRF antagonist had no significant effect on the total distance travelled in the elevated zero maze (EZM). (k) Graph showing that local administration of the α-helical CRF antagonist reversed the decrease in the number of entries into the open arms observed in MA4W rats receiving the vehicle alone. B, baseline.
Health Parameters, Nociceptive and Anxiety-Like Behavior
The body weight (g) and rectal temperature (°C) were recorded weekly, and nociceptive mechanical allodynia (automated von Frey test) and hyperalgesia (paw-pinch test) were assessed as described in the supplementary Material. Anxiety-like behavior was evaluated in the elevated zero maze (EZM) test, which consisted of a black circular platform divided into 4 quadrants, with 2 opposing open quadrants with 1-cm-high clear curbs to prevent falls and 2 opposing closed quadrants with 27-cm-high black walls. A 5-minute trial under the same lighting conditions began with the animal placed in the center of a closed quadrant. The SMART software was used to analyze the time spent in the open arms and the total distance travelled by each rat. Increases in the time spent in the closed areas were correlated with anxiety-like behavior (Borges et al., 2014).
Immunohistochemistry
Another set of control and MA rats (4 weeks) was used for quantification of the expression of pERK1/2 in the PVN and CRFI/II receptors in the LC (supplementary Material).
Statistical Analysis
All data are represented as mean ± SEM and were analyzed using STATISTICA 10.0 or GraphPad Prism 5 software, using either an unpaired Student t test (2-tailed) or 1-way, 2-way, or repeated-measures ANOVA followed by the appropriate posthoc tests. The level of significance was considered P<.05.
Results
Effect of MA on Health Parameters and Nociceptive Responses
The behavior of control animals was normal, with no signs of an inflammatory reaction. CFA injection produced a stable MA, with the signs of inflammation restricted to the injected joint and evident a few hours after induction, persisting into the fourth week. The weight gain of MA rats was significantly lower than that of the control animals 1 and 3 weeks after CFA injection (repeated-measures, Bonferroni test; P<.05) (Figure 1b), while their body temperature remained normal during the experiment (Figure 1c). Regarding the pain threshold, a significant decrease in the withdrawal threshold of the ipsilateral paw was evident when compared with the contralateral paw, indicative of mechanical hyperalgesia (repeated-measures, Bonferroni test; P<.05 for weeks 1, 2, and 4, P<.01 for week 3) (Figure 1d). Additionally, there was a significant decrease in the paw withdrawal threshold to von Frey stimulation by the ipsilateral-inflamed paw of MA rats compared with the contralateral paw, indicative of mechanical allodynia (repeated-measures, Bonferroni test; P<.001) (Figure 1e).
Effect of Intra-LC Microinjection of an α-Helical CRF Receptor Antagonist on the pERK1/2 Levels in the LC
The administration of the α-helical CRF receptor antagonist in the LC normalized the pERK1/2 values in MA4W rats at both doses of the compound used (1-way ANOVA, Dunnett’s test; P>.05 MA4W 28ng and MA4W 34ng vs control) (Figure 1f), suggesting that CRF acts through the ERK1/2 signaling cascade in LC neurons.
Effect of Intra-LC Microinjection of an α-Helical Receptor Antagonist on MA-Induced Pain
In the paw withdrawal threshold, a significant increase in pain sensitivity was observed when the ipsilateral paw was compared with the contralateral paw in the MA4W rats before (pre-drug, 2-way ANOVA, Bonferroni test; P<.001) and after microinjection of the αCRF into the LC (2-way ANOVA, Bonferroni test; P<.01) (Figure 1g). Thus, the intra-LC administration of the α-helical CRF receptor antagonist (28ng) had no significant effect on the paw withdrawal threshold of MA4W rats (P>.05) (Figure 1g). In the force threshold, microinjection of the αCRF receptor antagonist into the LC had no effect on the ipsilateral sensitivity to innocuous stimulation. Indeed, the significant decrease in the force supported by the ipsilateral paw of MA4W rats when compared with the contralateral paw was present before and after administration of the drug (2-way ANOVA, Bonferroni test; P<.001) (Figure 1h).
Effect of Intra-LC Microinjection of an α-Helical CRF Receptor Antagonist on MA-Induced Anxiety
In the EZM, MA4W rats that received the vehicle alone (MA4W-vehicle) spent significantly less time in the open arms than control animals, indicative of anxiety-like behavior (2-way ANOVA, Bonferroni test; P<.05) (Figure 1i). By contrast, those animals that received an intra-LC microinjection of the αCRF receptor antagonist (MA4W-αCRF) spent significantly longer time in the open arms compared with the MA4W-vehicle animals (2-way ANOVA, Bonferroni test; P<.05) (Figure 1i). No effect of microinjecting the αCRF receptor antagonist into the LC was observed in the control rats. Moreover, no differences were observed in the total distance travelled in the EZM (Figure 1j), ruling out any influence of locomotor impairment on the experimental results. Regarding the number of entries into the open arms, the MA4W-vehicle rats appeared to enter these arms less frequently than the control animals that received the vehicle alone, although this difference was not significant. No such difference was observed in the MA4W-αCRF rats (Figure 1k).
Expression of CRF Receptors in the LC and pERK1/2 in the PVN
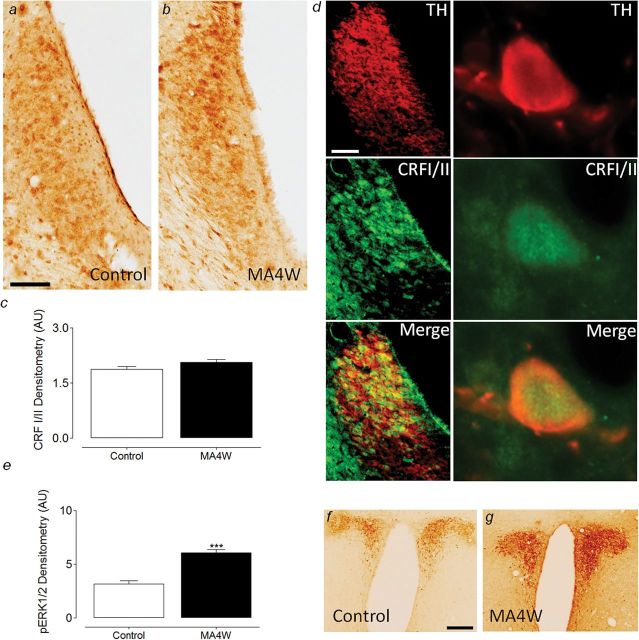
When the expression of the CRF I and II receptors was studied in the LC, no differences were observed between the control and MA4W rats (Figure 2a-c). Most of the neurons expressing CRFI/II receptors also expressed tyrosine hydroxylase (TH, the rate-limiting enzyme in the biosynthesis of noradrenaline, marker of noradrenergic neurons), indicating their noradrenergic nature and demonstrating that the CRFI/II receptors were expressed by neurons in the LC area (Figure 2d).
Figure 2.
Expression of corticotropin-releasing factor (CRFI/II) receptors in the locus coeruleus (LC) of control and week 4 monoarthritic (MA4W) rats. (a-b) Photomicrographs showing the expression of CRFI/II receptors in control and MA4W rats, respectively. (c) Graph showing that there were no significant changes between control and MA4W rats in terms of CRFI/II receptor expression in the LC. (d) Immunofluorescence photomicrographs showing that almost all the neurons expressing CRFI/II receptors (green) are noradrenergic neurons, since they colocalize with tyrosine hydroxylase (TH) immunolabeling. (e) Graph showing the increase in phosphorylated extracellular signal-regulated kinases 1/2 (pERK1/2) expression in the paraventricular nucleus (PVN) nucleus of MA4W rats: ***P<.001 (unpaired 2-tailed Student’s t test). (f-g) Photomicrographs of pERK1/2 expression in control and MA4W rats, respectively. Scale bar=100 µm.
The expression of pERK1/2 was also studied in the PVN of the hypothalamus to determine the activity of this structure as a readout of CRF stimulation in the central nervous system. Interestingly, an increase in pERK1/2 was observed in MA4W rats compared with control rats (unpaired Student’s t test; P<.001) (Figure 2e-g).
Discussion
This study shows that the action of CRF on LC neurons is involved in the development of anxiety-like symptoms associated with prolonged inflammatory pain. As expected, 4 weeks after CFA injection, rats displayed signs of pain and anxiety, consistent with previous reports (Borges et al., 2014). We also observed a significant increase in ERK1/2 phosphorylation in the LC, in accordance with previous data (Borges et al., 2014), and this increased ERK1/2 activation in the LC seems to be related with the development of anxiety-like behaviors in chronic inflammatory conditions. This raises the question as to what produces this increase in ERK1/2 activation in the LC when painful conditions develop. CRF is a molecule linked with the endocrine and behavioral response to stress (Bale and Vale, 2004), and the role of CRF in different pain conditions has been studied (Lariviere and Melzack, 2000), although not its effects after prolonged times of inflammation (eg, 4 weeks). Here, we studied the PVN nucleus, a CRF-producing structure, and we found that pERK1/2 levels increase in MA4W rats compared with control rats, suggesting that PVN hyperactivation occurs in association with chronic inflammatory pain. As the PVN and LC have reciprocal excitatory connections (Perez et al., 2006), we hypothesized that this would underpin the ERK1/2 activation in the LC of MA4W rats. Indeed, the LC is rich in CRF receptors (Reyes et al., 2006; Mousa et al., 2007), and it has already been shown that CRF activates LC neurons (Valentino and Foote, 1988). Here, we found no significant differences in CRFI/II receptor expression in the LC of control and MA4W rats, which indicates that while enhanced neurotransmission might originate in the PVN when chronic inflammatory pain is established, it is not accompanied by changes in the expression of the CRFI/II receptors in the LC. The colocalization of CRFI/II receptors with the TH protein, as previously described (Reyes et al., 2006), confirmed the specificity of this labeling.
To better understand how CRF neurotransmission influences the role of the LC in nociception and anxiety behavior, an antagonist blocking the CRF receptors was microinjected into the contralateral LC. This strategy was adopted to study the ascending pain pathway passing through the LC given its important projections to corticolimbic areas (Figure 1a). The dose of the α-helical CRF antagonist used was based on previous studies (Mousa et al., 2007), and at both 28ng and 34ng, this antagonist successfully dampened pERK1/2 expression in the LC of MA4W rats. Thus, the effects of the lower dose alone (28ng) were evaluated on behavior. This procedure had no effect on pain sensitivity in the ipsilateral/inflamed or contralateral paws of MA4W rats.
In contrast, microinjection of the α-helical CRF receptor antagonist reverses the anxiety-like behavior observed in MA4W rats without interfering with locomotor activity. Indeed, the decrease in the time MA4W rats spent in the open arms was no longer observed when they received this antagonist. These results suggest that the increased CRF neurotransmission in chronic inflammatory conditions enhances the LC-driven activation of corticolimbic areas, which may be responsible for the development of anxiety. Indeed, it has already been shown that CRF infused into the LC increases anxiety, a behavioral effect of CRF associated with increased noradrenergic neurotransmission in LC terminal areas like the amygdala and hypothalamus (Butler et al., 1990; Weiss et al., 1994). Moreover, the α-helical CRF receptor antagonist prevents the development of anxiety induced by a neuropeptide Y receptor antagonist, while not producing any significant change when administered in a nonanxious state (Kask et al., 1997; Donatti and Leite-Panissi, 2011). Similarly, we did not find a significant effect of the α-CRF antagonist in control noninflamed animals. Overall, the effects in the ipsilateral paw and on anxiety-like behavior in MA rats are consistent with studies showing that administration of the α-helical CRF receptor antagonist to the basolateral or central nuclei of the amygdala has no effect on the nociceptive threshold but that it reduced innate fear behavior (Donatti and Leite-Panissi, 2013). Nevertheless, the lack of changes in nociception might be related to the use of a broad-spectrum CRF antagonist that blocks nonspecifically the signaling of CRF1 and CRF2 receptors. Indeed, when NBI27914, a specific CRF1 receptor antagonist, was microinjected into the amygdala, the withdrawal thresholds of the arthritic rats as well and the anxiety-like behavior were reversed (Ji et al., 2007).
Concluding, CRF signaling through the ERK1/2 cascade in the LC appears to be an important mechanism related with anxious behavior associated with chronic inflammatory conditions.
Interest Statement
None.
Supplementary Material
Acknowledgments
We would like to acknowledge the help provided by Raquel Rey-Brea, José Antonio García Partida, Jesus Gallego-Gamo, Paula Reyes Perez, Santiago Muñoz, and Elisa Galvão. This work was supported by “Cátedra Externa del Dolor Fundación Grünenthal/Universidad de Cádiz,” which paid a grant to the first author; “Cátedra em Medicina da Dor from Fundação Grünenthal-Portugal and Faculdade de Medicina da Universidade do Porto”; Instituto de Salud Carlos III and FEDER (European Union), cofinanced by Fondo Europeo de Desarrollo Regional “Una manera de hacer Europa” (PI12/00915, PI13/02659); CIBERSAM G18; Junta de Andalucía (CTS-510, Proyectos de Excelencia: CTS-7748); Fundación Española de Dolor (travel fellowship granted to Gisela Borges–1536).
References
- Bale TL, Vale WW. (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557. [DOI] [PubMed] [Google Scholar]
- Bomholt SF, Harbuz MS, Blackburn-Munro G, Blackburn-Munro RE. (2004) Involvement and role of the hypothalamo-pituitary-adrenal (HPA) stress axis in animal models of chronic pain and inflammation. Stress 7:1–14. [DOI] [PubMed] [Google Scholar]
- Borges G, Neto F, Mico JA, Berrocoso E. (2014) Reversal of monoarthritis-induced affective disorders by diclofenac in rats. Anesthesiology 120:1476–1490. [DOI] [PubMed] [Google Scholar]
- Butler PD, Weiss JM, Stout JC, Nemeroff CB. (1990) Corticotropin-releasing factor produces fear-enhancing and behavioral activating effects following infusion into the locus coeruleus. J Neurosci 10:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SH, Godefroy F, Besson JM, Weil-Fugazza J. (1992) A limited arthritic model for chronic pain studies in the rat. Pain 48:73–81. [DOI] [PubMed] [Google Scholar]
- Covic T, Cumming SR, Pallant JF, Manolios N, Emery P, Conaghan PG, Tennant A. (2012) Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS). BMC Psychiatry 12:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatti AF, Leite-Panissi CRA. (2011) Activation of corticotropin-releasing factor receptors from the basolateral or central amygdala increases the tonic immobility response in guinea pigs: an innate fear behavior. Behav Brain Res pp 23–30. [DOI] [PubMed] [Google Scholar]
- Donatti AF, Leite-Panissi CRA. (2013) Activation of the corticotropin-releasing factor receptor from the basolateral or central amygdala modulates nociception in guinea pigs. Adv Biosci Biotechnol 4:7. [DOI] [PubMed] [Google Scholar]
- Gyurcsik NC, Cary MA, Sessford JD, Flora PK, Brawley LR. (2014) Pain anxiety and negative outcome expectations for activity: negative psychological profiles differ between the inactive and active. Arthritis Care Res (Hoboken). [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. (2006) Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets 5:453–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel M, Cummons T, Lu P, Mark L, Harrison JE, Kennedy JD, Whiteside GT. (2010) Pain is a salient “stressor” that is mediated by corticotropin-releasing factor-1 receptors. Neuropharmacology 59:160–166. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Ruppert KA, Neugebauer V. (2007) Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kask A, Rägo L, Harro J. (1997) α‐Helical CRF9-41 prevents anxiogenic-like effect of NPY Y1 receptor antagonist BIBP3226 in rats. NeuroReport 8:3645–3647. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Melzack R. (2000) The role of corticotropin-releasing factor in pain and analgesia. Pain 84:1–12. [DOI] [PubMed] [Google Scholar]
- Mousa SA, Bopaiah CP, Richter JF, Yamdeu RS, Schafer M. (2007) Inhibition of inflammatory pain by CRF at peripheral, spinal and supraspinal sites: involvement of areas coexpressing CRF receptors and opioid peptides. Neuropsychopharmacology 32:2530–2542. [DOI] [PubMed] [Google Scholar]
- Perez H, Ruiz S, Nunez H, White A, Gotteland M, Hernandez A. (2006) Paraventricular-coerulear interactions: role in hypertension induced by prenatal undernutrition in the rat. Eur J Neurosci 24:1209–1219. [DOI] [PubMed] [Google Scholar]
- Reyes BA, Fox K, Valentino RJ, Van Bockstaele EJ. (2006) Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci 23:2991–2998. [DOI] [PubMed] [Google Scholar]
- Snyder K, Wang WW, Han R, McFadden K, Valentino RJ. (2012) Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology 37:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Wehby RG. (1988) Corticotropin-releasing factor: evidence for a neurotransmitter role in the locus ceruleus during hemodynamic stress. Neuroendocrinology 48:674–677. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Foote SL. (1988) Corticotropin-releasing hormone increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci 8:1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. (2008) Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierck CJ, Green M, Yezierski RP. (2010) Pain as a stressor: effects of prior nociceptive stimulation on escape responding of rats to thermal stimulation. Eur J Pain 14:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. (1994) Depression and anxiety: Role of the locus coeruleus and corticotropin-releasing factor. Brain Res Bull 35:561–572. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Parrish BP, Van Puymbroeck CM, Tennen H, Davis MC, Reich JW, Irwin M. (2007) Depression history, stress, and pain in rheumatoid arthritis patients. J Behav Med 30:187–197. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.