Abstract
The latest Neoproterozoic extinction of the Ediacara biota has been variously attributed to catastrophic removal by perturbations to global geochemical cycles, ‘biotic replacement’ by Cambrian-type ecosystem engineers, and a taphonomic artefact. We perform the first critical test of the ‘biotic replacement’ hypothesis using combined palaeoecological and geochemical data collected from the youngest Ediacaran strata in southern Namibia. We find that, even after accounting for a variety of potential sampling and taphonomic biases, the Ediacaran assemblage preserved at Farm Swartpunt has significantly lower genus richness than older assemblages. Geochemical and sedimentological analyses confirm an oxygenated and non-restricted palaeoenvironment for fossil-bearing sediments, thus suggesting that oxygen stress and/or hypersalinity are unlikely to be responsible for the low diversity of communities preserved at Swartpunt. These combined analyses suggest depauperate communities characterized the latest Ediacaran and provide the first quantitative support for the biotic replacement model for the end of the Ediacara biota. Although more sites (especially those recording different palaeoenvironments) are undoubtedly needed, this study provides the first quantitative palaeoecological evidence to suggest that evolutionary innovation, ecosystem engineering and biological interactions may have ultimately caused the first mass extinction of complex life.
Keywords: Ediacaran, Cambrian, extinction, ecology, diversity, ecosystem engineers
1. Introduction
The terminal Neoproterozoic (Ediacaran: 635–541 Ma) Ediacara biota was an enigmatic assemblage of large, morphologically complex eukaryotes that represent the first major radiation of multicellular life. The biological affinities of these organisms have been much debated, but recent work suggests they represent a mixture of stem- and crown-group animals, as well as extinct higher order clades with no modern representatives [1–3]. With the exception of a few isolated occurrences [4,5], Ediacara-type fossils are absent from Cambrian and younger strata. Three competing hypotheses have been proposed to explain their disappearance around the Ediacaran–Cambrian boundary [6]: (1) a ‘catastrophic’ extinction event precipitated by perturbations to global geochemical cycles in the terminal Ediacaran [7–12]; (2) the result of ‘biotic replacement’, whereby members (or precursors) of the Cambrian evolutionary fauna gradually outcompeted Ediacaran biotas through ecological engineering of Ediacaran ecosystems [6,13]; and (3) a taphonomic artefact, whereby the conditions required for Ediacaran preservation disappeared at the Ediacaran–Cambrian boundary [6]. This third model has been convincingly rejected [12], however, few studies have attempted to directly test predictions stemming from the two more plausible models.
The ‘biotic replacement’ model implies a gradual palaeoecological change through the Ediacaran, and therefore makes two predictions: (1) latest Ediacaran assemblages should be ecologically and taxonomically depauperate when compared to those in older assemblages; and (2) evidence for ecosystem engineering, such as bioturbation, should be more abundant in terminal Ediacaran sections. In this model, the extinction event is protracted and begins earlier in the Ediacaran with the first appearance of metazoan ecosystem engineers. Abundant evidence supporting the second prediction of the ‘biotic replacement’ model is provided by the relatively high diversity of metazoan traces in the uppermost Ediacaran and lowermost Cambrian rocks [6,14–16], however, the first prediction of this model has yet to be critically examined. In this study, we test the first prediction of the ‘biotic replacement’ model. We perform palaeoecological analyses of the latest Ediacaran (‘Nama’ assemblage: approx. 545–542 Ma) fossil localities preserved in Farm Swartpunt, southern Namibia, and compare the resulting diversity indices with older Ediacaran assemblages worldwide, which form a time series through the Mid- to End-Ediacaran. Discovery of lower species richness and evenness in terminal Ediacara fossil assemblages would support the predictions of the ‘biotic replacement’ hypothesis. Alternatively, finding equivalent richness and diversity metrics relative to older assemblages would instead support the ‘catastrophe’ hypothesis and suggest that Edicaran ecosystems suffered abrupt extinction at the Ediacaran–Cambrian boundary.
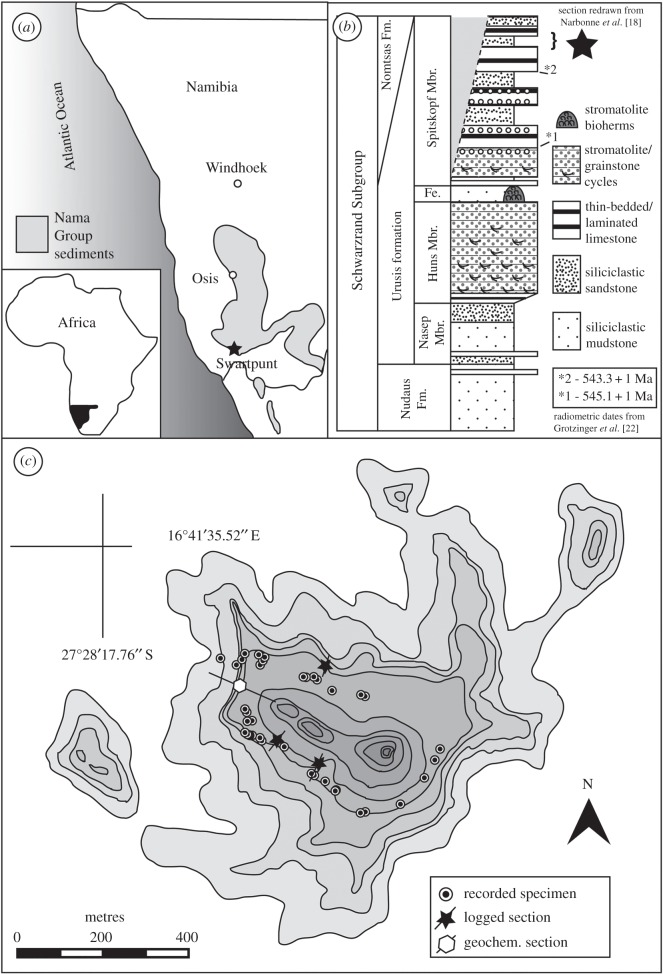
The fossil-bearing horizons at Farm Swartpunt are part of the latest Ediacaran Nama Group, Urusis Formation (Spitskopf Member), of southern Namibia (figure 1). The Nama Group records mixed siliciclastic–carbonate sedimentation into a foreland basin related to convergence along the Damara and Gariep deformational belts and was deposited into two sub-basins separated by the palaeo-topographic high of the Osis Arch [17,19–21]. Farm Swartpunt belongs to the southernmost of these two basins—the Witputs sub-basin—and preserves rocks that regionally dip approximately 1° to the west. An ash bed in the lower carbonate package of the Urusis Formation has been dated by U–Pb geochronology at 545.1 ± 1 Ma, and an ash bed approximately 85 m below the investigated fossil beds at 543.3 ± 1 Ma ([22]—see figure 1; recalculated to 540.61 ± 0.67 Ma in [23]). An erosive unconformity overlain by complex valley-filling deposits of the earliest Cambrian Nomtsas Formation cuts down through the Ediacaran strata, although the physical unconformity itself is not well exposed on Swartpunt Farm [18,22,24]. Nomtsas strata in the Swartkloofberg Farm directly north of Swartpunt contain an ash bed dated to 539.4 ± 1 Ma ([22]; recalculated to 538.18 ± 1.11 Ma in [23]). These ages are effectively identical to ages for the inferred Ediacaran–Cambrian boundary in Oman [25] and Siberia [26], confirming that the Ediacara biota at Swartpunt existed in the last approximately 1 Myr before the Ediacaran–Cambrian boundary.
Figure 1.
(a) Distribution of Nama-Group sediments in southwestern Africa (adapted from [17]); (b) generalized stratigraphic column for the latest Ediacaran Urusis Formation (Schwarzrand Subgroup) in the southernmost Witputs sub-basin (from [18]); and (c) the distribution of Ediacaran fossils and logged sections treated in this study. Graduations represent contours.
Latest Ediacaran fossil assemblages are thought to have unusually low diversity [18], however, diversity estimates from fossil data can be heavily influenced by worker effort (number of original taxonomic papers published on a single fossil site—see [27]) and sampling intensity [28], both of which are rarely accounted for in assessments of Ediacaran diversity (although see [29]). This first bias is especially true for Ediacaran sites (electronic supplementary material, S1) and emphasizes the need for sample-standardization from original field data, as opposed to global compilations of taxa. We therefore undertook an intensive survey of the latest Ediacaran fossil-bearing horizons preserved on Farm Swartpunt and performed rarefaction analyses to investigate richness estimates at a range of sampling intensities. We recovered 106 individual fossils from the surveyed area, both in place and as float specimens (from numerous horizons—see electronic supplementary material, S2 and S3), 79 of which were readily attributable to known Ediacaran taxa (complete dataset given in electronic supplementary material, S4). In addition to Swartpuntia and Pteridinium, we recovered numerous Aspidella, an erniettomorph taxon provisionally assigned to Ernietta, and a rangeomorph form provisionally assigned to Bradgatia (electronic supplementary material, S5). At least one of our Aspidella specimens preserves the trace of a segmented stem structure readily attributable to Swartpuntia (electronic supplementary material, S5). Of the 79 identifiable fossil specimens, 28 were found in place on the top surface of one stratigraphic horizon (‘Bed 1’—see electronic supplementary material, S2), allowing single bed comparisons with other datasets.
In order to test whether these latest Ediacaran assemblages are relatively depauperate, we performed the same analyses on three older Ediacaran assemblages, from Mistaken Point, Newfoundland (‘Avalon’ assemblage, dating between approx. 579 and approx. 565 Ma and comprising eight fossiliferous surfaces, using data from [30]), Nilpena, South Australia (‘White Sea’ assemblage, between approx. 555–550 Ma, comprising five facies associations, using data from [31]), and the White Sea, Russia (‘White Sea’ assemblage, using data from [32]). Locality summaries are given in electronic supplementary material, S6. Richness estimates from fossil data can be heavily influenced by stratigraphic (i.e. counted from in situ populations on a single bedding plane, versus collected from loose material and therefore likely aggregated over several fossiliferous horizons), and taphonomic (i.e. two-dimensional versus three-dimensional preservation) contexts. We therefore performed additional comparisons after adjusting the Mistaken Point, Nilpena and White Sea datasets to account for these differences, and thus form more realistic comparisons with our dataset from Swartpunt. In terms of stratigraphic context, we aggregated the Mistaken Point D, E and G surfaces (which in Newfoundland are separated by approx. 10 m of stratigraphy—[33]), so that our sampling protocol simulates random fossil sampling across several surfaces, and thus matches the stratigraphic context of fossil data from Swartpunt. In terms of taphonomic context, Ediacaran preservation can preserve frondose taxa either as holdfasts with associated fronds, or holdfasts without stems and fronds [34]. This latter taphonomic mode results in severe loss of taxonomic resolution. To account for these potential taphonomic differences between datasets, we simulated a taphonomic ‘worst case’ scenario, whereby all frondose taxa possessing holdfast structures in all datasets were re-assigned to Aspidella, thereby simulating poor preservation across all samples and eliminating between-locality differences in taxonomic resolution. We also performed an additional analysis and sensitivity test excluding Aspidella, which tested to what extent the observed patterns are controlled by frondose taxa.
Finally, fossil biotas may show low diversity and/or evenness not due to evolutionary factors, but because of palaeoenvironmental conditions. At least among metazoans, both low oxygen levels and euxinia are considerable barriers to colonization, and often lead to low diversity communities dominated by opportunistic taxa with broad niche tolerances and/or small-sized organisms with reduced oxygen requirements [35,36]. Communities with high organic carbon loading also generally exhibit low evenness. We therefore integrated our diversity analyses with a multi-proxy geochemical study to determine the redox state and organic carbon contents of the surrounding sediment at the time of deposition. This combination of palaeobiological and geochemical analyses allowed us to test whether: (i) diversity patterns at Swartpunt support either the ‘catastrophe’ or ‘biotic replacement’ model for the end of the Ediacara biota, and (ii) diversity patterns are more likely a consequence of ongoing biotic replacement (e.g. [6,13]) or environmental (i.e. abiotic) stress.
2. Material and methods
(a). Fossil collection
Because the lowermost approximately 16 m of the siliciclastic interval preserving fossils form a relatively steep cliff-forming unit, many of these lower horizons had to be excluded from surveying. As a result, the surveyed area mostly encompassed approximately 10 m of stratigraphy spanning from the top of the main cliff-forming unit (equivalent to fossil bed ‘A’ of [18]), up to a ridge-forming layer composed of thin-bedded sandstone with calcareous matrix/cement (approx. 5 m above fossil bed ‘B’ of [18]—electronic supplementary material, S2 and S3). All discovered fossils were identified in the field and recorded along with latitude and longitude, lithology, and stratigraphic context (i.e. in float or in place). In addition, each in situ specimen was photographed, measured and a long-axis orientation recorded. These fossil occurrences were used to construct a database that served as the basis for rarefaction analyses. In addition to surveying, we measured three sections around the rim of the outcrop to investigate the stratigraphic distribution of fossils within the key siliciclastic horizons at the top of the Spitskopf Member (see electronic supplementary material, S2). The total area surveyed at Farm Swartpunt was estimated as 20 359 m2 (=0.02 km2) using the polygon tool in Google Earth (electronic supplementary material, S3).
(b). Data treatment
Substantial work has re-described many of the organisms preserved around Mistaken Point. Consequently, a number of modifications were made to the original Clapham et al. [30] datasets to bring the taxonomy and nomenclature up to date (electronic supplementary material, S7; see also [37]). We assigned ‘discs/stems’, ‘discs’ and ‘holdfasts’ recorded on all Mistaken Point surfaces to Aspidella for two reasons: (1) Aspidella is thought to represent the holdfast structure to a frondose organism, but cannot yet be convincingly tied to any one specific taxon (and thus an assemblage of Aspidella may represent any number of six frondose taxa reported from Mistaken Point); and (2) this allows easy comparisons with the Nilpena, White Sea and Swartpunt localities, which also preserve holdfast structures without associated fronds. Lumping Aspidella in this fashion will therefore likely underestimate the real diversity of all four localities, but is preferable to excluding it entirely.
(c). Controlling for differences in taphonomic context between datasets
To control for taphonomic differences between datasets, we simulated a taphonomic ‘worst case’ scenario, whereby all frondose taxa possessing holdfast structures in the Mistaken Point, White Sea and Nilpena datasets (including Beothukis, Charnia, Charniodiscus, Culmofrons, ‘Dusters’, Primocandelabrum, Trepassia and Swartpuntia) were re-assigned to Aspidella, thereby simulating poor preservation across all samples and eliminating between-locality differences in taxonomic resolution.
(d). Rarefaction analyses
All palaeoecological analyses were performed using the open access statistical software R. For sampling intensity 1 : n (where n = the number of individuals within each dataset), individuals were randomly selected (without replacement) from each dataset, and the number of unique species calculated. This process was iterated 100 times for each dataset, and the final richness estimates taken as the mean value of all iterations. The distribution of iterated values for each n were also used to calculate 95% confidence intervals around mean values, to allow statistical comparison between localities for any given sampling intensity; if confidence intervals for two localities do not overlap at any given sampling intensity, then estimated richness at that sample size is significantly different between the two localities. All analyses treated Ediacaran fossil data at genus, rather than species level, due to the wide disparity in taxonomic resolution between the three treated sites. However, patterns are virtually identical for species-level analyses (see electronic supplementary material, S8).
(e). Geochemical analyses
Twenty-seven collected samples were first crushed to flour in a tungsten-carbide shatterbox. Iron speciation measurements for these samples are reported in [38], but are plotted and fully discussed here in their stratigraphic context (see also electronic supplementary material, S8). The iron speciation proxy has been well calibrated in modern anoxic environments, and samples with ratios of highly reactive iron (FeHR) to total iron (FeT) more than 0.38 are taken to represent deposition under an anoxic water column [39] (FeHR = iron in pyrite plus iron that is reactive to sulfide on early diagenetic timescales, including iron oxides, iron carbonates and magnetite). Values between 0.38 and 0.22 generally represent oxic conditions, but in certain cases (such as rapid deposition) anoxic water columns may result in lower enrichments [39,40]. Values beneath 0.22 are conservatively taken to indicate oxygenated conditions. In modern and ancient anoxic basins, values for total iron, as well as redox-sensitive trace metals, are enriched compared to crustal values [41,42]. Major, minor and trace-element abundances for 33 elements (total iron reported in [38]) were analysed by ICP-AES following standard four-acid digestion: hydrofluoric, hydrochloric, perchloric and nitric—results given in electronic supplementary material, S9). These new data allow for independent tests of iron speciation results using Fe/Al ratios and concentrations of trace metals such as molybdenum and vanadium. Specifically, Fe/Al ratios compared to oxic shale can be used to identify anoxic conditions even if highly reactive iron phases have been converted to poorly reactive clays (e.g. [43]) and redox-sensitive trace metals can be expected to accumulate under reducing conditions, with enrichments of each specific metal corresponding to different palaeoenvironmental conditions [42]. Per cent total inorganic carbon was determined via mass loss on acidification, and total organic carbon and organic carbon isotope values were measured on acidified samples by combustion within a Carlo Erba NA 1500 Analyser attached to a Thermo Scientific Delta V Advantage isotope ratio mass spectrometer. Extended methodological details of the analyses conducted can be found in the supplement of Sperling et al. [44].
3. Results
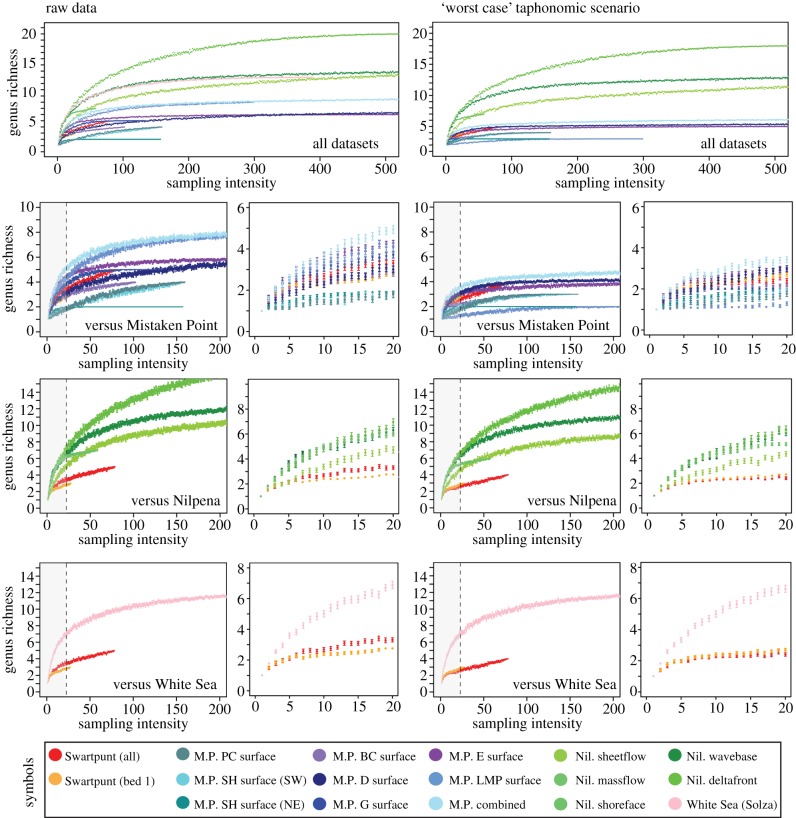
Our rarefaction curves (figure 2) illustrate estimated genus richness as a function of sampling intensity, and therefore provide a way of comparing diversity estimates between sites with differing total sample numbers. Our results show that, even after extensive surveying, the fossil assemblage at Farm Swartpunt is still undersampled, and that continued surveying may produce more rare taxa. This inference is supported by the discovery of another erniettomorph taxon, Nasepia (see electronic supplementary material, S5), during a preliminary survey in 2013, but not re-discovered during this study. Despite this, the species discovery curve for Swartpunt displays a notable flattening between sampling intensities 20–70, suggesting that relatively few rare taxa await discovery at the site. In comparison to the Mistaken Point datasets adapted from [30], aggregated data from Swartpunt suggests higher diversity than many individual horizons, but still lower than two of the Mistaken Point surfaces. When single-bed data (‘Bed 1’) from Swartpunt are used, richness estimates are higher than only three of the Mistaken Point surfaces. Likewise, when surfaces are aggregated (to simulate random sampling of several superimposed fossil horizons), richness estimates for Mistaken Point increase, becoming approximately 50% higher than aggregated data for Swartpunt. Comparing estimates between Swartpunt and the Nilpena/White Sea datasets reveals that aggregated Swartpunt diversity is significantly lower, at virtually any given sampling intensity, than any of the Australian or Russian localities. At sampling intensities between 50 and 70, Swartpunt diversity is between approximately 40% and approximately 60% lower than any Nilpena sites, and approximately 100% lower than the White Sea. In sum, aggregated data for Swartpunt indicate lower diversity than all other aggregated datasets. Single-bed data for Swartpunt indicate lower diversity than all except three of the Mistaken Point beds.
Figure 2.
Results of rarefaction analyses, comparing diversity estimates for raw data (left) and taphonomically adjusted (right) data. Top panels illustrate all datasets. Middle and lower panels illustrate contrasts between Swartpunt and Mistaken Point, Nilpena and White Sea datasets; error bars have been added to these panels as 95% CIs around mean diversity values. Areas of low sampling intensity (shaded in grey) have been expanded in adjacent panels to better illustrate differences in richness at low sample numbers.
This pattern is strengthened after applying our ‘worst case’ taphonomic scenario where all frondose taxa are re-assigned to Aspidella (figure 2). Although aggregated Swartpunt data now display higher taxonomic richness than any of the individual surfaces at Mistaken Point, it is still significantly less rich than the aggregated Newfoundland data at sampling intensities n > 5, even though the surveyed area at Swartpunt is far greater (see electronic supplementary material, S7), negating an explanation in terms of richness-area effects. Single-bed data from Swartpunt do show an increase in relative richness, but remain lower than the D and E surfaces at sampling intensities n > 15 (although error bars show some overlap). Richness comparisons between Swartpunt and the Nilpena/White Sea datasets remain virtually unchanged, although richness estimates for Nilpena decrease. For any given sampling intensity, Nilpena and White Sea localities remain between 50 and 100% richer than Swartpunt. Results of rarefaction analyses that exclude Aspidella entirely are identical to those of the raw data (electronic supplementary material, S9), illustrating that our results are not dependent on the relative abundance of frondose taxa at any site.
Our geochemical analyses illustrate that the redox environment was relatively uniform across the sampled stratigraphy (figure 3; electronic supplementary material, S10 and S11). The highly reactive iron pool for the fossiliferous Spitskopf Member strata is dominated by iron oxides (0.15 ± 0.08 weight per cent) with lesser amounts of iron carbonate (0.06 ± 0.02 weight per cent) and magnetite (0.04 ± 0.02 weight per cent) and negligible iron sulfide (pyrite). As total iron contents averaged 4.41 ± 0.86 weight per cent, this resulted in low overall highly reactive (FeHR) to total (FeT) ratios (mean of 0.06 ± 0.025; maximum of 0.14). These results are consistent with the limited sampling at this locality in the regional study of Wood et al. [45]. Total aluminium averaged 9.28 ± 1.20 weight per cent, resulting in iron to aluminium ratios (Fe/Al) of 0.47 ± 0.06. This result overlaps with the average Palaeozoic normal (oxic) marine shale value of 0.53 ± 0.11 [41]. The redox-sensitive trace metal contents of fossiliferous Spitskopf sediments for molybdenum (1.2 ± 0.5 ppm), vanadium (102.7 ± 28.0 ppm), chromium (49.1 ± 16.6 ppm) and cobalt (19.5 ± 4.7 ppm) are also at or below average shale values [46] both as absolute abundances and when normalized to a biogeochemically conservative element such as aluminium. Of these four elements, no individual sample enrichments, either absolute or Al-normalized, were seen for Mo, V and Cr, and two samples, SWP −8.8 and 11.6, were slightly enriched in Co (30 ppm for both, 3.49 and 3.50 Co ppm/Al weight per cent ratio).
Figure 3.
Geochemical profile for studied section (‘geochem section’ in figure 1). From left to right, columns illustrate highly reactive iron to total iron (FeHR/FeT) ratios, iron to aluminium (FeT/Al) ratios and total organic carbon weight per cent (TOC) values. The FeHR/FeT ratio of 0.38 separating anoxic from oxic water columns [39] and the average Palaeozoic oxic shale value of Fe/Al = 0.53 [41] are shown as vertical blue bars on the first two columns. Relative standard deviations are estimated at less than 5% for pooled FeHR sequential extractions, FeT and Al [44], and a replicate TOC sample differed by 0.009 wt%. Bracketed interval corresponds to measured ‘Section 1’ illustrated in electronic supplementary material, S2. For description of stratigraphy (ridges 1–3) and sampling, see electronic supplementary material, S2.
4. Discussion
Our results illustrate that, even after accounting for differences in sampling intensity and taphonomic variation between sites, estimated species richness at Farm Swartpunt is significantly lower than older assemblages from Mistaken Point, South Australia and Russia. Although applying our ‘worst case’ taphonomic scenario brings richness estimates for Swartpunt closer to those of Mistaken Point (figure 2), we believe that this scenario is overly conservative, as Swartpunt preserves both abundant fronds and holdfasts (see electronic supplementary material, S5). As such, we consider it unlikely that a large number of additional frondose taxa (represented by isolated Aspidella) existed at Swartpunt without being preserved. This is despite the fact that the Mistaken Point surfaces record a relatively deep-water fauna well below the photic zone, and so might be expected to possess lower richness than the majority of shallow-water communities (although see [47]). This supports the inference that the soft-bodied Ediacaran assemblage at Swartpunt possesses unusually low diversity and, more generally, that the latest Ediacaran communities preserved in Namibia are depauperate when compared to those found in older Ediacaran deposits. The results of rarefaction curves are consistent with calculated palaeoecological indices for each dataset (electronic supplementary material, S7), which support the inference of relatively high dominance, low evenness and low diversity Ediacaran communities existing approximately 1 Myr before the Ediacaran–Cambrian boundary.
This inference of general low diversity in the latest Ediacaran communities at Swartpunt supports the first prediction of the ‘biotic replacement’ model and is consistent with interpretation as a low richness ecosystem in the process of being marginalized by ecosystem engineers. This finding comes with a number of caveats; first, other latest Ediacaran localities preserve taxa not described here, such as Rangea and Nemiana from the nearby Farm Aar [48,49]. However, these localities are also typically considered to have low diversity and are moreover largely transported assemblages (preserved in channels and along the base of gutter casts), meaning that richness estimates are likely to be artificially inflated [31,50]. Second, we acknowledge that the outcrop at Swartpunt represents only one site (and moreover a site that reconstructs at high palaeo-latitudes, see [6], where relatively low species richness would be expected given a latitudinal biodiversity gradient), and so interpreting diversity patterns at global scales comes with some risk. In addition, modern ecological communities are subject to a variety of stochastic processes that affect community structure; relative abundance data from additional sites will be required to strengthen and confirm the inference that Nama-aged Ediacaran assemblages are universally depauperate. However, review of other Nama-aged fossil sites does not reveal a large number of Ediacaran taxa absent from Swartpunt, even at palaeo-equatorial latitudes [6], and Ediacara biota do not exhibit any perceptible latitudinal gradient in diversity [51]. As such, we are confident that our analyses are likely representative of global patterns, rather than just southern Namibia. The high abundance of erniettomorph fossils at Swartpunt also suggests that low ecological diversity is unlikely the result of a taphonomic or Signor–Lipps effect. Given that the diversity at Swartpunt comprises surficial (Pteridinium), erect (Swartpuntia) and potentially semi-infaunal (Ernietta—[48]) organisms, there is no reason to suspect that other iconic Ediacaran groups such as the Bilteromorpha, Triradialomorpha or Dickinsonimorpha were originally present, but not preserved. Given the environmental breadth and taphonomic integrity of the Dickinsonimorpha in particular, it is highly likely that this group became extinct before the end of the Ediacaran [31]. With relatively high sample numbers (79 individuals), both at Swartpunt and elsewhere [6,49], it is also unlikely that Signor–Lipps effects can explain the low diversity (and predominance of erniettomorphs) in latest Ediacaran sections worldwide.
Our field data (see electronic supplementary material, S2) support previous interpretations of these sections (e.g. [18,22]) as recording a quiet and open-marine palaeoenvironment near fair weather wave base, characterized by ripple-cross lamination and seafloor microbial mats, and showing evidence for occasional disruption by storms [18]. We suggest that the facies characteristics at Swartpunt are similar to many of the palaeoenvironments of South Australia (in particular, the delta-front and wave-base sand facies recorded at Nilpena—[31,52]), which possess similar sedimentological features; specifically, thin-bedded sandstones with ripple marks (wave-base sands) and laminated horizons with significant silt component (delta-front sands). Moreover, we find no evidence for a stressed palaeoenvironment at Swartpunt in either the sedimentological or geochemical record. Sedimentologically, the absence of any exposure surfaces or evaporitic minerals such as gypsum makes a hypersaline environment unlikely. Geochemically, highly reactive iron to total iron ratios of less than 0.38, and even more conservatively 0.22, are taken to represent an oxygenated environment [39,40], and thus the geochemical data (figure 3) indicate persistently oxygenated conditions during the lifetime of these organisms. These results are supported by the total iron/aluminium ratio and the abundances of redox-sensitive trace metals, both of which are at or below average shale values. Total organic carbon percentages are also low (0.07 ± 0.01 weight per cent), and do not provide evidence for organic carbon loading driving diversity patterns. Although some caveats exist on the interpretation of the geochemical data (electronic supplementary material, S12), particularly the difficulty in distinguishing degrees of dysoxia [53], these represent the most reliable current proxies of local redox chemistry, and illustrate that the fossiliferous strata at Farm Swartpunt show no evidence for stressed conditions across multiple proxies. This contrasts, for instance, with Early Ediacaran strata of the Eastern European Platform, which contain an assemblage of large ornamented acritarchs but no macroscopic body fossils, and exhibit evidence of a stressed environment manifested by fluctuating oxic-to-anoxic conditions [54]. Thus, while geochemical data cannot unambiguously rule out stressed conditions, the best available geochemical tests provide no support for such a scenario. As such, the low diversity of terminal Ediacaran assemblages at Farm Swartpunt most likely represents a genuine ecological and evolutionary signal, rather than a sampling-, taphonomic- or environment-based artefact.
The significant reduction in assemblage diversity between the older and apex-diversity assemblages preserved at Nilpena, and the depauperate Nama-aged assemblages represented at Swartpunt, supports the ‘biotic replacement’ model for the end of the Ediacara biota. This in turn suggests that the extinction was likely a protracted event; beginning sometime in the interval separating the White Sea and Nama Ediacaran assemblages, and which preferentially removed iconic Ediacaran clades such as the Dickinsonimorphs, Triradialomorphs and Bilateralomorphs [2,6]. We note that this model does not preclude the existence of another (and more sudden) extinction event at the Ediacaran–Cambrian boundary; however, our data suggest that Ediacaran communities were depauperate and ‘stressed’ long before 541 Ma. The existence at Nilpena of many Ediacaran taxa characteristic of the Nama assemblage (principally the Erniettomorpha and Rangeomorpha), together with taxa more typical of the White Sea assemblage [31], illustrates that overall low diversity in the latest Ediacaran is due to the removal of White Sea-type taxa, rather than the evolutionary replacement of one ecological association of organisms with another. In this model, latest Ediacaran associations therefore represent the survivors of a post-White Sea episode of extinction that removed the majority of known Ediacaran diversity. Although Phanerozoic extinction events have been shown to exhibit wide variation in ecological selectivity [55], this hypothesis might also predict that surviving taxa represent ecological generalists or opportunists with broad niche tolerances, or taxa otherwise readily able to colonize ecological refugia (perhaps in the sediment subsurface—[48]). In support of this, it should be noted that rangeomorphs represent the longest ranging Ediacaran clade, dominating both deep- and shallow-water facies (especially in the absence of other Ediacaran groups). This points to the overall high-tolerance of rangeomorphs to a broad diversity of environments and suggests a high tolerance to conditions that may be limiting to other Ediacarans.
In summary, palaeoecological analysis of the latest Ediacaran fossil localities at Farm Swartpunt confirm that communities had abnormally low diversity when compared with older Ediacaran assemblages, even after correcting for a variety of potential sampling and taphonomic biases. Although we cannot altogether rule out abiotic stressors (such as minor hyposalinity, temperature or other climatic factors), our geochemical data illustrate that the low observed species richness is unlikely to be the consequence of a restricted environment or fluctuating redox conditions. The discovery of complex trace fossils attributable to active metazoan substrate mining in the same locality [14] supports this inference. Together with the observation that latest Ediacaran to earliest Cambrian fossil localities in southern Namibia also contain evidence for increased diversity of bilaterian infauna and putative ecosystem engineers, these data provide the first quantitative support for the ‘biotic replacement’ model for the end of the Ediacara biota. In this scenario, soft-bodied Ediacara biota were slowly marginalized by newly evolving members of the Cambrian evolutionary fauna, which would have competed for resources, mixed the consistency and redox profile of the sediment and potentially changed the delivery or distribution of organic carbon to the seafloor [13,56–58]. This in turn suggests that the end of the Ediacara biota may have begun long before the Ediacaran–Cambrian boundary; the depauperate nature of communities preserved in southern Namibia indicates that the influence of ecosystem engineers likely stretches farther back into the Ediacaran. As such, future fossil discoveries that span the critical interval between ‘White Sea’ and ‘Nama’-aged assemblages should provide further evidence for extinction, and reveal earlier evidence for ecosystem engineering. In addition, this suggests that the first mass extinction of complex life may have been largely biologically mediated—ultimately caused by a combination of evolutionary innovation, ecosystem engineering and biological interactions—making this event unique in comparison with the much more heavily studied (and largely abiotically driven) Phanerozoic ‘Big Five’.
Supplementary Material
Acknowledgements
We extend thanks to the Geological Survey of Namibia, and in particular Helke Mocke, Charlie Hoffmann, Roger Swart and Gabi Schneider for logistical help in conducting fieldwork. We also thank Mr Lothar Gessert for access to Farm Swartpunt.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
S.A.F.D., D.H.E. and M.L. designed the research. S.A.F.D., T.B., R.A.R., S.M., S.T. and M.L. collected the data for palaeoecological analyses. E.A.S., A.S.M., P.M. and D.T.J. collected samples, measured sections and performed geochemical analyses. S.A.F.D., E.A.S., D.T.J. and M.L. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
S.A.F.D. and R.A.R. thank the Yale Peabody Museum of Natural History for support. M.L., S.T. and D.H.E. thank the NASA Astrobiology Institute; M.L. thanks the Connaught Foundation, National Science and Engineering Research Council of Canada and National Geographic Society for generous funding. E.A.S. was supported by a NAI Postdoctoral Fellowship. Geochemical analyses were supported by NSF-EAR 1324095.
References
- 1.Xiao S, Laflamme M. 2009. On the eve of animal radiation: phylogeny, ecology, and evolution of the Ediacara biota. Trends Ecol. Evol. 24, 31–40. ( 10.1016/j.tree.2008.07.015) [DOI] [PubMed] [Google Scholar]
- 2.Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097. ( 10.1126/science.1206375) [DOI] [PubMed] [Google Scholar]
- 3.Brasier MD, Antcliffe JB, Liu AG. 2012. The architecture of Ediacaran fronds. Palaeontology 55, 1105–1124. ( 10.1111/j.1475-4983.2012.01164.x) [DOI] [Google Scholar]
- 4.Jenson S, Gehling JG, Droser ML. 1998. Ediacara-type fossils in Cambrian-aged sediments. Nature 393, 567–569. ( 10.1038/31215) [DOI] [Google Scholar]
- 5.Hagadorn JW, Fedo CM, Waggoner B. 2000. Early Cambrian Ediacaran-type fossils from California. J. Palaeontol. 74, 731–740. () [DOI] [Google Scholar]
- 6.Laflamme M, Darroch SAF, Tweedt S, Peterson KJ, Erwin DH. 2013. The end of the Ediacara biota: extinction, biotic replacement, or Cheshire cat? Gondwana Res. 23, 558–573. ( 10.1016/j.gr.2012.11.004) [DOI] [Google Scholar]
- 7.Knoll AH, Carroll SB. 1999. Early animal evolution: emerging views from comparative biology and geology. Science 284, 2129–2137. ( 10.1126/science.284.5423.2129) [DOI] [PubMed] [Google Scholar]
- 8.Kimura H, Watanabe Y. 2001. Ocean anoxia at the Precambrian–Cambrian boundary. Geology 29, 995–998. () [DOI] [Google Scholar]
- 9.Amthor JE, Grotzinger JP, Schroder S, Bowring SA, Ramezani J, Martin MW, Matter A. 2003. Extinction of Cloudina and Namacalathus at the Precambrian–Cambrian boundary in Oman. Geology 31, 431–434. () [DOI] [Google Scholar]
- 10.Schroder S, Grotzinger JP. 2007. Evidence for anoxia at the Ediacaran–Cambrian boundary: the record of redox-sensitive trace elements and rare earth elements in Oman. J. Geol. Soc. Lond. 164, 175–187. ( 10.1144/0016-76492005-022) [DOI] [Google Scholar]
- 11.Wille M, Nagler TF, Lehmann B, Schroder S, Kramers JD. 2008. Hydrogen sulphide release to surface waters at the Precambrian/Cambrian boundary. Nature 453, 767–769. ( 10.1038/nature07072) [DOI] [PubMed] [Google Scholar]
- 12.Buatois LA, Narbonne GM, Mangano MG, Carmona NB, Myrow P. 2014. Ediacaran matground ecology persisted into the earliest Cambrian. Nat. Commun. 5, 3544 ( 10.1038/ncomms4544) [DOI] [PubMed] [Google Scholar]
- 13.Erwin DH, Tweedt SM. 2012. Ecological drivers of the Ediacaran–Cambrian diversification of Metazoa. Evol. Ecol. 26, 417–433. ( 10.1007/s10682-011-9505-7) [DOI] [Google Scholar]
- 14.Jenson S, Runnegar BN. 2005. A complex trace fossil from the Spitskop Member (terminal Ediacaran–? Lower Cambrian) of southern Namibia. Geol. Mag. 142, 561–569. ( 10.1017/S0016756805000853) [DOI] [Google Scholar]
- 15.Carbone C, Narbonne GM. 2014. When life got smart: the evolution of behavioral complexity through the Ediacaran and Cambrian of NW Canada. J. Palaeontol. 88, 309–330. ( 10.1666/13-066) [DOI] [Google Scholar]
- 16.Macdonald FA, Pruss SB, Strauss JV. 2014. Trace fossils with spreiten from the late Ediacaran Nama Group, Namibia: complex feeding patterns five million years before the Precambrian–Cambrian boundary. J. Palaeontol. 88, 299–308. ( 10.1666/13-042) [DOI] [Google Scholar]
- 17.Saylor BZ, Kaufman AJ, Grotzinger JP, Urban F. 1998. A composite reference section for the terminal Proterozoic strata of southern Namibia. J. Sedimentary Res. 68, 1223–1235. ( 10.2110/jsr.68.1223) [DOI] [PubMed] [Google Scholar]
- 18.Narbonne GM, Saylor BZ, Grotzinger JP. 1997. The youngest Ediacaran fossils from Southern Africa. J. Palaeontol. 71, 953–967. [DOI] [PubMed] [Google Scholar]
- 19.Germs GJB. 1983. Implications of a sedimentary facies and depositional environmental analysis of the Nama Group in South West Africa/Namibia. Geol. Soc. South. Afr. Spec. Publ. 11, 89–114. [Google Scholar]
- 20.Saylor BZ, Grotzinger JP, Germs GJB. 1995. Sequence stratigraphy and sedimentology of the Neoproterozoic Kuibis and Schwarzrand Subgroups (Nama Group), southwestern Namibia. Precambrian Res. 73, 153–171. ( 10.1016/0301-9268(94)00076-4) [DOI] [Google Scholar]
- 21.Grotzinger JP, Adams EW, Schröder S. 2005. Microbial–metazoan reefs of the terminal Proterozoic Nama Group (c. 550–543 Ma), Namibia. Geol. Mag. 142, 499–517. ( 10.1017/S0016756805000907) [DOI] [Google Scholar]
- 22.Grotzinger JP, Bowring BZ, Saylor BZ, Kaufman AJ. 1995. Biostratigraphic and geochronologic constraints on early animal evolution. Science 270, 598–604. ( 10.1126/science.270.5236.598) [DOI] [Google Scholar]
- 23.Narbonne GM, Xiao S, Shields G.. 2012. Ediacaran Period, ch. 18 In Geologic timescale 2012 (eds Gradstein F, Ogg J, Ogg G), pp. 427–449. Boston, MA: Elsevier. [Google Scholar]
- 24.Wilson JP, et al. 2012. Deep-water incised valley deposits at the Ediacaran–Cambrian boundary in southern Namibia contain abundant Treptichnus pedum. Palaios 27, 252–273. ( 10.2110/palo.2011.p11-036r) [DOI] [Google Scholar]
- 25.Bowring SA, Grotzinger JP, Condon DJ, Ramezani J, Newall M, Allen PA. 2007. Geochronologic constraints of the chronostratigraphic framework of the Neoproterozoic Huqf Supergroup, Sultanate of Oman. Am. J. Sci. 307, 1097–1145. ( 10.2475/10.2007.01) [DOI] [Google Scholar]
- 26.Bowring SA, Grotzinger JP, Isachsen CE, Knoll AH, Pelechaty SM, Kolosov P. 1993. Calibrating rates of early Cambrian evolution. Science 261, 1293–1298. ( 10.1126/science.11539488) [DOI] [PubMed] [Google Scholar]
- 27.Dunhill AM, Benton MJ, Twitchett RJ, Newell AJ. 2012. Completeness of the fossil record and the validity of sampling proxies at outcrop level. Palaeontology 55, 1155–1175. ( 10.1111/j.1475-4983.2012.01149.x) [DOI] [Google Scholar]
- 28.Darroch SAF, Wagner PJ. 2015. Response of beta diversity to pulses of Ordovician–Silurian mass extinction. Ecology 96, 532–549. ( 10.1890/14-1061.1) [DOI] [PubMed] [Google Scholar]
- 29.Droser ML, Gehling JG, Jensen SR. 2006. Assemblage palaeoecology of the Ediacara biota: the unabridged edition? Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 131–147. ( 10.1016/j.palaeo.2005.12.015) [DOI] [Google Scholar]
- 30.Clapham ME, Narbonne GM, Gehling JG. 2003. Palaeoecology of the oldest-known animal communities: Ediacaran assemblages at Mistaken Point, Newfoundland. Palaeobiology 29, 527–544. () [DOI] [Google Scholar]
- 31.Gehling JG, Droser ML. 2013. How well do fossil assemblages of the Ediacara biota tell time? Geology 41, 447–450. ( 10.1130/G33881.1) [DOI] [Google Scholar]
- 32.Zakrevskaya M. 2013. Paleoecological reconstruction of the Ediacaran benthic macroscopic communities of the White Sea (Russia). Palaeogeogr. Palaeoclimatol. Palaeoecol. 410, 27–38. ( 10.1016/j.palaeo.2014.05.021) [DOI] [Google Scholar]
- 33.Wood DA, Dalrymple RW, Narbonne GM, Gehling JG, Clapham ME. 2003. Palaeoenvironmental analysis of the late Neoproterozoic Mistaken Point and Trepassey formations, southeastern Newfoundland. Can. J. Earth Sci. 40, 1375–1391. ( 10.1139/e03-048) [DOI] [Google Scholar]
- 34.Narbonne GM. 2005. The Ediacara biota: Neoproterozoic origin of animals and their ecosystems. Ann. Rev. Earth Planet. Sci. 33, 421–442. ( 10.1146/annurev.earth.33.092203.122519) [DOI] [Google Scholar]
- 35.Diaz RJ, Rosenberg R. 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. Annu. Rev. 33, 245–303. [Google Scholar]
- 36.Levin L, Ekau W, Gooday A, Jorissen F, Middelburg J, Naqvi W, Neira C, Rabalais N, Zhang J. 2009. Effects of natural and human-induced hypoxia on coastal benthos. Biogeosci. Discuss. 6, 3563–3654. ( 10.5194/bgd-6-3563-2009) [DOI] [Google Scholar]
- 37.Darroch SAF, Laflamme M, Clapham ME. 2013. Population structure of the oldest known macroscopic communities from Mistaken Point, Newfoundland. Palaeobiology 39, 591–608. ( 10.1666/12051) [DOI] [Google Scholar]
- 38.Sperling EA, Wolock CJ, Morgan AS, Gill BC, Kunzmann M, Halverson GP, Macdonald FA, Knoll AH, Johnston DT. 2015. Statistical analysis of iron geochemical data suggests limited Late Proterozoic oxygenation. Nature 523, 451–454. ( 10.1038/nature14589) [DOI] [PubMed] [Google Scholar]
- 39.Raiswell R, Canfield DE. 1998. Sources of iron for pyrite formation in marine sediments. Am. J. Sci. 298, 219–245. ( 10.2475/ajs.298.3.219) [DOI] [Google Scholar]
- 40.Poulton SW, Canfield DE. 2011. Ferruginous conditions: a dominant feature of the ocean through Earth's history. Elements 7, 107–112. ( 10.2113/gselements.7.2.107) [DOI] [Google Scholar]
- 41.Raiswell R, Newton R, Bottrell SH, Coburn PM, Briggs DEG, Bond DPG, Poulton SW. 2008. Turbidite depositional influences on the diagenesis of Beecher's Trilobite Bed and the Hunsruck Slate; sites of soft tissue pyritization. Am. J. Sci. 308, 105–129. ( 10.2475/02.2008.01) [DOI] [Google Scholar]
- 42.Tribovillard N, Algeo TJ, Lyons T, Riboulleau A. 2006. Trace metals as palaeoredox and palaeoproductivity proxies: an update. Chem. Geol. 232, 12–32. ( 10.1016/j.chemgeo.2006.02.012) [DOI] [Google Scholar]
- 43.Cummings VM, Poulton SW, Rooney AD, Selby D. 2013. Anoxia in the terrestrial environment during the late Mesoproterozoic. Geology 41, 583–586. ( 10.1130/G34299.1) [DOI] [Google Scholar]
- 44.Sperling EA, Halverson GP, Knoll AH, Macdonald FA, Johnston DT. 2013. A basin redox transect at the dawn of animal life. Earth Planet. Sci. Lett. 371–372, 143–155. ( 10.1016/j.epsl.2013.04.003) [DOI] [Google Scholar]
- 45.Wood RA, Poulton SW, Prave AR, Hoffmann K-H, Clarkson MO, Guilbaud RJ, Lyne W, Curtis AS, Kasemann A. 2015. Dynamic redox conditions control late Ediacaran ecosystems in the Nama Group, Namibia. Precambrian Res. 261, 252–271. ( 10.1016/j.precamres.2015.02.004) [DOI] [Google Scholar]
- 46.Turekian KK, Wedepohl KH. 1961. Distribution of the elements in some major units of the Earth's crust. Geol. Soc. Am. Bull. 72, 175–192. (doi:10.1130%2F0016-7606%281961%2972%5B175%3ADOTEIS%5D2.0.CO%3B2) [Google Scholar]
- 47.Gage JD, Tyler PA. 1992. Deep sea biology: a natural history of organisms at the deep-sea floor. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 48.Grazhdankin D, Seilacher A. 2002. Underground Vendobionta from Namibia. Palaeontology 45, 57–78. ( 10.1111/1475-4983.00227) [DOI] [Google Scholar]
- 49.Vickers-Rich P, et al. 2013. Reconstructing Rangea: new discoveries from the Ediacaran of Southern Namibia. J. Palaeontol. 87, 1–15. ( 10.1666/12-074R.1) [DOI] [Google Scholar]
- 50.Olszewski TA, Kidwell SM. 2007. The preservational fidelity of evenness in molluscan death assemblages. Paleobiology 33, 1–23. ( 10.1666/05059.1) [DOI] [Google Scholar]
- 51.Waggoner BM. 2003. The Ediacaran biotas in space and time. Integr. Comp. Biol. 43, 104–113. ( 10.1093/icb/43.1.104) [DOI] [PubMed] [Google Scholar]
- 52.Gehling JG. 2000. Environmental interpretation and a sequence stratigraphic framework for the terminal Proterozoic Ediacara Member within the Rawnsley Quartzite, South Australia. Precambrian Res. 1000, 65–95. ( 10.1016/S0301-9268(99)00069-8) [DOI] [Google Scholar]
- 53.Boyer DL, Owens JD, Lyons TW, Droser ML. 2011. Joining forces: combined biological and geochemical proxies reveal a complex but refined high-resolution palaeo-oxygen history in Devonian epeiric seas. Palaeogeogr. Palaeoclimatol. Palaeoecol. 306, 134–146. ( 10.1016/j.palaeo.2011.04.012) [DOI] [Google Scholar]
- 54.Johnston D, Poulton S, Goldberg T, Sergeev V, Podkovyrov V, Vorob'eva N, Bekker A, Knoll A. 2012. Late Ediacaran redox stability and metazoan evolution. Earth Planet. Sci. Lett. 335, 25–35. ( 10.1016/j.epsl.2012.05.010) [DOI] [Google Scholar]
- 55.Twitchett RJ. 2006. The palaeoclimatology, palaeoecology and palaeoenvironmental analysis of mass extinction events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 190–213. ( 10.1016/j.palaeo.2005.05.019) [DOI] [Google Scholar]
- 56.Logan GA, Hayes JM, Hieshima GB, Summons RE. 1995. Terminal Proterozoic reorganization of biogeochemical cycles. Nature 376, 53–57. ( 10.1038/376053a0) [DOI] [PubMed] [Google Scholar]
- 57.Butterfield NJ. 2009. Oxygen, animals and oceanic ventilation: an alternative view. Geobiology 7, 1–7. ( 10.1111/j.1472-4669.2009.00188.x) [DOI] [PubMed] [Google Scholar]
- 58.Sperling EA, Peterson KJ, Laflamme M. 2011. Rangeomorphs, Thectardis (Porifera?) and dissolved organic carbon in the Ediacaran oceans. Geobiology 9, 24–33. ( 10.1111/j.1472-4669.2010.00259.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.