Abstract
Background:
Magnetic resonance imaging studies have provided evidence of structural modifications in cortical-limbic regions in major depressive disorder. To date, however, few studies have tracked structural changes in patients during treatment. This prospective, longitudinal imaging study investigated associations between brain structure and clinical responsiveness in a sample of patients with treatment-resistant major depressive disorder during an approximate 1-year follow-up period.
Methods:
FreeSurfer software was used to extract volume or cortical thickness values from 6 regions of interest (hippocampus, rostral middle frontal gyrus, orbitofrontal cortex, rostral and caudal anterior cingulate cortices, and inferior temporal gyrus) in patients (n = 26) and matched healthy controls (n = 28). Regions of interest were selected based on previous evidence of potential associations between morphometric characteristics in these regions and treatment response or remission. Analyses were conducted to compare volume and cortical thickness in patients and controls at baseline imaging, determine whether patients’ brain structure at treatment initiation was associated with response over follow-up, and compare longitudinal changes in volume and cortical thickness in patients who achieved sustained 6-month remission (Montgomery-Åsberg Depression Rating Scale Score ≤12) with nonremitters.
Results:
Patients and controls showed no structural differences at baseline. Among patients, thicker right caudal anterior cingulate cortex at baseline was associated with greater symptom improvement over follow-up. Remitters and nonremitters showed subtle changes in volume and thickness over time in opposing directions, with increased hippocampal volume and cortical thickness in the rostral middle frontal gyrus, orbitofrontal cortex, and inferior temporal gyrus in remitters, and decreased volume or thickness in these regions in nonremitters.
Conclusions:
The results suggest that longitudinal structural trajectories may differ in major depressive disorder patients according to their clinical response to treatment.
Keywords: Major depressive disorder, magnetic resonance imaging, hippocampal volume, cortical thickness, remission
Introduction
Mounting evidence suggests that major depressive disorder (MDD) is associated with abnormalities in cortical and subcortical brain structures and their connective circuits. Volume-based magnetic resonance imaging (MRI) studies have primarily localized brain volume differences in patients with depression relative to healthy individuals to frontal and limbic brain regions. Specifically, meta-analyses of cross-sectional imaging studies have most commonly reported volume reductions in the prefrontal, orbitofrontal, and cingulate cortices, the hippocampus, and striatum in patients with MDD (Koolschijn et al., 2009; Arnone et al., 2012). Multimodal imaging has provided further evidence of dysfunction of the cortical-limbic circuit in depression with findings of altered anatomical connectivity in this network by diffusion tensor tractography (Fang et al., 2012) and abnormal effective connectivity by positron emission tomography measures of brain glucose metabolism (Seminowicz et al., 2004). Cytoarchitectural abnormalities in the cerebral cortex in MDD have also been widely reported in postmortem studies. Specifically, reduced neuronal and glial cell densities, neuronal size, and cortical thickness have been found in the dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortices of patients with depression (Ongür et al., 1998; Rajkowska et al., 1999; Cotter et al., 2001, 2002). Collectively, this evidence implicates the cortical-limbic regions in the structural modifications observed in depressed patients.
Recent studies that have compared first-episode MDD patients and healthy controls have found regional increases in cortical thickness (van Eijndhoven et al., 2013; Qiu et al., 2014) and reductions in cortical volume and altered white matter integrity (Han et al., 2013) in several cortico-limbic areas, suggesting structural differences in patients close to illness onset. Studies involving chronic medicated MDD patients have revealed relationships between regional structural abnormalities and clinical course in depression. Meta-analyses have shown that longer illness duration in patients is associated with greater gray matter (GM) volume decrease in the rostral anterior cingulate cortex and dorsomedial frontal cortex (Bora et al., 2012), and smaller hippocampal volume (McKinnon et al., 2009). The number of prior depressive episodes is also associated with patients’ total hippocampal volume (Videbech and Ravnkilde, 2004), dentate gyrus volume, and thickness of the medial prefrontal cortex (Treadway et al., 2015). Evidence of the relationship between structural brain changes and illness burden suggests that these modifications may be state-related rather than (or in addition to) trait-related markers and thus may be reversible upon treatment or remission. In fact, meta-regression analysis of cross-sectional studies has shown larger hippocampal volume among remitted patients compared with currently depressed patients (Kempton et al., 2011).
Longitudinal structural imaging studies may determine whether atrophic changes are amenable to treatment, but to date few such studies have been conducted in depression.
Initial longitudinal research has revealed a depression-specific pattern of brain volume decline consistent with the areas identified as affected in cross-sectional studies (Frodl et al., 2008a). Moreover, these same regions appear to respond over time to antidepressant treatment and/or remission. Frodl and colleagues (2008a) found less GM volume decline in the hippocampus, prefrontal, and anterior cingulate cortices in patients who achieved remission over 3-year follow-up compared with nonremitters. In a previous study, we found increased whole brain volume, and increased GM volume in the orbitofrontal cortex and inferior temporal gyrus in patients who achieved 6-month sustained remission (Phillips et al., 2012). These studies provide evidence that the extent of brain atrophy in depression may be lessened or reversed in patients who achieve remission.
Finally, since treatment responders and nonresponders seemingly follow different structural trajectories over time, it is possible that structural differences present in individuals at treatment initiation (eg, smaller brain volume or thinner cortex) may predispose patients to poorer treatment response or vulnerability for relapse during treatment. Previous studies have shown smaller volume of the hippocampus (Frodl et al., 2004; MacQueen et al., 2008; Hoogenboom et al., 2013) and rostral middle frontal gyrus (Hoogenboom et al., 2013) and thinner posterior cingulate gyrus (Järnum et al., 2011) in pretreatment scans of patients who failed to achieve remission over follow-up compared with remitters.
In the present study, we prospectively examined cortical thickness and volume changes in specific cortical-limbic regions of interest (ROIs) in the same sample of patients with treatment-resistant depression previously examined by our group using whole-brain imaging techniques (Phillips et al., 2012). ROIs were selected a priori based on previous evidence of structural differences in these areas in MDD patients and comparison subjects and potential associations between morphometric characteristics in these regions and treatment response or remission. The objectives were to determine whether the patients demonstrated cortical thickness or volume differences relative to healthy matched controls at baseline imaging, whether patients’ brain structure at treatment initiation was associated with their clinical response over follow-up, and to compare longitudinal changes in cortical thickness and volume in patients who achieved sustained remission with nonremitters.
Methods
Participants
Twenty-eight outpatients with treatment-resistant depression (aged 18–65 years) were recruited from the Mood Disorders Research Unit at the Royal Ottawa Mental Health Centre, Ottawa, Ontario, Canada. This patient sample was previously reported by Phillips et al. (2012). Diagnosis of MDD was determined by psychiatric consultation on the basis of DSM-IV criteria (American Psychiatric Association, 1994). Treatment resistance was defined as current episode illness duration of at least 6 months, failure to achieve remission after treatment with at least 2 antidepressants at adequate dosage for at least 6 weeks each, and presence of depressive symptoms corresponding to a Hamilton Rating Scale for Depression (Hamilton, 1960) score ≥18 and a Montgomery-Åsberg Depression Rating Scale (MADRS; Montgomery and Åsberg, 1979) score ≥22. Exclusionary criteria for patients included diagnosis of posttraumatic stress disorder, any psychotic disorder, anorexia nervosa, or a history of manic, hypomanic, or mixed episode. Twenty-nine age-, gender-, and handedness-matched healthy control participants were recruited through community advertisement. Controls were free of psychiatric disorders confirmed through administration of the Scheduled Clinical Interview for DSM-IV-Nonpatient Edition (First et al., 2002), and reported no history of mood or anxiety disorders among their first-degree relatives. Exclusion criteria for all participants were presence of major medical illnesses, neurological disorders, history of head injury with loss of consciousness, diagnosis of substance abuse or dependence, exposure to oral or intravenous steroids, IQ <80, or contraindications to MRI. Handedness was evaluated with the Edinburgh Handedness Inventory (Oldfield, 1971).
All participants underwent baseline MRI at study inclusion (controls were examined with MRI only once at baseline). Patients underwent a second follow-up MRI scan after either a 6-month period of sustained remission (defined as MADRS score ≤12 at each visit) or after a 12-month period of failure to remit. All patients were receiving antidepressant treatment at time of image acquisition. Patients were followed longitudinally and treated with intensive pharmacotherapy under the care of study investigators with the goal of attaining remission. At study visits (once every 2 weeks), patients were assessed by administration of the MADRS if an approximate 20% symptom improvement was not detected (Szegedi et al., 2009), an increase in doses (if tolerated), a change in medication, or an augmentation strategy was implemented. Medication choices were based on their different mechanisms of action and potential synergies on the serotonin, norepinephrine, and dopamine systems (Blier, 2006). During follow-up, patients received individualized treatment with medications from the following classes: tricyclic antidepressants, monoamine oxidase inhibitors, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, selective norepinephrine reuptake inhibitors, dopamine agonists, atypical antipsychotics, and other medications including bupropion, mirtazapine, pindolol, trazodone, and lithium (Phillips et al., 2012). No patients received electroconvulsive therapy during follow-up. The research protocol was approved by the Research Ethics Board of the Royal Ottawa Mental Health Centre. After complete description of the study to subjects, informed written consent was obtained.
Image Acquisition and Processing
T1-weighted magnetic resonance images were obtained at 1.5-T (Seimens Magnetom Symphony Systems, Siemens, Erlangen, Germany) using the same magnetization-prepared rapid gradient echo acquisition protocol (with TE =4.38ms, TR =1500ms, flip angle =15º, field of view =250mm, matrix =256×256, slice thickness =1mm). Most baseline scans and all follow-up scans were obtained on the same scanner at St-Joseph MRI, Gatineau, Quebec, Canada. The baseline scans of 3 patients were obtained at the Ottawa Hospital, Ottawa, Ontario, Canada. MRI scans were reviewed by a licensed radiologist to rule out clinically significant neuroanatomical abnormalities.
Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer image analysis suite (version 4.5, http://surfer.nmr.mgh.harvard.edu) to derive measures of cortical thickness (Fischl and Dale, 2000), subcortical volume (Fischl et al., 2002), and total intracranial volume (Buckner et al., 2004). The technical details of these processing pipelines have been previously described in detail (Dale et al., 1999; Fischl et al., 1999). The cortical reconstructions for each participant were visually inspected for inaccuracies in segmentation and manually corrected as necessary by a single rater (J.L.P.) blind to subject identity, diagnostic group, and time point. To measure changes in cortical thickness and volume in patients between baseline and follow-up scans, images were processed using the FreeSurfer longitudinal pipeline (Reuter et al., 2012).
ROI
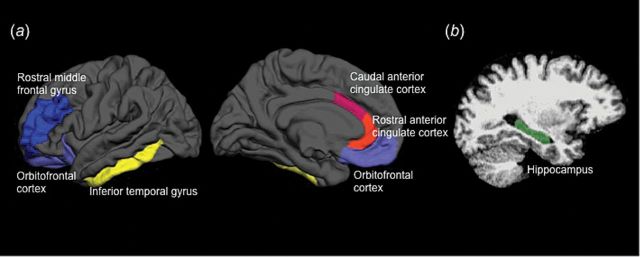
For this study, data was analyzed from 5 cortical and 1 subcortical ROI (Figure 1). Cortical surface gyri were labeled using the automated FreeSurfer cortical parcellation procedure (Desikan et al., 2006). Based on previous studies (Desikan et al., 2006; Kikinis et al., 2010), the rostral middle frontal gyrus was chosen as the gyral-based neuroanatomical representative of the dorsolateral prefrontal cortex (DLPFC, a functionally defined area). The rostral middle frontal gyrus includes Brodmann area 46, the core component of the DLPFC (Rajkowska and Goldman-Rakic, 1995). For the orbitofrontal cortex, prior to data extraction, the medial and lateral orbitofrontal cortical parcellation labels were combined to create a customized orbitofrontal cortex ROI. The rostral anterior cingulate cortex (ACC), caudal ACC, and inferior temporal gyrus parcellation labels were used with no modifications. Mean cortical thickness values (calculated as the mean distance between the pial and gray/white matter surfaces across the specified region) were extracted from these 5 ROIs for each hemisphere. Volume was extracted from a single subcortical ROI, the hippocampus, defined using the FreeSurfer automated subcortical segmentation procedure (Fischl et al., 2002).
Figure 1.
Regions of interest (ROIs) examined in this study. (a) Cortical surface-based ROIs rendered on the lateral (left) and medial (center) pial surface representations of the left hemisphere on an average brain. (b) The single subcortical ROI, the hippocampus, overlaid on a sagittal slice from a single participant (right).
Statistical Analysis
Statistical analyses were conducted using PASW Statistics version 18.0 (SPSS Inc., Chicago, IL). Demographic and clinical variables of patient and control groups, and remitter and nonremitter groups were compared with 2-tailed independent samples t tests (for continuous variables) or chi-square tests (for dichotomous variables). Within resultant patient groups (remitters and nonremitters), baseline and follow-up MADRS scores were compared with paired t tests.
All neuroanatomical measures were examined for normality using the Shapiro-Wilk test. Cross-sectional analyses of volumetric and thickness data from each ROI were conducted to compare patient and control groups at baseline imaging. Baseline left and right hippocampal volumes were investigated using MANCOVA with diagnosis (patient or control) as the independent variable and total intracranial volume (TIV) and baseline scanner as covariates. Baseline mean cortical thickness values for each cortical ROI (rostral middle frontal gyrus, rostral ACC, caudal ACC, orbitofrontal cortex, inferior temporal gyrus) were examined by individual MANCOVAs adjusted for baseline scanner. The cortical thickness analyses were not adjusted for TIV, since it does not affect the thickness of the cortical mantle (Buckner et al., 2004).
Among patients, exploratory analyses were conducted to test for correlations between baseline volume or mean cortical thickness measures in each ROI and change in MADRS scores over follow-up using Pearson correlation statistics.
Longitudinal analyses were conducted to compare volume and cortical thickness changes in patients who remitted over follow-up and nonremitters. Repeated-measures ANCOVAs were conducted for each ROI with volume or mean cortical thickness as the dependent variable, hemisphere (left or right) and time (baseline or follow-up) as the within-subject variables, and remission status (nonremitter or remitter) as the between-subject variable, with interscan interval (number of days between scans), baseline scanner, baseline age, and TIV (for volume analyses only) as covariates. The main effects for remission status and time and the remission status × time interaction are reported for each ROI. Simple effects were obtained by deconstructing significant interactions.
A 2-tailed P<.05 was considered statistically significant for all comparisons unless otherwise noted. For cortical thickness analyses, main effects and interactions were considered significant after application of a Bonferroni correction for multiple comparisons with a significance level of P<.01 (P<.05/5 number of comparisons (cortical ROIs) in each hemisphere). For exploratory correlation analyses, results were reported at uncorrected P values (P<.05).
Results
Demographic and Clinical Characteristics
Following MR image acquisition, 3 participants were excluded from the study: one patient due to poor quality MRI data, one patient due to lack of follow-up data, and one control subject due to evidence of brain tumor. The final sample for this study consisted of 54 participants, including 26 patients with treatment-resistant MDD examined at baseline and follow-up and 28 matched healthy controls examined at baseline only. Patient and control groups did not differ significantly on demographic variables (age, gender, or handedness) or baseline total intracranial volume (TIV) (Table 1).
Table 1.
Demographic, Clinical, and Volumetric Characteristics of Study Participants (N = 54)
| Group mean (±SD)a | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Controls (n = 28) | Patients (n = 26) | P value b | Nonremitters (n = 14) | Remitters (n = 12) | P value c |
| Age, y | 45.7 (10.6) | 46.0 (10.4) | .91 | 44.7 (10.5) | 47.5 (10.6) | .50 |
| Gender, n male/female | 10/18 | 8/18 | .70 | 3/11 | 5/7 | .40 |
| Handedness, n right/leftd | 25/3 | 22/4 | .61 | 13/1 | 9/3 | .31 |
| Baseline TIV, mm3 | 1529301 (126250) | 1526607 (163255) | .95 | 1511361 (196469) | 1544395 (119641) | .62 |
| Age at illness onset, ye | --- | 30.3 (14.1) | --- | 29.5 (14.5) | 31.2 (13.5) | .76 |
| No. depressive episodes, A/B/Cf | --- | 10/6/10 | --- | 6/3/5 | 4/3/5 | .88 |
| Baseline MADRS score | --- | 34.6 (7.0) | --- | 38.2 (5.2) | 30.3 (6.4) | .002 |
| Follow-up MADRS score | --- | 16.7 (12.7) | --- | 26.6 (8.6) | 5.3 (3.3) | <.001 |
| Change in MADRS score | --- | -17.9 (11.8) | --- | -11.7 (11.3) | -25.0 (8.0) | <.001 |
| Interscan intervalg | --- | 379.4 (88.3) | --- | 421.1 (36.6) | 330.8 (106.5) | .007 |
Abbreviations: MADRS, Montgomery-Åsberg Depression Rating Scale; TIV, total intracranial volume.
aUnless otherwise indicated.
bControls versus patients, Independent samples t test or X 2 test.
cNonremitters versus remitters, Independent samples t test or X 2 test.
dHandedness was measured using Edinburgh Handedness Inventory (Hamilton, 1960).
eData missing for 1 participant.
fNumber of episodes prior to study enrollment expressed as categories: A = 1–2 episodes, B = 3–4 episodes, C = 5+ episodes.
gNumber of days between scans.
Mean (±SD) duration of follow-up for patients was 379 (±88) days. During follow-up, 12 patients achieved sustained 6-month remission, while 14 patients failed to achieve sustained remission. Comparison of remitter and nonremitter groups (Table 1) revealed no differences in demographic variables, age at onset, or number of previous depressive episodes, highlighting the homogeneity of the patient sample. Remitters had significantly lower MADRS scores at baseline and follow-up imaging relative to nonremitters. However, within-group paired t tests revealed significant decreases in MADRS scores over follow-up in both groups (remitters t 11 = 10.76, P<.001; nonremitters t 13 = 3.89, P = .002). Remitters also had shorter interscan intervals (days between MRI scans) compared with nonremitters (this variable was included as a covariate for all longitudinal analyses).
Cross-Sectional Imaging Results
Morphometric data were normally distributed. Baseline hippocampal volumes and mean cortical thickness values for each ROI are presented in Table 2. Cross-sectional comparisons of patients and controls at baseline revealed no between-group differences in hippocampal volume or mean cortical thickness in the rostral middle frontal gyrus, rostral ACC, caudal ACC orbitofrontal cortex, or inferior temporal gyrus (for each ROI no significant main effect of diagnosis).
Table 2.
Cross-Sectional Comparisons of Volumetric and Thickness Data of Study Participants at Baseline Imaging (N = 54)
| Mean (±SE) | ||||||
|---|---|---|---|---|---|---|
| Region of Interest | Patients (n = 26) | Controls (n = 28) | F | df | P value a | |
| Volume (mm3) | ||||||
| Hippocampus | LH | 4307.0 (67.3) | 4344.6 (64.8) | 0.16 | 1,50 | .70 |
| RH | 4363.4 (63.1) | 4394.7 (60.7) | 0.12 | 1,50 | .73 | |
| Mean cortical thickness (mm) | ||||||
| Rostral middle frontal gyrus | LH | 2.452 (0.03) | 2.482 (0.03) | 0.59 | 1,51 | .45 |
| RH | 2.436 (0.02) | 2.474 (0.02) | 1.17 | 1,51 | .28 | |
| Rostral anterior cingulate cortex | LH | 2.935 (0.05) | 3.014 (0.05) | 1.40 | 1,51 | .24 |
| RH | 2.910 (0.05) | 2.978 (0.04) | 1.15 | 1,51 | .29 | |
| Caudal anterior cingulate cortex | LH | 2.677 (0.05) | 2.814 (0.05) | 4.06 | 1,51 | .05 |
| RH | 2.628 (0.04) | 2.711 (0.04) | 1.96 | 1,51 | .17 | |
| Orbitofrontal cortex | LH | 2.648 (0.03) | 2.675 (0.03) | 0.44 | 1,51 | .51 |
| RH | 2.613 (0.03) | 2.615 (0.03) | 0.001 | 1,51 | .97 | |
| Inferior temporal gyrus | LH | 2.884 (0.03) | 2.948 (0.03) | 1.96 | 1,51 | .17 |
| RH | 2.891 (0.03) | 2.950 (0.03) | 2.04 | 1,51 | .16 | |
Abbreviations: LH, left hemisphere; RH, right hemisphere.
aFor each anatomical region of interest, individual multivariate analyses of covariance were conducted with volume or mean cortical thickness as the dependent variable, diagnosis (patient or control) as the independent variable and baseline scanner and total intracranial volume (volumetric analyses only) as covariates.
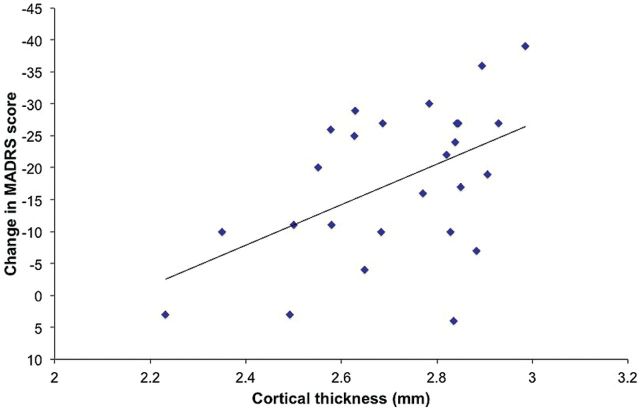
Among patients, exploratory analyses were conducted to test for correlations between baseline volume or thickness measures and change in symptoms over follow-up. There was a significant negative correlation between patients’ mean right caudal anterior cingulate cortical thickness and change in MADRS score over follow-up (r = -0.50, P = .009) (Figure 2). This indicates that thicker right caudal ACC at baseline was associated with greater symptom improvement (larger decrease in MADRS scores). No significant correlations were observed between change in MADRS and baseline volume or mean cortical thickness in the other ROIs.
Figure 2.
Correlation between patients’ baseline mean cortical thickness of the right caudal anterior cingulate cortex and their change in Montgomery-Åsberg Depression Rating Scale (MADRS) score over follow-up (where decrease in MADRS score indicates clinical improvement).
Longitudinal Imaging Results
For the longitudinal imaging analyses, volume and cortical thickness changes over follow-up in the ROIs were examined in patients with repeated-measures ANCOVAs (Table 3). Averaged across both time points, volume and thickness measures did not differ in remitted and nonremitted patient groups (there was no significant main effect of remission status for any ROI). Similarly, the changes in volume or thickness over follow-up were not statistically significant when considering the entire patient sample (both remitters and nonremitters; no significant main effect of time for any ROI).
Table 3.
Longitudinal Analyses of Volumetric and Thickness Data of Patients over Follow-Up (n = 26)a
| Main Effect of Remission Status | Main Effect of Time | Time x Remission Status Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Morphometric variable | F | df | P value | F | df | P value | F | df | P value |
| Volume | |||||||||
| Hippocampus | 0.09 | 1,20 | .926 | 3.36 | 1,20 | .082 | 15.79 | 1,20 | .001 |
| Mean cortical thickness | |||||||||
| Rostral middle frontal gyrus | 1.55 | 1,21 | .228 | 1.67 | 1,21 | .210 | 12.06 | 1,21 | .002 |
| Rostral anterior cingulate cortex | 0.001 | 1,21 | .973 | 1.19 | 1,21 | .289 | 4.14 | 1,21 | .055 |
| Caudal anterior cingulate cortex | 1.48 | 1,21 | .237 | 0.64 | 1,21 | .431 | 0.53 | 1,21 | .473 |
| Orbitofrontal cortex | 2.25 | 1,21 | .148 | 2.80 | 1,21 | .109 | 11.74 | 1,21 | .003 |
| Inferior temporal gyrus | 0.06 | 1,21 | .816 | 0.33 | 1,21 | .573 | 8.72 | 1,21 | .008 |
aFor each anatomical region of interest (ROI), individual repeated measures analyses of covariance were conducted with interscan interval, baseline scanner, baseline age, and total intracranial volume (for volumetric analyses only) as covariates.
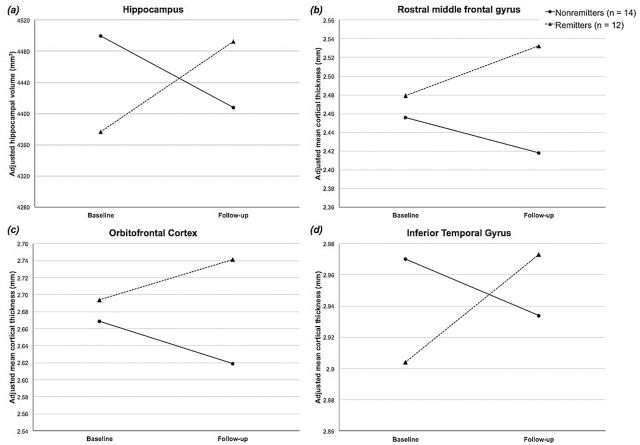
There were, however, significant remission status × time interaction effects for hippocampal volume and rostral middle frontal gyrus, orbitofrontal cortex, and inferior temporal gyrus cortical thickness. These significant interaction terms indicate that the mean paired differences in volume or cortical thickness from baseline to follow-up in remitters differed significantly from nonremitters (structural change over time differed according to patients’ remission status). Graphical representation of patients’ imaging measures at baseline and follow-up for each ROI for which the interaction effect was detected demonstrate morphological changes in opposing directions with a consistent pattern of increased volume or cortical thickness in remitters and decreased volume or thickness in nonremitters (Figure 3). Posthoc simple effects analyses revealed that the slight changes in volume or thickness seen in each group over follow-up were themselves not statistically significant (no significant main effect of time within either group [remitters or nonremitters] for any ROI showing a significant remission status × time interaction).
Figure 3.
Graphical representation of the significant remission status × time interaction effects on volume in the hippocampus (a), and mean cortical thickness in the rostral middle frontal gyrus (b), orbitofrontal cortex (c), and inferior temporal gyrus (d).
Discussion
There were 3 main findings in this study: (1) patients did not differ from control participants in volume or cortical thickness in any of the specified ROIs at baseline imaging; (2) among patients, thicker right caudal anterior cingulate cortex at baseline was associated with greater symptom improvement over follow-up; and (3) the direction of longitudinal hippocampus volume and rostral middle frontal gyrus, orbitofrontal cortex, and inferior temporal gyrus cortical thickness changes in patients differed according to their remission status, with increasing volume or thickness in remitters over time and decreasing volume or thickness in nonremitters.
In contrast to meta-analyses demonstrating smaller hippocampal volume among MDD patients relative to controls (Campbell et al., 2004; Videbech and Ravnkilde, 2004; Koolschijn et al., 2009; Arnone et al., 2012), the cross-sectional comparison of patients and controls at baseline imaging revealed no differences in hippocampal volume. There were also no between-group differences in mean cortical thickness in the surface ROIs examined in this study. In recent years, several groups have found reduced cortical thickness in patients in prefrontal regions, including the orbitofrontal cortex (Järnum et al., 2011; Tu et al., 2012; Grieve et al., 2013; Na et al., 2014), DLPFC (van Tol et al., 2014), dorsal ACC (Li et al., 2014), and rostral middle frontal gyrus (Tu et al., 2012; Na et al., 2014). However, all these studies used vertex-based analyses (where cortical thickness values are compared at each vertex across the entire cortical surface), whereas this study employed a ROI approach and examined mean cortical thickness values. Reported clusters of decreased cortical thickness in vertex-based analyses may be very regionally specific, and it is possible that such differences may not be detectable when cortical thickness values are averaged across regions.
The exploratory correlation analyses demonstrated an association between patients’ cortical thickness in the right caudal ACC at treatment initiation and their change in MADRS scores over follow-up, with thicker caudal ACC at baseline predicting greater symptom improvement. Numerous functional imaging modalities (electroencephalogram, functional MRI, and positron emission tomography) have demonstrated the importance of pretreatment rostral ACC activity in the prediction of pharmacological response in MDD patients (reviewed in Pizzagalli, 2011). Regarding structural imaging, there is some evidence for the predictive potential of the structural correlates of the ACC for antidepressant treatment response. Previous studies have shown prediction of clinical response to pharmacotherapy among patients in association with increased cortical depth of the subgenual ACC (Coryell et al., 2005), greater GM volume in the right rostral ACC (Costafreda et al., 2009), and diffusion tensor imaging measures of white matter connectivity between the subgenual ACC and limbic regions (Korgaonker et al., 2014). Within the other ROIs, there were no further correlations identified between any neuroanatomical measure and change in clinical symptoms. Thus, the effect appears specific to the caudal ACC although as the analyses were not corrected for multiple comparisons, these results should be interpreted with caution.
In our previous report of whole-brain volume change in this patient sample, sustained remitters showed overall brain volume increase while nonremitters lost brain volume over follow-up (Phillips et al., 2012). In the longitudinal analyses, similar results were seen in several ROIs; with remitted and nonremitted patients demonstrating subtle increases and decreases, respectively, in hippocampal volume, and in cortical thickness in the rostral middle frontal gyrus, orbitofrontal cortex, and inferior temporal gyrus. Posthoc analyses revealed that the magnitude of the changes in volume and cortical thickness observed over time within each group were themselves not statistically significant. Rather, it was the finding that the structural changes occurred in opposing directions according to patients’ remission status over follow-up that was significant.
The volume and thickness changes were small, which may indicate that a longer follow-up period is necessary to elicit marked structural changes and to determine whether the trajectories of morphometric change observed in remitters and nonremitters continue as time progresses. Frodl and colleagues (2004) investigated hippocampal volume changes in a cohort of MDD patients after 1- and 3-year follow-up periods. At 1-year, they found no changes in hippocampal volume, yet at 3-year follow-up, they found a modest increase in hippocampal volume in patients who had received consistent antidepressant treatment over the entire study period (Frodl et al., 2008b). In the present study, all patients received intensive pharmacotherapy over follow-up, yet only remitters showed increasing volume or thickness over time, suggesting that remission itself may be driving structural recovery rather than antidepressant treatment. This distinction may be important for the hippocampus, as the only other studies to document hippocampal volume increases over time in MDD patients failed to include either nonremitted patients (Ahdidan et al., 2011) or treatment nonresponders (Schermuly et al., 2011). Nevertheless, although we found increasing orbitofrontal cortical thickness only in remitters (with cortical thinning in nonremitters), Järnum et al. (2011) found increased cortical thickness in the orbitofrontal cortices over 6-month follow-up in their full patient sample that included both remitted and nonremitted patients. This finding supports an effect of antidepressant treatment on cortical thickness changes in the orbitofrontal cortex. However, the consistent pattern of decreasing volume or cortical thickness in nonremitters in this study suggests that for certain brain regions or morphometric measures, antidepressant-mediated structural recovery may only occur if patients respond clinically to the treatment.
Several questions persist regarding the relationship between structural changes and treatment response in depression. It is not known whether normalization of atrophic changes are necessary for patient response to antidepressants or whether response to treatment, and thus, alleviation of continuing depressive symptoms, contribute to the structural recovery seen in remitted patients. Furthermore, while there are a number of potential mechanisms that may contribute to volume or cortical thickness increases observed with MRI in antidepressant-treated remitted patients (including regeneration of neurons, increase in glial cell numbers, synaptogenesis, larger neuropil volume, more blood vessels, or less apoptosis), any discussion of these possibilities remains speculative and further research is necessary to address these questions.
The design of this study permitted the investigation of longitudinal volume and cortical thickness changes in patients according to their response to treatment. It elicited different information than would be obtained by cross-sectional comparison of remitted patients with currently ill patients or comparison of how patients’ brain structure changes over time relative to a healthy control group without consideration of their clinical outcome. Despite these strengths, this study has certain limitations that merit comment. There was no follow-up imaging of control participants, which precludes the possibility of studying normal progressive volume and cortical thickness changes in healthy individuals over the time period investigated. Further, as all patients were treated at baseline imaging and received pharmacotherapy throughout the follow-up period, the longitudinal effects of untreated depression on brain structure (separate from any pharmacological effects) could not be studied. Similarly, due to the naturalistic treatment approach used, the individual effects of any particular medications or augmentation strategies on volume or thickness changes could not be analyzed. The latter is less of a concern for this particular study, as the goal was to investigate the effects of overall clinical outcome (remission status) on longitudinal structural change. Finally, the study contained a relatively small sample size, although the patient group was very homogenous and well characterized clinically.
In summary, our findings suggest that cortical thickness of the right caudal ACC may be associated with treatment-resistant MDD patients’ change in depressive symptoms over follow-up. Further, the results indicate that while the patient sample did not differ from healthy controls on baseline volume or cortical thickness in the ROIs examined, over time, patients did demonstrate subtle structural changes, the direction of which varied according to their remission status over follow-up. Remitted patients showed a pattern of increasing volume or thickness over follow-up in several ROIs, while nonremitters demonstrated decreasing volume and cortical thinning over time. Although the longitudinal changes themselves were small in magnitude, the opposing direction of change seen in the 2 patient groups is an interesting finding that warrants further study.
Interest Statement:
Dr. Blier received grant funding and/or honoraria for lectures and/or participation in advisory boards for Astra Zeneca, Bristol Myers Squibb, Eli Lilly, Euthymics, Forest, Janssen, Lundbeck, Merck, Otsuka, Pfizer, Pierre Fabre, Servier, Shire, Takeda, and Valeant. Dr. Tremblay has served as a consultant to Lundbeck. All other authors declare no conflicts of interest.
Acknowledgments
This work was supported in part by a grant from the University of Ottawa Medical Research Fund to Dr. Blier. Dr. Phillips was supported by doctoral scholarships from the Canadian Institutes of Health Research, the Ontario Ministry of Colleges and Universities, and the University of Ottawa. The authors thank Andra Smith, PhD, and Guylaine Veillette, RTNM, MR for their technical assistance, and Chantal Hebert, RN, and Maria da Silva for study coordination and administrative support, respectively.
References
- Ahdidan J, Hviid LB, Chakravarty MM, Ravnkilde B, Rosenberg R, Rodell A, Stødkilde-Jørgensen H, Videbech P. (2011) Longitudinal MR study of brain structure and hippocampus volume in major depressive disorder. Acta Psychiatr Scand 123:211–219. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edition, text revision (DSM-IV-TR). Washington, DC: American Psychiatric Press. [Google Scholar]
- Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. (2012) Magnetic resonance imaging studies in unipolar depression: systemic review and meta-regression analyses. Eur Neuropsychopharm 22:1–16. [DOI] [PubMed] [Google Scholar]
- Blier P. (2006) Medication combination and augmentation strategies in the treatment of major depression. In: Stein DJ, Kupfer DJ, Schatzberg AF, editors. The American Psychiatric Publishing Textbook of Mood Disorders. Arlington (VA): Psychiatric Publishing Inc, pp. 509–524. [Google Scholar]
- Bora E, Fornito A, Pantelis C, Yücel M. (2012) Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord 138:9–18. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. (2004) A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 23:724–738. [DOI] [PubMed] [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. (2004) Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 161:598–607. [DOI] [PubMed] [Google Scholar]
- Coryell W, Nopoulos P, Drevets W, Wilson T, Andreasen NC. (2005) Subgenual prefrontal cortex volumes in major depressive disorder and schizophrenia: diagnostic specificity and prognostic implications. Am J Psychiatry 162:1706–1712. [DOI] [PubMed] [Google Scholar]
- Costafreda SG, Chu C, Ashburner J, Fu CH. (2009) Prognostic and diagnostic potential of the structural neuroanatomy of depression. PLoS One 4:e6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. (2002) Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 12:386–394. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Fang P, Zeng LL, Shen H, Wang L, Li B, Liu L, Hu D. (2012) Increased cortical-limbic anatomical network connectivity in major depression revealed by diffusion tensor imaging. PLoS One 7:e45972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First C, Spitzer R, Gibson M, Williams JBW. (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fischl B, Sereno MI, Dale AM. (1999) Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Frodl T, Meisenzahl EV, Zetzsche T, Höhne T, Banac S, Schorr C, Jäger M, Leinsinger G, Bottlender R, Reiser M, Möller H-J. (2004) Hippocampal and amygdala changes in patients with major depressive disorder and healthy controls during 1-year follow-up. J Clin Psychiatry 65:492–499. [DOI] [PubMed] [Google Scholar]
- Frodl T, Koutsouleris N, Bottlender R, Born C, Jäger M, Scupin I, Reiser M, Möller H-J, Meisenzahl EV. (2008a) Depression-related variation in brain morphology over 3 years. Effects of stress? Arch Gen Psychiatry 65:1156–1165. [DOI] [PubMed] [Google Scholar]
- Frodl T, Jäger M, Smajstrlova I, Born C, Bottlender R, Palladino T, Reiser M, Möller H-J, Meisenzahl EM. (2008b) Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci 33:423–430. [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM. (2013) Widespread reductions in gray matter volume in depression. Neuroimage Clin 3:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K-M, Choi S, Jung J, Na K-S, Yoon H-K, Lee M-S, Ham B-J. (2014) Cortical thickness, cortical and subcortical volume, and white matter integrity in patients with their first episode of major depression. J Affect Disord 155:42–48. [DOI] [PubMed] [Google Scholar]
- Hoogenboom WS, Perlis RH, Smoller JW, Zeng-Treitler Q, Gainer VS, Murphy SN, Churchill SE, Kohane IS, Shenton ME, Iosifescu DV. (2014) Feasibility of studying brain morphology in major depressive disorder with structural magnetic resonance imaging and clinical data from the electronic medical record: a pilot study. Psychiatry Res 211:202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järnum H, Eskildsen SF, Steffensen EG, Lundbye-Christensen S, Simonsen CW, Thomsen IS, Fründ E-T, Théberge J, Larsson E-M. (2011) Longitudinal MRI study of cortical thickness, perfusion, and metabolic levels in major depressive disorder. Acta Psychiatr Scand 124:435–446. [DOI] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, Williams SC. (2011) Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68:675–690. [DOI] [PubMed] [Google Scholar]
- Kikinis Z, Fallon JH, Niznikiewicz M, Nestor P, Davidson C, Bobrow L, Pelavin PE, Fischl B, Yendiki A, McCarley RW, Kikinis R, Kubicki M, Shenton ME. (2010) Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophr Res 123:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. (2009) Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp 30:3719–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgaonkar MS, Williams LM, Song LM, Usherwood T, Grieve SM. (2014) Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br J Psychiatry 205:321–328. [DOI] [PubMed] [Google Scholar]
- Li M, Metzger CD, Li W, Safron A, van Tol M-J, Lord A, Krause AL, Borchardt V, Dou W, Genz A, Heinze H-J, He H, Walter M. (2014) Dissociation of glutamate and cortical thickness is restricted to regions subserving trait but not state markers in major depressive disorder. J Affect Disord 169:91–100. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Yucel K, Taylor VH, Macdonald K, Joffe R. (2008) Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol Psychiatry 64:880–883. [DOI] [PubMed] [Google Scholar]
- McKinnon MC, Yucel K, Nazarov A, MacQueen GM. (2009) A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 34:41–54. [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. (1979) A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389. [DOI] [PubMed] [Google Scholar]
- Na KS, Chang HS, Won E, Han KM, Choi S, Tae WS, Yoon HK, Kim YK, Joe SH, Jung IK, Lee MS, Ham BJ. (2014) Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS One 9:e85425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. (1971) The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9:97–113. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci USA 95:13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Batten LA, Aldosary F, Tremblay P, Blier P. (2012) Brain-volume increase with sustained remission in patients with treatment-resistant unipolar depression. J Clin Psychiatry 73:615–631. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. (2011) Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology 36:138–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui L, Lui S, Kuang W, Huang X, Li J, Li J, Zhang J, Chen H, Sweeney JA, Gong Q. (2014) Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry 4:e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. (1995) Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex 5:307–322. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. (1999) Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098. [DOI] [PubMed] [Google Scholar]
- Reuter M, Schmansky NJ, Rosas HD, Fischl B. (2012) Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 61:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. (2004) Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 22:409–418. [DOI] [PubMed] [Google Scholar]
- Schermuly I, Wolf D, Lieb K, Stoeter P, Fellgiebel A. (2011) State dependent posterior hippocampal volume increases in patients with major depressive disorder. J Affect Disord 135:405–409. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Jansen WT, van Willigenburg AP, van der Meulen E, Stassen HH, Thase ME. (2009) Early improvement in the first 2 weeks as a predictor of treatment outcome in patients with major depressive disorder: a meta-analysis including 6562 patients. J Clin Psychiatry 70:344–353. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Waskom ML, Dillon DG, Holmes AJ, Park MTM, Chakravarty MM, Dutra SJ, Polli FE, Iosifescu DV, Fava M, Gabrieli JDE, Pizzagalli DA. (2015) Illness progression, recent stress, and morphometry of hippocampal subfields and medial prefrontal cortex in major depression. Biol Psychiatry 77:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu P-C, Chen L-F, Hsieh J-C, Bai Y-M, Li C-T, Su T-P. (2012) Regional cortical thinning in patients with major depressive disorder: a surface-based morphometry study. Psychiatry Res 202:206–213. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, Katzenbauer M, Grown W, Tepest R, Fernandez G, Buitelaar J, Tendolkar I. (2013) Paralimbic cortical thickness in first-episode depression: evidence for trait-related differences in modd regulation. Am J Psychiatry 170:1477–1486. [DOI] [PubMed] [Google Scholar]
- van Tol MJ, Li M, Metzger CD, Hailla N, Horn DI, Li W, Heinze HJ, Bogerts B, Steiner J, He H, Walter M. (2014) Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med 44:2053–2065. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. (2004) Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966. [DOI] [PubMed] [Google Scholar]