Abstract
For vertebrates, annual cycles are organized into a series of breeding and non-breeding periods that vary in duration and location but are inextricably linked biologically. Here, we show that our understanding of the fundamental ecology of four vertebrate classes has been limited by a severe breeding season research bias and that studies of individual and population-level responses to natural and anthropogenic change would benefit from a full annual cycle perspective. Recent emergence of new analytical and technological tools for studying individual and population-level animal movement could help reverse this bias. To improve understanding of species biology and reverse the population declines of many vertebrate species, a concerted effort to move beyond single season research is vital.
Keywords: annual cycle, seasonal interaction, research bias
1. Introduction: periods of the annual cycle are inextricably linked
Physical cycles as significant as the rotation of the tilted Earth around the Sun entrain living things into cycles. The ecology and evolution of vertebrates are organized into annual cycles that include reproductive, non-breeding and migration/dispersal periods that vary in duration and location. Spotted salamander (Ambystoma maculatum) breeding occurs over several days in early spring when adults migrate to vernal ponds, mate and lay eggs [1]. Soon after mating, spotted salamanders return to terrestrial foraging areas, where they remain for more than 95% of the annual cycle. Leatherback sea turtles (Dermochelys coriacea) spend most of their adult life at sea, moving vast distances in search of jellyfish. Every few years, individuals mate at sea then females lay eggs on beaches [2]. Ovenbirds (Seiurus aurocapilla) are on breeding areas from May until August, during which time they raise young. Pair bonds then disintegrate, and individuals moult and migrate to tropical wintering areas where they spend more than 70% of the annual cycle [3]. Although there is enormous variation in annual cycles across vertebrate taxa, what is consistent is that the breeding period composes a relatively small proportion of the cycle.
The duration and location of annual events depend on a multitude of interacting endogenous and exogenous factors. A growing body of literature demonstrates that, although periods of the annual cycle are often temporally and/or geographically separate, they are inextricably linked [4]. Events occurring during one period often continue to influence individuals and populations during subsequent periods, profoundly influencing both ecological and evolutionary processes. These effects are termed ‘seasonal interactions’ and can occur at two scales—the individual and population levels. At the individual level, non-fatal effects, such as poor physical condition or delayed phenology, carry-over from one season to the next to influence vital rates such as reproductive success or survival. At the population level, numerical changes between seasons can drive a density-dependent effect such as reduced winter survival altering densities and recruitment in the breeding period [5]. Both individual- and population-level effects can influence animals simultaneously and are not necessarily mutually exclusive.
Although this terminology is relatively new, the notion of seasonal interactions has been in the literature for a long time. Darwin noted that events prior to breeding can influence female fecundity in migratory birds [6]. Fretwell [5] argued that population dynamics of organisms living in seasonal environments result from events occurring between seasons [5]. He presented a theoretical case that breeding densities are determined in part by overwinter survival, which in turn, is related to events occurring during the preceding breeding season. Seasonal interactions can also result from interspecific relationships, such as in predator–prey dynamics when high survival in a prey species in one season influences predator dynamics in a subsequent season [7]. Collectively, several studies illustrate how full annual cycle research is necessary to understand fundamental biology across multiple species groups (e.g. amphibians [8], reptiles [9], birds [10], mammals [11]). Research not incorporating prior seasons may misclassify mechanisms underlying individual variation in the season under consideration and numerical changes in populations. Furthermore, full annual cycle approaches are essential for interpreting potential effects of major stressors like climate change [12,13].
2. Methods: has research been biased towards breeding studies?
We conducted a literature review to quantitatively assess when during the annual cycle ecological research has been conducted and if seasonal interactions have been considered. We asked: (i) are there seasonal biases in vertebrate ecology research?, (ii) to what degree has research incorporated more than one period of the annual cycle?, (iii) to what degree has research looked for seasonal interactions? and (iv) have any observed biases changed over time?
The focus of our literature survey was ecological studies of amphibians, reptiles, birds and mammals. We carefully reviewed all articles published during 1994, 2000, 2006 and 2012 in five high impact (more than 3.0 IF) ecology journals (Ecology, Conservation Biology, Oecologia, Journal of Animal Ecology, Behavioral Ecology) and four taxa-specific journals (Herpetologia, The Auk, Journal of Avian Biology, Journal of Mammalogy). We excluded papers that had no new data collection (e.g. commentaries, reviews).
Periods of the annual cycle were categorized as (i) breeding, (ii) in transit (i.e. migration, dispersal) or (iii) stationary non-breeding. For papers that did not explicitly state the period studied, we used the seasonal information reported (e.g. months or seasons of inquiry). If a paper focused on multiple species of the same taxon, it was recorded once but if it focused on more than one taxonomic group we recorded separate entries for each taxon.
To test for seasonal biases, we used Pearson χ2-tests with expected values being equal proportions to represent the null hypothesis that no bias exists. This conservative assumption is that seasons are equally important or that the importance is relative to the length of the season in their contribution to fitness variation. We recognize the simplicity of this underlying assumption and future research should test its validity. Seasons vary in length and in the number and importance of life-history events. For example, the stationary non-breeding period is often longer but typified by foraging, resting and survival, whereas migration can be quick, stressful and individuals can experience high mortality. The breeding period can be short, is characterized by reproduction, offspring rearing and other physiologically demanding processes.
3. Results: research in animal ecology is severely biased towards the breeding period
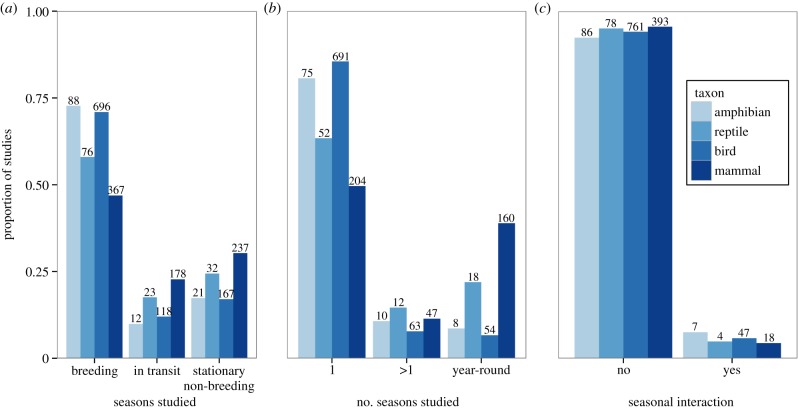
Across all taxa, we found a strong seasonal bias (χ2 = 96.5, N = 2015, p < 0.0001; figure 1a), with 61% of research conducted during breeding. Seasonal biases towards breeding were significant for all orders: amphibians (χ2 = 85.5, N = 121, p < 0.0001), reptiles (χ2 = 36.8, N = 131, p < 0.0001), birds (χ2 = 629.9, N = 980, p < 0.0001) and mammals (χ2 = 72.4, N = 783, p < 0.0001).
Figure 1.
Proportion of studies on four vertebrate orders conducted (a) during each period of the annual cycle: breeding, in transit (i.e. migration or a similar seasonal movement between breeding and non-breeding), or stationary non-breeding, (b) during one annual cycle period, more than one, or year-round, and (c) examining a seasonal interaction.
Few studies incorporated multiple periods. Across all taxa, 73% (N = 1022) of studies were conducted during only one period of the annual cycle, 9% (N = 33) incorporated more than one period and 17% (N = 60) were year-round (χ2 = 1015.8, n = 1394, p < 0.0001; figure 1b). Differences were significant for: amphibians, (χ2 = 93.7, n = 93, p < 0.0001), reptiles (χ2 = 34.1, n = 82, p < 0.0001), birds (χ2 = 995.1, n = 808, p < 0.0001) and mammals (χ2 = 95.1, n = 411, p < 0.0001).
Few studies (5.5%; χ2 = 1107.6, N = 1394, p < 0.0001; figure 1c) have looked for seasonal interactions (amphibians: χ2 = 68.1, N = 93, p < 0.0001; reptiles: χ2 = 66.8, N = 82, p < 0.0001; birds: χ2 = 634.5, N = 808, p < 0.0001; mammals: χ2 = 338.5, N = 411, p < 0.0001). In addition, there has been little change in when research is conducted, and the proportion of year-round studies has remained constant (figure 2). While the number of studies incorporating seasonal interactions doubled from 1994 to 2012, the overall proportion in 2012 was only 5% of all studies.
Figure 2.
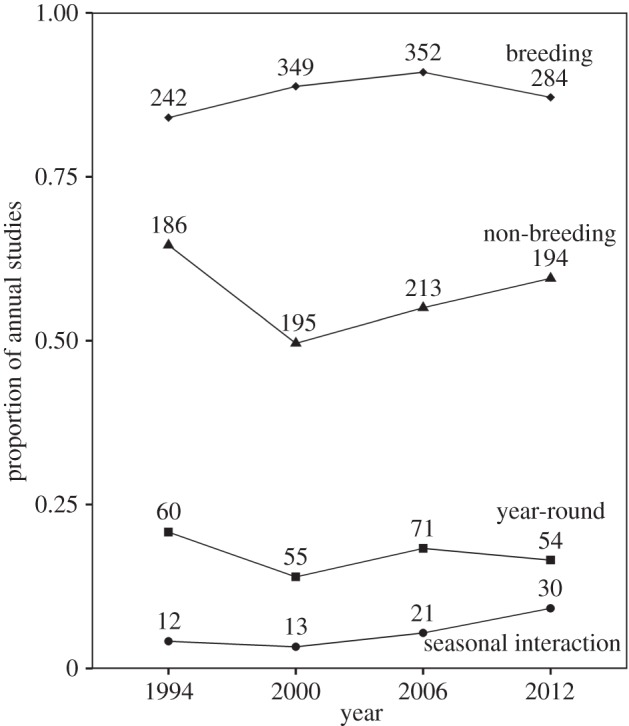
Change in annual proportion of studies published from 1994 to 2012 that included research during the breeding period, during the stationary non-breeding period or year-round, or that included seasonal interactions.
4. Moving forward–reversing the bias
Such biases likely occurred because of the: (i) longstanding belief that events during the breeding season are paramount to other periods of the annual cycle [14], (ii) logistical complexities of collecting annual or full life cycle data, including cost, time and an academic calendar, and (iii) technical obstacles associated with following individual animals and mass movements of populations across vast distances, or to inaccessible places (e.g. marine mammals at sea). Individual tracking devices have been too expensive, unreliable or large for the vast majority of species, making it challenging to follow animals as they move throughout the annual cycle.
Fortunately, limitations that once hindered our ability to track individuals and populations throughout the year, or even over lifetimes, are disappearing. New analytical, computing and technological tools are advancing the pace and magnitude of data collection, analysis and modelling of animal movements. Satellite and cell phone transmitters, geolocators [15], stable isotope analyses [16] and genomics [17] are advancing our ability to track animals for longer periods and at higher resolutions. When combined with complementary demographic data, quantification of habitat quality and/or measures of physiology, these advancements are revolutionizing our understanding of movement ecology and fundamental biology. For instance, in birds they have revealed how interactions between stages of the annual cycle can limit fitness [18], seasonal variation in mortality [19] and previously unknown distributions [20]. Many of these approaches involve large, expensive equipment or lack precision, so it remains essential that we continue to push for improvements to reduce costs, improve accuracy and precision, and increase utility for animals of all sizes. Finally, the refinement of remote sensing tools (e.g. weather radar) [21] and citizen science approaches (e.g. eBird) [22] are recent advances allowing observations of mass movements of populations over large spatial scales and across periods of the annual cycle.
The increase in seasonal interaction studies is a positive development but is still far from where we need to be. Such information is vital for management and conservation, as these fields rely on detailed understanding of limitation and regulation of populations, particularly those in decline. For example, full annual cycle approaches are necessary to establish whether mortality factors induce compensatory or additive mortality [23] and large-scale perspectives quantifying mass movements of animals across the annual cycle will be essential for understanding large-scale land-use patterns [24]. Clearly, full annual cycle approaches, at the individual and population level, are required for a vast array of conservation questions and are an essential step to implementing effective approaches to reverse population declines and keep common species common.
If we as scientists and conservation biologists are to reverse the alarming declines of many vertebrate populations [25], in a rapidly changing world, then we need to develop and implement more strategic, effective and efficient conservation strategies. These strategies must involve a full annual cycle perspective and improved technologies for the remote, dynamic and continuous assessments of the biology of animals for longer periods and over larger spatial scales.
Acknowledgements
The manuscript benefited from the comments of Autumn-Lynn Harrison, Colin Studds and three anonymous reviewers.
Data accessibility
The dataset is available at: http://nationalzoo.si.edu/scbi/migratorybirds/research/data/.
Authors' contributions
All authors conceived, designed and executed the study. P.P.M. wrote the first draft and all authors provided comments. All authors have read and approve the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by NSF, US Fish and Wildlife Service's Migratory Bird Program, Upper Midwest/Great Lakes LCC and Smithsonian's Didden Fellowship.
References
- 1.Petranka JW. 1998. Salamanders of the United States and Canada. Washington, DC: Smithsonian Books. [Google Scholar]
- 2.Eckert KL, Wallace BP, Frazier JG, Eckert SA, Pritchard PCH. 2012. Synopsis of the biological data on the leatherback sea turtle. (Dermochelys coriacea). US Dept Interior Fish Wildl. Serv. Biol. Tech. Publ. BTP-R4015-2012. See http://digitalmedia.fws.gov/cdm/ref/collection/document/id/1519. [Google Scholar]
- 3.Porneluzi P, Van Horn MA, Donovan TM. 2011. Ovenbird (Seiurus aurocapilla). In The birds of North America (ed. Poole A.). Ithaca, NY: Cornell Laboratory of Ornithology. [Google Scholar]
- 4.Harrison XA, Blount JD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 5.Fretwell SD. 1972. Populations in a seasonal environment. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 6.Darwin C. 1871. In The descent of man, and selection in relation to sex, p. 260, London: Murray. [Google Scholar]
- 7.Messier F. 1994. Ungulate population models with predation: a case study with the North American moose. Ecology 75, 478–488. ( 10.2307/1939551) [DOI] [Google Scholar]
- 8.Touchon JC, McCoy MW, Vonesh JR, Warkentin KM. 2013. Effects of plastic hatching timing carry over through metamorphosis in red-eyed treefrogs. Ecology 94, 850–860. ( 10.1890/12-0194.1) [DOI] [Google Scholar]
- 9.Hare KM, Caldwell AJ, Cree A. 2012. Effects of early postnatal environment on phenotype and survival of a lizard. Oecologia 168, 639–649. ( 10.1007/s00442-011-2145-3) [DOI] [PubMed] [Google Scholar]
- 10.Morrissette M, Bêty J, Gauthier G, Reed A, Lefebvre J. 2010. Climate, trophic interactions, density dependence and carry-over effects on the population productivity of a migratory Arctic herbivorous bird. Oikos 119, 1181–1191. ( 10.1111/j.1600-0706.2009.18079.x) [DOI] [Google Scholar]
- 11.English S, Bateman AW, Mares R, Ozgul A, Clutton-Brock TH. 2014. Maternal, social and abiotic environmental effects on growth vary across life stages in a cooperative mammal. J. Anim. Ecol. 83, 332–342. ( 10.1111/1365-2656.12149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ådahl E, Lundberg P, Jonzén N. 2006. From climate change to population change: the need to consider annual life cycles. Glob. Change Biol. 12, 1627–1633. ( 10.1111/j.1365-2486.2006.01196.x) [DOI] [Google Scholar]
- 13.Small-Lorenz SL, Culp LA, Ryder TB, Will TC, Marra PP. 2013. A blind spot in climate change vulnerability assessments. Nat. Clim. Change 3, 91–93. ( 10.1038/nclimate1810) [DOI] [Google Scholar]
- 14.Pulliam HR, Millikan GC. 1982. Social organization in the nonreproductive season. Avian Biol. 6, 169–197. ( 10.1016/B978-0-12-249406-2.50012-5) [DOI] [Google Scholar]
- 15.Bridge ES, et al. 2011. Technology on the move: recent and forthcoming innovations for tracking migratory birds. BioScience 61, 689–698. ( 10.1525/bio.2011.61.9.7) [DOI] [Google Scholar]
- 16.Hobson KA, Wassenaar LI. 2008. Tracking animal migration with stable isotopes. Amsterdam, The Netherlands: Academic Press. [Google Scholar]
- 17.Jonker RM, et al. 2013. Genetic consequences of breaking migratory traditions in barnacle geese Branta leucopsis. Mol. Ecol. 22, 5835–5847. ( 10.1111/mec.12548) [DOI] [PubMed] [Google Scholar]
- 18.Reudink MW, Marra PP, Kyser TK, Boag PT, Langin KM, Ratcliffe LM. 2009. Non-breeding season events influence sexual selection in a long-distance migratory bird. Proc. R. Soc. B 276, 1619–1626. ( 10.1098/rspb.2008.1452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klaassen RH, et al. 2014. When and where does mortality occur in migratory birds? Direct evidence from long-term satellite tracking of raptors. J. Anim. Ecol. 83, 176–184. ( 10.1111/1365-2656.12135) [DOI] [PubMed] [Google Scholar]
- 20.Beason JP, Gunn C, Potter KM, Sparks RA, Fox JW. 2012. The Northern Black Swift: migration path and wintering area revealed. Wilson J. Ornithol. 124, 1–8. ( 10.1676/11-146.1) [DOI] [Google Scholar]
- 21.Kelly JF, Shipley JR, Chilson PB, Howard KW, Frick WF, Kunz TH. 2012. Quantifying animal phenology in the aerosphere at a continental scale using NEXRAD weather radars. Ecosphere 3, 16 ( 10.1890/ES11-00257.1) [DOI] [Google Scholar]
- 22.Sullivan BL, et al. 2014. The eBird enterprise: an integrated approach to development and application of citizen science. Biol. Conserv. 169, 31–40. ( 10.1016/j.biocon.2013.11.003) [DOI] [Google Scholar]
- 23.Loss SR, Will T, Marra PP. 2012. Direct human-caused mortality of birds: improving quantification of magnitude and assessment of population impact. Front. Ecol. Environ. 10, 357–364. ( 10.1890/110251) [DOI] [Google Scholar]
- 24.La Sorte FA, et al. 2015. Documenting stewardship responsibilities across the annual cycle for birds on U.S. public lands. Ecol. Appl. 25, 39–51. ( 10.1890/14-0702.1) [DOI] [PubMed] [Google Scholar]
- 25.Dirzo R, Young HS, Galetti M, Ceballos G, Isaac NJ, Collen B. 2014. Defaunation in the Anthropocene. Science 345, 401–406. ( 10.1126/science.1256585) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available at: http://nationalzoo.si.edu/scbi/migratorybirds/research/data/.