Abstract
Background:
Inducible or neuronal nitric oxide synthase gene deletion increases or decreases anxiety-like behavior in mice, respectively. Since nitric oxide and endocannabinoids interact to modulate defensive behavior, the former effect could involve a compensatory increase in basal brain nitric oxide synthase activity and/or changes in the endocannabinoid system. Thus, we investigated the expression and extinction of contextual fear conditioning of inducible nitric oxide knockout mice and possible involvement of endocannabinoids in these responses.
Methods:
We evaluated the effects of a preferential neuronal nitric oxide synthase inhibitor, 7-nitroindazol, nitric oxide synthase activity, and mRNA changes of nitrergic and endocannabinoid systems components in the medial prefrontal cortex and hippocampus of wild-type and knockout mice. The effects of URB597, an inhibitor of the fatty acid amide hydrolase enzyme, which metabolizes the endocannabinoid anandamide, WIN55,212-2, a nonselective cannabinoid agonist, and AM281, a selective CB1 antagonist, on contextual fear conditioning were also evaluated.
Results:
Contextual fear conditioning expression was similar in wild-type and knockout mice, but the latter presented extinction deficits and increased basal nitric oxide synthase activity in the medial prefrontal cortex. 7-Nitroindazol decreased fear expression and facilitated extinction in wild-type and knockout mice. URB597 decreased fear expression in wild-type and facilitated extinction in knockout mice, whereas WIN55,212-2 and AM281 increased it in wild-type mice. Nonconditioned knockout mice showed changes in the mRNA expression of nitrergic and endocannabinoid system components in the medial prefrontal cortex and hippocampus that were modified by fear conditioning.
Conclusion:
These data reinforce the involvement of the nitric oxide and endocannabinoids (anandamide) in stress-related disorders and point to a deregulation of the endocannabinoid system in situations where nitric oxide signaling is increased.
Keywords: nitric oxide, 7-nitroindazole, URB597, WIN55, 212-2, AM281, anandamide, CB1 receptors, fear conditioning, extinction
Introduction
Aversive stimuli increase glutamate release in brain structures involved in stress-related disorders, such as the medial prefrontal cortex (MPFC), amygdala, hippocampus (HIP), and dorsal periaqueductal gray matter (Moghaddam, 1993; Musazzi et al., 2011; Riaza Bermudo-Soriano et al., 2012). Glutamate, by acting on NMDA receptors and increasing calcium influx, can activate the neuronal nitric oxide synthase (nNOS) enzyme, increasing NO production (Contestabile, 2000). Due to its high liposolubility, NO can act presynaptically and increase neurotransmitter release (Esplugues, 2002).
NO seems to be involved in stress-related disorders (Guimaraes et al., 2005), such as posttraumatic stress disorder (PTSD) (Oosthuizen et al., 2005). Several studies have shown that interference with NO and glutamate signaling can attenuate the behavioral consequences of stress exposure in rodents (Forestiero et al., 2006; Joca and Guimaraes, 2006; Spolidorio et al., 2007; Resstel et al., 2008; Aguiar and Guimaraes, 2009; Tonetto et al., 2009; Lisboa, 2011 , 2013; ).
Supporting NO involvement in anxiety, nNOS knockout (KO) mice present anxiolytic-like behavior in the elevated plus maze (EPM) test (Wultsch et al., 2007), decreased auditory fear conditioning, and a marked impairment of contextual fear conditioning (CFC) (Kelley et al., 2009). This phenotype was pharmacologically mimicked by administration of preferential nNOS inhibitors to wild-type (WT) mice or rescued by an NO donor in nNOS KO mice (Kelley et al., 2010).
On the other hand, mice with deletion of the inducible NOS gene (iNOS KO) seem to be more susceptible to stress, showing anxiogenic-like behavior in the EPM (Buskila et al., 2007). Moreover, this behavioral change is exacerbated 7 days after exposure to a predator odor (Abu-Ghanem et al., 2008). This anxiogenic-like effect was prevented by nonselective NOS inhibitor L-NAME treatment, suggesting that this behavioral change could involve a compensatory increase in the activity of other NOS isoforms (ie, nNOS or endothelial [eNOS]). In fact, these animals showed increased basal levels of NOS activity in the amygdala and cortex, the latter effect being attenuated by inhibition of NOS constitutive isoforms (Buskila et al., 2007; Gilhotra and Dhingra, 2009).
Recent results indicate that the nitrergic and the endocannabinoid (ECB) systems could interact during stressful or aversive situations (Lisboa and Guimaraes, 2012; Lisboa et al., 2013; Lisboa et al., 2014). ECBs are lipids synthesized from cellular membranes that behave as natural agonists for cannabinoid receptors (Battista et al., 2006; Di Marzo and Petrosino, 2007; Maccarrone et al., 2007). Similar to NO, ECBs are synthesized “on-demand” in postsynaptic neurons after neuronal stimulation and are not stored in vesicles, being characterized as atypical neurotransmitters (Piomelli, 2003; Ligresti et al., 2005). After their synthesis, ECBs diffuse to presynaptic terminals where they can activate cannabinoid receptors type 1 (CB1) or 2 (CB2) and decrease the release of neurotransmitters such as glutamate and GABA (Wilson and Nicoll, 2002; De Petrocellis et al., 2004; Fernandez-Ruiz et al., 2007, 2008). ECBs are metabolized postsynaptically by the enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). It has been suggested that CB1 receptors inhibit NOS activity. Indeed, NOS activity is enhanced in the cortex of CB1 KO mice (Kim et al., 2006). In addition, anandamide inhibited anxiety and panic-like behaviors induced by administration of an NO donor into the dorsal periaqueductal gray matter (Lisboa and Guimaraes, 2012; Lisboa et al., 2013).
Based on these pieces of evidence, the present study investigated the possible involvement of the nitrergic system in behavioral changes stress-related behavioral changes by testing the hypothesis that: 1) 7-nitroindazole (7-NI), a preferential nNOS inhibitor, would attenuate CFC in WT mice, 2) iNOS KO mice would show increased CFC, 3) the enhanced CFC observed in iNOS KO mice would be associated to increased NOS activity and NOS mRNA expression in the MPFC and HIP, and 4) the behavioral changes observed in iNOS KO mice would be attenuated by 7-NI. We further investigated the involvement of the cannabinoid system in the modulation of CFC by testing the hypothesis that facilitation of ECB signaling and CB1 antagonism would attenuate and increase CFC, respectively. Moreover, to verify a possible interaction between cannabinoids and NO, we tested the hypothesis that cannabinoid drugs would modulate CFC and that iNOS KO mice would present changes in mRNA expression of genes associated to the ECB system in the MPFC and HIP.
Methods
Animals
All experiments were performed using male C57BL/6J and iNOS KO (C57BL/6J background) mice (8–12 weeks old). Breeding homozygous pairs of mice with targeted deletion of the iNOS gene were obtained from Jackson Laboratories (no. 002609, Bar Harbor, ME) and maintained in our local animal farm facility. The animals were housed in groups of 5 animals per cage in a temperature-controlled room (24±1oC) under standard laboratory conditions (12h light/12h dark, lights on at 6:30 am) with food and water available ad libitum until they had reached the appropriate age for the experimental procedures. Animals that received the same treatment were kept in pairs until the end of the experiments. Procedures were conducted in conformity with the guidelines of the Brazilian Council for the care and use of laboratory animals (COBEA), which comply with international laws and politics, and were approved by our local ethical committee. Experiments were conducted between 9:00 am and 3:00 pm.
Drugs and Treatment
7-NI (15, 30, and 60mg/kg, Sigma-Aldrich), a preferential nNOS inhibitor, was dissolved in 5% Tween 80 in NaCl 0.9%; WIN55,212-2 (Win; 0.1, 0.3, and 1.0mg/kg, Sigma-Aldrich), a nonselective cannabinoid agonist, and AM281 (1, 2, and 4mg/kg), a potent and selective CB1 antagonist, were dissolved in 10% dimethylsulfoxide (DMSO) in NaCl 0.9% and administered i.p. to WT mice 30 minutes before the first reexposure to the context chamber (Maren, 1998; Rutkowska et al., 2006; Gilhotra and Dhingra, 2009; Gomes et al., 2011). URB597 (URB; 0.3, 1, and 3mg/kg), an inhibitor of the FAAH enzyme that metabolizes the ECB anandamide, was dissolved in 10% DMSO in NaCl 0.9% and administered i.p. to WT mice 1 hour before the first reexposure to the context chamber (Gomes et al., 2011). All drugs were administered in a fixed volume of 10mL/kg of body weight. The animals were also reexposed to the same context 48, 72, and 96 hours after the first chamber exposure.
For evaluation of freezing behavior, independent groups of WT and iNOS KO mice received i.p. injections of 7-NI (effective dose, 30mg/kg) or URB (effective dose, 3.0mg/kg) 30 minutes or 1 hour, respectively, before the first reexposure to the context chamber previously paired with electric footshocks.
CFC
Prior to the beginning of the conditioning procedure, each animal remained in the conditioning chamber for 2 minutes for habituation. They remained there for an additional 2 minutes period after the procedure. The CFC procedure consisted of submitting the animals to 3 inescapable electrical footshocks (0.75 mA, 2 seconds), randomly delivered. Each animal was reexposed to the same chamber for 5 minutes for evaluation of freezing behavior 24, 48, 72, and 96 hours after the conditioning session. Freezing time was manually registered by an observer, who was unaware to the animal condition and treatment, using a stopwatch. Additionally, for the molecular analysis, nonconditioned animals were exposed to the chamber during the same time period, but no footshock was delivered.
Quantification of Nitrite and Nitrate (NOx) Levels
MPFC tissue punch and HIP dissection were performed according to the Mouse Brain Atlas (Paxinos and Franklin, 2004). MPFC and HIP tissue samples of WT and KO naïve mice were used for quantifying the levels of NO2 -/NO3 - products (NOx) from the spontaneous oxidation of NO under physiological conditions. These levels were used as indirect measurements of NO production, as previously described (Moraes-Neto et al., 2014). iNOS KO and WT naive animals were anesthetized with chloral hydrate (5%, Sigma-Aldrich), decapitated, and had their brain removed. The MPFC and HIP were dissected and immediately immersed in lysis buffer solution (137nM NaCl, 20mM Tris-HCl, pH 8, 10% [v/v] glycerol). The tissues were homogenized in this buffer and centrifuged (15 minutes, 10000rpm, 4°C). The supernatants were collected and subjected to quantification of total protein by the Bradford method (Bradford, 1976) using bovine serum albumin (Sigma) as standard. The absorbance was read at 595nm, and the line equation provided by the standard curve was used to calculate the amount of total protein present in each sample.
The reduction of NO3 - (nitrite) to NO2 - (nitrate) were performed by a β-NADPH–dependent enzyme reductase from Aspergillus sp using the Griess’ method. Briefly, samples were incubated overnight (37°C) with the β-NADPH enzyme (Sigma-Aldrich). The following day, NO3 - was determined by adding Griess colorimetric reagent (Invitrogen) according to the manufacturer’s instructions. After 10 minutes, absorbance was read at 540nm, and the results were calculated based on the standard curve of sodium nitrite (NaNO3), corrected by total protein. Data are shown as relative percentage of NOx levels compared with the WT group (mean control group’s value was set to 100% and the individual animal’s values were normalized to the control mean).
Reverse Transcription and mRNA Quantification by Real-Time Polymerase Chain Reaction (PCR)
Independent groups of nonconditioned and conditioned WT and iNOS KO mice were used for evaluation of the mRNA expression of CB1 and CB2 receptor and nNOS, eNOS, FAAH, and MAGL enzyme genes in the MPFC and HIP 24 hours after the conditioning session.
The MPFC and HIP of WT and iNOS KO naive mice were collected as mentioned before in RNAse-free conditions. The collected tissues were maintained in microtubes (1.5mL) containing RNAlater (Ambion) and frozen at -80ºC until RNA extraction. Total RNA was isolated using TRizol reagent (Invitrogen) following the manufacturer’s instructions. Briefly, 750 µL of TRIzol was added to the samples, agitated for 30 seconds, and incubated in dry ice for 5 minutes. For each milliliter of suspension, 200 µL of chloroform (Sigma) and 10 µL of glycogen (20mg/mL; glycogen source oyster, USB) were added, vortexed, incubated at room temperature for 5 minutes, and centrifuged (14000rpm, 25 minutes, 4ºC). The supernatant was transferred to a new tube and 500 µL of iced isopropanol was added. The samples were incubated overnight at -80ºC. The samples were centrifuged (14000rpm, 15 minutes, 4ºC), the pellet suspended in ethanol 70%, and centrifuged again and allowed to dry at room temperature. RNA was suspended in 15 µL of ultra-pure water and maintained at -80ºC. RNA concentration was determined by UV spectrophotometer and 500ng was used for cDNA synthesis (High Capacity cDNA Reverse Transcription, Applied Biosystems). mRNA quantification was performed by real-time quantitative PCR (StepOne Plus, Applied Biosystems) using Taqman PCR arrays for the following genes: CnR1 (CB1 receptor, Mm01212171_s1), CnR2 (CB2 receptor, Mm02620087_s1), mgII (MAGL, Mm00449274_m1), faah (Mm00515684_m1), Nos1 (nNOS, Mm00435175_m1), Nos3 (eNOS, Mm00435217_m1) and ACTB (β-actin, reference gene, Mm00607939_s1). Determination of gene transcript in each sample was obtained by the ΔΔCq method. For each sample, the quantification cycle of mRNA was measured and normalized by the quantification cycle of reference gene. The fold change of mRNA in the sample relative to control group was determined by 2-ΔΔCq. Data are shown as a relative percentage of mRNA expression compared with the control group (mean control group’s value was set to 100% and the individual animal’s values were normalized to the control mean).
Statistical Analysis
Fear conditioning data are expressed as the mean±SEM and were analyzed by repeated-measures ANOVA, with time as the repeated measure, treatment as the independent factor, and genotype as the dependent factor. Student Newman-Keuls (S-N-K) posthoc test was used for evaluating overall treatment effects and individual differences in case of significant interactions between factors. The data from Griess reaction and real-time quantitative PCR data are represented as percentage relative to the mean of control group and were analyzed by Student’s t test. Differences were considered significant at P≤.05.
Results
Administration of the nNOS Inhibitor 7-NI Before the First Reexposure to the Aversive Context Attenuated CFC and Facilitated Fear Extinction in WT Animals
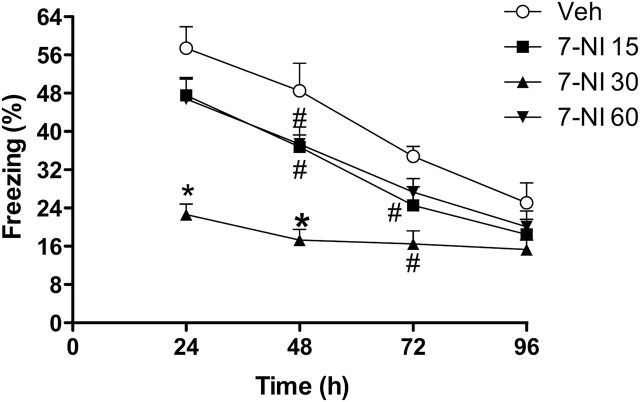
There was a significant effect of treatment (F3,26=15.8, P<.0001), time (F3,24=42.8, P<.0001), and interaction between them (F9,68=2.6, P<.05). During reexposure to the aversive chamber after conditioning, 7-NI 30mg/kg significantly attenuated freezing behavior, facilitating extinction (24 hours: F3,26=15.8, P<.0001, S-N-K, P<.05; 48 hours: F3,26=12.8, P<.0001, S-N-K, P<.05; 72 hours: F3,26=5.7, P<.01, S-N-K, P<.05; n=6–8/group) (Figure 1). Moreover, at 96 hours after conditioning, all groups presented decreased freezing behavior compared to the 24-hour time point (veh: t=5.3, d.f.=10, P<.0005; 7-NI 15: t=6.3, d.f.=14, P<.0001; 7-NI 30: t=2.5, d.f.=14, P<.05; 7-NI 60: t=4.8, d.f.=14, P<.0005), indicating that fear extinction had occurred. There were no differences between the groups at this time (P>.05).
Figure 1.
Dose-response curve of 7-nitroindazole (7-NI) on extinction of contextual fear conditioning (CFC) in WT mice. 7-NI was administered 30 minutes before the first reexposure to the aversive context. The 15-, 30-, and 60-mg/kg doses facilitate extinction compared with vehicle (n=6–8/group). Results are expressed as means±SEM. S-N-K *P<.05 different from other groups; #P<.05 different from vehicle.
The High Dose of the Nonselective Cannabinoid Agonist, Win, Administered Before the First Reexposure to the Aversive Context Increased CFC
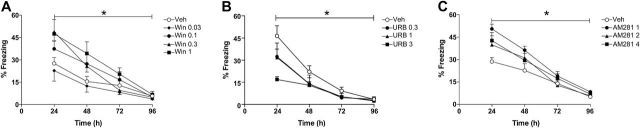
There was a significant effect of time (F3,26=48.2, P<.0001) and treatment (F4,28=4.1, P<.01) and a tendency to interaction between them (F12,74=1.7, P=.08). Win 1.0mg/kg increased freezing behavior (S-N-K, P<.05, n=5–9/group) (Figure 2A). Moreover, at 96 hours after conditioning, all groups presented decreased freezing behavior compared to the 24-hour time point (veh: t=5.1, d.f.=16, P<.0005; Win 0.03: t=2.7, d.f.=14, P<.05; Win 0.1: t=6.1, d.f.=10, P<.0005; Win 0.3: t=7,1, d.f.=8, P<.0005; Win 1.0: t=4.1, d.f.=8, P<.005). There were no differences between the groups at this time (P>.05).
Figure 2.
Dose-response curves for WIN55,212-2 (Win) (A), URB597 (URB) (B), and AM281 (C) on extinction of contextual fear conditioning (CFC) in WT mice. Win and AM281 were administered 30 minutes and URB 1 hour before the first reexposure to the aversive context. A) WIN 1mg/kg increased freezing behavior (Student Newman-Keuls [S-N-K], P<.05; n=5–9/group). B) URB 3mg/kg attenuated freezing behavior (S-N-K, P<.05; n=5–8/group). C) AM281 1mg/kg increased freezing behavior (S-N-K, P<.05; n=7/group). Results are expressed as means±SEM. *P<.05 main effect of time and drug.
The Inhibitor of the Anandamide Hydrolase Enzyme (FAAH), URB, Administered Before the First Reexposure to the Aversive Context Attenuated CFC
There was a significant effect of time (F3,2=33.4, P<.0001) and treatment (F3,24=3.3, P<.05), but no interaction between them (P>.05). URB 3mg/kg attenuated freezing behavior (S-N-K, P<.05, n=6–8/group) (Figure 2B). Again, at 96 hours after conditioning, all groups presented reduced freezing behavior compared with the 24-hour time point (veh: t=6.2, d.f.=14, P<.0001; URB 0.3: t=3.1, d.f.=10, P<.05; URB 1.0: t=5.0, d.f.=10, P<.001; URB 3.0: t=5.6, d.f.=14, P<.0001), indicating that fear extinction had occurred. There were no differences between the groups at this time (P>.05).
The CB1 Antagonist, AM281, Administered Before the First Reexposure to the Aversive Context Increased CFC
There was a significant effect of time (F3,22=89.7, P<.0001) and treatment (F3,24=3.0, P=.05), but no interaction between them (P>.05). The dose of 1mg/kg of AM281 significantly increased freezing behavior (S-N-K, P<.05, n=7/group) (Figure 2C). Moreover, at 96 hours after conditioning, all groups presented decreased freezing behavior compared with the 24-hour time point (veh: t=7.5, d.f.=12, P<.0001; AM281 1mg/kg: t=12.4, d.f.=12, P<.0001; AM281 2mg/kg: t=5.6, d.f.=12, P<.005; AM281 4mg/kg: t=6.3, d.f.=12, P<.0001). There were no differences between the groups at this time (P>.05).
iNOS KO Mice Showed Increased CFC
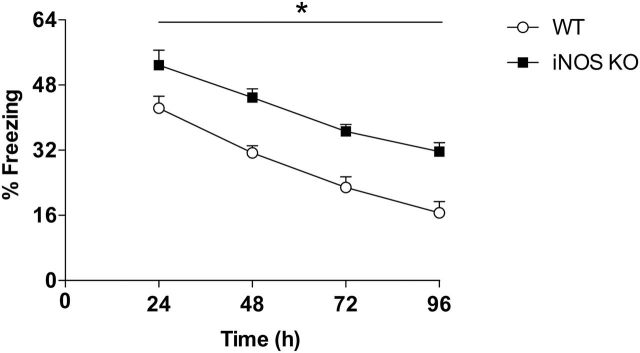
There was a significant effect of time (F3,16=41.9, P<.0001) and genotype (F1,18=26.0, P<.0001) but no interaction between them. iNOS KO mice presented increased freezing behavior during fear expression at the 24-hour time point and during all extinction trials (n=10/group) (Figure 3). Moreover, at 96 hours after fear conditioning, there was no difference between the groups compared with the 24-hour time point (WT: t=6.4, d.f.=18, P<.0001; KO: t=4.9, d.f.=18, P=.0001), indicating that both WT and iNOS KO mice had extinguished fear.
Figure 3.
Evaluation of extinction of contextual fear conditioning (CFC) in inducible nitric oxide synthase (iNOS) knockout (KO) mice. iNOS KO mice presented increased fear behavior compared with wild type (WT) (n=10/group) during all sessions. Results are expressed as means±SEM. *P<.05, main effect of time and genotype.
iNOS KO Mice Presented Increased NOx Levels in the MPFC but Not in the HIP
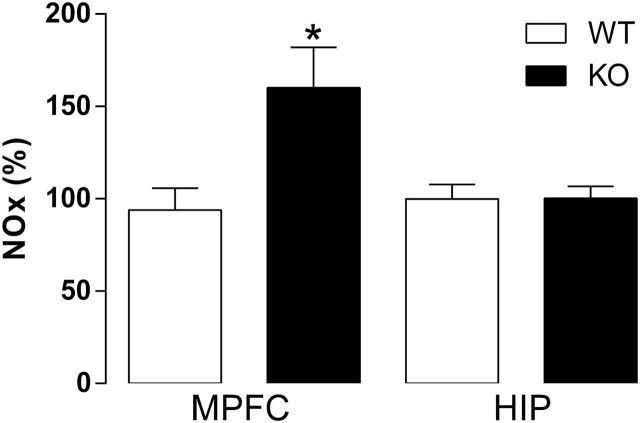
Naïve iNOS KO mice presented increased percentage of NOx in the MPFC (t15=2.8, P<.05) (Figure 4), but not in the HIP (P>.05).
Figure 4.
NO2 -/NO3 - products (NOx) levels in the medial prefrontal cortex (MPFC) and hippocampus (HIP) of wild-type (WT) and nitric oxide synthase (iNOS) knockout (KO) mice. iNOS KO mice have increased NOx levels compared with WT in the MPFC, but not in the HIP (n=8–9/group). Results are expressed as percentage means±SEM of control values. Student’s t test, *P<.05.
7-NI Decreased CFC in WT and iNOS KO Mice
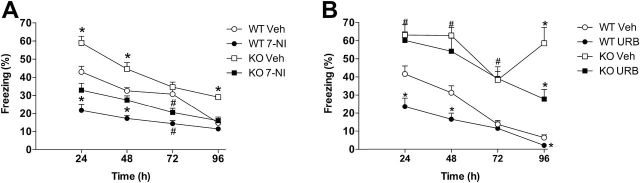
There was a significant effect of time (F3,49=86.3, P<.0001), treatment (F2,51=44.6, P<.0001), and genotype (F1,51=50.0, P<.0001), interactions between time and treatment (F6,96=3.6, P<.01) and time and genotype (F3,49=3.0, P<.05), and a tendency of interaction among the 3 factors (F6,96=1.9, P=.09). 7-NI facilitated extinction (Figure 5A), attenuating freezing behavior in both the WT and KO mice 24 hours (F3,33=19.9, P<.0001, S-N-K, P<.05), 48 hours (F3,33=20.0, P<.0001, S-N-K, P<.05), and 72 hours (F3,33=15.2, P<.0001, S-N-K, P<.05) after conditioning. Moreover, at 96 hours after fear conditioning, all groups presented less freezing than at the 24-hour time point (WT veh: t12=7.3, P<.0001; WT 7-NI: t16=2.9, P<.05; KO veh: t18=7.8, P<.0001; KO 7-NI: t20=3.9, P<.001), indicating the occurrence of fear extinction. At this point, only KO veh mice still presented increased freezing behavior, indicating extinction resistance (F3,33=21.3, P<.0001, S-N-K, P<.05).
Figure 5.
7-Nitroindazole (7-NI) and URB597 (URB) attenuated fear behavior in nitric oxide synthase (iNOS) knockout (KO) mice. A) 7-NI 30mg/kg administered before the first reexposure to the chamber attenuated fear behavior in wild-type (WT) and KO mice. Results are expressed as means±SEM. Student Newman-Keuls (S-N-K) *P<.05 different from other groups; #P<.05 compared with respective control group; n=7=11/group. B) KO mice presented extinction deficits and URB 3mg/kg facilitate this behavior. URB 3mg/kg also facilitated extinction in WT mice. Results are expressed as means±SEM. S-N-K *P<.05 different from other groups; #P<.05 KO mice different from WT mice; n= 7–9/group.
URB Facilitated Extinction of CFC in WT and iNOS KO Mice
There was a significant effect of time (F3,26=25.9, P<.0001), treatment (F1,28=7.7, P<.05), and genotype (F1,28=78.0, P<.0001) and interaction between time and treatment (F3,26=3.2, P<.05) as well as between time, treatment, and genotype (F3,26=4.1, P<.05) (Figure 5B). At all time points after conditioning, KO veh mice presented increased freezing behavior compared with WT veh mice. URB 3mg/kg facilitated fear extinction in both WT and KO mice (24 hours: F3,28=13.4, P<.0001, S-N-K P<.05; 48 hours: F3,28=20.3, P<.0001, S-N-K, P<.05; 72 hours: F3,28=13.5, P<.0001; 96 hours: F3,28=42.7, P<.0001, S-N-K, P<.05). Moreover, at 96 hours after conditioning, all groups except the KO veh group presented decreased freezing behavior compared with the 24-hour time point (WT veh: t=7.5, d.f.=16, P<.0001; WT URB3: t=4.4, d.f.=14, P<.001; KO veh: P>.05; KO URB3: t=4.6, df.=12, P<.001), indicating that KO veh did not extinguish fear, whereas the other groups did.
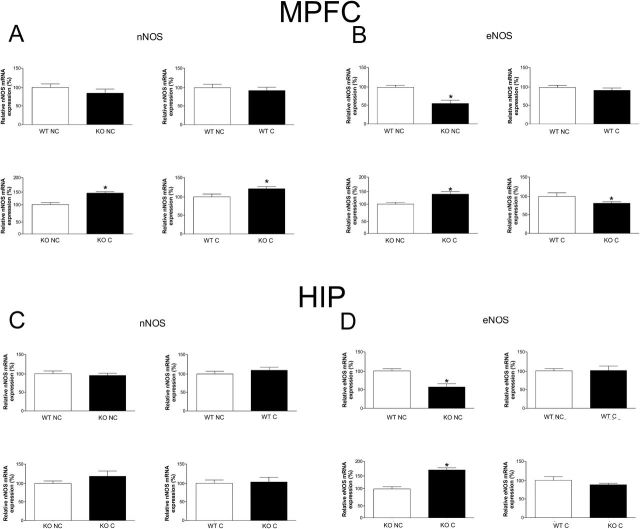
CFC Changed nNOS and eNOS mRNA Expression in iNOS KO Mice
Nonconditioned iNOS KO mice did not show changes in nNOS mRNA expression in the MPFC (P>.05) (Figure 6A) or in the HIP (P>.05) (Figure 6C). However, 24 hours after conditioning, there was a significant increase in nNOS mRNA expression in the MPFC (Figure 6A), but not in the HIP (Figure 6C), of iNOS KO mice compared with nonconditioned KO mice (t12=2.8, P<.05) or with conditioned WT mice (t12=2.3, P<.05). Fear conditioning did not increase nNOS mRNA expression in the WT animals (P>.05). In addition, although nonconditioned iNOS KO mice presented decreased eNOS mRNA expression compared to WT animals in the MPFC (WT nonconditioned vs KO nonconditioned t13=3.8, P<.01; WT conditioned vs KO C t12=3.1, P<.01) (Figure 6B) and HIP (WT nonconditioned vs KO nonconditioned t13=4.4, P<.001) (Figure 6D), the conditioning procedure increased this expression 24 hours later in iNOS KO mice compared with nonconditioned KO mice in the MPFC (t10=2.4, P<.05) (Figure 6B). This expression, however, was still lower than WT conditioned mice in the MPFC (t12=3.1, P<.01) (Figure 6B).
Figure 6.
Expression of neuronal nitric oxide synthase (nNOS) and endothelial nitric oxide synthase (eNOS) mRNA in the medial prefrontal cortex (MPFC) (A-B) and hippocampus (HIP) (C-D) of wild-type (WT) and inducible nitric oxide synthase (iNOS) KO mice. A) In the MPFC, conditioning (C) increase nNOS mRNA in KO mice compared with KO nonconditioned mice (NC), and KO C presented higher mRNA nNOS levels than WT C (n=7–8/group). B) In the MPFC, KO NC presented lower eNOS mRNA than WT NC, and conditioning increased eNOS mRNA in KO mice compared with KO NC, although KO C presented lower eNOS mRNA than WT C (n=6–8/group). C) In the HIP, there was no difference in the expression of nNOS mRNA. D) In the HIP, KO NC presented lower eNOS mRNA than WT NC, whereas conditioning increased eNOS mRNA in KO mice compared with KO NC (n=5–8/group). Results are expressed as percentage means±SEM of control values. Student’s t test, *P<.05.
Changes in mRNA Expression of ECB-Related Genes in the MPFC and HIP of iNOS KO Mice in Basal Conditions and 24 Hours After Conditioning
Nonconditioned iNOS KO mice showed increased CB1 and CB2 receptors mRNA expression in the MPFC (CB1: t12=2.8, P<.05 [Figure 7A]; CB2: t8=3.6, P<.01, [Figure 7B]) and decreased in the HIP (CB1: t12=5.3, P<.001 [Figure 7E]; CB2: t11=3.02, P<.05 [Figure 7F]). These animals also presented decreased MAGL (t11=3.8, P<.005 [Figure 7C]) and FAAH mRNA expression (t11=3.9, P<.005; [Figure 7D]) in the MPFC when compared with nonconditioned WT mice. In the MPFC, the conditioning procedure induced opposite effects in iNOS KO mice compared with nonconditioned KO, decreasing CB1 (t12=2.3, P<.05) (Figure 7A) and CB2 receptor (t10=4.5, P<.01) (Figure 7B) and increasing MAGL (t12=2.5, P<.05) (Figure 7C) and FAAH mRNA expression (t10=3.3, P<.01) (Figure 7D). Fear conditioning also tended to decrease CB1 mRNA expression in the MPFC of WT mice (t14=1.8, P=.09). In the HIP, conditioning decreased CB2 mRNA expression compared with nonconditioned WT mice (t12=2.5, P<.05) (Figure 7F) and increased both MAGL (t13=2.5, P<.05) (Figure 7G) and FAAH mRNA levels (t13=2.9, P<.05) (Figure 7H). There was a tendency to decreased MAGL mRNA levels in nonconditioned KO compared with nonconditioned WT mice (t13=1.9, P=.07) (Figure 7G) and a tendency to decreased MAGL mRNA levels in conditioned KO mice compared with conditioned WT mice (t12=1.7, P=.1) (Figure 7G). There was also a tendency to increased FAAH mRNA levels in conditioned KO mice compared with nonconditioned KO mice (t12=1.7, P=.1) (Figure 7H).
Figure 7.
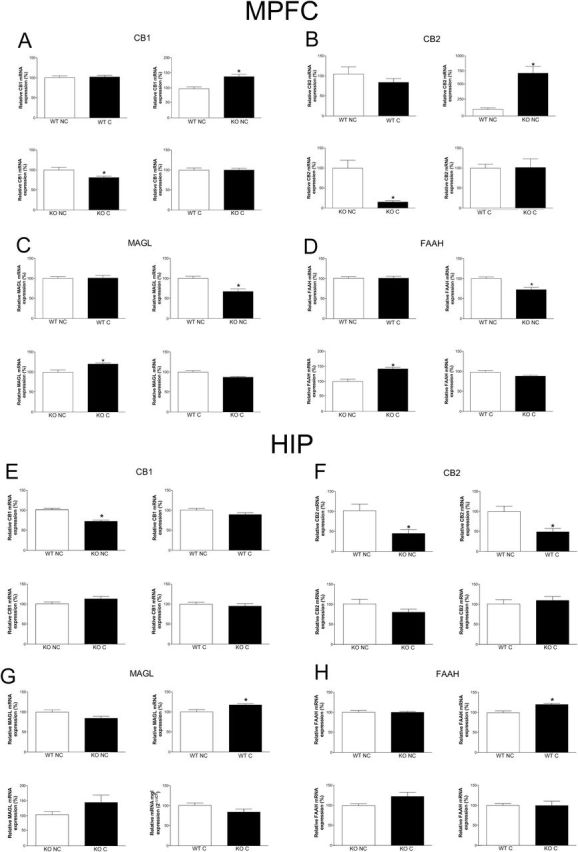
Expression of cannabinoid receptors type 1 (CB1) and 2 (CB2), monoacylglycerol lipase (MAGL), and fatty acid amide hydrolase (FAAH) mRNA in the medial prefrontal cortex (MPFC) (A-D) and hippocampus (HIP) (E-H) of wild-type (WT) and inducible nitric oxide synthase (iNOS) knockout (KO) mice. In the MPFC, KO nonconditioned (NC) presented higher CB1 (A) and CB2 (B) mRNA than WT NC, whereas conditioning decreased both CB1 and CB2 mRNA in KO mice compared with KO NC (n=5–8/group), KO NC presented lower MAGL (C) and FAAH (D) mRNA than WT NC, whereas conditioning increased both MAGL and FAAH mRNA in KO conditioned (C) compared with KO NC (n=6–8/group). Results are expressed as means±SEM. Student’s t test, *P<.05. In the HIP, KO NC presented lower CB1 (E) and CB2 (F) mRNA than WT NC, and conditioning decreased the CB2 mRNA level in WT compared with WT NC (n=6–8/group); conditioning increased MAGL (G) and FAAH (H) mRNA in WT mice compared with WT NC (n=7–8/group). Results are expressed as percentage means±SEM of control values. Student’s t test, *P<.05.
Discussion
Our results were, to the best of our knowledge, the first to show that animals with genetic iNOS delection present increased freezing behavior in the CFC model. These mice also showed increased NOS activity in the MPFC and changes in nNOS and eNOS mRNA expression. The increased freezing behavior in iNOS KO mice was attenuated by the preferential nNOS inhibitor 7-NI, which also decreased fear behavior in WT mice. In addition, inhibition of the FAAH enzyme by URB attenuated freezing behavior, whereas the higher dose of a nonselective cannabinoid agonist, Win, and a CB1 antagonist, AM281, increased this behavior. iNOS KO mice also showed changes in mRNA expression of genes associated with ECB signaling molecules. URB facilitated fear extinction in these mice, suggesting that the ECB and NO systems interact to modulate CFC.
iNOS has been related to inflammatory conditions, since different inflammatory stimuli induce its expression in several brain areas (for review, see Heneka and Feinstein, 2001), where this enzyme is highly expressed in astrocytes and microglia (for reviews, see Murphy et al., 1993; Brosnan et al., 1997; Minghetti and Levi, 1998). iNOS inhibition or its genetic deletion attenuates inflammatory conditions (Wei et al., 1995; Cuzzocrea et al., 1998; Herencia et al., 2001; Camuesco et al., 2004). More recently, it has been recognized that inflammatory insults can also induce behavioral changes that resemble psychiatric conditions such as depression (Capuron and Miller, 2011; Maes et al., 2011, 2012) and schizophrenia (Monji et al., 2009; ).
Psychological stress can also increase peripheral and brain levels of enzymatic sources of inflammatory mediators, including iNOS (Garcia-Bueno et al., 2008; Munhoz et al., 2008). Some of these mediators are important to plastic processes, such as synaptic changes in the HIP (Stellwagen and Malenka, 2006), and can influence learning, memory, and stress coping (Goshen and Yirmiya, 2009; Miller et al., 2013). In rodents, chronic psychological stressors enhance NOS activity and iNOS protein and mRNA expression in cortical neurons and increase plasmatic nitrites levels. These effects are blocked by a selective iNOS inhibitor (Olivenza et al., 2000; Peng et al., 2012). iNOS inhibitors or genetic deletion of this enzyme also attenuate behavioral consequences of chronic stress (Gilhotra and Dhingra, 2009) and induce antidepressant-like effects in mice (Montezuma et al., 2012). Corroborating the idea of iNOS involvement in stress disorders, an iNOS gene polymorphism was proposed to be a risk factor for the development of depression in humans (Galecki et al., 2010, 2011). Finally, increased NO signaling, mainly by iNOS, has been associated with PTSD (Harvey et al., 2004; ). Together, these pieces of evidence suggest that the absence of iNOS could be protective in stress- and inflammatory-related conditions, such as depression and PTSD.
Contrary to this proposition, however, iNOS KO mice show anxiogenic-like behavior in the EPM (Buskila et al., 2007), which was exacerbated 7 days later after exposing these animals to a predator odor (Abu-Ghanem et al., 2008). In both cases, a nonselective NOS inhibitor prevented the anxiogenic-like effect, suggesting that this behavioral change could be due to a compensatory NO increase from other NOS isoforms. Similar to those observations, in the present study we observed that iNOS KO mice exhibited increased freezing behavior in the CFC and that the preferential nNOS inhibitor 7-NI attenuated this behavior. Although it is not possible to completely disregard the interference of learning deficits in our results, previous study failed to find deficits in iNOS KO mice tested in the Morris water maze paradigm (Medeiros et al., 2007).
iNOS is not only expressed in the central nervous system during inflammatory conditions but is also present at basal levels in certain brain regions (Amitai, 2010) such as the HIP (Montezuma et al., 2011). Considering iNOS basal expression in the brain, the participation of NO during brain development (Contestabile, 2000), and the presence of other constitutive, calcium-dependent isoforms in the brain (nNOS and eNOS), the absence of iNOS could lead to an overcompensation by the nitrergic system to the lack of this enzyme during development, as previously discussed (Mashimo and Goyal, 1999). Supporting this hypothesis, iNOS KO mice have increased basal NOS activity in both amygdala and cortex, an effect attenuated, at least in the cortex, by systemic administration of L-NNA, an inhibitor of constitutive NOS isoforms (Buskila et al., 2007; Gilhotra and Dhingra, 2009). In the present study, in addition to increased freezing behavior, we also observed increased basal NOS activity in the MPFC of iNOS KO mice.
Even if PTSD is a complex disorder with several clinical manifestations, changes in processes related to conditioned fear, in which responses could be exaggerated and/or resistant to extinction, have been associated with the vulnerability to develop this disorder (Amstadter et al., 2009; ). Memory processing could be altered by increased stress sensitivity (Mahan and Ressler, 2012). PTSD patients present decreased neuronal activity in HIP and MPFC, 2 brain regions associated with CFC and extinction, suggesting that neurotransmitter alterations in these structures could be involved in extinction of aversive memories (Pissiota et al., 2002; Shin et al., 2004a, 2004b; Vermetten et al., 2007). A recent study found that a polymorphism in the nitric oxide synthase-1 adaptor protein (NOS1AP) gene that codifies NOS1AP, which binds to nNOS and reduces NMDA receptor signaling, was associated with increased depression severity in PTSD patients (Lawford et al., 2013). nNOS is involved in processes altered in PTSD, such as emotional responses, memory formation, and cognitive performance. nNOS KO mice are less anxious (Wultsch et al., 2007; Walton et al., 2013), present impaired cognitive performance (Kirchner et al., 2004; Weitzdoerfer et al., 2004; Wultsch et al., 2007; Walton et al., 2013), impaired short- and long-term olfactory fear conditioning memory (Pavesi et al., 2013), and attenuated contextual and cue-fear behavior (Kelley et al., 2009). Similar effects were induced by the administration of nNOS inhibitors systemically (Holscher et al., 1996; Kelley et al., 2010; Pavesi et al., 2013) or locally into brain regions associated with memory and emotional processing such as the HIP or MPFC (Resstel et al., 2008; Fabri et al., 2014). These results suggest that NO plays an important role in memory formation and the normal expression of conditioned fear. Corroborating this proposal, systemic administration of a preferential nNOS inhibitor to WT mice before the first context reexposure attenuated freezing behavior.
Considering that 7-NI is a preferential nNOS inhibitor compared with eNOS (IC50 bovine eNOS 0.7±0.2 µM, IC50 rat nNOS 0.47 µM) (Ji et al., 2009), increased nNOS activity could be responsible for the behavioral changes observed in iNOS KO mice. Nonetheless, in basal conditions, we failed to find altered mRNA expression of nNOS and eNOS in the MPFC or HIP of iNOS KO mice. However, 24 hours after the conditioning session, the mRNA expression of both isoforms increased, suggesting that both could participate in the observed effects. Corroborating the proposal that genetic alterations of the NO system could also induce overcompensation of eNOS expression/activity, O′Dell and coworkers (1994) showed that blockade of hippocampal LTP by NOS inhibitors was still present in nNOS KO mice and that eNOS was expressed in the hippocampal CA1 region of these animals. This is consistent with previous observations showing eNOS expression in pyramidal cells of this region (Dinerman et al., 1994) and its involvement in LTP (Wilson et al., 1997), supporting that, at least in LTP, eNOS could play an important role in the absence of nNOS. In addition, impairment in cognitive performance in Wistar rats is associated with increased eNOS expression in the HIP (Gokcek-Sarac et al., 2012). Although the involvement of eNOS in emotional behavior is much less investigated, anxiogenic (Frisch et al., 2000), antidepressant (Reif et al., 2004), or no effect (Demas et al., 1999; Dere et al., 2002) have already been reported.
Like NO, ECBs are also atypical neurotransmitters, being synthetized on demand on postsynaptic neuron and acting in a retrograde fashion in presynaptic terminals (Esplugues, 2002; Piomelli, 2003). Also similar to the nitrergic, the ECB system plays an important role important in emotion control (Lafenetre et al., 2007; Moreira and Wotjak, 2010) and processing of aversive memories in rodents (Lafenetre et al., 2007; Resstel et al., 2009). In the present study, administration of the anandamide metabolism inhibitor, URB, to WT mice attenuated freezing behavior and facilitated fear extinction, whereas the selective CB1 antagonist, AM281, increased freezing behavior. Even if there are contradictory results (Mikics et al., 2006), several studies show that pharmacological antagonist or genetic deletion of CB1 receptors increases freezing behavior and impairs extinction in fear conditioning models (Marsicano et al., 2002; Suzuki et al., 2004; Varvel et al., 2005; Kamprath et al., 2006; Niyuhire et al., 2007; Reich et al., 2008).
In contrast to these results, the higher dose of the nonselective cannabinoid receptor agonist Win increased freezing behavior. These latter data corroborate previous results showing that high doses of WIN can increase CFC in mice (Mikics et al., 2006) and fear induced by predator exposure in rats (Lisboa et al., 2014). WIN at lower doses usually induces anxiolytic-like effect (Viveros et al., 2005; Patel and Hillard, 2006), decreases fear induced by predator exposure (Lisboa et al., 2014), and facilitates fear extinction (Pamplona et al., 2006) in rodents, probably by activation of CB1 receptors and decreased of glutamate release (Rey et al., 2012). However, at higher doses this drug could also activate TRPV1 receptors facilitating glutamate release (Moreira et al., 2012). Therefore, similar to blockade of CB1 receptors, WIN at high doses could disinhibit glutamate release, activate NO production, and increase freezing behavior. In fact, CB1 KO mice have increased cortical NOS activity (Kim et al., 2006) and CB1 antagonist in vitro increased, whereas CB1 agonist decreased NMDA-elicited intracellular calcium in the HIP (Hampson et al., 2011).
In addition to behavioral changes, iNOS KO mice presented several alterations in mRNA expression of components of the ECB system (CB1 and CB2 receptors, FAAH and MAGL enzymes) in the HIP and MPFC. If translated into protein changes, the increased mRNA expression of MAGL and FAAH, associated with the decreased gene expression of CB1 and CB2 receptors, observed in the MPFC of KO mice after conditioning could be reflected in a decrease in ECB signaling in this region. This might facilitate NO signaling by decreasing the inhibitory effects of ECBs on glutamate release. NO signaling would be further enhanced by the increased nNOS and eNOS mRNA expression found in this region.
These results agree with the proposal that the MPFC NO system is activated during aversive memory reactivation, a moment where a local decrease in the activity of the cannabinoid system and increase in NO release would facilitate fear responses. Corroborating this proposal, administration of the FAAH inhibitor URB in iNOS KO mice prevented the increased freezing behavior observed in the vehicle-treated iNOS KO mice 96 hours after conditioning, indicating a facilitation of fear extinction. This result suggests that increasing anandamide levels could counteract increased NO signaling and deregulated ECB system in these animals. Since no pharmacological inhibition of MAGL was performed in the present study, it is not possible to exclude the involvement of 2-arachidonoylglycerol in these effects.
In conclusion, the data presented here reinforce the proposal that NO and EBCs (at least anandamide) are involved in the modulation of stress-related disorders and point to a possible deregulation of the ECB system in situations where NO signaling is increased. All together, these results suggest a potential therapeutic role for NOS inhibitors and modulators of the ECB system in stress-related disorders.
Interest Statement
None.
Acknowledgments
This work was supported by São Paulo Research Foundation FAPESP (2011/22523-0; 2012/17626-2) and National Counsel of Technological and Scientific Development (CNPq). S.F.L., F.V.G., A.L.S., S.R.L.J., F.S.G., and L.B.M.R. are recipients of a FAPESP fellowship. F.S.G. is a recipient of a CNPq fellowship. The authors thank José C. de Aguiar and Eleni T. Gomes for their excellent technical assistance.
References
- Abu-Ghanem Y, Cohen H, Buskila Y, Grauer E, Amitai Y. (2008) Enhanced stress reactivity in nitric oxide synthase type 2 mutant mice: findings in support of astrocytic nitrosative modulation of behavior. Neuroscience 156:257–265. [DOI] [PubMed] [Google Scholar]
- Aguiar DC, Guimaraes FS. (2009) Blockade of NMDA receptors and nitric oxide synthesis in the dorsolateral periaqueductal gray attenuates behavioral and cellular responses of rats exposed to a live predator. J Neurosci Res 87:2418–2429. [DOI] [PubMed] [Google Scholar]
- Amitai Y. (2010) Physiologic role for “inducible” nitric oxide synthase: a new form of astrocytic-neuronal interface. Glia 58:1775–1781. [DOI] [PubMed] [Google Scholar]
- Amstadter AB, Nugent NR, Koenen KC. (2009) Genetics of PTSD: Fear conditioning as a model for future research. Psychiatr Ann 39:358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista N, Fezza F, Finazzi-Agro A, Maccarrone M. (2006) The endocannabinoid system in neurodegeneration. Ital J Biochem 55:283–289. [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal biochem 72:248–254. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Lee SC, Liu J. (1997) Regulation of inducible nitric oxide synthase expression in human glia: implications for inflammatory central nervous system diseases. Biochem Soc Trans 25:679–683. [DOI] [PubMed] [Google Scholar]
- Buskila Y, Abu-Ghanem Y, Levi Y, Moran A, Grauer E, Amitai Y. (2007) Enhanced astrocytic nitric oxide production and neuronal modifications in the neocortex of a NOS2 mutant mouse. PloS one 2:e843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camuesco D, Comalada M, Rodriguez-Cabezas ME, Nieto A, Lorente MD, Concha A, Zarzuelo A, Galvez J. (2004) The intestinal anti-inflammatory effect of quercitrin is associated with an inhibition in iNOS expression. Br J Pharmacol 143:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Miller AH. (2011) Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130:226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contestabile A. (2000) Roles of NMDA receptor activity and nitric oxide production in brain development. Brain Res Brain Res Rev 32:476–509. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Zingarelli B, Hake P, Salzman AL, Szabo C. (1998) Antiinflammatory effects of mercaptoethylguanidine, a combined inhibitor of nitric oxide synthase and peroxynitrite scavenger, in carrageenan-induced models of inflammation. Free Radic Biol Med 24:450–459. [DOI] [PubMed] [Google Scholar]
- Demas GE, Kriegsfeld LJ, Blackshaw S, Huang P, Gammie SC, Nelson RJ, Snyder SH. (1999) Elimination of aggressive behavior in male mice lacking endothelial nitric oxide synthase. J Neurosci 19:RC30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. (2004) The endocannabinoid system: a general view and latest additions. Br J Pharmacol 141:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, De Souza Silva MA, Topic B, Fiorillo C, Li JS, Sadile AG, Frisch C, Huston JP. (2002) Aged endothelial nitric oxide synthase knockout mice exhibit higher mortality concomitant with impaired open-field habituation and alterations in forebrain neurotransmitter levels. Genes Brain Behav 1:204–213. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. (2007) Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol 18:129–140. [DOI] [PubMed] [Google Scholar]
- Dinerman JL, Dawson TM, Schell MJ, Snowman A, Snyder SH. (1994) Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci U S A 91:4214–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues JV. (2002) NO as a signalling molecule in the nervous system. Br J Pharmacol 135:1079–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabri DR, Hott SC, Reis DG, Biojone C, Correa FM, Resstel LB. (2014) The expression of contextual fear conditioning involves activation of a NMDA receptor-nitric oxide-cGMP pathway in the dorsal hippocampus of rats. Eur Neuropsychopharmacol 24:1676–1686. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Romero J, Velasco G, Tolon RM, Ramos JA, Guzman M. (2007) Cannabinoid CB2 receptor: a new target for controlling neural cell survival? Trends Pharmacol Sci 28:39–45. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Pazos MR, Garcia-Arencibia M, Sagredo O, Ramos JA. (2008) Role of CB2 receptors in neuroprotective effects of cannabinoids. Mol Cell Endocrinol 286:S91–96. [DOI] [PubMed] [Google Scholar]
- Forestiero D, Manfrim CM, Guimaraes FS, de Oliveira RM. (2006) Anxiolytic-like effects induced by nitric oxide synthase inhibitors microinjected into the medial amygdala of rats. Psychopharmacology (Berl) 184:166–172. [DOI] [PubMed] [Google Scholar]
- Frisch C, Dere E, Silva MA, Godecke A, Schrader J, Huston JP. (2000) Superior water maze performance and increase in fear-related behavior in the endothelial nitric oxide synthase-deficient mouse together with monoamine changes in cerebellum and ventral striatum. J Neurosci 20:6694–6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galecki P, Maes M, Florkowski A, Lewinski A, Galecka E, Bienkiewicz M, Szemraj J. (2010) An inducible nitric oxide synthase polymorphism is associated with the risk of recurrent depressive disorder. Neurosci Lett 486:184–187. [DOI] [PubMed] [Google Scholar]
- Galecki P, Maes M, Florkowski A, Lewinski A, Galecka E, Bienkiewicz M, Szemraj J. (2011) Association between inducible and neuronal nitric oxide synthase polymorphisms and recurrent depressive disorder. J Affect Disord 129:175–182. [DOI] [PubMed] [Google Scholar]
- Garcia-Bueno B, Caso JR, Leza JC. (2008) Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 32:1136–1151. [DOI] [PubMed] [Google Scholar]
- Gilhotra N, Dhingra D. (2009) Involvement of NO-cGMP pathway in anti-anxiety effect of aminoguanidine in stressed mice. Prog Neuropsychopharmacol Biol Psych 33:1502–1507. [DOI] [PubMed] [Google Scholar]
- Gokcek-Sarac C, Karakurt S, Adali O, Jakubowska-Dogru E. (2012) Correlation between hippocampal levels of neural, epithelial and inducible NOS and spatial learning skills in rats. Behav Brain Res 235:326–333. [DOI] [PubMed] [Google Scholar]
- Gomes FV, Casarotto PC, Resstel LB, Guimaraes FS. (2011) Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Prog Neuropsychopharmacol Biol Psych 35:434–438. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. (2009) Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol 30:30–45. [DOI] [PubMed] [Google Scholar]
- Guimaraes FS, Beijamini V, Moreira FA, Aguiar DC, de Lucca AC. (2005) Role of nitric oxide in brain regions related to defensive reactions. Neurosci Biobehav Rev 29:1313–1322. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Miller F, Palchik G, Deadwyler SA. (2011) Cannabinoid receptor activation modifies NMDA receptor mediated release of intracellular calcium: implications for endocannabinoid control of hippocampal neural plasticity. Neuropharmacology 60:944–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey BH, Oosthuizen F, Brand L, Wegener G, Stein DJ. (2004) Stress-restress evokes sustained iNOS activity and altered GABA levels and NMDA receptors in rat hippocampus. Psychopharmacology (Berl) 175:494–502. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Feinstein DL. (2001) Expression and function of inducible nitric oxide synthase in neurons. J Neuroimmunol 114:8–18. [DOI] [PubMed] [Google Scholar]
- Herencia F, Ferrandiz ML, Ubeda A, Guillen I, Dominguez JN, Charris JE, Lobo GM, Alcaraz MJ. (2001) 4-dimethylamino-3’,4’-dimethoxychalcone downregulates iNOS expression and exerts anti-inflammatory effects. Free Radic Biol Med 30:43–50. [DOI] [PubMed] [Google Scholar]
- Holscher C, McGlinchey L, Anwyl R, Rowan MJ. (1996) 7-Nitro indazole, a selective neuronal nitric oxide synthase inhibitor in vivo, impairs spatial learning in the rat. Learn Mem 2:267–278. [DOI] [PubMed] [Google Scholar]
- Ji H, Tan S, Igarashi J, Li H, Derrick M, Martasek P, Roman LJ, Vasquez-Vivar J, Poulos TL, Silverman RB. (2009) Selective neuronal nitric oxide synthase inhibitors and the prevention of cerebral palsy. Ann Neurol 65:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joca SR, Guimaraes FS. (2006) Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl) 185:298–305. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. (2010) Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 27:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. (2006) Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci 26:6677–6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. (2010) Pharmacological modulators of nitric oxide signaling and contextual fear conditioning in mice. Psychopharmacology (Berl) 210:65–74. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Balda MA, Anderson KL, Itzhak Y. (2009) Impairments in fear conditioning in mice lacking the nNOS gene. Learn Mem 16:371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Ledent C, Jin K, Greenberg DA. (2006) Role for neuronal nitric-oxide synthase in cannabinoid–induced neurogenesis. J Pharmacol Exp Ther 319:150–154. [DOI] [PubMed] [Google Scholar]
- Kirchner L, Weitzdoerfer R, Hoeger H, Url A, Schmidt P, Engelmann M, Villar SR, Fountoulakis M, Lubec G, Lubec B. (2004) Impaired cognitive performance in neuronal nitric oxide synthase knockout mice is associated with hippocampal protein derangements. Nitric oxide 11:316–330. [DOI] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. (2007) The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res 56:367–381. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Morris CP, Swagell CD, Hughes IP, Young RM, Voisey J. (2013) NOS1AP is associated with increased severity of PTSD and depression in untreated combat veterans. J Affect Disord 147:87–93. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Cascio MG, Di Marzo V. (2005) Endocannabinoid metabolic pathways and enzymes. Curr Drug Targets CNS Neurol Disord 4:615–623. [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Guimaraães FS, Resstel LBM. (2011) Anxiety-behavior modulated by ventral medial prefrontal cortex of rats submitted to the vogel conflict test involves a local NMDA receptor and nitric oxide. J Behav Brain Sci 1:181–187. [Google Scholar]
- Lisboa SF, Guimaraes FS. (2012) Differential role of CB1 and TRPV1 receptors on anandamide modulation of defensive responses induced by nitric oxide in the dorsolateral periaqueductal gray. Neuropharmacology 62:2455–2462. [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Magesto AC, Aguiar JC, Resstel LB, Guimaraes FS. (2013) Complex interaction between anandamide and the nitrergic system in the dorsolateral periaqueductal gray to modulate anxiety-like behavior in rats. Neuropharmacology 75C:86–94. [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Camargo LH, Magesto AC, Resstel LB, Guimaraes FS. (2014) Cannabinoid modulation of predator fear: involvement of the dorsolateral periaqueductal gray. Int J Neuropsychopharmacol 17:1193–1206. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Battista N, Centonze D. (2007) The endocannabinoid pathway in Huntington’s disease: a comparison with other neurodegenerative diseases. Prog Neurobiol 81:349–379. [DOI] [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. (2011) The new ‘5-HT’ hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Prog Neuropsychopharmacol Biol Psych 35:702–721. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Ringel K. (2012) Activation of cell–mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog Neuropsychopharmacol Biol Psych 36:169–175. [DOI] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. (2012) Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci 35:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. (1998) Effects of 7-nitroindazole, a neuronal nitric oxide synthase (nNOS) inhibitor, on locomotor activity and contextual fear conditioning in rats. Brain Res 804:155–158. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418:530–534. [DOI] [PubMed] [Google Scholar]
- Mashimo H, Goyal RK. (1999) Lessons from genetically engineered animal models. IV. Nitric oxide synthase gene knockout mice. Am J Physiol 277:G745–750. [DOI] [PubMed] [Google Scholar]
- Medeiros R, Prediger RD, Passos GF, Pandolfo P, Duarte FS, Franco JL, Dafre AL, Di Giunta G, Figueiredo CP, Takahashi RN, Campos MM, Calixto JB. (2007) Connecting TNF-alpha signaling pathways to iNOS expression in a mouse model of Alzheimer’s disease: relevance for the behavioral and synaptic deficits induced by amyloid beta protein. J Neurosci 27:5394–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Haroon E, Raison CL, Felger JC. (2013) Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety 30:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghetti L, Levi G. (1998) Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog Neurobiol 54:99–125. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. (1993) Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem 60:1650–1657. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S. (2009) Cytokines and schizophrenia: microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci 63:257–265. [DOI] [PubMed] [Google Scholar]
- Montezuma K, Biojone C, Lisboa SF, Cunha FQ, Guimaraes FS, Joca SR. (2011) Inhibition of iNOS induces antidepressant-like effects in mice: Pharmacological and genetic evidence. Neuropharmacology. Neuropharmacol 62:485–491. [DOI] [PubMed] [Google Scholar]
- Moraes-Neto TB, Scopinho AA, Biojone C, Correa FM, Resstel LB. (2014) Involvement of dorsal hippocampus glutamatergic and nitrergic neurotransmission in autonomic responses evoked by acute restraint stress in rats. Neuroscience 258:364–373. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Wotjak CT. (2010) Cannabinoids and anxiety. Curr Top Behav Neurosci 2:429–450. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Terzian AL, Guimaraes FS, Wotjak CT. (2012) Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 204:186–192. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Garcia-Bueno B, Madrigal JL, Lepsch LB, Scavone C, Leza JC. (2008) Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Braz J Med Biol Res 41:1037–1046. [DOI] [PubMed] [Google Scholar]
- Murphy S, Simmons ML, Agullo L, Garcia A, Feinstein DL, Galea E, Reis DJ, Minc-Golomb D, Schwartz JP. (1993) Synthesis of nitric oxide in CNS glial cells. Trends Neurosci 16:323–328. [DOI] [PubMed] [Google Scholar]
- Musazzi L, Racagni G, Popoli M. (2011) Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int 59:138–149. [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH. (2007) The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 191:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, Fishman MC. (1994) Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science 265:542–546. [DOI] [PubMed] [Google Scholar]
- Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, Bosca L, Leza JC. (2000) Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochemistry 74:785–791. [DOI] [PubMed] [Google Scholar]
- Oosthuizen F, Wegener G, Harvey BH. (2005) Nitric oxide as inflammatory mediator in post-traumatic stress disorder (PTSD): evidence from an animal model. Neuropsychiatr Disease Treatment 1:109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. (2006) The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 188:641–649. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. (2006) Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318:304–311. [DOI] [PubMed] [Google Scholar]
- Pavesi E, Heldt SA, Fletcher ML. (2013) Neuronal nitric-oxide synthase deficiency impairs the long-term memory of olfactory fear learning and increases odor generalization. Learn Mem 20:482–490. [DOI] [PubMed] [Google Scholar]
- Peng YL, Liu YN, Liu L, Wang X, Jiang CL, Wang YX. (2012) Inducible nitric oxide synthase is involved in the modulation of depressive behaviors induced by unpredictable chronic mild stress. J Neuroinflammation 9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. (2003) The molecular logic of endocannabinoid signalling. Nature reviews Neuroscience 4:873–884. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H, Fredrikson M. (2002) Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci 252:68–75. [DOI] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. (2008) Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J Psychopharmacol 22:769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif A, Schmitt A, Fritzen S, Chourbaji S, Bartsch C, Urani A, Wycislo M, Mossner R, Sommer C, Gass P, Lesch KP. (2004) Differential effect of endothelial nitric oxide synthase (NOS-III) on the regulation of adult neurogenesis and behaviour. Eur J Neurosci 20:885–895. [DOI] [PubMed] [Google Scholar]
- Resstel LB, Correa FM, Guimaraes FS. (2008) The expression of contextual fear conditioning involves activation of an NMDA receptor-nitric oxide pathway in the medial prefrontal cortex. Cereb Cortex 18:2027–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Moreira FA, Guimaraes FS. (2009) Endocannabinoid system and fear conditioning. Vitam Horm 81:421–440. [DOI] [PubMed] [Google Scholar]
- Rey AA, Purrio M, Viveros MP, Lutz B. (2012) Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37:2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaza Bermudo-Soriano C, Perez-Rodriguez MM, Vaquero-Lorenzo C, Baca-Garcia E. (2012) New perspectives in glutamate and anxiety. Pharmacol Biochem Behav 100:752–774. [DOI] [PubMed] [Google Scholar]
- Rutkowska M, Jamontt J, Gliniak H. (2006) Effects of cannabinoids on the anxiety-like response in mice. Pharmacol Rep 58:200–206. [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK.(2004a) Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry 61:168–176. [DOI] [PubMed] [Google Scholar]
- Shin LM, Shin PS, Heckers S, Krangel TS, Macklin ML, Orr SP, Lasko N, Segal E, Makris N, Richert K, Levering J, Schacter DL, Alpert NM, Fischman AJ, Pitman RK, Rauch SL.(2004b) Hippocampal function in posttraumatic stress disorder. Hippocampus 14:292–300. [DOI] [PubMed] [Google Scholar]
- Smythies J. (2013) Schizophrenia: one coat of many colors. Front Psychiatry 4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidorio PC, Echeverry MB, Iyomasa M, Guimaraes FS, Del Bel EA. (2007) Anxiolytic effects induced by inhibition of the nitric oxide–cGMP pathway in the rat dorsal hippocampus. Psychopharmacology (Berl) 195:183–192. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. (2006) Synaptic scaling mediated by glial TNF–alpha. Nature 440:1054–1059. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24:4787–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetto LL, Terzian AL, Del Bel EA, Guimaraes FS, Resstel LB. (2009) Inhibition of the NMDA receptor/Nitric Oxide pathway in the dorsolateral periaqueductal gray causes anxiolytic-like effects in rats submitted to the Vogel conflict test. Behav Brain Funct 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. (2005) Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology (Berl) 179:863–872. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Schmahl C, Southwick SM, Bremner JD. (2007) Positron tomographic emission study of olfactory induced emotional recall in veterans with and without combat-related posttraumatic stress disorder. Psychopharmacol Bull 40:8–30. [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. (2005) Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81:331–342. [DOI] [PubMed] [Google Scholar]
- Walton JC, Selvakumar B, Weil ZM, Snyder SH, Nelson RJ. (2013) Neuronal nitric oxide synthase and NADPH oxidase interact to affect cognitive, affective, and social behaviors in mice. Behav Brain Res 256:320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, Xu D, Muller W, Moncada S, Liew FY. (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375:408–411. [DOI] [PubMed] [Google Scholar]
- Weitzdoerfer R, Hoeger H, Engidawork E, Engelmann M, Singewald N, Lubec G, Lubec B. (2004) Neuronal nitric oxide synthase knock-out mice show impaired cognitive performance. Nitric oxide 10:130–140. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Yanovsky J, Godecke A, Stevens DR, Schrader J, Haas HL. (1997) Endothelial nitric oxide synthase and LTP. Nature 386:338. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. (2002) Endocannabinoid signaling in the brain. Science 296:678–682. [DOI] [PubMed] [Google Scholar]
- Wultsch T, Chourbaji S, Fritzen S, Kittel S, Grunblatt E, Gerlach M, Gutknecht L, Chizat F, Golfier G, Schmitt A, Gass P, Lesch KP, Reif A. (2007) Behavioural and expressional phenotyping of nitric oxide synthase-I knockdown animals. J Neural Transm Suppl 69–85. [DOI] [PubMed] [Google Scholar]