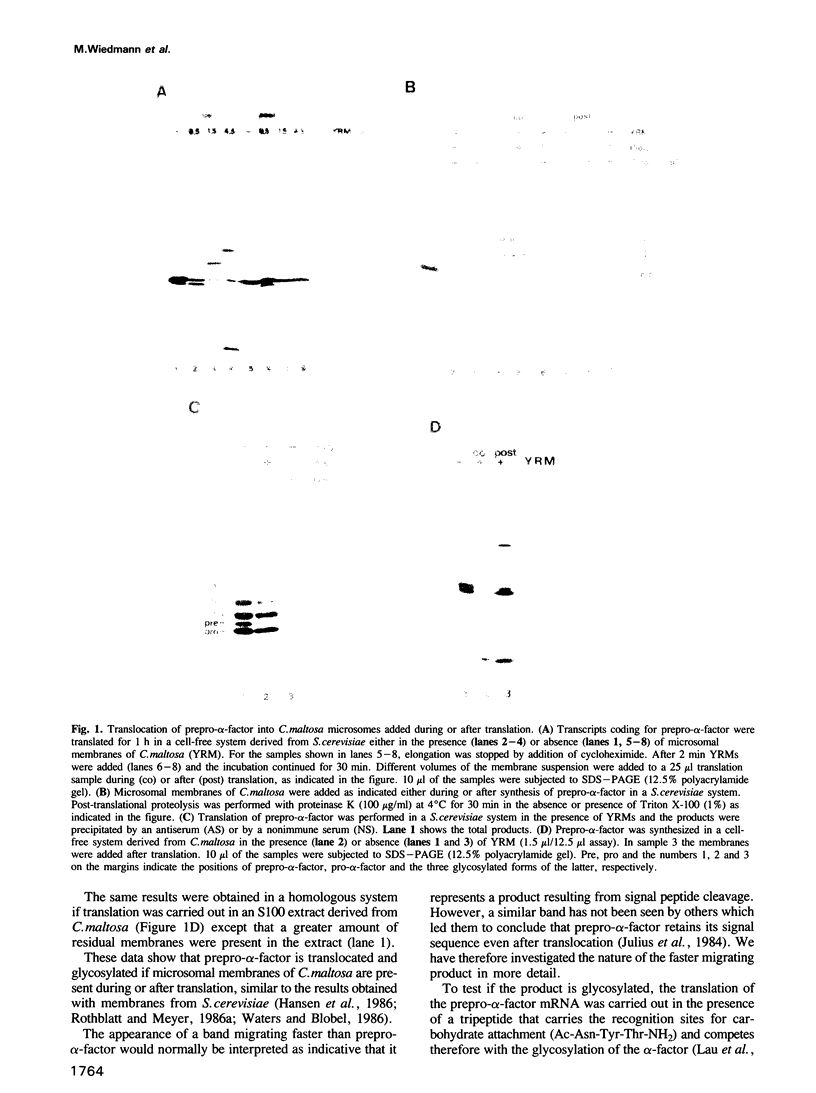
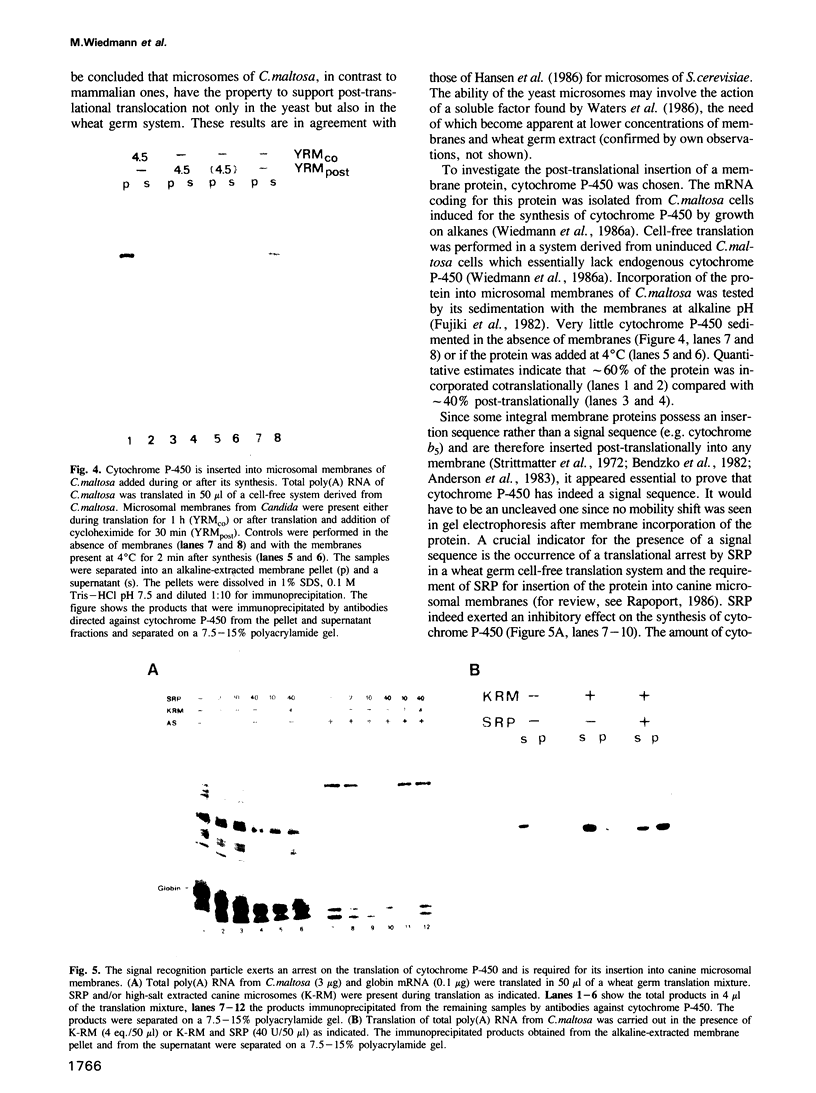
Abstract
We have isolated from the yeast Candida maltosa microsomal membranes that are active in the translocation of proteins synthesized in cell-free systems derived from C. maltosa, Saccharomyces cerevisiae or wheat germ. Translocation and core glycosylation of prepro-alpha-factor, a secretory protein, were observed with yeast microsomes added during or after translation. The signal peptide is cleaved off. Cytochrome P-450 from C. maltosa, the first integral membrane protein studied in a yeast system, is also inserted both co- and post-translationally into Candida microsomal membranes. Its insertion into canine microsomes occurs efficiently only in a co-translational manner and is dependent on the function of the signal recognition particle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Mostov K. E., Blobel G. Mechanisms of integration of de novo-synthesized polypeptides into membranes: signal-recognition particle is required for integration into microsomal membranes of calcium ATPase and of lens MP26 but not of cytochrome b5. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7249–7253. doi: 10.1073/pnas.80.23.7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendzko P., Prehn S., Pfeil W., Rapoport T. A. Different modes of membrane interactions of the signal sequence of carp preproinsulin and of the insertion sequence of rabbit cytochrome b5. Eur J Biochem. 1982 Mar;123(1):121–126. doi: 10.1111/j.1432-1033.1982.tb06507.x. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell. 1983 Dec;35(3 Pt 2):677–685. doi: 10.1016/0092-8674(83)90100-9. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Walter P., Blobel G. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol. 1982 Nov;95(2 Pt 1):470–477. doi: 10.1083/jcb.95.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen W., Garcia P. D., Walter P. In vitro protein translocation across the yeast endoplasmic reticulum: ATP-dependent posttranslational translocation of the prepro-alpha-factor. Cell. 1986 May 9;45(3):397–406. doi: 10.1016/0092-8674(86)90325-9. [DOI] [PubMed] [Google Scholar]
- Julius D., Schekman R., Thorner J. Glycosylation and processing of prepro-alpha-factor through the yeast secretory pathway. Cell. 1984 Feb;36(2):309–318. doi: 10.1016/0092-8674(84)90224-1. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Kurzchalia T. V., Wiedmann M., Girshovich A. S., Bochkareva E. S., Bielka H., Rapoport T. A. The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature. 1986 Apr 17;320(6063):634–636. doi: 10.1038/320634a0. [DOI] [PubMed] [Google Scholar]
- Lau J. T., Welply J. K., Shenbagamurthi P., Naider F., Lennarz W. J. Substrate recognition by oligosaccharyl transferase. Inhibition of co-translational glycosylation by acceptor peptides. J Biol Chem. 1983 Dec 25;258(24):15255–15260. [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Müller G., Zimmermann R. Import of honeybee prepromelittin into the endoplasmic reticulum: structural basis for independence of SRP and docking protein. EMBO J. 1987 Jul;6(7):2099–2107. doi: 10.1002/j.1460-2075.1987.tb02476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport T. A. Protein translocation across and integration into membranes. CRC Crit Rev Biochem. 1986;20(1):73–137. doi: 10.3109/10409238609115901. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: reconstitution of the translocation and glycosylation of alpha-factor and invertase in a homologous cell-free system. Cell. 1986 Feb 28;44(4):619–628. doi: 10.1016/0092-8674(86)90271-0. [DOI] [PubMed] [Google Scholar]
- Rothblatt J. A., Meyer D. I. Secretion in yeast: translocation and glycosylation of prepro-alpha-factor in vitro can occur via an ATP-dependent post-translational mechanism. EMBO J. 1986 May;5(5):1031–1036. doi: 10.1002/j.1460-2075.1986.tb04318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt J. A., Webb J. R., Ammerer G., Meyer D. I. Secretion in yeast: structural features influencing the post-translational translocation of prepro-alpha-factor in vitro. EMBO J. 1987 Nov;6(11):3455–3463. doi: 10.1002/j.1460-2075.1987.tb02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi M., Mihara K., Sato R. Signal recognition particle is required for co-translational insertion of cytochrome P-450 into microsomal membranes. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3361–3364. doi: 10.1073/pnas.81.11.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G., Zimmermann R. Import of frog prepropeptide GLa into microsomes requires ATP but does not involve docking protein or ribosomes. EMBO J. 1987 Mar;6(3):699–703. doi: 10.1002/j.1460-2075.1987.tb04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter P., Rogers M. J., Spatz L. The binding of cytochrome b 5 to liver microsomes. J Biol Chem. 1972 Nov 25;247(22):7188–7194. [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle: a ribonucleoprotein required for cotranslational translocation of proteins, isolation and properties. Methods Enzymol. 1983;96:682–691. doi: 10.1016/s0076-6879(83)96057-3. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Blobel G. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol. 1986 May;102(5):1543–1550. doi: 10.1083/jcb.102.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters M. G., Chirico W. J., Blobel G. Protein translocation across the yeast microsomal membrane is stimulated by a soluble factor. J Cell Biol. 1986 Dec;103(6 Pt 2):2629–2636. doi: 10.1083/jcb.103.6.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C., Wickner W., Zimmermann R. M13 procoat and a pre-immunoglobulin share processing specificity but use different membrane receptor mechanisms. Proc Natl Acad Sci U S A. 1983 May;80(10):2809–2813. doi: 10.1073/pnas.80.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann B., Wiedmann M., Kärgel E., Schunck W. H., Müller H. G. N-alkanes induce the synthesis of cytochrome P-450 mRNA in Candida maltosa. Biochem Biophys Res Commun. 1986 May 14;136(3):1148–1154. doi: 10.1016/0006-291x(86)90454-7. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Huth A., Rapoport T. A. Internally transposed signal sequence of carp preproinsulin retains its functions with the signal recognition particle. FEBS Lett. 1986 Jan 1;194(1):139–145. doi: 10.1016/0014-5793(86)80065-5. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Huth A., Rapoport T. A. Xenopus oocytes can secrete bacterial beta-lactamase. Nature. 1984 Jun 14;309(5969):637–639. doi: 10.1038/309637a0. [DOI] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Bielka H., Rapoport T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J Cell Biol. 1987 Feb;104(2):201–208. doi: 10.1083/jcb.104.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Hartmann E., Rapoport T. A. A signal sequence receptor in the endoplasmic reticulum membrane. 1987 Aug 27-Sep 2Nature. 328(6133):830–833. doi: 10.1038/328830a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Mollay C. Import of honeybee prepromelittin into the endoplasmic reticulum. Requirements for membrane insertion, processing, and sequestration. J Biol Chem. 1986 Sep 25;261(27):12889–12895. [PubMed] [Google Scholar]
- Zwizinski C., Date T., Wickner W. Leader peptidase is found in both the inner and outer membranes of Escherichia coli. J Biol Chem. 1981 Apr 10;256(7):3593–3597. [PubMed] [Google Scholar]