Abstract
Background:
Atypical antipsychotic augmentation was demonstrated to be efficacious in treatment-resistant depression (TRD) in previous meta-analyses. We investigate whether there are differences in the effect size of atypical antipsychotic augmentation in major depressive disorder according to the degree of treatment resistance.
Methods:
A comprehensive search of four databases identified 11 randomized controlled trials. The 11 trials, which included 3 341 participants, were pooled using a random-effects meta-analysis.
Results:
Atypical antipsychotic augmentation of antidepressant therapy showed superior efficacy compared to antidepressant monotherapy in TRD in terms of both response and remission rates (response, risk ratio [RR] = 1.38, 95% confidence interval [CI] = 1.25 to 1.53; remission, RR = 1.62, 95% CI = 1.42 to 1.85). In addition, regarding response rates in the TRD trials, atypical antipsychotic augmentation exhibited significantly different effect sizes according to the degree of treatment resistance (TRD 1: RR = 1.24; TRD 2: RR = 1.37; TRD 2–4: RR = 1.58). In non-TRD trials, atypical antipsychotic augmentation failed to show superior efficacy over antidepressant monotherapy in terms of remission rates (RR = 0.89; 95% CI = 0.69 to 1.14). Atypical antipsychotic augmentation of antidepressant therapy exhibits greater effect size in patients with a higher degree of treatment resistance.
Conclusions:
This finding strengthens the rationale for considering atypical antipsychotic augmentation among depressed patients with multiple previous treatment failures in clinical practice. The efficacy of atypical antipsychotic augmentation for non-TRD seems to be different from that for TRD and, thus, further studies of non-TRD populations are needed.
Keywords: atypical antipsychotics, augmentation, major depressive disorder, treatment resistance
Introduction
Since the introduction of newer antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors, there have been great advances in treatments for major depressive disorder (MDD; Garnock-Jones and McCormack, 2010; Nichols et al., 2010; Choi et al. 2012; Rocha et al., 2012; Citrome, 2013). However, many patients with MDD have shown inadequate responses to standard antidepressant therapy (Rush et al., 2006; Nelson and Papakostas, 2009; Vieta and Colom, 2011; Turner et al., 2014). As a result, many researchers have sought to develop better treatment strategies for treatment-resistant MDD (Bauer et al., 2014; Carvalho et al., 2014; Fond et al., 2014; McIntyre et al., 2014; McNamara et al., 2014); one such is augmentation treatment with atypical antipsychotic agents (Nelson and Papakostas, 2009). Previous articles include three meta-analyses (Papakostas et al., 2007; Nelson and Papakostas, 2009; Spielmans et al., 2013), one Cochrane review (Komossa et al., 2010), and several other review articles (Chen et al., 2011; Kato and Chang, 2013; Wright et al., 2013) that have demonstrated the efficacy of atypical antipsychotic augmentation among patients with MDD.
Previous meta-analyses found that different atypical antipsychotics had no significant differences in efficacy. It was also found that the duration of augmentation treatment did not significantly affect the pooled efficacy results (Papakostas et al., 2007; Nelson and Papakostas, 2009). However, the impact of variables such as the baseline severity of depression or the degree of treatment resistance on the effects of atypical antipsychotic augmentation in patients with MDD remains unknown.
In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial, patients who were more treatment resistant showed lower response and remission rates when treated with standard pharmacotherapy, suggesting that patients who required more treatment steps differed from those who required fewer steps in terms of treatment response to pharmacotherapy (Rush et al., 2006). Even though atypical antipsychotic augmentation was demonstrated to be efficacious for treatment-resistant depression (TRD), there has been no systematic review or meta-analysis so far that investigated whether the effect size of atypical antipsychotic augmentation differs according to the degree of treatment resistance or TRD stage.
The primary objective of this meta-analysis is to investigate whether there is a difference in the effect sizes of atypical antipsychotic augmentation according to the degree of treatment resistance, defined as the number of treatment failures during the index episode among TRD patients. The secondary objective is to determine whether there is a difference in the effect sizes of atypical antipsychotic augmentation between non-TRD and TRD populations. We hypothesized that atypical antipsychotic augmentation in TRD would have greater effect sizes for higher degrees of treatment resistance. We also hypothesized that atypical antipsychotic augmentation would show a difference in effect size between TRD and non-TRD populations.
Methods
We performed a comprehensive literature search using multiple databases investigating the efficacy of atypical antipsychotic augmentation added to standard antidepressant therapy among patients with MDD who are either treatment resistant or not. We conducted this meta-analysis using the Cochrane review methods (Higgins and Green, 2008).
Data Source and Literature Source
We searched the Cochrane Library, EMBASE, MEDLINE, and KoreaMed on May 30, 2014. There was no restriction on language in our literature search. The following MeSH terms and keywords were used for the search through MEDLINE: atypical antipsychotic agent, neuroleptic, antipsychotic, aripiprazole, asenapine, clozapine, olanzapine, quetiapine, risperidone, sulpiride, ziprasidone, zotepine, blonanserin, iloperidone, lurasidone, paliperidone, major depression, and depression. Please see Supplementary Table S1 for the full search formula. For the other databases, we employed the same search strategies. After the electronic search, we also hand-searched the reference lists of identified articles.
Study Selection
Two reviewers (Drs Wang and Bahk) decided independently whether the identified studies met the selection criteria. The assessment of study inclusion was made with two levels of screening. We screened the titles and abstracts at the first level, and then we screened the full text at the second level. Studies were included in our meta-analysis if they: (1) were double-blind, placebo-controlled, randomized, acute-phase trials investigating the efficacy of atypical antipsychotic augmentation; (2) included subjects with non-psychotic MDD, regardless of the degree of treatment resistance; and (3) were published in a peer-reviewed journal. There were no restrictions regarding the dosage or the duration of atypical antipsychotic augmentation. The studies were excluded if: (1) they included subjects who had other comorbid major psychiatric disorders, other anxiety or somatic symptoms, or other comorbid medical diseases; (2) in a treatment arm, subjects were given both placebo and atypical antipsychotics (for example, during the first part of a double-blind phase, subjects were given a placebo, and then during the second part, they were given a low-dose atypical antipsychotic agent); or (3) they investigated the efficacy of atypical antipsychotic augmentation for relapse prevention.
Data Extraction
Two reviewers (Drs Wang and Bahk) extracted data independently from each study based on a predefined data extraction form. Any disagreements were resolved by discussion between the two reviewers. The following data were extracted from each study: (1) remission rate, with remission defined in each trial by either a Montgomery-Asberg Depression Rating Scale (MADRS; Montgomery and Asberg, 1979) score ≤8, 10, or 12 or a Hamilton Rating Scale for Depression (HAMD; Hamilton, 1960) score ≤7; (2) response rate, with response defined as a ≥50% reduction of baseline MADRS or HAMD scores; (3) type of atypical antipsychotic agent; (4) medication dose; (5) duration of augmentation treatment with atypical antipsychotics; (6) degree of treatment resistance (defined as the number of failed attempts to show adequate response to standard antidepressant therapy during the current index episode, not including any previous episodes); and (7) other demographic variables, including participant age and sex.
Assessment of Methodological Quality
The two reviewers (Drs Wang and Bahk) assessed the quality of each included study based on their risk of bias as defined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2008). Any disagreements between the two reviewers were resolved with discussion.
We did not assess publication bias because our analysis only included nine TRD trials and two non-TRD trials.
Statistical Analysis
The primary outcome variables of our meta-analysis were response rate and remission rate in patients with MDD treated with atypical antipsychotics in addition to standard antidepressant therapy. Response rate was defined by at least a 50% reduction of MADRS or HAMD scores from baseline. Remission rate was defined as either MADRS ≤8, 10, or 12, or HAMD ≤7 at the endpoint, and we used the authors’ definition of remission from their original articles. If possible, the data from the intention-to-treat sample in each trial were used for analysis. For dichotomous outcomes in response rate and remission rate, results were expressed as RR with their 95% CIs.
For heterogeneity estimation, we used the I2 statistic. The meanings of the I2 values are as follows: 25% for low, 50% for moderate, and 75% for high heterogeneity. Considering the heterogeneity of the population characteristics in each trial and the use of various types of atypical antipsychotics, we used a random effects model.
We conducted subgroup analyses according to the degree of treatment resistance, defined as the number of failed trials during the current index episode. To investigate whether different definitions of remission could affect the outcome of our meta-analysis, we conducted a sensitivity analysis comparing subgroups with different definitions of remission. For these analyses, we used RevMan version 5.2.
To investigate whether there are significant differences in effect sizes according to the degree of treatment resistance within TRD, we conducted a meta-regression for response and remission efficacy data using STATA.
Results
Identification of Studies
Searches of the databases identified 6 007 articles for possible inclusion (Figure 1). Of these, 5 063 publications were excluded because it was clear from the title and abstract that they did not meet the selection criteria. For the remaining 44 articles, we obtained full manuscripts, and following scrutiny of these, we excluded 34 publications because nine were open-label studies, six were post hoc analysis studies, five were poster abstracts, five were review articles, and four used a previous history of treatment failure and were not confined to the current index episodes. Two trials were excluded from this meta-analysis because in one treatment arm, participants were given a placebo for the first 30 days prior to switching to aripiprazole for the last 30 days; thus, both the placebo and the active drug were administered in the same treatment arm during double-blind phases. We excluded three further studies for other reasons. Therefore, the total number of articles included in the review was 10 (Figure 1). Among these 10 studies, two trials identical in study design were included in Thase et al.’s study (2007), and thus, a total of 11 trials were included in this meta-analysis.
Figure 1.
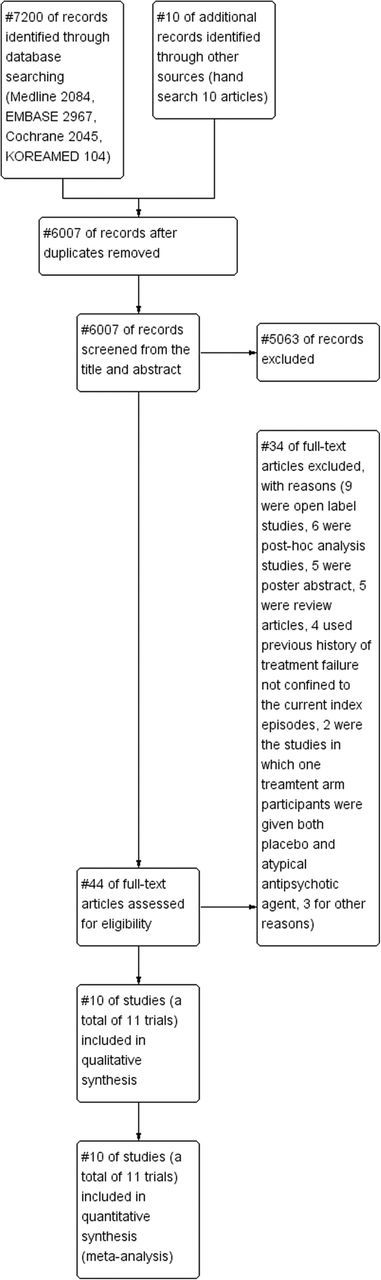
PRISMA
We classified the 11 trials into four groups according to the degree of treatment resistance, defined by the number of trials with failure to show adequate response to standard antidepressant therapy within the current index depressive episode: we defined non-TRD as no history of treatment failure to standard antidepressant therapy (SAT) within the current depressive episode (Garakani et al., 2008; Lin et al., 2011); TRD 1 as a history of one treatment failure to SAT within the current depressive episode (Mahmoud et al., 2007; Bauer et al., 2009; El-Khalili et al., 2010); TRD 2 as a history of two treatment failures to SAT within the current depressive episode (Thase et al., 2007); and TRD 2–4 as a history of two to four treatment failures to SAT within the current depressive episode (Berman et al., 2007, 2009; Marcus et al., 2008; Kamijima et al., 2013). There were no significant differences in the SAT used in the included studies (see Supplementary Table S2 to identify control medications used in each study). All four of the TRD 2–4 trials were aripiprazole trials, in which subjects with a history of one to three treatment failures within the index episode were included, and those who failed to respond to prospective antidepressant therapy again were randomized to either atypical antipsychotics or placebo added to ongoing antidepressants. None of the TRD 2–4 trials provided efficacy results separately for those with two, three, or four treatment failures. In all four TRD 2–4 trials, the proportion of subjects with two treatment failures was the highest, followed by those with three, and then four, treatment failures.
The data of all 11 trials were included in the remission analysis. However, two non-TRD trials were excluded from the response analysis because only one of them (Lin et al., 2011) provided the data for responses, and we thought it was meaningless to show the response rate results from only one non-TRD trial. Thus, a total of nine TRD trials were included in the analysis of response rates to determine whether there was a difference in the effect sizes for response rate according to the degree of treatment resistance.
Study Characteristics and Patient Populations
The 11 trials included one trial with risperidone (Mahmoud et al., 2007), three with quetiapine (Garakani et al., 2008; Bauer et al., 2009; El-Khalili et al., 2010), two with an olanzapine-fluoxetine combination (Thase et al., 2007), and five with aripiprazole (Berman et al., 2007, 2009; Marcus et al., 2008; Lin et al., 2011; Kamijima et al., 2013). Two trials investigated the efficacy of atypical antipsychotics in the non-TRD population (Garakani et al., 2008; Lin et al., 2011), and the remaining nine trials did so in the TRD population. In the 11 trials, data from a total of 3 341 participants were included for the remission analysis, and among them, 1 931 were given atypical antipsychotics and the other 1 410 were given placebos. In nine trials, data from a total of 3 239 participants were included for the response analysis; among them, 1 882 were given atypical antipsychotics and the other 1 357 were given placebo. The characteristics of the included 11 trials are summarized in Table 1.
Table 1.
Characteristics of the Trials Included in this Meta-Analysis
| Study | TRD stage | Atypical antipsychotics | Dosage of atypical antipsychotics (mg) | Total number of failed antidepressant trials during current episode before AAP augmentation | Number of historical failure | Presence of prospective antidepressant trial | Duration of prospective antidepressant trial, if any (weeks) | Duration of double- blind phase (weeks) |
|---|---|---|---|---|---|---|---|---|
| Garakani et al., 2008 | Non-TRD | quetiapine | 25–100 | 0 | 0 | no | n/a | 8 |
| Lin et al., 2011 | Non-TRD | aripiprazole | 2.5 | 0 | 0 | no | n/a | 4 (a priori 10 weeks) |
| Mahmoud et al., 2007 | TRD 1 | risperidone | 1 until week 4, 1 or 2 since after | 1 | 1 | yes | 4 | 6 |
| Bauer et al., 2009 | TRD 1 | quetiapine fumarate extended-release | 150, 300 | 1 | 1 | no | n/a | 6 |
| El-Khalili et al., 2010 | TRD 1 | quetiapine fumarate extended-release | 150, 300 | 1 | 1 | no | n/a | 6 |
| Thase et al., 2007 (1) | TRD 2 | OFC | OFC (O 6–18, F 50–100) | 2 | 1 | yes | 8 | 8 |
| Thase et al., 2007 (2) | TRD 2 | OFC | OFC (O 6–18, F 50–100) | 2 | 1 | yes | 8 | 8 |
| Kamijima et al., 2013 | TRD 2–4 | aripiprazole | 3, 3–15 | 2–4 | 1–3 | yes | 8 | 6 |
| Berman et al., 2007 | TRD 2–4 | aripiprazole | 2–20 | 2–4 | 1–3 | yes | 8 | 6 |
| Marcus et al., 2008 | TRD 2–4 | aripiprazole | 2–20 | 2–4 | 1–3 | yes | 8 | 6 |
| Berman et al., 2009 | TRD 2–4 | aripiprazole | 2–20 | 2–4 | 1–3 | yes | 8 | 6 |
AAP, atypical antipsychotic agent; non-TRD, non-treatment resistant depression; OFC, olanzapine-fluoxetine combination; SAT, standard antidepressant therapy; TRD, treatment resistant depression; TRD 1, defined by no history of treatment failure to SAT within the current depressive episode; TRD 2, defined by history of two treatment failures to SAT within the current depressive episode; TRD 2–4, defined by history of two to four treatment failures to SAT within the current depressive episode.
Quality of the Included Studies
We assessed the quality of the included studies using the risk of bias. The details regarding random sequence generation and blinding were rarely reported. Thus, it is unclear whether blinding and random sequence generation was actually appropriately conducted. Overall, we consider the quality of the included studies to be unclear. The results are summarized in a risk of bias graph (Figure 2).
Figure 2.
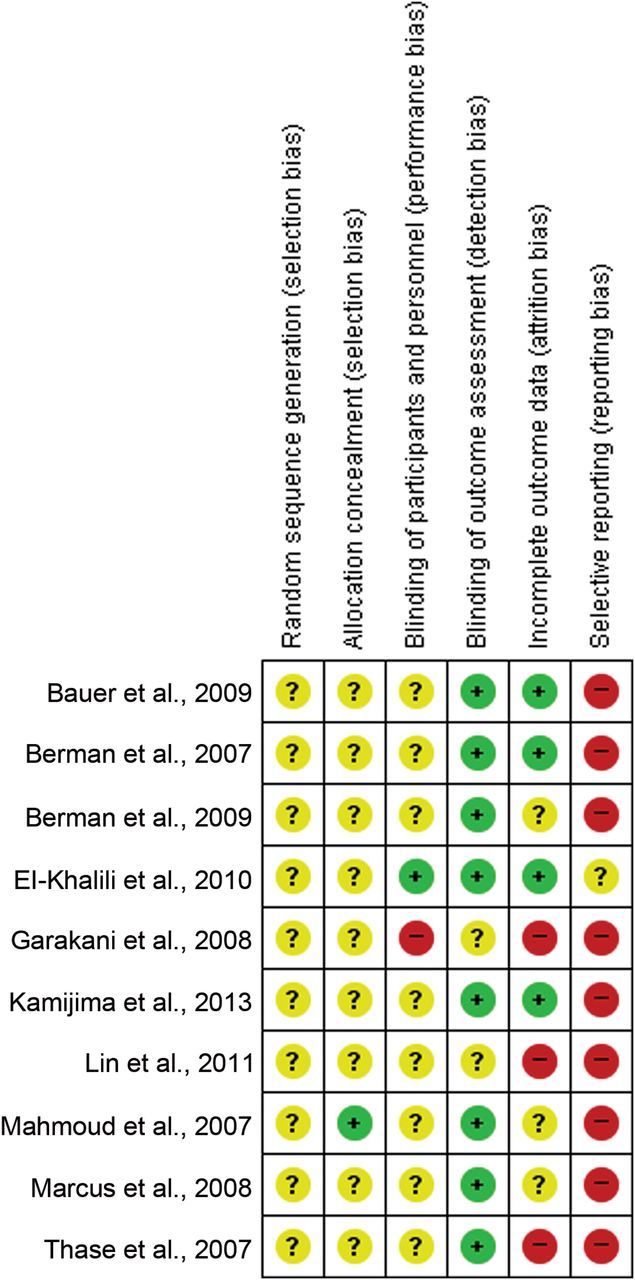
Risk of Bias Graph of Included Studies. The colors represent the quality of the studies included in this meta-analysis: red for high risk, yellow for uncertain, and green for low risk. The overall risk is unclear.
Response
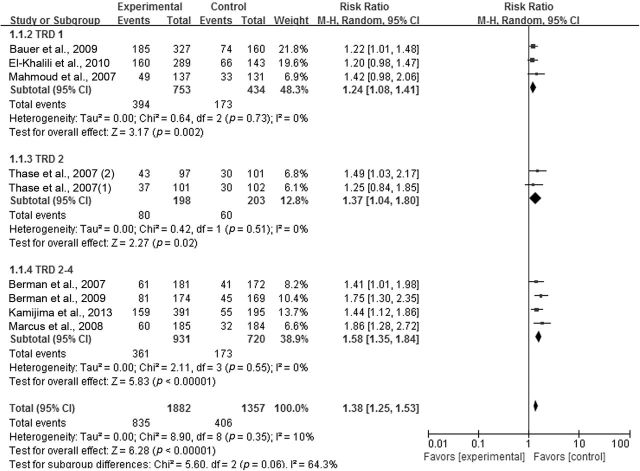
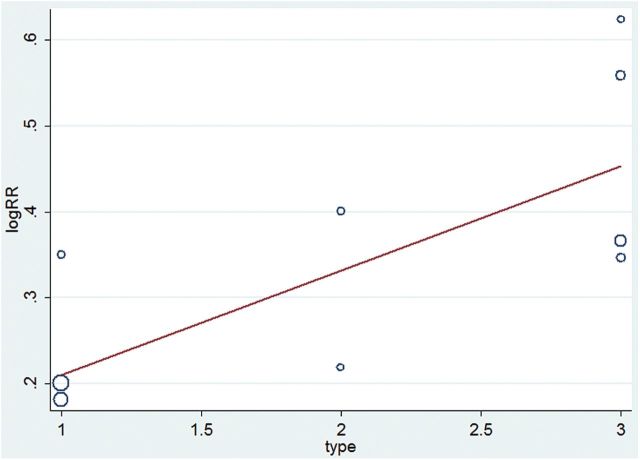
We present the results of the meta-analysis regarding response rate as a forest plot (Figure 3). The pooled risk ratio for atypical antipsychotic augmentation versus antidepressant monotherapy in total TRD trials (including the TRD 1, 2, and 2–4 groups) was 1.38 (95% CI = 1.25 to 1.53), confirming the superior efficacy of atypical antipsychotic augmentation in TRD. The risk ratios for atypical antipsychotic augmentation versus antidepressant monotherapy according to the degree of treatment resistance were 1.24 (95% CI = 1.08 to 1.41) for TRD 1; 1.37 (95% CI = 1.04 to 1.80) for TRD 2; and 1.58 (95% CI = 1.35 to 1.84) for TRD 2–4. The test for subgroup differences showed moderate heterogeneity between TRD groups (TRD 1 vs. TRD 2 vs. TRD 2–4; Chi2 = 5.6, I2 = 64.3%). The meta-regression analysis showed that there were statistically significant differences in effect sizes according to the degree of treatment resistance, suggesting that the higher the resistance to treatment, the greater the effect size is (Z = 0.121257, p = 0.050; Figure 4).
Figure 3.
Relative risk ratios of response rates for atypical antipsychotic augmentation in treatment-resistant depression. CI, confidence interval; M-H, Mantel Haenszel method; SAT, standard antidepressant therapy; TRD, treatment resistant depression; TRD 1, defined by no history of treatment failure to SAT within the current depressive episode; TRD 2, defined by history of two treatment failures to SAT within the current depressive episode; TRD 2–4, defined by history of two to four treatment failures to SAT within the current depressive episode.
Figure 4.
Meta-regression analysis of response rate in treatment-resistant depression. Type 1 indicates the TRD 1 group, 2 indicates the TRD 2 group, and 3 indicates the TRD 2–4 group. The coefficient is 0.121257, and the p value is 0.050. RR, risk ratio; SAT, standard antidepressant therapy; TRD, treatment resistant depression; TRD 1, defined by no history of treatment failure to SAT within the current depressive episode; TRD 2, defined by history of two treatment failures to SAT within the current depressive episode; TRD 2–4, defined by history of two to four treatment failures to SAT within the current depressive episode.
Remission
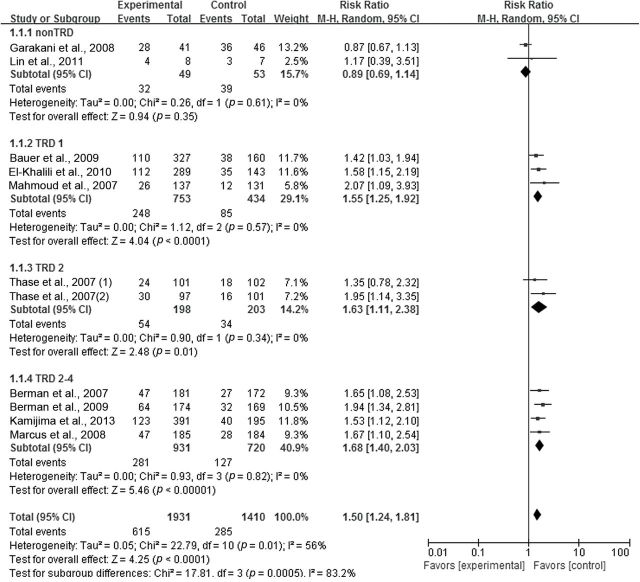
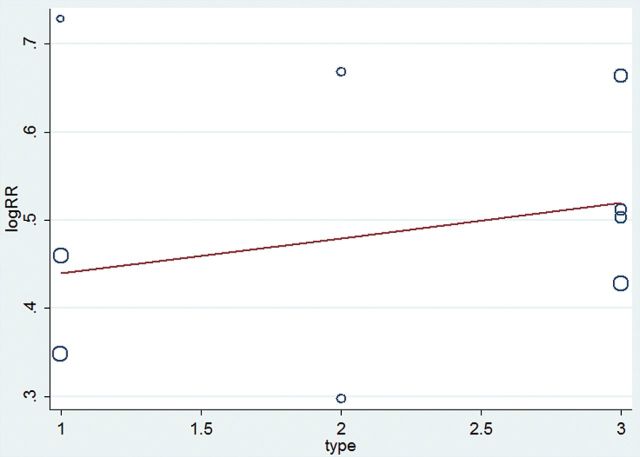
We present the result of the meta-analysis regarding remission rate as a forest plot (Figure 5). The pooled risk ratio for atypical antipsychotic augmentation versus antidepressant monotherapy in total TRD trials (including the TRD 1, 2, and 2–4 groups) was 1.62 (95% CI = 1.42–1.85, z = 7.21, n = 9, p < 0.00001), confirming the superior efficacy of atypical antipsychotic augmentation. The test for heterogeneity within TRD trials showed no heterogeneity (Chi2 = 3.26, p = 0.92, I2 = 0%). Risk ratios according to the degree of treatment resistance were 1.55 (95% CI = 1.25–1.92) for TRD 1; 1.63 (95% CI = 1.11–2.38) for TRD 2; and 1.68 (95% CI = 1.40–2.03) for TRD 2–4. Testing the differences among TRD subgroups (TRD 1 vs. TRD 2 vs. TRD 2–4) showed no heterogeneity (Chi2 = 0.31, p = 0.86, I2 = 0%; Figure 5). The meta-regression analysis showed that there were no significant differences in effect sizes according to the degree of treatment resistance in TRD trials in terms of remission rate (Z = 0.0402108, p = 0.595; Figure 6).
Figure 5.
Relative risk ratios of remission rates for atypical antipsychotic augmentation in depression. CI, confidence interval; M-H, Mantel Haenszel method; Non-TRD, non-treatment resistant depression; SAT, standard antidepressant therapy; TRD, treatment resistant depression; TRD 1, defined by no history of treatment failure to SAT within the current depressive episode; TRD 2, defined by history of two treatment failures to SAT within the current depressive episode; TRD 2–4, defined by history of two to four treatment failures to SAT within the current depressive episode.
Figure 6.
Meta-regression analysis of remission rate in treatment resistant depression. Type 1 indicates the TRD 1 group, 2 indicates the TRD 2 group, and 3 indicates the TRD 2–4 group. The coefficient is 0.0402108, and p value is 0.595. RR, risk ratio; SAT, standard antidepressant therapy; TRD, treatment resistant depression; TRD 1, defined by no history of treatment failure to SAT within the current depressive episode; TRD 2, defined by history of two treatment failures to SAT within the current depressive episode; TRD 2–4, defined by history of two to four treatment failures to SAT within the current depressive episode.
Meanwhile, the pooled risk ratio for atypical antipsychotic augmentation versus antidepressant monotherapy among non-TRD trials was 0.89 (95% CI = 0.69 to 1.14, z = 0.94, n = 2, p = 0.35), failing to show any superior efficacy of atypical antipsychotic augmentation to antidepressant monotherapy. The test for heterogeneity showed no heterogeneity (Chi2 = 0.26, df = 1, p = 0.49, I2 = 0%) within non-TRD trials (Figure 5).
To investigate whether the different definitions of remission adopted in each study could affect the outcome of our meta-analysis, we conducted a sensitivity analysis according to the definitions. We originally used the authors’ definition (MADRS ≤8, 10, or HAMD ≤7) to calculate pooled risk ratios of remission rates; however, the majority of the included studies provided information regarding remission rates defined as MADRS ≤10. Thus, we classified them into two subgroups (using the authors’ definition vs. MADRS ≤10), and compared the pooled risk ratios between the different definitions of remission. The pooled risk ratio when using the authors’ definition (MADRS ≤8) was 1.50 (95% CI = 1.19 to 1.88), and when using MADRS ≤10 was 1.38 (95% CI = 1.14 to 1.66). There was no significant subgroup difference (I2 = 0%, p = 0.57), suggesting that different definitions of remission did not significantly affect the outcome of our meta-analysis (see Supplementary Figure S1).
Discussion
In our meta-analysis, data from a total of 10 reports of 11 trials were analyzed. In both remission and response analyses among TRD groups, this meta-analysis confirms the superior efficacy of atypical antipsychotic augmentation compared to antidepressant monotherapy, as was demonstrated in previous meta-analyses (Papakostas et al., 2007; Nelson and Papakostas, 2009).
In addition, in terms of response rates among TRD groups, this meta-analysis revealed significant differences in effect sizes of atypical antipsychotic augmentation according to the degree of treatment resistance. The effect sizes of atypical antipsychotic augmentation became larger as the degree of treatment resistance became higher. The heterogeneity between TRD subgroups was moderate, with I2 values of 64.3%. The results from the meta-regression demonstrate that the difference in effect sizes according to the degree of treatment resistance is statistically significant (Z = 0.121257, p = 0.050). In the STAR*D report, as participants required more and more treatment steps, the response and remission rates became lower (Nierenberg et al., 2006; Rush et al., 2006). These studies showed that multiple treatment failures (in other words, higher degrees of treatment resistance) were associated with worse prognoses for subsequent antidepressant trials. A systematic review regarding the switching of antidepressant strategies after administration of a first SSRI found that the number of previous antidepressant treatments was negatively correlated with the treatment outcome (Ruhe et al., 2006). As the first study to investigate whether the degree of treatment resistance can predict the magnitude of the effect sizes for atypical antipsychotic augmentation, our meta-analysis yields findings with valuable meaning. The positive correlation between effect size and the degree of treatment resistance has the important clinical implication that atypical antipsychotic augmentation is more effective in reducing subsequent treatment failures among patients with a higher degree of treatment resistance. This means that atypical antipsychotic augmentation could be an especially good alternative treatment strategy for patients with relatively high numbers of treatment failures within the index episodes. However, we cannot definitively state that the difference in effect sizes between TRD groups shown in our meta-analysis results only from the difference in the number of treatment failures within the index episode. It is possible that other socio-demographic or disease-related characteristics of the populations, other than the number of treatment failures within the index episode, could affect the actual treatment refractoriness of each population. There are previous reports that patients with concurrent hypomanic/manic symptoms, somatic symptoms, or anxiety symptoms could respond to atypical antipsychotics differently than those without those symptoms (Altamura et al., 2004; Karp et al., 2005; Pae et al., 2012; Patkar et al., 2012). Thus, in the present situation where there is a lack of information regarding those population characteristics in the included trials, we should be careful in explaining the significantly different effect sizes according to the degree of treatment resistance.
In terms of remission rate among TRD groups, this meta-analysis also showed the tendency that, as the degree of treatment resistance became higher, the effect size became larger. However, this trend was not statistically significant. We think the reason why the results from the analysis of remission rates are not as significant as those from the analysis of response rates is as follows. The definition of remission requires the absolute values of MADRS or HAMD scores at the endpoint to be lower than a certain point, whereas the definition of response requires a ≥50% reduction from baseline MADRS or HAMD scores. The definition of the degree of treatment resistance, in our meta-analysis, depends on the number of treatment failures (failure to achieve ≥25–50% symptom reduction with standard antidepressant therapy) within the index episodes. Thus, we think the results from the analysis of response rate, compared to remission rate, showed a statistically stronger correlation between the effect size and the degree of treatment resistance. To identify whether pooled response and remission rates may differ according to the degree of resistance that we defined here, we calculated pooled response and remission rates among control groups (antidepressant monotherapy groups) according to the degree of treatment resistance among the TRD trials. The control response rates were 39.90% for the TRD 1 group, 29.6% for the TRD 2 group, and 24.0% for the TRD 2–4 group, showing a tendency for higher degrees of resistance to be associated with lower response rates. The control remission rates were 19.0% for the TRD 1 group, 16.7% for the TRD 2 group, and 17.6% for TRD the 2–4 group. This negative correlation between the degree of treatment resistance and control response rates is in line with the previous findings in STAR*D (Rush et al., 2006). To put it another way, even though we used arbitrary definitions for grouping TRD in this meta-analysis by using the number of previous treatment failures within the index episode, more treatment-resistant patients are classified into higher TRD groups (TRD 2 or TRD 2–4 rather than TRD 1). The negative correlation between the control remission rate and the degree of treatment resistance was not as prominent as that between the control response rate and the degree of resistance. This might be explained by the fact that the mean baseline severity of depression in the TRD 2–4 control group was lower than that in the TRD 2 control group, which could be related to the cut-off point difference of the HAMD in the inclusion criteria between the TRD 2 and TRD 2–4 groups.
A previous meta-analysis for MDD showed that the magnitude of clinical benefits of antidepressant therapy, compared to a placebo, increased with the baseline severity of depressive symptoms (Fournier et al., 2010). In addition, a retrospective study showed that baseline severity of depressive symptoms was a predictor of response for lithium augmentation among patients with TRD (Bschor et al., 2001). However, interestingly, in this study, the mean MADRS total scores of the control groups at randomization were lower in TRD 2–4 trials compared to those in TRD 1 or TRD 2 (27.6–28.2 for TRD 1; 29.7–30.1 for TRD 2; 25.5–27.1 for TRD 2–4). This might be partly due to the lower cut-off points of the HAMD in inclusion criteria for the augmentation phases of the TRD 2–4 trials. Nevertheless, the control response rates in TRD 2–4 were lowest among the TRD groups. These findings suggest that among patients with TRD, the response rate to pharmacotherapy is associated more with the degree of treatment resistance than with the baseline severity of depressive symptoms. If this is true, these findings provide good evidence showing that patients with a high degree of treatment resistance, but with relatively low symptom severity, could have clinical benefit from atypical antipsychotic augmentation. However, to confirm this, further studies are needed.
Meanwhile, this meta-analysis revealed that the efficacy of atypical antipsychotic augmentation differed according to whether or not the population was treatment-resistant. This meta-analysis failed to show the superior efficacy of atypical antipsychotic augmentation compared to antidepressant monotherapy among non-TRD populations in terms of remission. However, this negative efficacy result for non-TRD should be interpreted with caution. First, the dosages of atypical antipsychotics used in both non-TRD studies were relatively small (quetiapine 25–100mg/day, aripiprazole 2.5mg/day), and these low dosages could have led to the negative efficacy results. Second, there are some limitations in the non-TRD studies. Especially in Lin et al.’s study (2011), the most critical limitation is a high drop-out rate, leading to a broken balance design at week 6, and thus, the authors assessed the efficacy of low-dose aripiprazole augmentation using the 4-week efficacy data, rather than the a priori 10-week efficacy data. Considering these issues and the results from the two non-TRD trials, we can say that the efficacy of low-dose atypical antipsychotic augmentation among non-TRD patients remains unclear, and the existing evidence is not enough to recommend the use of low-dose atypical antipsychotics among non-TRD patients. Further, larger-sample, high-quality studies that investigate the efficacy of atypical antipsychotic augmentation among the non-TRD population and investigate effective dosages of atypical antipsychotics in that population are warranted.
There are several limitations to our meta-analysis. First, we used an arbitrary classification regarding treatment resistance that depended on the number of failed antidepressant trials during the index episode. The definitions of treatment-resistant depression are various and still evolving (Thase and Rush, 1997; Fava, 2003). The definition by Thase and Rush, which is commonly used, employs the failure to tricyclic antidepressant (TCA) or monoamine oxidase inhibitor (MAOI) as a major cut-off point, and thus, to apply this definition in current clinical practice, where TCA and MAOI are not used as commonly as before, seems to be rather impractical. Second, we only included randomized controlled trials and excluded trials in which the participants had comorbid psychiatric symptoms or medical conditions; thus, our results cannot be generalized to community populations with depression. Third, we only included two non-TRD trials, thus hampering the generalizability of our results to the entire non-TRD population. Fourth, there were not enough studies enabling comparison of effect sizes of different atypical antipsychotics within a same TRD stage. However, the finding of previous meta-analyses that different atypical antipsychotics did not have significantly different efficacy (Papakostas et al., 2007; Nelson and Papakostas, 2009) suggests that the use of different atypical antipsychotics does not have a significant influence on the pooled effect sizes, depending on the degree of treatment resistance. Even with these limitations, our meta-analysis draws the important conclusion that atypical antipsychotic augmentation is more efficacious in reducing subsequent treatment failure in those with higher degrees of treatment resistance. In addition, this meta-analysis suggests that the degree of treatment resistance during the index depressive episode could be a useful predictor of the effect of atypical antipsychotic augmentation. The findings of this meta-analysis may strengthen the rationale for the use of atypical antipsychotic augmentation among depressed patients with higher degrees of treatment resistance in clinical practice. We think that future studies directly comparing the effect sizes of various alternative treatment strategies (for example, atypical antipsychotic augmentation versus lithium augmentation) for TRD according to the degree of treatment resistance will help clinicians choose appropriate treatment strategies for each level of treatment resistance.
Supplementary Material
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S00000000000000
Statement of Interest
None.
Supplementary Material
Acknowledgments
None.
References
- Altamura AC, Montresor C, Salvadori D, Mundo E. (2004) Does comorbid subthreshold anxiety affect clinical presentation and treatment response in depression? A preliminary 12-month naturalistic study. Int J Neuropsychop 7:481–487. [DOI] [PubMed] [Google Scholar]
- Bauer M, Pretorius HW, Constant EL, Earley WR, Szamosi J, Brecher M. (2009) Extended-release quetiapine as adjunct to an antidepressant in patients with major depressive disorder: results of a randomized, placebo-controlled, double-blind study. J Clin Psychiatry 70:540–549. [DOI] [PubMed] [Google Scholar]
- Bauer M, Adli M, Ricken R, Severus E, Pilhatsch M. (2014) Role of lithium augmentation in the management of major depressive disorder. CNS Drugs 28:331–342. [DOI] [PubMed] [Google Scholar]
- Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, Khan A. (2007) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 68:843–853. [DOI] [PubMed] [Google Scholar]
- Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, Carson WH, Adson D, Taylor L, Hazel J, Marcus RN. (2009) Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr 14:197–206. [DOI] [PubMed] [Google Scholar]
- Bschor T, Canata B, Muller-Oerlinghausen B, Bauer M. (2001) Predictors of response to lithium augmentation in tricyclic antidepressant-resistant depression. J Affect Disord 64:261–265. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Berk M, Hyphantis TN, McIntyre RS. (2014) The integrative management of treatment-resistant depression: a comprehensive review and perspectives. Psychother Psychosom 83:70–88. [DOI] [PubMed] [Google Scholar]
- Chen J, Gao K, Kemp DE. (2011) Second-generation antipsychotics in major depressive disorder: update and clinical perspective. Curr Opin Psychiatry 24:10–17. [DOI] [PubMed] [Google Scholar]
- Choi E, Zmarlicka M, Ehret MJ. (2012) Vilazodone: a novel antidepressant. Am J Health Syst Pharm 69:1551–1557. [DOI] [PubMed] [Google Scholar]
- Citrome L. (2013) Levomilnacipran for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant--what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract 67:1089–1104. [DOI] [PubMed] [Google Scholar]
- El-Khalili N, Joyce M, Atkinson S, Buynak RJ, Datto C, Lindgren P, Eriksson H. (2010) Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychop 13:917–932. [DOI] [PubMed] [Google Scholar]
- Fava M. (2003) Diagnosis and definition of treatment-resistant depression. Biol Psychiatry 53:649–659. [DOI] [PubMed] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, Micoulaud-Franchi JA, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L. (2014) Ketamine administration in depressive disorders: a systematic review and meta-analysis. Psychopharmacology (Berl) 231:3663–3676. [DOI] [PubMed] [Google Scholar]
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, Fawcett J. (2010) Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 303:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garakani A, Martinez JM, Marcus S, Weaver J, Rickels K, Fava M, Hirschowitz J. (2008) A randomized, double-blind, and placebo-controlled trial of quetiapine augmentation of fluoxetine in major depressive disorder. Int Clin Psychopharmacol 23:269–275. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones KP, McCormack PL. (2010) Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs 24:769–796. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S. (2008) Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. Oxford: The Cochrane Collaboration.
- Kamijima K, Higuchi T, Ishigooka J, Ohmori T, Ozaki N, Kanba S, Kinoshita T, Koyama T. (2013) Aripiprazole augmentation to antidepressant therapy in Japanese patients with major depressive disorder: a randomized, double-blind, placebo-controlled study (ADMIRE study). J Affect Disord 151:899–905. [DOI] [PubMed] [Google Scholar]
- Karp JF, Scott J, Houck P, Reynolds CF, 3rd, Kupfer DJ, Frank E. (2005) Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry 66:591–597. [DOI] [PubMed] [Google Scholar]
- Kato M, Chang CM. (2013) Augmentation treatments with second-generation antipsychotics to antidepressants in treatment-resistant depression. CNS Drugs 27(Supp 1):S11–19. [DOI] [PubMed] [Google Scholar]
- Komossa K, Depping AD, Gaudchau A, Kissling W, Leucht S. (2010) Second-generation antipsychotics for major depressive disorder and dysthymia. Cochrane Database Syst Rev. Advance online publication. Retrieved 8 Dec 2010. 10.1002/14651858.CD008121.pub2. [DOI] [PubMed] [Google Scholar]
- Lin CH, Lin SH, Jang FL. (2011) Adjunctive low-dose aripiprazole with standard-dose sertraline in treating fresh major depressive disorder: a randomized, double-blind, controlled study. J Clin Psychopharmacol 31:563–568. [DOI] [PubMed] [Google Scholar]
- Mahmoud RA, Pandina GJ, Turkoz I, Kosik-Gonzalez C, Canuso CM, Kujawa MJ, Gharabawi-Garibaldi GM. (2007) Risperidone for treatment-refractory major depressive disorder: a randomized trial. Ann Intern Med 147:593–602. [DOI] [PubMed] [Google Scholar]
- Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM. (2008) The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 28:156–165. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, Barakat M, Miguelez M. (2014) Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord 156:1–7. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Strimpfel J, Jandacek R, Rider T, Tso P, Welge JA, Strawn JR, Delbello MP. (2014) Detection and Treatment of Long-Chain Omega-3 Fatty Acid Deficiency in Adolescents with SSRI-Resistant Major Depressive Disorder. PharmaNutrition 2:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI. (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psych 166:980–991. [DOI] [PubMed] [Google Scholar]
- Nichols AI, Tourian KA, Tse SY, Paul J. (2010) Desvenlafaxine for major depressive disorder: incremental clinical benefits from a second-generation serotonin-norepinephrine reuptake inhibitor. Expert Opin Drug Metab Toxicol 6:1565–1574. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Fava M, Trivedi MH, Wisniewski SR, Thase ME, McGrath PJ, Alpert JE, Warden D, Luther JF, Niederehe G, Lebowitz B, Shores-Wilson K, Rush AJ. (2006) A comparison of lithium and T(3) augmentation following two failed medication treatments for depression: a STAR*D report. Am J Psych 163:1519–1530. [DOI] [PubMed] [Google Scholar]
- Pae CU, Patkar AA, Gilmer W, Holtzman N, Thommi SB, Ghaemi SN. (2012) Predictors of response to ziprasidone: results from a 6-week randomized double-blind, placebo-controlled trial for acute depressive mixed state. Pharmacopsychiatry 45:152–155. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Shelton RC, Smith J, Fava M. (2007) Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry 68:826–831. [DOI] [PubMed] [Google Scholar]
- Patkar A, Gilmer W, Pae CU, Vohringer PA, Ziffra M, Pirok E, Mulligan M, Filkowski MM, Whitham EA, Holtzman NS, Thommi SB, Logvinenko T, Loebel A, Masand P, Ghaemi SN. (2012) A 6 week randomized double-blind placebo-controlled trial of ziprasidone for the acute depressive mixed state. PLOS ONE 7:e34757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha FL, Fuzikawa C, Riera R, Hara C. (2012) Combination of antidepressants in the treatment of major depressive disorder: a systematic review and meta-analysis. J Clin Psychopharmacol 32:278–281. [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Huyser J, Swinkels JA, Schene AH. (2006) Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry 67:1836–1855. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psych 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. (2013) Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLOS Med 10:e1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thase ME, Rush AJ. (1997) When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry 58(Supp 13):23–29. [PubMed] [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, Watson SB, Dube S. (2007) A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry 68:224–236. [DOI] [PubMed] [Google Scholar]
- Turner P, Kantaria R, Young AH. (2014) A systematic review and meta-analysis of the evidence base for add-on treatment for patients with major depressive disorder who have not responded to antidepressant treatment: a European perspective. J Psychopharmacol 28:85–98. [DOI] [PubMed] [Google Scholar]
- Vieta E, Colom F. (2011) Therapeutic options in treatment-resistant depression. Ann Med 43:512–530. [DOI] [PubMed] [Google Scholar]
- Wright BM, Eiland EH, 3rd, Lorenz R. (2013) Augmentation with atypical antipsychotics for depression: a review of evidence-based support from the medical literature. Pharmacotherapy 33:344–359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.