Abstract
Mutualisms affect the biodiversity, distribution and abundance of biological communities. However, ecological processes that drive mutualism-related shifts in population structure are often unclear and must be examined to elucidate how complex, multi-species mutualistic networks are formed and structured. In this study, we investigated how the presence of key marine mutualistic partners can drive the organisation of local communities on coral reefs. The cleaner wrasse, Labroides dimidiatus, removes ectoparasites and reduces stress hormones for multiple reef fish species, and their presence on coral reefs increases fish abundance and diversity. Such changes in population structure could be driven by increased recruitment of larval fish at settlement, or by post-settlement processes such as modified levels of migration or predation. We conducted a controlled field experiment to examine the effect of cleaners on recruitment processes of a common group of reef fishes, and showed that small patch reefs (61–285 m2) with cleaner wrasse had higher abundances of damselfish recruits than reefs from which cleaner wrasse had been removed over a 12-year period. However, the presence of cleaner wrasse did not affect species diversity of damselfish recruits. Our study provides evidence of the ecological processes that underpin changes in local population structure in the presence of a key mutualistic partner.
Keywords: cleaning mutualism, recruitment, ectoparasites, reef fish behaviour
1. Introduction
On coral reefs, cleaning symbioses are key mutualistic networks that increase abundance and promote species diversity in local fish communities [1,2]. Cleaner organisms, such as the bluestreak cleaner wrasse, Labroides dimidiatus, interact with multiple species of fish, and provide fitness benefits to their clients by reducing stress hormones [3] and ectoparasite loads such as gnathiid isopods [4]. The presence of cleaner wrasse also affects fish population structure, size–frequency distribution and growth [2,4,5]. Abundance and diversity of fishes, both adult damselfishes (site-attached) [2,6] and visitors (moving between patch reefs) [1] increase, as does the abundance of juvenile visitor fishes [2] on reefs with cleaner wrasse.
Damselfishes (Pomacentridae) are one of the most abundant groups of fishes found on coral reefs. Damselfishes play a critical role in structuring coral reef benthic communities [7], with their presence indirectly increasing the survival of juvenile fishes [8]. Although the abundance and diversity of adult damselfishes are higher on reefs where cleaner wrasse are present, it is not known if this difference occurs during recruitment, as damselfishes are relatively sedentary after settlement (when fish first join the reef assemblage after a pelagic larval stage). Whether differences in reef fish population structure in the presence of cleaning organisms involve regulatory processes such as migration or predation during settlement, or post-settlement during the juvenile and adult life stages, has not been investigated. Understanding recruitment, a critical demographic process, is important as it determines the structure of many marine and terrestrial communities [9]. In this study, we examined whether the presence of cleaner wrasses influences recruitment patterns of larval coral reef fishes on patch reefs, which could explain differences in the abundance and diversity of local assemblages of adult fishes. As previous studies have shown a decrease in abundance and diversity of reef fishes on reefs where cleaner wrasse are absent [1,2,6], we predict a similar trend occurring for damselfish recruits.
2. Material and methods
Our study was conducted on 16 small patch reefs in shallow (3−7 m depth) areas in lagoon and back-reef habitats near Lizard Island, Great Barrier Reef, Australia (electronic supplementary material, figure S1). Since 2000, ‘removal’ reefs (n = 7) were inspected at three-month intervals and cleaner wrasse removed with hand and barrier nets; ‘control’ reefs (n = 9) were surveyed for cleaner wrasse (see the electronic supplementary material, table S1 for numbers encountered).
To investigate whether the abundance and species richness of damselfish recruits differed between removal and control reefs, we counted recruits (less than 20 mm TL) for 5 days in each of three lunar cycles, beginning 4 days after the new moon (18−22 November 2012, 17−21 December 2012 and 16−20 January 2013). These times were selected because settlement of fishes to coral reefs peak around the time of the new moon during these summer months [10]. Counts alternated between randomly selected control and removal reefs and fish were identified to species where possible. Fish were counted once per reef by the same observer (D.S.) on SCUBA for 60−120 min, depending on reef size. As damselfishes are most active from morning to midday [11], reefs were surveyed between 09.00 and 12.00. The observer circled approximately 1 m above the reef counting an abundant, or several less-abundant, species (in the same order for each reef) on each pass. For analysis, fish were categorized either as common, wherein recruits of these species were found on 90% of reefs across all three months (of which there were five species: Pomacentrus adelus, P. amboinensis, P. moluccensis, P. nagasakiensis and Chrysiptera rollandi), or uncommon (all other damselfishes). We compared the abundance and three measures of diversity of damselfish recruits on control and removal reefs.
We conducted generalized linear mixed-effect models with a Poisson distribution error, to examine whether the total abundance of all the five common damselfishes combined (and also separately by species) and the total abundance of uncommon damselfishes differed relative to the presence of cleaner wrasse. We also used full linear mixed-effect models with an REML procedure to analyse species richness and Simpson's indices of diversity [12] and used a permutational multivariate analysis of variance and principle coordinates analysis for the species composition. A Bonferroni correction (α = 0.01) was applied to account for multiple comparisons. All statistical analyses were conducted using R v. 3.0.1 [13], except for the species composition which was analysed using PRIMER v. 6.0 [14]. For further information on statistical analyses, see the electronic supplementary material.
3. Results
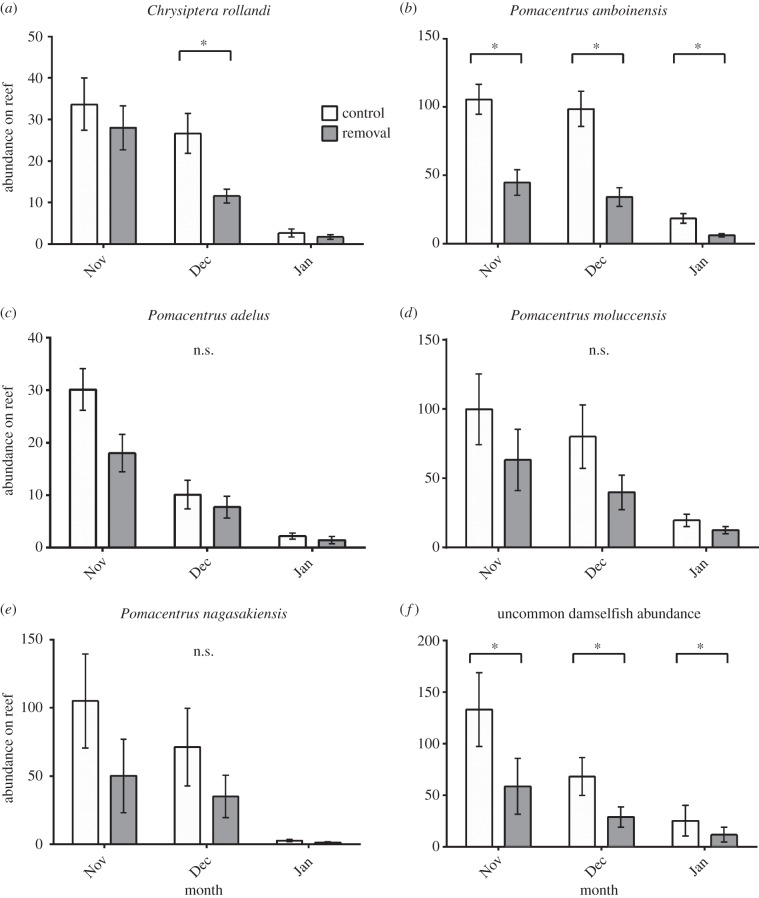
Thirty-one species of damselfish recruits were identified (electronic supplementary material, table S2). The total abundance of common damselfishes was lower on removal than on control reefs (p = 0.006; electronic supplementary material, figure S2 and table S3). At the species level, the abundances of C. rollandi (p = 0.0001, December only) and P. amboinensis (p < 0.0001; figure 1a–b; electronic supplementary material, table S3) were significantly lower on reefs where cleaner wrasse had been removed than on control reefs. Although there were similar trends in the average abundance of the other common species, P. adelus, P. moluccensis and P. nagasakiensis, these differences were not significant (p = 0.0769, 0.0689 and 0.0573, respectively; figure 1c–e). Total abundance of uncommon damselfishes was also lower on removal reefs (p = 0.0070, figure 1f).
Figure 1.
Mean (±s.e.) abundance of recruiting damselfish species. Control and cleaner wrasse removal reefs: (a–e) abundance of individual common species, (f) abundance of uncommon species. Asterisk (*) denotes significant difference in cleaner wrasse treatment (p < 0.01), n.s. = p > 0.01.
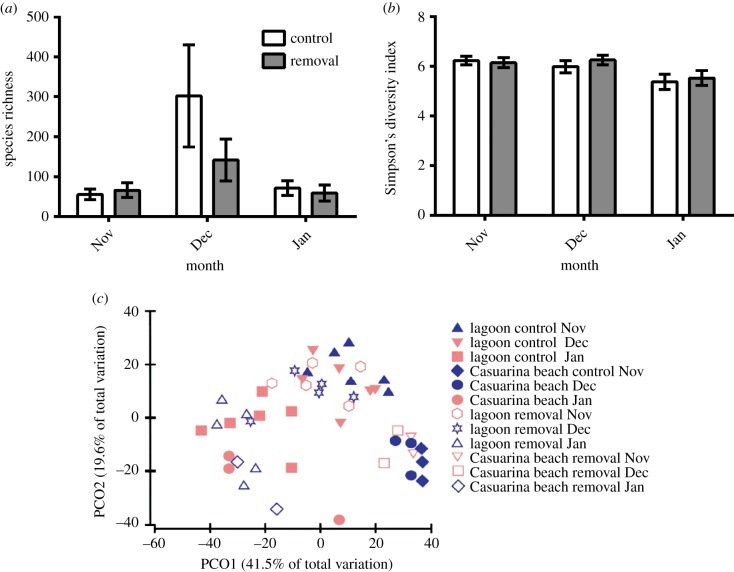
We found no differences in measures of diversity between removal and control reefs (species richness: p = 0.3578; Simpson's diversity index: p = 0.8418; species composition: PERMANOVA, F1−36 = 1.83, p = 0.1029; figure 2a–c; electronic supplementary material, table S4).
Figure 2.
Diversity of all damselfish recruits per reef. (a) Species richness, (b) Simpson's diversity index and (c) species composition on control and cleaner wrasse removal reef, represented by a principle coordinate analysis (PCO). (Online version in colour.)
4. Discussion
The combined abundances of the five common species, the individual abundances of two common species (C. rollandi and P. amboinensis), and the total abundance of uncommon recruited damselfishes were higher on reefs with cleaner wrasse present than on those without. There was also a strong (but not statistically significant) trend for abundances of the other common damselfishes (P. adelus, P. moluccensis and P. nagasakiensis) to be higher on reefs with cleaners. These results suggest that the presence of a key mutualistic species creates changes in population structure around the time of settlement and recruitment. This in turn could explain the observed changes in abundance and distribution of coral reef fish populations in relation to cleaner wrasse presence [2].
The presence of cleaner wrasse could influence recruitment processes by a number of direct or indirect mechanisms. If young fish use visual or olfactory cues to locate potential microhabitats, then sensory cues may enable them to locate cleaners directly. Indeed, a recent study suggests that recently settled fish can recognize and preferentially choose a microhabitat near a cleaner [15]. Alternatively, cleaner wrasse could influence recruitment processes indirectly by reducing the abundance of ectoparasites in the local environment. Higher abundances of parasites in a system have the potential to detrimentally affect the recruitment of juveniles, as demonstrated in lower recruitment success of juvenile cricket frogs (Acris crepitans) in ponds with high rates of parasitic infection [16]. Although both adults and recruit damselfishes are known to be infected with gnathiids, cleaner wrasse rarely clean recruits and tend to clean larger individuals [15]. However, with gnathiids decreasing swimming performance and increasing mortality of settling fish [17] the removal of even a single gnathiid can increase the survival of an individual. Cleaner wrasse may also indirectly promote fish recruitment by influencing the abundance of conspecifics, which have been shown to influence microhabitat selection by settling fish, as settling fish may use the presence of conspecifics as a measure of habitat quality [18] or the behaviour of predators [19]. The abundance of all (adult and juvenile) conspecifics for P. amboinensis, P. moluccensis and P. nagasakiensis are also higher on reefs with cleaner wrasse than those without (D Sun 2012, unpublished data). Further investigation is required to disentangle the relative importance of these direct and indirect mechanisms.
Interestingly, there was no difference in species richness, Simpson's diversity index, or composition of damselfish recruits between control and removal reefs, suggesting that the presence of cleaner wrasse affects the abundance of individual species but not the overall community diversity of recruit damselfishes. Previous studies have shown an increase in the abundance and species richness of resident adult fishes in the presence of cleaner wrasse [2,6]. Our results suggest that such patterns in diversity are not likely due to differential recruitment, but due to post-settlement events, including migration in response to cleaner wrasse presence or the effects of parasitism [2]. Parasitic infection by gnathiid isopods can make recruits more susceptible to the high rates of predation that occur immediately after settlement (typically 56% within 2 days of settlement) [20]. Ultimately, this process could affect the diversity of fishes in the presence of cleaner wrasse.
In summary, we have demonstrated for the first time that the presence of cleaner wrasse has the potential to influence the structure and demography of reef fish populations via effects on the process of recruitment. Thus, mutualism is an important mechanism that contributes to the already-complex recruitment processes of reef fishes.
Supplementary Material
Acknowledgements
We thank Lizard Island Research Station staff, field volunteers and S. P. Blomberg for statistical advice.
Ethics
Experimental procedures were approved by The University of Queensland Animal Ethics Committee.
Data accessibility
The data are available as the electronic supplementary material.
Authors' contributions
D.S. collected data, designed the study, carried out statistical analyses and drafted the manuscript; K.L.C. contributed to experimental design and helped edit the manuscript; J.W. helped collect data and edit the manuscript; M.G.M., M.I.M. and T.H.C. helped with interpretation of the data and edited the manuscript; A.S.G. designed the study, carried out statistical analyses and helped edit the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by Sea World Research and Rescue Foundation, Australia, to D.S., T.H.C. and A.S.G. and an Australian Research Council Discovery Grant to A.S.G. and M.G.M.
References
- 1.Grutter AS, Murphy J, Choat H. 2003. Cleaner fish drives local fish diversity on coral reefs. Curr. Biol. 13, 64–67. ( 10.1016/S0960-9822(02)01393-3) [DOI] [PubMed] [Google Scholar]
- 2.Waldie PA, Blomberg SP, Cheney KL, Goldizen AW, Grutter AS. 2011. Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS ONE 6, e21 201–e21 207. ( 10.1371/journal.pone.0021201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soares MC, Oliveira R, Ros AFH, Grutter AS, Bshary R. 2011. Tactile stimulation lowers stress in fish. Nat. Commun. 2, 534 ( 10.1038/ncomms1547) [DOI] [PubMed] [Google Scholar]
- 4.Grutter AS. 1999. Cleaner fish really do clean. Nature 398, 672–673. ( 10.1038/19443) [DOI] [Google Scholar]
- 5.Clague GE, Cheney KL, Goldizen AW, McCormick MI, Waldie PA, Grutter AS. 2011. Long-term cleaner fish presence affects growth of a coral reef fish. Biol. Lett. 6, 863–865. ( 10.1098/rsbl.2011.0458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bshary R. 2003. The cleaner wrasse, Labroides dimidiatus, is a key organism for reef fish diversity at Ras Mohammed National Park, Egypt. J. Anim. Ecol. 72, 169–176. ( 10.1046/j.1365-2656.2003.00683.x) [DOI] [Google Scholar]
- 7.Ceccarelli D, Jones G, McCook L. 2005. Effects of territorial damselfish on an algal-dominated coastal coral reef. Coral Reefs 24, 606–620. ( 10.1007/s00338-005-0035-z) [DOI] [Google Scholar]
- 8.McCormick MI, Meekan MG. 2007. Social facilitation of selective mortality. Ecology 88, 1562–1570. ( 10.1890/06-0830) [DOI] [PubMed] [Google Scholar]
- 9.Caley MJ, Carr MH, Hixon MA, Hughes TP, Jones GP, Menge BA. 1996. Recruitment and the local dynamics of open marine populations. Annu. Rev. Ecol. Syst. 27, 477–500. ( 10.1146/annurev.ecolsys.27.1.477) [DOI] [Google Scholar]
- 10.Milicich MJ, Doherty PJ. 1994. Larval supply of coral reef fish populations—magnitude and synchrony of replenishment to Lizard Island, Great Barrier Reef. Mar. Ecol. Prog. Ser. 110, 121–134. ( 10.3354/meps110121) [DOI] [Google Scholar]
- 11.Bosiger YJ, McCormick MI. 2014. Temporal links in daily activity patterns between coral reef predators and their prey. PLoS ONE 9, e111723 ( 10.1371/journal.pone.0111723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krebs CJ. 1999. Ecological methodology. Menlo Park, CA: Benjamin/Cummings. [Google Scholar]
- 13.R Development Core Team. 2013. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org. [Google Scholar]
- 14.Anderson MJ, Gorley RN, Clarke KR. 2008. PERMANOVA+ for Primer: guide to software and statistical methods. Plymouth, MN: PRIMER-E. [Google Scholar]
- 15.Sun. et al. Submitted. Cleaner wrasse influence habitat selection of young damselfish.
- 16.Beasley VR, et al. 2005. Risk factors and the decline of the northern cricket frog, Acris crepitans: evidence for involvement of herbicides, parasitism, and habitat modifications. In Amphibian declines: the conservation status of United States species (ed. Lanoo MJ.), pp. 75–86. Berkeley, CA: University of California Press. [Google Scholar]
- 17.Grutter AS, Crean AJ, Curtis LM, Kuris AM, Warner RR, McCormick MI. 2011. Indirect effect of an ectoparasite reduce successful establishment of a damselfish at settlement. Funct. Ecol. 25, 586–594. ( 10.1111/j.1365-2435.2010.01798.x) [DOI] [Google Scholar]
- 18.Coppock AG, Gardiner NM, Jones GP. 2013. Olfactory discrimination in juvenile coral reef fishes: responses to conspecifics and corals. J. Exp. Mar. Biol. Ecol. 443, 21–26. ( 10.1016/j.jembe.2013.02.026) [DOI] [Google Scholar]
- 19.Cheney KL, Bshary R, Grutter AS. 2008. Cleaner fish cause predators to reduce aggression toward bystanders at cleaning stations. Behav. Ecol. 19, 1063–1067. ( 10.1093/beheco/arn067) [DOI] [Google Scholar]
- 20.Almany GR, Webster MS. 2006. The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25, 19–22. ( 10.1007/s00338-005-0044-y) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available as the electronic supplementary material.