Abstract
Background:
Early life stress has been demonstrated to increase the risk of developing depression in adulthood. However, the roles and associated molecular mechanisms of stresses in the onset and relapse of depression have yet to be fully elucidated.
Methods:
Depression-like behaviors were induced in rats by maternal deprivation and chronic unpredictable stress. Depression- and anxiety-like behaviors of rats, dopamine receptor D2 level, and microRNAs expression in rats’ brain tissues were measured.
Results:
Chronic unpredictable stress alone induced depression-like behaviors in rats, but maternal deprivation enhanced the effect of chronic unpredictable stress. Escitalopram significantly decreased depression-like behaviors in chronic unpredictable stress rats but was less effective in maternal deprivation with chronic unpredictable stress rats. Maternal deprivation increased dopamine receptor D2 messenger RNA expression and decreased microRNA-9 expression in the striatum. Chronic unpredictable stress increased dopamine receptor D2 mRNA and protein levels and decreased microRNA-9 expression in the nucleus accumbens. Furthermore, maternal deprivation enhanced the effect of chronic unpredictable stress on dopamine receptor D2 gene and microRNA-9 expression. Chronic unpredictable stress increased the expression of microRNA-326 in the nucleus accumbens but decreased it in the striatum, whereas maternal deprivation elevated microRNA-326 expression in the striatum. Escitalopram normalized microRNA-326 expression but had no effect on the expression of microRNA-9, dopamine receptor D2 mRNA, and dopamine receptor D2 protein in both the nucleus accumbens and striatum. The in vitro study showed that only microRNA-9 directly targeted the 3’ untranslated region of dopamine receptor D2 mRNA and inhibited dopamine receptor D2 protein expression.
Conclusion:
Early life stress enhanced the susceptibility to late life stress and resistance to escitalopram treatment through decreasing microRNA-9 expression and subsequently upregulating dopamine receptor D2 expression in the nucleus accumbens. microRNA-326 may be a novel target of escitalopram.
Keywords: depression, dopamine receptor D2, microRNAs, stress
Introduction
Depression is a common psychiatric illness that is often described as a stress-related disorder. There is compelling evidence suggesting that both the onset and relapse of depression are associated with environmental stresses (Monroe et al., 2006; Pittenger and Duman, 2007). Moreover, preclinical and clinical studies have shown that early life stress can affect neural development and lead to changes in psychopathology, subsequently increasing the risk of developing depression in adulthood (Nestler et al., 2002; Korosi and Baram, 2009). However, the neurobiological mechanisms associated with the vulnerability induced by adverse stress during early life remain unclear.
The mesolimbic dopamine (DA) system is involved in the regulation of motivation, reward, volition, attention, and mood, all of which are likely to be impaired in patients with depression (Dunlop and Nemeroff, 2007). A large body of evidence has consistently revealed that DA system dysfunction is associated with depression-like behaviors (Lucas et al., 2004; Zhu et al., 2011). Moreover, DA receptor agonists can enhance the antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) (Basso et al., 2005). Although SSRIs are the most frequently used antidepressants in the clinic, they do not principally address the DA system (Basso et al., 2005). In addition, psychological stress affects not only the release and metabolism of DA but also the density of DA receptors, such as dopamine receptor D2 (DRD2), which is abundantly expressed within the nucleus accumbens (NAc) and striatum (Cabib and Puglisi-Allegra, 2012). Rats exposed to chronic stress exhibit increased DRD2 expression in the striatum and NAc (Lucas et al., 2004). Moreover, early postnatal life is a critical period for the development of the DA system (Sillivan et al., 2011). Our previous study showed that maternal deprivation, an animal model of early life adversity, leads to dysregulation in DRD2 mRNA expression in the striatum of rats with depression-like behaviors (Zhu et al., 2011). However, the molecular mechanisms regulating DRD2 expression have yet to be fully understood.
microRNAs (miRNAs) are small (approximately 22bp), single-stranded, noncoding RNAs involved in posttranscriptional regulation of genes. Several studies identified aberrant miRNA expression in patients with depression and in rodents with depression-like behavior (O’connor et al, 2012; Zhang et al, 2013). A previous study revealed that antidepressants can actually target miRNA (Baudry et al., 2010). Furthermore, miRNAs can mediate the development and function of the nervous system, including the DA system. For example, microRNA-133b regulates the maturation and function of midbrain DA neurons within a negative feedback circuit (Heyer et al, 2012). microRNA-504 has been found to upregulate the expression of DRD1 (Huang et al, 2009). However, the miRNAs that regulate DRD2 expression in the brains of stressed animals have yet to be identified.
We therefore hypothesize that early life stress enhances vulnerability to chronic stress through regulating miRNAs’ expression and subsequent DRD2 gene expression in the NAc and striatum of adult rats. To verify this hypothesis, maternal deprivation (MD) and chronic unpredictable stress (CUS), 2 classic stress paradigms that reflect the stresses present in early life and adulthood, were used to establish animal models of stress. Behavioral data were obtained from the open field test, sucrose preference test, and forced swimming test. The relationships between the expression of DRD2 and the associated miRNAs in the NAc and striatum, as well as the behavioral consequences, were investigated. Escitalopram, one of the most effective SSRIs for the treatment of depression (Kirino, 2012), was used to investigate the effects of an SSRI on depression-like behaviors and the expression of DRD2 and miRNAs.
Methods
Animals
Pregnant adult Sprague-Dawley rats (Slac Laboratory Animal Inc., Shanghai, China) were housed on a 12-h-light/-dark cycle with food and water provided ad libitum and checked daily until delivery. The day of delivery was designated postnatal day (PND) 0. Newborn male pups were randomly assigned to 4 groups: normal control (NOR, n=15), maternal deprivation (MD, n=12), chronic unpredictable stress (CUS, n=11), and MD with CUS (MD+CUS, n=11). Pups in the MD group received MD from PND1 to PND14, rats in the CUS group received CUS for 28 days at 10 weeks old, and rats in the MD+CUS group received both MD and CUS. Rats in NOR group were housed with their mothers until PND21. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and Chinese legislation on the use and care of laboratory animals.
MD
The MD paradigm was conducted by following our established protocol (Bai et al., 2012). Briefly, pups were separated from their mothers and placed in a single cell for 6 hours each day from 9:00 am to 3:00 pm and from PND 1 to PND 14. After 6 hours, pups were returned to their respective cages with their mothers. Pups were weaned at PND 21 and housed in groups of 2.
CUS
The CUS paradigm was executed as previously described (Zhang et al., 2013). Briefly, rats at 10 weeks old were randomly exposed to one stressor once a day for 28 days. The stressors included water deprivation for 24 hours, food deprivation for 24 hours, exposure to an elevated open platform (10 cm×10cm, 160cm in height) for 2 hours, restraint stress for 2 hours, and electric foot shock for 20 seconds (0.8mA, 1-second duration, average 1 shock/10 s). Stressors were given at different times of the day to establish unpredictability.
Escitalopram Treatment
At week 14, rats from each group were randomly given treatments of 5mg/kg of escitalopram (manufactured by H. Lundeck A/S, Copenhagen, Denmark) or saline. Escitalopram (dissolved in 0.9% saline at 1mg/mL) or saline was administered intraperitoneally each day in the morning for 4 weeks. To maintain depression-like behavior, CUS was continuously given to rats in the CUS and MD+CUS groups during escitalopram treatment. Behaviors of all rats were tested after escitalopram treatment.
Open Field Test
Animals were first placed in the center of a rectangular area (50×83×56cm) and allowed to explore freely. Rats’ activities were monitored for 5 minutes using a video camera connected to a computer. A computerized tracking system (Ethovision 1.50, Noldus IT, Wageningen, Netherlands) was used to record the ambulatory distance, number of vertical movements, and number of fecal pellets to assess locomotor activity, exploration, and anxiety-like behaviors, respectively. After each trial, the arena was thoroughly cleaned with 75% ethanol.
Sucrose Preference Test
The sucrose preference test spanned a total of 3 days and rats were housed individually (Lin et al., 2005). On the first day, rats were given free access to 2 bottles of sucrose solution (1%, wt/vol and 100g). On the second day, 1 bottle of sucrose solution was replaced with water. On the third day, rats were deprived of water and food for 23 hours and then given free access to 2 preweighed bottles of solution for 1 hour. The positions of the water and sucrose bottles were switched. The volume of liquid consumed from both bottles was recorded. The sucrose preference rate was calculated to assess the degree of anhedonia in rats using the following formula: sucrose preference rate=sucrose consumption (g)/[water consumption (g) +sucrose consumption (g)] ×100%.
Forced Swimming Test
Two swimming sessions were conducted: a 15-minute pretest on the first day followed by a 5-minute test the next day (Weaver et al., 2005). During the test, rats were placed individually in glass cylinders (21cm in diameter × 46cm in height) filled with water (25°C, 30cm in depth). After swimming for 15 minutes on day 1, rats were dried with towels and placed back in their home cages. At the end of the trial, the water in the cylinders was emptied and refilled. Twenty-four hours after the first trial, rats were placed in the swimming apparatus again for a 5-minute test trial. A video camera was used to record the activities of rats. The immobility time (time a rat spent in keeping its head above water with only slight movements) was calculated to measure behavioral despair.
Real-Time Reverse Transcription Quantitative PCR
After behavioral tests, rats were euthanized and whole NAc and the striatum were immediately collected as previously described (Zhu et al., 2011; Zhang et al., 2013). Total RNA was isolated from dissected brain tissue and cells using TRIzol reagent (Life Technologies) with 1 additional extraction with chloroform. Reverse transcription was performed using One Step PrimeScript miRNA cDNA Synthesis Kit (Perfect Real Time) and PrimeScript RT reagent Kit (Perfect Real Time) (both TaKaRa, Japan) for miRNA and mRNA, respectively. Real-time quantitative PCR was performed using SYBR Premix Ex Taq II (TaKaRa, Japan). The primers used for miRNAs and U6, DRD2, and β-actin are listed in Table 1. Relative quantification of gene expression was conducted using the Applied Biosystems 7500 Fast Real Time PCR System, and data analysis was performed using the comparative ΔΔCT method. U6 and β-actin mRNAs were used as internal control for miRNA and mRNA expression, respectively.
Table 1.
Sequences for Primers or miRNAs
| Sequences | |
|---|---|
| Primer for Real time RT-PCR | |
| Rat | |
| miR-9 | Forward: 5’-TCTTTGGTTATCTAGCTGTATGA-3’ |
| miR-141 | Forward: 5’-TAACACTGTCTGGTAAAGATGG-3’ |
| miR-200a | Forward: 5’-TAACACTGTCTGGTAACGATGT-3’ |
| miR-326 | Forward: 5’-CAAACGCCCTTCCTCCAGTA-3’ |
| U6 | Forward: 5’-GCAAGGATGACACGCAAATTC-3’ |
| DRD2 | Forward: 5’-CTTGATAGTCAGCCTTGCTGTG-3’ |
| Reverse: 5’-AGGGCACGTAGAATGAGACAAT-3’ | |
| β-actin | Forward: 5’-GGAGATTACTGCCCTGGCTCCTA-3’ |
| Reverse: 5’-GACTCATCGTACTCCTGCTTGCTG-3’ | |
| Human | |
| miR-9 | Forward: 5’-CTCTTTGGTTATCTAGCTGTATGA-3’ |
| miR-326 | Forward: 5’-GGCCCTTCCTCCAGAAAAAA-3’ |
| U6 | Forward: 5’-GCTTCGGCAGCACATATACTAAAAT-3’ |
| DRD2 | Forward: 5’-CCAGAGATTCCCAAGCCAAA-3’ |
| Reverse: 5’-GGTAAGAGGGCAAAGGATGAG-3’ | |
| β-actin | Forward: 5’-GCGGAAGTATTCTGTTTGGATTG -3’ |
| Reverse: 5’-GAGGACCAGCCTCATCATATTC -3’ | |
| Primer for PCR (Human) | |
| DRD2 3’UTR WT | Forward: 5’-AATCTCGAGCTCTGCTGCCTGCCCGCAC-3’ |
| Reverse: 5’-GGAGCGGCCGCGAAGGTGACTCGTCAAAG-3’ | |
| DRD2 3’UTR MUT | Forward: 5’-CCTTGGCCAATGTTCTGCAGCCGCCTTCCTTGAC-3’ |
| Reverse: 5’-GGCTGCAGAACATTGGCCAAGGATAGGGGGACTG-3’ | |
| miRNAs Mimics | |
| NC | Sense: 5’-UUCUCCGAACGUGUCACGUTT-3’ |
| Antisense 5’-ACGUGACACGUUCGGAGAATT-3’ | |
| miR-9 | Sense: 5’- UCUUUGGUUAUCUAGCUGUAUGA-3’ |
| Antisense 5’-AUACAGCUAGAUAACCAAAGAUU-3’ | |
| miR-326 | Sense: 5’-CCUCUGGGCCCUUCCUCCAG-3’ |
| Antisense 5’-GGAGGAAGGGCCCAGAGGUU-3’ | |
| miRNAs Inhibitor | |
| NC | 5’-CAGUACUUUUGUGUAGUACAA-3’ |
| miR-9 | 5’-UCAUACAGCUAGAUAACCAAAGA-3’ |
| miR-326 | 5’-CUGGAGGAAGGGCCCAGAGG-3’ |
Abbreviations: DRD2, dopamine receptor D2; MUT, mutation; WT, wild-type.
*The reverse primers of miRNAs and U6 were included in the kit, but no sequence was provided.
Western Blot
The rabbit polyclonal anti-DRD2 antibody was purchased from Santa Cruz Biotechnology (San Diego, CA). The mouse monoclonal anti-β-actin antibody and horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG were purchased from Sigma-Aldrich (St. Louis, MO). Total protein was extracted and Western blot was conducted as previously described (Zhang et al., 2006). Thirty micrograms of total protein was loaded onto a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. The same blot was reprobed for β-actin to serve as a loading control. The intensity of each band was quantified with Bio-Rad Quantity One software (Bio-Rad, Hercules, CA).
Bioinformatic Sequence Analysis
Prediction of miRNA binding to the DRD2 (NM_000795, NM_016574) 3’ untranslated region (3’-UTR) was performed by algorithms obtained from TargetScan (http://www.targetscan.org), PicTar (http://pictar.mdc-berlin.de), miRanda (http://www.microrna.org), and miRWalk (http://www.umm.uniheidelberg.de/apps/zmf/mirwalk). Results of predicted targets were checked with previous findings in neurons associated with psychopathologies. In this study, miR-9, miR-141, miR-200a, and miR-326 were identified as candidate miRNAs of the DRD2 gene.
Cell Culture
SH-SY5Y and 293T cell lines were obtained from Shanghai Institute of Cell Biology (China) and cultured in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 plus 10% fetal calf serum, 2mM l-glutamine, 100U/mL penicillin, and 100 µg/mL streptomycin (Gibco-BRL) at 37°C with 5% CO2.
Silencing of DRD2 Expression by miRNA
MiRNA-mimics and miRNA-inhibitors (sequences shown in Table 1) were synthesized (Gene Pharma) in the form of small interfering RNA duplexes or single strands according to Park’s study (Park et al., 2009). SH-SY5Y cells in each 6-well plate were transfected with 150ng miRNA and 1.5mL of Hepfect reagent according to the manufacturer’s instructions (Qiagen, Germany). SH-SY5Y cell line was chosen, because SH-SY5Y cells express human DRD2 mRNA and protein.
Dual-Luciferase Reporter Assay
Human DRD2 mRNA 3’-UTR (1132bp) (wild-type, WT) containing the putative binding site of miR-9 and its identical sequence with a mutation of the miR-9 seed sequence (mutation, MUT) were amplified by PCR (primer sequences listed in Table 1) from a human cDNA library, inserted into the XhoI and NotI sites of Firefly/Renilla luciferase reporter vector pmiR-RB-REPORT (RiboBio Co) and validated by sequencing. 293T cells were plated at 1×105 cells/well on 24-well plates 1 day before transfection and cotransfected with 30ng of pmiR-RB-DRD2 -3’UTR (or MUT) plasmid and 150ng of miR-9 mimics or NC mimics using lipofectamine 2000 transfection reagent according to the manufacturer’s instructions (Life Technologies). 293T cell line was used, because 293T cells do not express DRD2 but have a high transfection efficacy. Therefore, use of 293T cells can accurately reflect the interaction between the tested miRNA and the 3’-UTR of interest gene. Twenty-four hours posttransfection, firefly and renilla luciferase activities were consecutively measured according to the dual-luciferase assay manual (Promega, Madison, WI). The renilla luciferase signal was normalized to the firefly luciferase signal for each individual analysis. All transfections were performed in triplicate, and the experiment was replicated at least twice.
Statistical Analysis
The data were presented as M±SD. Data analysis was performed using the statistical software SPSS 17.0. Mean comparisons were performed using 2-way ANOVA or 1-way ANOVA. Linear regression analysis was used to investigate correlation between behavior indices (as dependent variables) and gene expression (as independent variables) as well as between DRD2 protein expression (as dependent variables) and miRNAs (as dependent variables). A P<.05 was considered statistically significant.
Results
The Behavioral Responses of MD Rats to CUS and Escitalopram
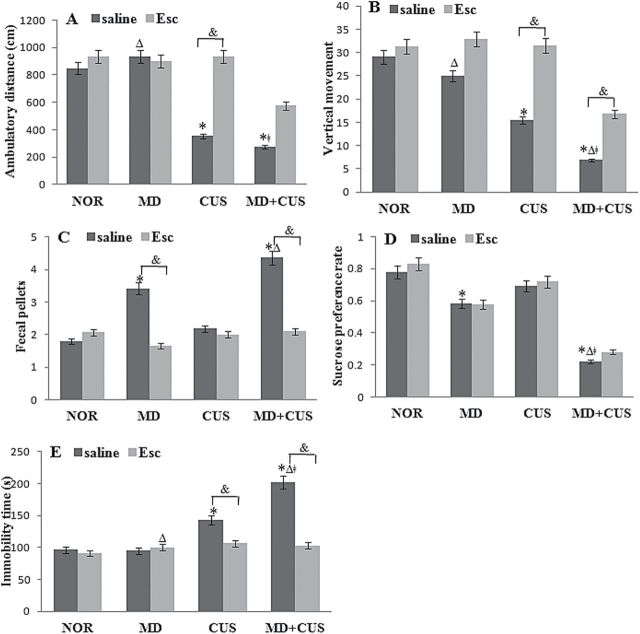
In the open field test, there was a significant main effect of CUS (F =41.59, P<.01) on the ambulatory distance. The ambulatory distance was significantly shorter in adult CUS rats (including CUS alone rats and MD+CUS rats) compared with adult non-CUS rats (including NOR rats and MD alone rats) (Figure 1A). There was a significant main effect of CUS (F =49.13, P<.01) and MD (F =7.93, P<.01) on the number of vertical movements. The number of vertical movements was significantly less in adult CUS rats than in adult non-CUS rats and significantly less in adult MD rats (including MD alone rats and MD+CUS rats) than in adult non-MD rats (including NOR rats and CUS alone rats). However, there were no interactions of MD×CUS on the ambulatory distance and number of vertical movements (Figure 1B). There was a significant main effect of MD (F =14.66, P<.01) on the number of fecal pellets. The number of fecal pellets was significantly higher in adult MD rats than in adult non-MD rats. There was neither a main effect of CUS nor the interaction of CUS and MD on the number of fecal pellets (Figure 1C). Escitalopram significantly reversed CUS-induced decreases in ambulatory distance (F=7.50, P<.01) and number of vertical movements (F=4.76, P<.05) while normalizing the number of fecal pellets (F=3.11, P<.05) in MD rats. The escitalopram increased the ambulatory distance (F=10.14, P<.01) and the number of vertical movements (F=17.18, P<.01) and fecal pellets (F=7.44, P<.01) in MD+CUS rats (Figure 1A-C).
Figure 1.
Comparison of behavioral indices. (A-E) Comparison of ambulatory distance (A), the number of vertical movements (B), fecal pellets (C), sucrose preference of rate (D), and immobility time (E) among groups. * P< .05 vs NOR for saline treatment; Δ P < .05 vs CUS for saline treatment; ǂ P < .05 vs MD for saline treatment; & P < .05 between 2 treatments.
In the sucrose preference test, significant main effects of MD (F=59.72, P<.01), CUS (F=25.97, P<.01), and their interaction (F=9.85, P<.01) on sucrose preference rate were observed. A significant difference in sucrose preference rate was observed between groups (Figure 1D). There was no significant effect of escitalopram on sucrose preference rate of rats in MD and MD+CUS group (Figure 1D).
In the forced swimming test, significant main effects of MD (F=7.45, P<.01), CUS (F=52.07, P<.01), and their interaction (F=8.06, P<.01) on immobility time were observed. A significant difference in immobility time was observed between groups (Figure 1E). Escitalopram significantly normalized the immobility time in CUS (F=7.58, P<.01) and MD+CUS (F=26.2, P<.01) rats (Figure 1E).
DRD2 Gene Expression in the NAc and Striatum of Stress-Induced Rats
A significant effect of CUS (F=126.15, P<.05) on DRD2 mRNA expression in the NAc was observed. The expression of DRD2 mRNA in the NAc of adult CUS rats was significantly higher than that in adult non-CUS rats (P<.05). However, there was neither a significant main effect of MD nor the interaction of MD×CUS on the expression of DRD2 mRNA in the NAc (Figure 2A). There were significant main effects of MD (F=37.15, P<.05), CUS (F=95.39, P<.05), and their interaction (F=27.62, P<.05) on the expression of DRD2 protein in the NAc. A significant difference in DRD2 protein levels was observed in the NAc between groups (Figure 2B). Escitalopram treatment did not normalize DRD2 mRNA and protein levels in CUS or MD+CUS rats (Figure2A-B).
Figure 2.
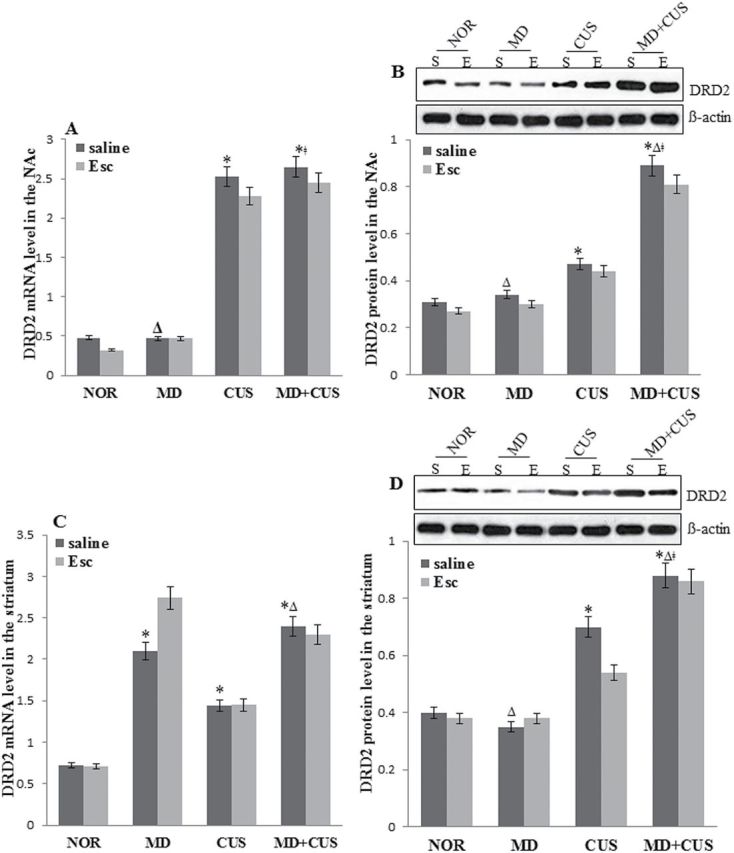
Dopamine receptor D2 (DRD2) protein and mRNA expression in the nucleus accumbens (NAc) and striatum. Comparison of DRD2 mRNA (A) and protein (B) expression in the NAc among groups. Comparison of DRD2 mRNA (C) and protein (D) expression in the striatum among groups. * P<.05 vs normal control (NOR) for saline treatment; Δ P<.05 vs chronic unpredictable stress (CUS) for saline treatment; ǂ P<.05 vs maternal deprivation (MD) for saline treatment. E, escitalopamine; S, saline.
In the striatum, significant main effects of MD (F=22.41, P<.05) and CUS (F=4.37, P<.05), but not the interaction between MD and CUS, on DRD2 mRNA was observed. The expression of DRD2 mRNA in the striatum in adult MD and adult CUS rats was significantly higher than that in adult non-MD and adult non-CUS rats, respectively (Figure 2C). Significant main effects of CUS (F=87.80, P<.05) and interaction of MD×CUS on the expression of DRD2 protein (F=7.41, P<.05) were observed. Significant difference in DRD2 protein levels was observed in the striatum between groups (Figure 2D). Escitalopram treatment did not show any effect on DRD2 mRNA and protein level in stressed rats (Figure 2C-D).
miRNAs Levels in the NAc and Striatum of Stress-Induced Rats
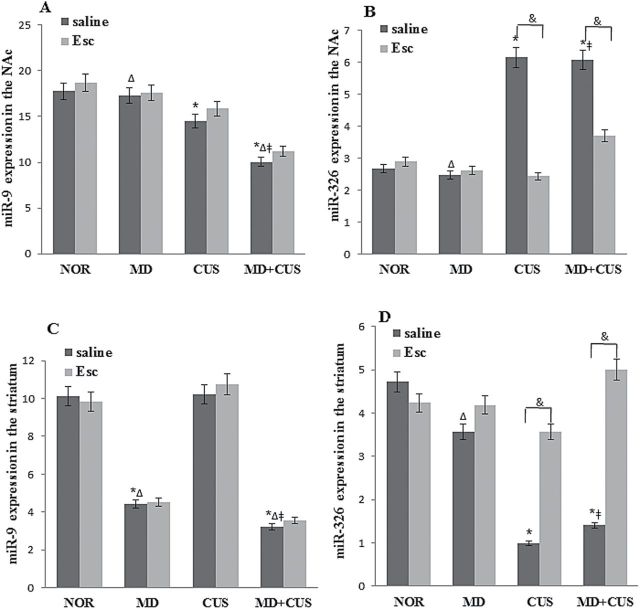
MiR-9, miR-141, miR-200a, and miR-326 were identified as the miRNAs targeting the 3’-UTR of the DRD2 gene by bioinformatic analysis, and their levels in the NAc and striatum were measured. Significant main effects of MD (F=8.06, P<.05), CUS (F=36.93, P<.05), and the interaction of MD×CUS (F=5.33, P<.05) on miR-9 expression were observed in the NAc. A significant difference in miR-9 levels was observed in the NAc between groups (Figure 3A). A significant main effect of CUS (F=84.88, P<.05) on miR-326 expression in the NAc of rats was observed. The adult CUS rats had a significantly higher level of miR-326 expression than adult non-CUS rats (P<.05), while there were neither a significant main effect of MD nor the interaction of MD×CUS on the expression of miR-326 in the NAc (Figure 3B). The decreased level of miR-9 in CUS and MD+CUS rats was not reversed by escitalopram treatments, while the elevated expression of miR-326 in CUS (F=37.74, P<.05) and MD+CUS (F=26.28, P<.05) rats was reversed by escitalopram to normal levels (Figure 3A-B).
Figure 3.
Expression of miRNAs in the nucleus accumbens (NAc) and striatum. Comparison of miR-9 (A) and miR-326 (B) expression in the NAc among groups. Comparison of miR-9 (C) and miR-326 (D) expression in the striatum among groups. * P<.05 vs normal control (NOR) for saline treatment; Δ P<.05 vs chronic unpredictable stress (CUS) for saline treatment; ǂ P<.05 vs maternal deprivation (MD) for saline treatment. & P<.05 between 2 treatments.
In the striatum, a significant main effect of MD on miR-9 (F=231.36, P<.05) was observed. The expression of miR-9 in the striatum of adult MD rats was significantly lower than that of adult non-MD rats. There was a significant main effect of CUS (F=311.39, P<.05) and MD (F=5.19, P<.05) as well as a significant interaction of MD×CUS (F=22.22, P<.05) on miR-326 level (Figure 3C). A significant difference in miR-326 levels was observed in the striatum between groups (Figure 3D). There was no difference in miR-9 expression between saline and escitalopram injection in MD alone and MD+CUS rats. The decreased expression of miR-326 in MD alone (F=4.13, P<.05), CUS alone (F=11.19, P<.05), and MD+CUS (F=24.24, P<.05) rats was normalized to the levels in NOR rats by escitalopram (Figure 3C-D). In addition, the expression of miR-141 and miR-200a in the NAc and striatum were not significantly affected by any treatment (stress or escitalopram) (data not shown).
Linear Regression Analysis of Depression-Like Behaviors and Gene Expression
Linear regression analysis of gene expression and behaviors measured in open field test was performed. The expression of miR-9 in the NAc and miR-326 expression in the striatum were positively correlated with ambulatory distance, while miR-326 in the NAc was negatively correlated with ambulatory distance. Moreover, the expression of DRD2 mRNA and protein in the NAc and DRD2 protein in the striatum were significantly negatively correlated with the ambulatory distance (Table 2). The miR-9 and miR-326 expression in the NAc and striatum were positively correlated with the number of vertical movements, except miR-326 expression in the striatum, which was negatively correlated with the number of vertical movements. Moreover, the expression of DRD2 mRNA and protein in both the NAc and striatum were significantly negatively correlated with the number of vertical movements (Table 2). The miR-9 expression in both the NAc and striatum were significantly correlated with the number of fecal pellets. The expression of DRD2 protein in the NAc and DRD2 mRNA in the striatum showed significantly positive correlation with the number of fecal pellets (Table 2)
Table 2.
Correlations between Behavioral Index and Gene Expression
| Ambulatory Distance | Vertical Counts | Fecal Pellets | Sucrose Preference Rate | Body Weight | Immobility Time | ||
|---|---|---|---|---|---|---|---|
| In the NAc | |||||||
| miR-9 | F(p) | 23.39 (.00) | 17.50 (.00) | 4.78 (.03) | 18.10 (.00) | 12.26 (.00) | 27.30 (.00) |
| R2 | 0.332 | 0.271 | .09 | 0.278 | 0.207 | 0.37 | |
| β(p) | 0.58 (.00) | 0.52 (.00) | -0.30 (.03) | 0.53 (.00) | 0.46 (.00) | -0.61 (.00) | |
| miR-326 | F(p) | 30.67 (.00) | 16.50 (.00) | 1.23 (0.27) | 8.11 (.01) | 3.00 (.09) | 14.49 (.00) |
| R2 | 0.395 | 0.260 | .026 | 0.147 | .060 | 0.236 | |
| β(p) | -0.63 (.00) | -0.51 (.00) | 0.16 (0.27) | -0.38 (.01) | -0.25 (.09) | 0.49 (.00) | |
| DRD2 mRNA | F(p) | 40.27 (.00) | 37.74 (.00) | 0.77 (0.39) | 14.76 (.00) | 5.50 (.02) | 33.28 (.00) |
| R2 | 0.461 | 0.432 | .016 | 0.239 | 0.105 | 0.415 | |
| β(p) | -0.68 (.00) | -0.66 (.00) | 0.13 (0.39) | -0.49 (.00) | -0.32 (.02) | 0.64 (.00) | |
| DRD2 Protein | F(p) | 21.11 (.00) | 31.70 (.00) | 5.41 (.02) | 40.99 (.00) | 12.58 (.00) | 37.66 (.00) |
| R2 | 0.310 | 0.403 | 0.103 | 0.466 | 0.211 | 0.445 | |
| β(p) | -0.56 (.00) | -0.64 (.00) | 0.32 (.02) | -0.68 (.00) | -0.46 (.00) | 0.67 (.00) | |
| In the striatum | |||||||
| miR-9 | F(p) | 0.26 (0.62) | 6.71 (.01) | 18.42 (.00) | 35.87 (.00) | 42.64 (.00) | 4.75 (.03) |
| R2 | .005 | 0.125 | 0.282 | 0.433 | 0.476 | .092 | |
| β(p) | .07 (0.62) | 0.35 (.01) | -0.53 (.00) | 0.66 (.00) | 0.69 (.00) | -0.30 (.03) | |
| miR-326 | F(p) | 29.14 (.00) | 32.59 (.00) | 2.65 (0.11) | 12.20 (.00) | 12.33 (.00) | 18.94 (.00) |
| R2 | 0.383 | 0.409 | .053 | 0.206 | 0.208 | 0.287 | |
| β(p) | 0.62 (.00) | 0.64 (.00) | -0.23 (0.11) | 0.45 (.00) | 0.46 (.00) | -0.54 (.00) | |
| DRD2 mRNA | F(p) | 0.55 (0.46) | 6.62 (.01) | 12.39(.00) | 13.00 (.00) | 24.21 (.00) | 2.91 (.01) |
| R2 | .011 | 0.123 | 0.209 | 0.217 | 0.34 | .058 | |
| β(p) | -0.11 (0.46) | -0.35 (.01) | 0.46 (.00) | -0.47 (.00) | -0.58 (.00) | 0.24 (.01) | |
| DRD2 Protein | F(p) | 13.68 (.00) | 25.52 (.00) | 2.38 (0.13) | 10.60 (.00) | 6.02 (.02) | 20.79 (.00) |
| R2 | 0.225 | 0.352 | .048 | 0.184 | 0.114 | 0.307 | |
| β(p) | -0.48 (.00) | -0.59 (.00) | 0.22 (0.13) | -0.43 (.00) | -0.34 (.02) | 0.55 (.00) | |
Abbreviations: NAc, nucleus accumbens.
Linear regression analysis of gene expression and behaviors in the sucrose preference test showed that the preference rate was positively correlated with the expression of miR-9 but negatively correlated with the expression of DRD2 mRNA and protein in the NAc and striatum. However, the preference rate was negatively correlated with miR-326 expression in the NAc but positively correlated with miR-326 expression in the striatum (Table 2).
Linear regression analysis of gene expression and behavior in the forced swimming test showed that the immobility time was significantly positively correlated with the expression of miR-326, DRD2 mRNA and protein in the NAc, and DRD2 protein in the striatum but negatively correlated with the expression of miR-9 in both the NAc and striatum and miR-326 in the striatum (Table 2).
In contrast, the expression of miR-141 and miR-200a in the NAc and striatum were not significantly correlated with any behaviors in the open field test, sucrose preference test, or forced swimming test (data not shown).
Linear Regression Analysis of miRNAs and DRD2 Protein Expression
The linear regression analysis showed that the expression of miR-9 and miR-326 was significantly correlated with the expression of DRD2 protein in the NAc (β =-0.670, P<.01 for miR-9; β=0.613, P<.01 for miR-326). In the striatum, the expression of miR-326 (β =-0.65, P<.01), but not miR-9 (β =-0.20, P=.16), was significantly negatively correlated with the expression of the DRD2 protein. The expression of miR-141 and miR-200a in both the NAc and striatum were not significantly correlated with the expression of DRD2 protein.
MiRNAs Targeted DRD2 in Vitro
To establish the direct relationship between miRNAs and DRD2 expression, SH-SY5Y cells were cotransfected with fluorescent RNA and miR-9 mimics, miR-9 inhibitor, miR-326 mimics, miR-326 inhibitor, or negative control (Figure 4A-B). Transfection with miR-9 mimics, but not the inhibitor, significantly increased miR-9 levels compared with the negative control (F=7.34, P<.05). Transfection with miR-326 mimics significantly increased, while transfection with miR-326 inhibitor significantly decreased, miR-326 levels compared with negative control (P<.01) (Figure 4C). MiR-9 mimics, but not miR-9 inhibitor, significantly suppressed DRD2 protein levels compared with negative control (F=48.16, P<.01). Neither miR-326 mimics nor the miR-326 inhibitor influenced DRD2 protein levels (Figure 4E). Moreover, the expression of DRD2 mRNA was stable and was not affected by any treatment (Figure 4D).
Figure 4.
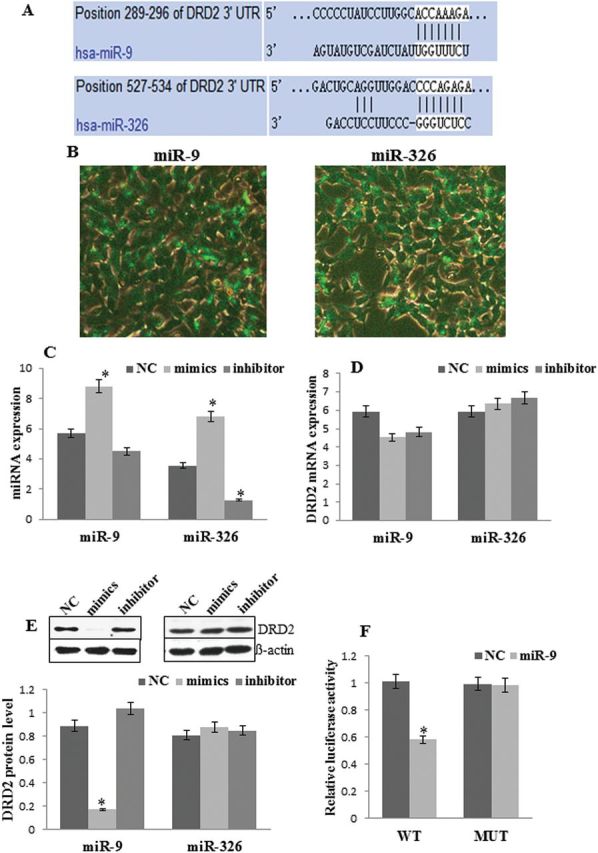
microRNAs (miRNAs) regulated dopamine receptor D2 (DRD2) expression in vitro. (A) Nucleotide base of DRD2 mRNA 3’ untranslated region (3’-UTR) pairs with miR-9 and miR-326. (B) The expression of FAM (green) after transfection of miR-9 and miR-326 into SH-SY5Y cell. (C) The expression of miR-9 and miR-326 after transfection of mimics or inhibitor into SH-SY5Y cell. (D) The expression of DRD2 mRNA after transfection of mimics or inhibitor into SH-SY5Y cell. (E) Western blot of DRD2 and ß-actin after transfection of mimics and inhibitor into SH-SY5Y cell. (F) Relative luciferase activity of DRD2 (wild-type, WT) 3’UTR and its mutation (MUT) reporter after cotransfection of plasmid and miR-9 mimics (or negative control mimics) into 293T cell. *P < .05.
Dual-Luciferase reporter assay system was used to investigate whether miR-9 regulated the expression of DRD2 via directly binding to the DRD2 mRNA 3’UTR. The results showed that cotransfection of pmiR-RB-DRD2-3’UTR WT plasmid with miR-9 mimics, but not cotransfection of pmiR-RB-DRD2 -3’UTR MUT with miR-9 mimics, significantly decreased luciferase reporter activity in 293T cells (F=24.63, P<.01) (Figure 4F).
Discussion
Exposure to stressful events has been reported to be one of the most powerful triggering factors for several psychiatric disorders (Friedman et al., 2011). Exposure to stresses in early life can disrupt stress-responsive biological regulatory systems, which might ultimately lead to an increased vulnerability towards developing depression later in life (Korosi et al., 2012). In this study, neither CUS nor MD alone altered the rate of sucrose preference, but MD followed by CUS significantly decreased the sucrose preference rate. CUS but not MD induced despair-like behavior, whereas MD enhanced the effect of response to CUS and increased immobility time compared with CUS, suggesting that MD enhanced the susceptibility to become depressed during adulthood (Zhang et al., 2013; Uchida et al., 2010). In addition, MD induced anxiety-like behaviors, whereas CUS decreased locomotor activity and exploration of rats. Therefore, early life stress affects the ability of rats to cope adequately with chronic stress in adulthood. However, MD or CUS can also induce depression-like behaviors. Thus, early life stress induced anxiety-like behavior and decreased exploration, while chronic stress during adulthood induced despair-like behavior and decreased locomotor activity and exploration.
Consistent with previous studies, depression-like and anxiety-like behaviors induced by CUS or MD were ameliorated by escitalopram treatment. In contrast, the anhedonia and despair in MD+CUS rats were not reversed by 4-week escitalopram treatment. The differential response to escitalopram could be explained by MD enhancing the risk for developing depression-like behaviors, but also increasing escitalopram treatment resistance at the same time. Second, perhaps because depression may also be associated with strong deficits in the DA system, SSRIs do not principally address depression, especially anhedonia (Scheggi et al., 2011; Landau et al., 2011).
Numerous studies have demonstrated that depression is associated with increased binding of DRD2 in the NAc and striatum where DRD2 is most abundantly expressed (Shah et al.,1997). A preclinical study revealed that chronic social stress increased DRD2, but not DRD1 receptor expression, in the striatum of rats (Lucas et al., 2004), Consistent with previous study, MD increased the expression of DRD2 mRNA in the striatum, while CUS increased DRD2 mRNA and protein in the NAc. This may suggest that DA receptor function varies across different brain regions in response to different stresses. Moreover, there was a higher level of DRD2 protein in MD+CUS rats than in CUS rats, suggesting that MD enhanced the effect of CUS on DRD2 gene expression. In addition, DRD2 overexpression was significantly associated with depression-like behaviors. These results suggested that up-regulation of DRD2 was a possible mechanism for the development of depression-like behaviors in maternally deprived rats. In addition, this study revealed that escitalopram did not reverse the aberrant expression of DRD2 mRNA and protein.
MicroRNAs (miRNAs) represent a class of small non-coding RNAs regulating gene expression by inducing RNA degradation or interfering with translation. Aberrant miRNA expression has been reported in several mood disorders. miR-9 is abundantly expressed in vertebrate brains (Packer et al., 2008) and was found to be aberrant in stress-induced rats or depressed patients (Uchida et al., 2010; Meerson and Cacheaux, 2010). In the present study, miR-9 was found to be downregulated in the NAc of CUS rats and in the striatum of MD rats, suggesting that miR-9 in the NAc was sensitive to stress in adulthood, but miR-9 in the striatum to early life stress. Moreover, we first demonstrated that CUS combined with MD more significantly decreased miR-9 expression in both the NAc and striatum compared to MD or CUS only. The decreased miR-9 expression was significantly associated with depressive phenotypes. Escitalopram did not reverse decreased miR-9 expression in either the NAc or striatum. These results indicated that downregulation of miR-9 may be responsible for development of stress susceptibility and escitalopram treatment resistance of rodents exposed to MD during early postnatal period. Furthermore, aberrant miR-9 expression in the NAc, but not in the striatum, negatively correlated with elevated expression of DRD2 protein. The in vitro study demonstrated that miR-9 directly targeted DRD2 mRNA 3’UTR and repressed the translation of mRNA, which resulted in decreased protein expression. These results implicated that upregulated DRD2 protein in the NAc may be a result of decreased miR-9 expression.
An interesting finding in this study was that CUS increased the expression of miR-326 in the NAc and decreased it in the striatum, whereas elevated miR-326 expression was only induced by MD in the striatum. In addition, aberrant miR-326 expression in the NAc positively correlated with depressive-like phenotypes. These observations reflect a distinct role of miR-326 expression in different brain areas in stress-induced depression-like phenotypes. In addition, all aberrant levels of miR-326 were normalized by 4-week treatment of escitalopram, suggesting that miR-326 in the NAc and striatum plays a crucial role in the escitalopram treatment. Moreover, the expression of miR-326 positively correlated with DRD2 protein in both the NAc and striatum. However, miR-326 was found to exhibit no effect on DRD2 gene expression in the in vitro study. This suggested that the involvement of miR-326 in stress-induced depression-like phenotypes is not directly mediated by the regulation of DRD2 gene expression. A previous study showed that miR-326 was downregulated in the rostroventral midbrain in depressed patients who committed suicide. Therefore, further studies are required to investigate which pathway regulates miR-326 expression and subsequently regulates stress-induced depression-like phenotypes.
In conclusion, MD enhanced the development of depression-like behaviors and increased resistance to escitalopram treatment in rats. One of the possible mechanisms is associated with the downregulation of miR-9 expression, and subsequent up-regulation of DRD2 expression in the NAc. However, miR-326 may be a novel target of escitalopram treatment. These findings add to our understanding of the psychopathologies of depression.
Interest Statement
None
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 81071097), Hunan Provincial Science and Technology plan projects (2014TT2005), and Hunan Provincial Innovation Foundation for Postgraduate (CX2013B113).
References
- Bai M, Zhu X, Zhang Y, Zhang S, Zhang L Xue L, Zhang X. (2012) Abnormal hippocampal BDNF and miR-16 expression is associated with depression-like behaviors induced by stress during early life. PloS One 7:e46921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso AM, Gallagher KB, Bratcher NA, Brioni JD, Moreland RB, Hsieh GC, Rueter LE. (2005) Antidepressant-like effect of D2/3 receptor-, but not D4 receptor-activation in the rat forced swim test. Neuropsychopharmacol 30:1257–1268. [DOI] [PubMed] [Google Scholar]
- Baudry A, Mouillet–Richard S, Schneider B, Launay JM, Kellermann O. (2010) miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science 329:1537–1541. [DOI] [PubMed] [Google Scholar]
- Cabib S, Puglisi-Allegra S. (2012) The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev 36:79–89. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Resick PA, Bryant RA, Strain J, Horowitz M, Spiegel D. (2011) Classification of trauma and stressor‐related disorders in DSM-5. Depress Anxiety 28:737–749. [DOI] [PubMed] [Google Scholar]
- Heyer MP, Pani AK, Smeyne RJ, Kenny PJ, Feng G. (2012) Normal midbrain dopaminergic neuron development and function in miR-133b mutant mice. J Neurosci 32:10887–10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li MD. (2009) Differential allelic expression of dopamine d1 receptor gene (DRD1) is modulated by microRNA miR-504. Biol Psychiatry 65:702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino E. (2012) Escitalopram for the management of major depressive disorder: a review of its efficacy, safety, and patient acceptability. Patient Preference and Adherence 6:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Baram TZ. (2009) The pathways from mother’s love to baby’s future. Front Beh Neurosc 3:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korosi A, Naninck EFG, Oomen CA, Schouten M, Krugers H, Fitzsimons C, Lucassen PJ. (2012) Early-life stress mediated modulation of adult neurogenesis and behavior. Behav Brain Res 227:400–409. [DOI] [PubMed] [Google Scholar]
- Landau AM, Chakravarty MM, Clark CM, Zis AP, Doudet DJ. (2010) Electroconvulsive therapy alters dopamine signaling in the striatum of non-human primates. Neuropsychopharmacol 36:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Liu AH, Xu Y, Tie L, Yu HM, Li XJ. (2005) Effect of chronic unpredictable mild stress on brain–pancreas relative protein in rat brain and pancreas. Behav Brain Res 165:63–71. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KLK, Blanchard RJ, Blanchard DC, Markham C, McEwen BS. (2004) Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neurosci 124:449–457. [DOI] [PubMed] [Google Scholar]
- Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D. (2010) Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci 40:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Torres LD, Guillaumot J, Harkness KL, Roberts JE, Frank E, Kupfer D. (2006) Life stress and the long-term treatment course of recurrent depression: III. Nonsevere life events predict recurrence for medicated patients over 3 years. J Consult Clin Psychol 74:112. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Gould E, Manji H. (2002) Preclinical models: status of basic research in depression. Biol Psychiatry 52:503–528. [DOI] [PubMed] [Google Scholar]
- O’connor RM, Dinan TG, Cryan JF. (2011) Little things on which happiness depends: microRNAs as novel therapeutic targets for the treatment of anxiety and depression. Mol Psychiatry 17:359–376. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee JH, Ha M, Nam JW, Kim VN. (2008) miR-29 miRNAs activate p53 by targeting p85α and CDC42. Nat Struct Mol Biol 16:23–29. [DOI] [PubMed] [Google Scholar]
- Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL. (2008) The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci 28:14341–14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger C, Duman RS. (2007) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacol 33:88–109. [DOI] [PubMed] [Google Scholar]
- Scheggi S, Marchese G, Grappi S, Secci ME, De Montis MG, Gambarana C. (2011) Cocaine sensitization models an anhedonia-like condition in rats. Int J Neuropsychopharmacol 14:333–346. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. (1997) Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med 27:1247–1256. [DOI] [PubMed] [Google Scholar]
- Sillivan SE, Black YD, Naydenov AV, Vassoler FR, Hanlin RP, Konradi C. (2011) Binge cocaine administration in adolescent rats affects amygdalar gene expression patterns and alters anxiety-related behavior in adulthood. Biol Psychiatry 70:583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Watanabe Y. (2010) Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci 30:15007–15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. (2005) Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci 25:11045–11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Lee TH, Davidson C, Lazarus C, Wetsel WC, Ellinwood EH. (2006) Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacol 32:377–387. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu X, Bai M, Zhang L, Xue L, Yi J. (2013) Maternal deprivation enhances behavioral vulnerability to stress associated with miR-504 expression in nucleus accumbens of rats. PloS One 8:e69934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Peng S, Zhang S, Zhang X. (2011) Stress-induced depressive behaviors are correlated with Par-4 and DRD2 expression in rat striatum. Behav Brain Res 223:329–335. [DOI] [PubMed] [Google Scholar]