Abstract
Retrovirus virions carry a diploid genome associated with a large number of small viral finger protein molecules which are required for encapsidation. Our present results show that finger protein p12 of Rous sarcoma virus (RSV) and p10 of murine leukaemia virus (MuLV) positions replication primer tRNA on the replication initiation site (PBS) at the 5' end of the RNA genome. An RSV mutant with a Val-Pro insertion in the finger motif of p12 is able to partially encapsidate genomic RNA but is not infectious because mutated p12 is incapable of positioning the replication primer, tRNATrp. Since all known replication competent retroviruses, and the plant virus CaMV, code for finger proteins analogous to RSV p12 or MuLV p10, the initial stage of reverse transcription in avian, mammalian and human retroviruses and in CaMV is probably controlled in an analogous way.
Full text
PDF
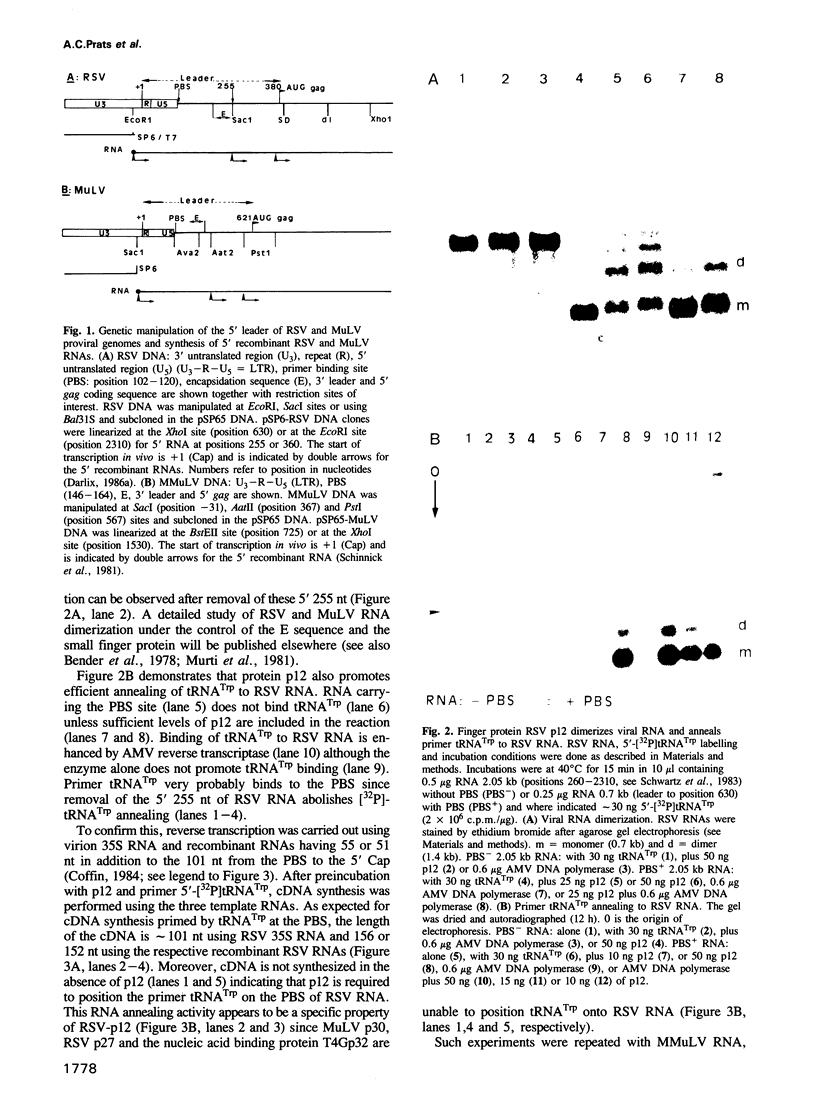





Images in this article
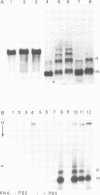
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araya A., Litvak S. Studies on the interaction of tRNA and avian myeloblastosis DNA polymerase. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):631–637. doi: 10.1101/sqb.1979.043.01.070. [DOI] [PubMed] [Google Scholar]
- Araya A., Sarih L., Litvak S. Reverse transcriptase mediated binding of primer tRNA to the viral genome. Nucleic Acids Res. 1979 Aug 24;6(12):3831–3843. doi: 10.1093/nar/6.12.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender W., Chien Y. H., Chattopadhyay S., Vogt P. K., Gardner M. B., Davidson N. High-molecular-weight RNAs of AKR, NZB, and wild mouse viruses and avian reticuloendotheliosis virus all have similar dimer structures. J Virol. 1978 Mar;25(3):888–896. doi: 10.1128/jvi.25.3.888-896.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. M. Viral DNA synthesized in vitro by avian retrovirus particles permeabilized with melittin. I. Kinetics of synthesis and size of minus- and plus-strand transcripts. J Virol. 1981 Jan;37(1):109–116. doi: 10.1128/jvi.37.1.109-116.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulay J. L., Dennefeld C., Alberga A. The Drosophila developmental gene snail encodes a protein with nucleic acid binding fingers. 1987 Nov 26-Dec 2Nature. 330(6146):395–398. doi: 10.1038/330395a0. [DOI] [PubMed] [Google Scholar]
- Brown P. O., Bowerman B., Varmus H. E., Bishop J. M. Correct integration of retroviral DNA in vitro. Cell. 1987 May 8;49(3):347–356. doi: 10.1016/0092-8674(87)90287-x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cesareni G., Muesing M. A., Polisky B. Control of ColE1 DNA replication: the rop gene product negatively affects transcription from the replication primer promoter. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6313–6317. doi: 10.1073/pnas.79.20.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. J., Stoltzfus C. M. Cloning and nucleotide sequences of cDNAs spanning the splice junctions of Rous sarcoma virus mRNAs. J Virol. 1985 Mar;53(3):969–972. doi: 10.1128/jvi.53.3.969-972.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey S. N. Amino acid sequence homology in gag region of reverse transcribing elements and the coat protein gene of cauliflower mosaic virus. Nucleic Acids Res. 1986 Jan 24;14(2):623–633. doi: 10.1093/nar/14.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Bromley P. A., Spahr P. F. Extensive in vitro transcription of rous sarcoma virus RNA by avian myeloblastosis virus DNA polymerase and concurrent activation of the associated RNase H. J Virol. 1977 Sep;23(3):659–668. doi: 10.1128/jvi.23.3.659-668.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L. Circularization of retroviral genomic RNA and the control of RNA translation, packaging and reverse transcription. Biochimie. 1986 Jul-Aug;68(7-8):941–949. doi: 10.1016/s0300-9084(86)81057-4. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Control of Rous sarcoma virus RNA translation and packaging by the 5' and 3' untranslated sequences. J Mol Biol. 1986 Jun 5;189(3):421–434. doi: 10.1016/0022-2836(86)90314-1. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F. Binding sites of viral protein P19 onto Rous sarcoma virus RNA and possible controls of viral functions. J Mol Biol. 1982 Sep 15;160(2):147–161. doi: 10.1016/0022-2836(82)90172-3. [DOI] [PubMed] [Google Scholar]
- Darlix J. L., Spahr P. F., Bromley P. A., Jaton J. C. In vitro, the major ribosome binding site on Rous sarcoma virus RNA does not contain the nucleotide sequence coding for the N-terminal amino acids of the gag gene product. J Virol. 1979 Feb;29(2):597–611. doi: 10.1128/jvi.29.2.597-611.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlix J. L., Zuker M., Spahr P. F. Structure-function relationship of Rous sarcoma virus leader RNA. Nucleic Acids Res. 1982 Sep 11;10(17):5183–5196. doi: 10.1093/nar/10.17.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Rueckert R. R. Properties of a ribonucleoprotein particle isolated from Nonidet P-40-treated Rous sarcoma virus. J Virol. 1972 Nov;10(5):1010–1020. doi: 10.1128/jvi.10.5.1010-1020.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M., Dorizzi M., Sarger C., Labouresse J. Purification of tRNATrp, tRNAVal, and partial purification of tRNAIle and tRNAMfet from beef liver. Biochimie. 1976;58(10):1159–1165. doi: 10.1016/s0300-9084(76)80114-9. [DOI] [PubMed] [Google Scholar]
- Grimsley N., Hohn T., Hohn B. Recombination in a plant virus: template-switching in cauliflower mosaic virus. EMBO J. 1986 Apr;5(4):641–646. doi: 10.1002/j.1460-2075.1986.tb04261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Copeland T. D., Sowder R. C., Smythers G. W., Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981 Aug 25;256(16):8400–8406. [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Katz R. A., Terry R. W., Skalka A. M. A conserved cis-acting sequence in the 5' leader of avian sarcoma virus RNA is required for packaging. J Virol. 1986 Jul;59(1):163–167. doi: 10.1128/jvi.59.1.163-167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Effect of polymerase mutations on packaging of primer tRNAPro during murine leukemia virus assembly. J Virol. 1981 Apr;38(1):403–408. doi: 10.1128/jvi.38.1.403-408.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Selective packaging of host tRNA's by murine leukemia virus particles does not require genomic RNA. J Virol. 1979 Jan;29(1):328–335. doi: 10.1128/jvi.29.1.328-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietz J. A., Grossman Z., Lueders K. K., Kuff E. L. Nucleotide sequence of a complete mouse intracisternal A-particle genome: relationship to known aspects of particle assembly and function. J Virol. 1987 Oct;61(10):3020–3029. doi: 10.1128/jvi.61.10.3020-3029.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Verma I. M. Two base changes restore infectivity to a noninfectious molecular clone of Moloney murine leukemia virus (pMLV-1). J Virol. 1984 Jan;49(1):214–222. doi: 10.1128/jvi.49.1.214-222.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moelling K., Sykora K. W., Dittmar K. E., Scott A., Watson K. F. The isolation of avian viral RNA and polypeptides. J Biol Chem. 1979 May 25;254(10):3738–3742. [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murti K. G., Bondurant M., Tereba A. Secondary structural features in the 70S RNAs of Moloney murine leukemia and Rous sarcoma viruses as observed by electron microscopy. J Virol. 1981 Jan;37(1):411–419. doi: 10.1128/jvi.37.1.411-419.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Darlix J. L., Spahr P. F. It is Rous sarcoma virus protein P12 and not P19 that binds tightly to Rous sarcoma virus RNA. J Mol Biol. 1984 Mar 15;173(4):531–538. doi: 10.1016/0022-2836(84)90396-6. [DOI] [PubMed] [Google Scholar]
- Méric C., Spahr P. F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986 Nov;60(2):450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panet A., Haseltine W. A., Baltimore D., Peters G., Harada F., Dahlberg J. E. Specific binding of tryptophan transfer RNA to avian myeloblastosis virus RNA-dependent DNA polymerase (reverse transcriptase). Proc Natl Acad Sci U S A. 1975 Jul;72(7):2535–2539. doi: 10.1073/pnas.72.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel I., Bastia D. A replication initiator protein enhances the rate of hybrid formation between a silencer RNA and an activator RNA. Cell. 1987 Nov 6;51(3):455–462. doi: 10.1016/0092-8674(87)90641-6. [DOI] [PubMed] [Google Scholar]
- Pugatsch T., Stacey D. W. Identification of a sequence likely to be required for avian retroviral packaging. Virology. 1983 Jul 30;128(2):505–511. doi: 10.1016/0042-6822(83)90279-9. [DOI] [PubMed] [Google Scholar]
- Schulein M., Burnette W. N., August J. T. Stoichiometry and specificity of binding of Rauscher oncovirus 10,000-dalton (p10) structural protein to nucleic acids. J Virol. 1978 Apr;26(1):54–60. doi: 10.1128/jvi.26.1.54-60.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Bailey J. M. The binding of an avian myeloblastosis virus basic 12,000 dalton protein to nucleic acids. Nucleic Acids Res. 1979 Dec 11;7(7):2055–2072. doi: 10.1093/nar/7.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora K. W., Moelling K. Properties of the avian viral protein p12. J Gen Virol. 1981 Aug;55(Pt 2):379–391. doi: 10.1099/0022-1317-55-2-379. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Som T. Control of ColE1 plasmid replication: enhancement of binding of RNA I to the primer transcript by the Rom protein. Cell. 1984 Oct;38(3):871–878. doi: 10.1016/0092-8674(84)90282-4. [DOI] [PubMed] [Google Scholar]
- Vincent A., Colot H. V., Rosbash M. Sequence and structure of the serendipity locus of Drosophila melanogaster. A densely transcribed region including a blastoderm-specific gene. J Mol Biol. 1985 Nov 5;186(1):149–166. doi: 10.1016/0022-2836(85)90265-7. [DOI] [PubMed] [Google Scholar]
- Vincent A. TFIIIA and homologous genes. The 'finger' proteins. Nucleic Acids Res. 1986 Jun 11;14(11):4385–4391. doi: 10.1093/nar/14.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]