Abstract
Providing key resources to animals may enhance both their biodiversity and the ecosystem services they provide. We examined the performance of annual flower strips targeted at the promotion of natural pest control in winter wheat. Flower strips were experimentally sown along 10 winter wheat fields across a gradient of landscape complexity (i.e. proportion non-crop area within 750 m around focal fields) and compared with 15 fields with wheat control strips. We found strong reductions in cereal leaf beetle (CLB) density (larvae: 40%; adults of the second generation: 53%) and plant damage caused by CLB (61%) in fields with flower strips compared with control fields. Natural enemies of CLB were strongly increased in flower strips and in part also in adjacent wheat fields. Flower strip effects on natural enemies, pests and crop damage were largely independent of landscape complexity (8–75% non-crop area). Our study demonstrates a high effectiveness of annual flower strips in promoting pest control, reducing CLB pest levels below the economic threshold. Hence, the studied flower strip offers a viable alternative to insecticides. This highlights the high potential of tailored agri-environment schemes to contribute to ecological intensification and may encourage more farmers to adopt such schemes.
Keywords: conservation biological control, ecosystem functioning, habitat management, landscape context, Oulema melanopus L., wildflower strip
1. Introduction
Meeting growing demands for agricultural products, while minimizing negative environmental impacts, is among the biggest challenges to mankind [1]. Productivity increase per unit area achieved by conventional agricultural intensification has come at the cost of adverse effects on the environment, including losses of farmland biodiversity and associated ecosystem services, which may even have negative feedbacks on sustainable crop production [2,3]. Plant protection measures are still predominantly based on chemical pesticides which, however, are costly in terms of monetary investment and their impact on biodiversity and the environment [4,5]. The often concomitant simplification of agricultural landscapes further tends to disrupt ecosystem services [6], with biological pest control considered as being one of the services most at risk [5].
Ecological intensification, by contrast, seeks environmentally friendly alternatives to anthropogenic chemical inputs by harnessing ecosystem services [7]. Effective promotion of natural enemy-mediated pest control through adequate habitat management, for example, may have a strong potential to increase yields at reduced levels of pesticide inputs [8]. Besides promoting farmland biodiversity, a key goal of many agri-environment schemes (AES) is to foster ecosystem services, such as biological pest control or animal provided pollination [9,10]. Whereas biodiversity effects of AES have been repeatedly studied in the last decade [11–13], effects of AES on ecosystem services such as natural pest control or pollination remained much less studied and the consequences of pest control on crop damage or yield were rarely quantified [14]. Sown wildflower strips tailored to the needs of functionally important arthropod groups such as crop pollinators or pests' natural enemies may effectively promote the delivery of ecosystem services in nearby crops [9,15].
Many service-providing arthropods depend on plant-provided resources (e.g. nectar, pollen and shelter) at least during some life stages. These resources have become rare in intensified agricultural landscapes, but may be effectively substituted by sown flower strips [15–18]. Maximizing ecosystem services through habitat management needs a refined selection of floral resources and a well-adapted management to ensure that the right resources are provided at the place and time they are needed. Annual flower strips within crop rotations can meet this objective and offer a flexible tool for practitioners to manage ecosystem services on the field scale. However, such transient habitat elements rely on the colonization by service providers from less disturbed perennial semi-natural habitats. Therefore, their effectiveness in providing pest-control services is expected to be contingent on the amount of perennial habitats in the agricultural landscape (i.e. landscape complexity [12,19,20]). Recent studies underline the role of floral resources for natural enemy performance on the plot and field scale [6,18,21,22]. In addition, landscape complexity can be an important driver of natural enemy and pest assemblages at large spatial scales [5]. Yet, there is a lack of studies simultaneously addressing the effects of targeted floral resources on natural enemies, pest suppression and the consequences on crops at various levels of landscape complexity ([5,23], but e.g. [24]).
Cereal leaf beetles (hereafter CLB), Oulema sp., are among the major cereal pests in Europe, Asia and North America, and cause economic damage at densities above 0.4 larvae per tiller (wheat shoot including stem, leaves and ear) [25,26]. To date, CLB control largely relies on insecticide use. Alternative control strategies are highly desired. To our knowledge, this is one of the first replicated studies exploring the potential of tailored agri-environmental measures to control CLB.
Here, we examined the effectiveness of experimentally established annual flower strips specifically designed to promote natural control of cereal pests along a gradient of landscape complexity. By focusing on CLB control by its natural enemies, we addressed the following questions: (i) do flower strips promote natural enemies of CLB? (ii) Do they reduce CLB densities in adjacent winter wheat? (iii) To what extent does this translate into lower plant damage? (iv) How does landscape complexity interact with flower strip effectiveness?
2. Material and methods
(a). Study design
Field experiments were conducted between April and July 2012. Thirty winter wheat fields (hereafter focal fields) were selected along a gradient of landscape complexity in the central Swiss plateau (cantons Zurich and Aargau). The region represents the typical agricultural landscape of the Swiss plateau consisting of a relatively small-scaled mosaic of arable crops (predominantly cereals, maize, sugar-beet, oilseed rape and potatoes), grasslands and forests (electronic supplementary material, table S1). Field size was 2.03 ha (±0.18 ha) on average and the minimum distance between focal fields was 900 m (mean ± s.e.: 7918 ± 232 m). All focal fields were managed without fungicides, insecticides or growth regulators (Swiss IP extenso; [27]). Along the full length of a randomly selected border of 15 focal fields, a standardized 3 m-wide flower strip was sown in April 2012. In the other 15 focal fields, a 3 m-wide winter wheat strip along the full length of a randomly chosen border served as a control strip.
The seed mixture of the flower strips consisted of the following annual plant species: Anethum graveolens L. (Apiaceae), Anthemis arvensis L. (Asteraceae), Anthriscus cerefolium (L.) Hoffm. (Apiaceae), Centaurea cyanus L. (Asteraceae), Coriandrum sativum L. (Apiaceae), Fagopyrum esculentum Moench (Polygonaceae) and Papaver rhoeas L. (Papaveraceae) (see electronic supplementary material, table S2 for quantities of seeds sown per area). These species were selected based on a review of existing evidence for positive effects of floral and extra-floral (C. cyanus) resources offered by these species on the performance, fitness or population dynamics of key natural enemies of major wheat pests, such as CLB and aphids, i.e. ladybirds (Coleoptera: Coccinellidae), lacewings (Neuroptera: Chrysopidae), parasitic wasps (Hymenoptera), predatory bugs (Hemiptera: Heteroptera) and hoverflies (Diptera: Syrphidae) [9,18,28–32]. A further criterion for the selection of the plant species was that the provision of floral and extra-floral resources, as well as shelter, matches the time at which crop pests are most effectively controlled by their natural enemies (April–July in the study area) along with agronomic (agronomical unproblematic species) and aesthetical considerations [33]. No pesticide treatments (except targeted herbicide application to individual plants), mowing or fertilization were conducted in the flower strips. Five flower strips had to be abandoned because they were overgrown by spontaneous weedy vegetation and/or the sown plant species failed to establish properly.
(b). Assessment of cereal leaf beetle density and plant damage
CLB, Oulema sp. are major cereal crop pests in Europe, Asia and North America [26,34]. Overwintering predominantly in woody habitats, CLB adults disperse into cereal crops in spring, where the larvae cause damage by removing the photosynthetic tissue of cereal plants [25]. The economic threshold has been estimated at 0.4 larvae per tiller [25]. Natural enemies comprise generalist predators such as ground beetles, rove beetles, ladybirds, predatory bugs and lacewing larvae, and specialized parasitic hymenoptera [34–37], but quantitative knowledge on the relative importance of different CLB natural enemies is largely lacking. In the study region, two CLB species, O. melanopus L. and O. gallaeciana Heyden occur in wheat crops. However, O. melanopus is by far more abundant than O. gallaeciana. As larvae of the two CLB species cannot be easily discriminated in the field, we did not analyse them separately. The two CLB species have a similar pest status [35].
CLB density and plant damage were assessed at two distances (near versus far) from the flower strips or wheat control strips following a stratified random approach. First, a ‘near’ sector ranging from 0.5 to 10.4 m from the strip border and a ‘far’ sector between 10.5 and 20.4 m from the strip border were defined. In a second step, we randomly selected a distance within the ‘near’ sector and then defined the ‘far’ distance as the near distance plus 10 m. This design allows the modelling of a ‘near’ and a ‘far’ distance category as well as distance as a continuous variable. All CLB larvae of 25 wheat tillers from two randomly selected plots at each distance and focal field were recorded twice during the peak of larval appearance [26,35] (end of May/mid-June; BBCH 40–70; electronic supplementary material, table S3). Adult CLB were sampled using standardized sweep netting (60 sweeps at each distance and focal field, 40 cm sweep net diameter). We assessed the second generation of beetles that develop from larvae at the beginning of July (BBCH 77–87; electronic supplementary material, table S3), which should, in contrast to the first generation of adult beetles colonizing fields, directly reflect the overall impacts of natural enemies on eggs, larvae and pupae. Plant damage caused by CLB was assessed as percentage leaf damage of the same 2 × 25 wheat tillers per distance used for the sampling of CLB larvae in mid-June (electronic supplementary material, table S3) within six categories (1: less than 1%; 2: 1–5%; 3: 5–10%; 4: 10–25%; 5: 25–50%; 6: greater than 50%) [35].
(c). Sampling of natural enemies
Natural enemies were sampled at the same distances as CLB and plant damage, and additionally in flower and control strips. Predatory bugs, ladybirds (adults and larvae) and lacewings (adults and larvae) were sampled using standardized sweep netting (sweep net diameter: 40 cm; 60 sweeps); ground beetles were sampled with pitfall traps (two pitfalls per distance; 10 cm funnel diameter; 70% ethanol). Sweep net sampling was carried out during two rounds in mid-June and at the beginning of July (electronic supplementary material, table S3). Pitfall sampling was carried out during three sampling rounds of one week from 5 May to 5 July (electronic supplementary material, table S3). All captured individuals were identified to species or, if not possible (e.g. Heteroptera nymphs), genus level. Hymenopteran parasitoids could not be analysed in this study.
(d). Landscape complexity
To examine the effects of landscape complexity and potential interactions with flower strip on natural enemies, CLB and wheat plant damage, the percentage of non-crop area was calculated in a radius of 750 m around focal fields (electronic supplementary material, table S1). This scale is considered adequate to study responses of specialist pests and natural enemies to the landscape context (e.g. [5]). Information on land-use classes was derived from official digital land-use maps (vector25 and TLM3D, swisstopo, Wabern) and verified using aerial photographs (SWISSIMAGE, swisstopo, Wabern). Where necessary, additional information about agricultural land use in the study year was acquired from local administration agencies (Office of Landscape, Agriculture and Environment of the canton of Zurich; Agrofutura AG, canton of Aargau). The calculation of non-crop area was performed with ArcMap 10.1 GIS software [38].
(e). Statistical analyses
Generalized linear mixed-effects models (GLMMs) were fitted to test the effect of flower strip on natural enemies (response variables: ground beetles, predatory bugs, adult ladybirds, ladybird larvae, adult lacewings and lacewing larvae; total number of individuals pooled from all sampling rounds) and CLB density (response variables: total CLB larvae per 50 wheat tillers and total number of CLB adults) within adjacent winter wheat fields. GLMMs with Poisson error distribution (log-link function) were used to analyse natural enemies, except for the number of ground beetles: these data were better fitted by a Gaussian error distribution with identity-link function. To account for overdispersion in the CLB density data, GLMMs with negative binomial error distributions (log-link function) were fitted using the Automatic Differentiation Model Builder (glmmADMB) package [39] in R. A linear mixed-effects model (LME) was used to model plant damage. Mean leaf damage was calculated for each distance per field using mean percentage values from categories attributed to each plot. Percentages were arcsine-square root-transformed to achieve normally distributed residuals and avoid heteroscedasticity. All full models contained the fixed effects flower strip (factor: focal field with flower strip versus focal field with wheat control strip), distance (continuous explanatory variable) and their interaction, as well as the covariates wheat variety, wheat density (number of wheat tillers per square metre) and focal field area, and field identity as a random blocking factor. The model for CLB larvae additionally included the crossed random factor sampling round. Collinearity among covariates was assessed using pairwise scatterplots, correlation coefficients and variance inflation factors (VIF). Wheat height, which was positively correlated with wheat density (correlation coefficient > |0.5| [40]) was excluded from the set of candidate models.
To additionally analyse natural enemies in the flower strips themselves compared with wheat control strips, negative binomial GLMs (log-link function) using the glm.nb function of the MASS package [41] with the explanatory variable flower strip and the covariate field area were fitted for each natural enemy group separately. Landscape complexity and its interaction with flower strip was included in the models described above in order to test the hypothesis that flower strip effects are contingent on landscape complexity. Moran's I similarity spline correlograms [42] indicated no spatial autocorrelation in the residuals of the models.
All numerical explanatory variables were standardized prior to the analyses (to get a predictor with mean of zero and standard deviation of one) to avoid numerical precision problems. Model selection based on likelihood-ratio tests followed recommendations by Zuur et al. [40] and minimum adequate models were used for statistical inference. Model assumptions were checked according to the graphical validation procedures recommended by Zuur et al. [40]. All statistical analyses were done using R v. 3.1.0 software [43].
3. Results
(a). Impact of flower strips on cereal leaf beetle density and plant damage
The number of CLB larvae was reduced by 40% in winter wheat fields with flower strips (hereafter flower strip fields) compared with winter wheat fields with winter wheat strips (hereafter control fields) (table 1, figure 1a). CLB larvae increased with distance from flower strips, but in a similar way as from wheat control strips. Consequently, significantly less adult CLB (−53%) re-emerged in flower strip fields than in control fields (table 1, figure 1b). Moreover, wheat plant damage caused by CLB was reduced by 61% in flower strip fields compared with control fields (table 1, figure 1c). The decrease in wheat plant damage in flower strip fields compared with control fields tended to be higher towards the field centres, with highest plant damage in the interior of control fields (table 1, figure 1c).
Table 1.
Summary of main fixed effects treatment (factor with two levels: flower strip or wheat control strip), distance from flower or control strip (continuous variable) and their interaction on wheat plant damage (arcsine-square root-transformed), cereal leaf beetle (CLB) density and the density of different natural enemy groups in adjacent winter wheat fields and within the strips themselves (only natural enemies). Degrees of freedom (d.f.), χ2-values and p-values from likelihood-ratio tests of the model selection procedure (see Material and methods section) are shown. p-values of explanatory variables that were included in the final model are in bold lettering.
| within winter wheat |
within strip |
|||||
|---|---|---|---|---|---|---|
| d.f. | χ2 | p | d.f. | χ2 | p | |
| pest density | ||||||
| CLB larvae | ||||||
| treatment | 1 | 4.93 | 0.026 | |||
| distance | 1 | 8.20 | 0.004 | |||
| treatment × distance | 1 | 0.51 | 0.474 | |||
| CLB adults | ||||||
| treatment | 1 | 4.51 | 0.034 | |||
| distance | 1 | 0.06 | 0.830 | |||
| treatment × distance | 1 | 1.61 | 0.205 | |||
| plant damage | ||||||
| wheat plant damage by CLB | ||||||
| treatment | 1 | 4.39 | 0.036 | |||
| distance | 1 | 1.13 | 0.288 | |||
| treatment × distance | 1 | 2.79 | 0.098 | |||
| natural enemy abundance | ||||||
| ground beetles (adults) | ||||||
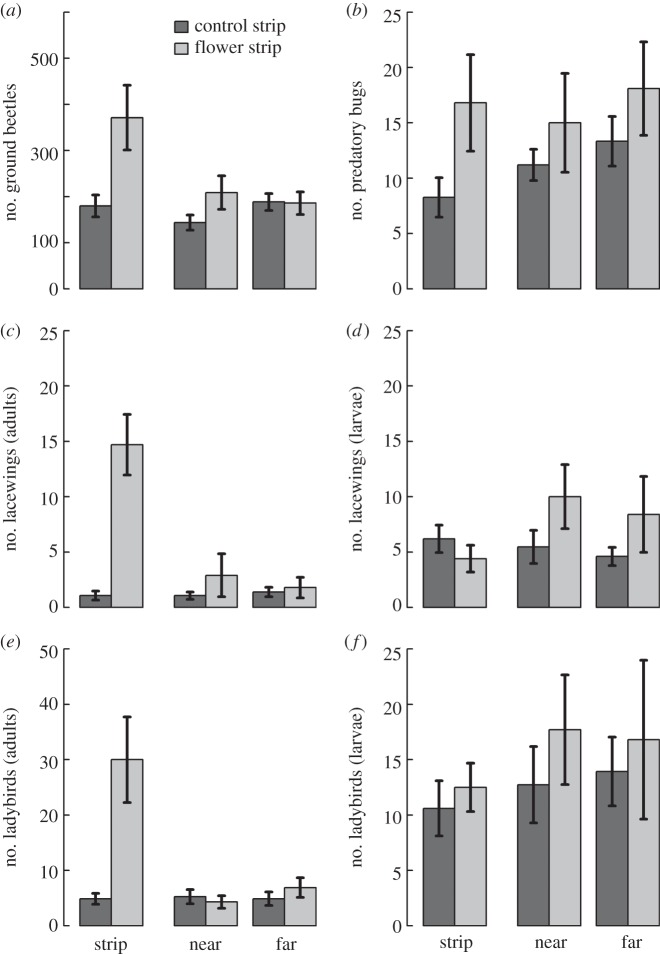
| treatment | 1 | 0.60 | 0.437 | 1 | 10.60 | 0.001 |
| distance | 1 | 0.51 | 0.478 | |||
| treatment × distance | 1 | 9.48 | 0.002 | |||
| predatory bugs (adults and nymphs) | ||||||
| treatment | 1 | 2.86 | 0.091 | 1 | 4.42 | 0.036 |
| distance | 1 | 0.38 | 0.540 | |||
| treatment × distance | 1 | 0.00 | 0.989 | |||
| lacewings (adults) | ||||||
| treatment | 1 | 0.14 | 0.714 | 1 | 50.58 | <0.0001 |
| distance | 1 | 0.05 | 0.818 | |||
| treatment × distance | 1 | 3.26 | 0.071 | |||
| lacewings (larvae) | ||||||
| treatment | 1 | 0.97 | 0.324 | 1 | 0.99 | 0.319 |
| distance | 1 | 0.11 | 0.744 | |||
| Treatment × Distance | 1 | 0.01 | 0.909 | |||
| ladybirds (adults) | ||||||
| treatment | 1 | 0.04 | 0.839 | 1 | 37.53 | <0.0001 |
| distance | 1 | 0.44 | 0.507 | |||
| treatment × distance | 1 | 2.24 | 0.134 | |||
| ladybirds (larvae) | ||||||
| treatment | 1 | 0.10 | 0.748 | 1 | 0.25 | 0.619 |
| distance | 1 | 11.99 | 0.001 | |||
| treatment × distance | 1 | 0.00 | 0.964 | |||
Figure 1.
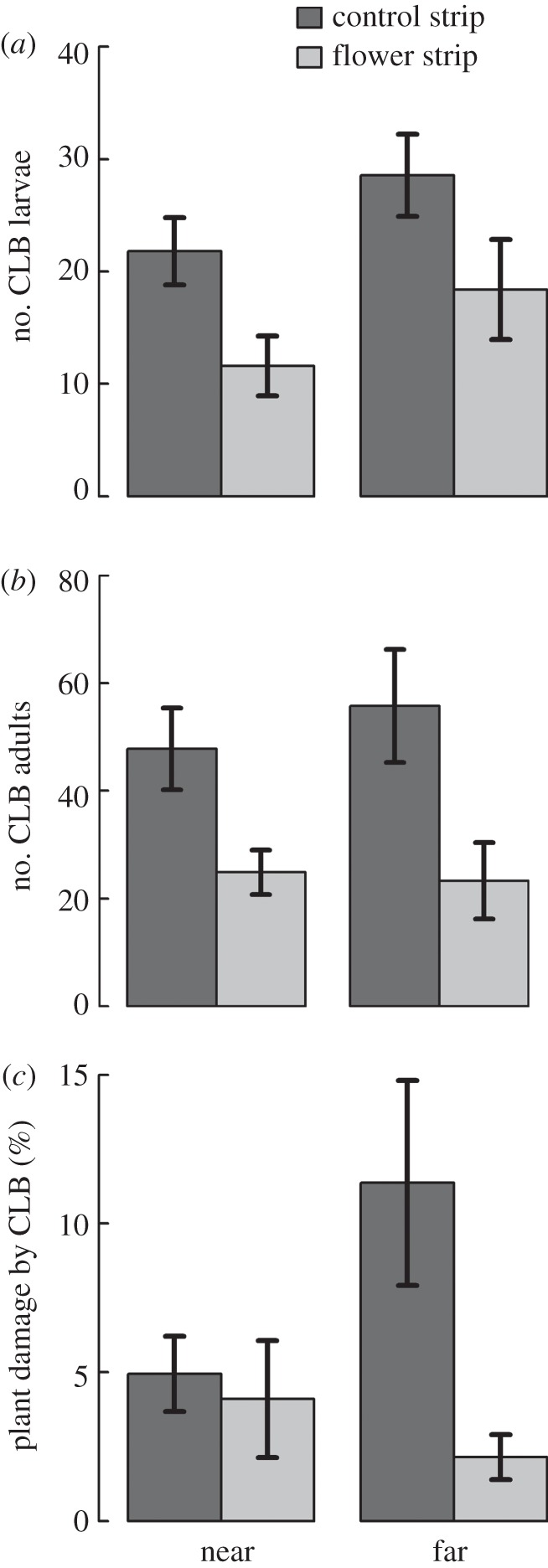
Effects of flower strips on pest density and wheat plant damage. Mean (±1 s.e.) (a) number of cereal leaf beetle (CLB) larvae, (b) number of adult CLBs (second generation) and (c) percentage leaf damage caused by CLBs in winter wheat fields with wheat control strips (dark grey; n = 15) and winter wheat fields with flower strips (light grey; n = 10). Near: mean = 4.75 m, range = 0.6–10.3 m; far: mean = 14.75 m, range = 10.6–20.3 m (see Material and methods section). Statistical test summaries are given in table 1.
(b). Impact of flower strips on natural enemies
Numbers of adults of all studied natural enemy groups increased strongly in flower strips compared with wheat control strips (table 1, figure 2a–c,e), while the number of larvae of ladybirds and lacewings did not significantly differ (table 1, figure 2d,f). In adjacent winter wheat, the number of predatory bugs tended to be higher in flower strip fields than control fields (table 1, figure 2b). Moreover, the number of ground beetles was significantly higher—and that of adult lacewings tended to be higher—in flower strip fields, but only near flower strips (significant flower strip × distance interaction: table 1, figure 2a,c). No significant flower strip effects were found for the numbers of other natural enemy groups (table 1, figure 2d–f).
Figure 2.
Effects of flower strips on natural enemy density. Mean (±1 s.e.) individual number of (a) ground beetles (adults), (b) predatory bugs (adults and nymphs), (c) adult lacewings, (d) lacewing larvae, (e) adult ladybirds and (f) ladybird larvae in winter wheat fields with wheat control strips (dark grey; n = 15) and winter wheat fields with flower strips (light grey; n = 10). Strip: centre of flower or wheat control strip; near: mean = 4.75 m, range = 0.6–10.3 m; far: mean = 14.75 m, range = 10.6–20.3 m (see Material and methods section). Statistical test summaries are given in table 1.
(c). Interactions with landscape complexity
Landscape complexity calculated as percentage non-crop area within 750 m radius around focal fields (mean = 47.0 ± 3.3, range = 8.0–74.7) did not significantly influence the numbers of CLB larvae (χ2 = 0.27, d.f. = 1, p = 0.603), CLB adults (χ2 = 0.00, d.f. = 1, p = 1.000) or wheat plant damage (χ2 = 0.56, d.f. = 1, p = 0.453). Moreover, there was no significant interactive effect of flower strip and landscape complexity on the number of CLB larvae (χ2 = 3.41, d.f. = 1, p = 0.065), CLB adults (χ2 = 0.06, d.f. = 1, p = 0.813) or wheat plant damage (χ2 = 0.13, d.f. = 1, p = 0.721).
Similarly, no significant effect of landscape complexity or the interaction of flower strip × landscape complexity was found for any of the natural enemy groups, except for a significant flower strip × landscape complexity interaction for ladybird larvae within winter wheat fields (χ2 = 4.99, d.f. = 1, p = 0.025; electronic supplementary material, table S4). Ladybird larvae in winter wheat fields adjoining flower strips tended to increase with landscape complexity (z = 1.82, p = 0.069), whereas the slope in winter wheat fields adjoining control strips was non-significant (z = −1.52, p = 0.127).
4. Discussion
This study demonstrates the high effectiveness of annual flower strips in reducing CLB density and crop plant damage in adjacent winter wheat. Among the studied natural enemies of CLB, ground beetles, predatory bugs and lacewings showed the strongest positive responses to flower strips, suggesting a prominent role of these predator groups in CLB control. To our knowledge, this is one of the first replicated studies demonstrating high effectiveness of flower strips in reducing crop damage beyond reductions in pest densities, highlighting the potential of tailored flower strips for conservation biological control.
The observed reductions in pest levels (CLB larvae: 40%, second generation CLB adults: 53%) and the crop plant damage (61%) in the presence of flower strips are remarkably strong. CLB larvae were reduced from an average of 0.50 (±0.05) individuals per tiller to 0.30 (±0.05) individuals, and thus below the economic threshold of 0.4 larvae per tiller [25]. Further, these high levels of pest control in wheat crops were not restricted to the immediate vicinity of the flower strips, but reached up to 20 m into the fields. This contrasts with earlier studies in which effects of field margins were restricted to their immediate vicinity (e.g. [44–46]). So far, studies investigating the effect of flower strips on pest control have mainly focused on parasitoid–host systems and parasitism [17,28,32,47]. Parasitism usually increased in the presence of flower strips. However, high parasitism does not necessarily translate into reductions of pest densities or crop damage [28]. Indeed, only few studies found decreased pest levels or reduced crop damage in adjacent crops [17,28,48]. Conservation biocontrol measures are far from universally successful. No effects, or even increasing levels of crop pests and/or damage close to flower strips, have been reported for other study systems [17,49]. These can arise, for example, if pests benefit similarly or even more strongly from the offered resources than their enemies [28,50] or from increased top-down control of pest's natural enemies through (hyper-)parasitoids and predators [21,51]. The first mechanism should not have compromised the effectiveness of flower strips in our study system because CLB are not expected to benefit from floral resources offered by herbaceous plant species [35]. This may partly explain the strong reductions in pest and crop damage.
At least two other factors may have contributed to the high effectiveness of the tested flower strips in reducing CLB densities and plant damage. (i) The careful selection of plant species offering a large amount of floral, extra-floral and structural resources that proved to benefit natural enemies [18,30,52] and (ii) the rather high diversity of flowering plants comprising the flower strips, characterized by a staggered provision of floral, extra-floral (C. cyanus) and other resources (e.g. shelter and alternative prey) were found to be complementary in terms of attractiveness and accessibility for different natural enemy groups (M. Tschumi 2013, unpublished data). Thus, species-rich flower strips may attract and benefit a higher diversity of natural enemies than species-poor or single-species strips [53], which may be associated with enhanced pest control [54].
Highly increased numbers of all observed natural enemies (except larvae) inside flower strips compared with wheat control strips confirm that the offered floral and other resources were attractive to a broad range of natural enemies. This may also apply to other natural enemy taxa beyond the predators assessed here that may have contributed to biological control (e.g. parasitic wasps, rove beetles or birds). Floral resource provisioning was dominated by F. esculentum, C. sativum and C. cyanus at the time of CLB control (May and June), indicating that these species were particularly relevant in the studied system. Yet, owing to bad weather conditions in early spring the seed mixture was sown slightly later in the season than in years with better weather during this time of the year. As a consequence, the onset of flowering was somewhat later than in typical years. However, in our study year the reduced abundance of CLB larvae started to appear before full flowering of the strips. Thus, in addition to floral resources, natural enemies are likely to have benefitted from other resources offered by flower strips, such as alternative prey, shelter and structural resources [18,55].
Contrary to the strong effects of bordering flower strips, landscape complexity did not appear to affect CLB densities or crop damage, neither directly nor by modulating impacts of flower strips. This contrasts studies that have found highest effectiveness of habitat management in landscapes with intermediate complexity [19]. The lack of effects of landscape complexity may be explained by the relatively small-scaled landscape structure and the resulting moderate to high landscape complexity of Swiss agricultural landscapes compared with other countries. In many European regions, arable landscapes comprise only 0–40% of non-crop habitats [56]. By contrast, the landscapes studied here embraced proportions of non-crop habitat between 8.0 and 74.7% (average 47.0 ± 3.3%), as is typical for the Swiss plateau [56]. Only one of our landscapes fell below the 20% threshold that has been suggested for structurally poor landscapes impoverished in natural pest enemies [19]. This suggests that species pools of natural enemies were large enough and perennial semi-natural habitats offering complementary resources—such as adequate overwintering sites—sufficiently connected to annual flower strips to support their high performance in providing pest-control services at the local (field) scale [52]. In cleared landscapes with low proportions of permanent semi-natural habitats, however, annual flower strips may be less effective. An alternative reason for the low importance of landscape complexity in our study is that the scale of 750 m radius may not be appropriate. Yet, analyses at the smaller scale of 250 m radius did not yield any significant effects on leaf beetles or crop damage either (results not shown). Nevertheless, we cannot exclude possible effects of landscape complexity at larger scales.
Economic viability of tailored flower strips depends on associated costs and on their benefits in terms of increased crop yield and/or insecticide savings (electronic supplementary material B). The observed CLB reductions may enhance wheat yield (or mitigate yield damage) by 2.5–10% ([25]; M. Tschumi 2013, unpublished data). Assuming a moderate to high yield increase in winter wheat (i.e. greater than or equal to 3.7%) or the substitution of insecticides, flower strips can become economically self-sustaining or even profitable, even if they are established on potential wheat cropping area (electronic supplementary material B). Concurrent benefits of tailored flower strips for aphid control, as observed for potato crops (M. Tschumi 2013, unpublished data), may further benefit yield.
Tailored flower strips can be particularly valuable for and facilitate the adoption of low-input or organic management, because they provide one of few effective alternatives to insecticides. In Switzerland and the EU, the creation of ecological focus areas by farmers, including flower strips, is supported by direct payments [57,58]. If tailored flower strips are included, these AES compensate land opportunity and management costs, and benefits through enhanced pest-control services could be an additional incentive for farmers to adopt these schemes.
5. Conclusion
We conclude that tailored flower strips are an effective tool for conservation biological control of CLBs in winter wheat at intermediate to high levels of landscape complexity. By reducing CLB larvae below the suggested economic threshold, tailored flower strips can contribute to a reduction in insecticide use in conventional winter wheat production, and thus to effective ecological intensification. In organic wheat production, tailored flower strips provide an effective tool to mitigate CLB caused crop damage. The direct link between flower strips, pest control and crop damage reduction should encourage farmers to adopt such pest-control measures, which may also benefit farmland biodiversity. We propose that existing AES should be complemented to include flower strips tailored at the provisioning of ecosystem services to sustainably assist agricultural food production.
Supplementary Material
Acknowledgements
We thank Lisa Eggenschwiler and Stephan Bosshart for valuable discussions and support in the field and all the people that helped collecting data. Furthermore, we thank Felix Herzog, Louis Sutter, Thomas Horvath, Daniel Karp and two anonymous reviewers for helpful comments on the manuscript; Roland Risch, Sergio Wicki, Jonas Winizki and Erich Szerencsits for GIS support; Markus Lips and Patrik Mouron for helping with cost–benefit analysis and Gisela Lüscher, Philippe Jeanneret, Manuel Schneider and Matthias Suter for statistical advice. We thank Ralf Heckmann for the identification of true bugs and Werner Marggi for identifying ground beetles. We owe special thanks to the farmers that helped in sowing flower strips and provided access to their fields and to the Hauser and Sur-La-Croix foundations for providing financial support.
Data accessibility
Data are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.51cj4
Authors' contributions
M.T. participated in the design of the study, collected field data, processed samples, analysed the data and drafted the manuscript; M.A. participated in the design of the study, collected field data and helped draft the manuscript; M.H.E. participated in the design of the study and helped draft the manuscript; K.J. conceived the study, participated in the design of the study, collected field data and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
Funding was provided by the Hauser and Sur-La-Croix foundations. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Godfray HCJ, et al. 2010. Food security: the challenge of feeding 9 billion people. Science 327, 812–818. ( 10.1126/science.1185383) [DOI] [PubMed] [Google Scholar]
- 2.Matson PA, Parton WJ, Power AG, Swift MJ. 1997. Agricultural intensification and ecosystem properties. Science 277, 504–509. ( 10.1126/science.277.5325.504) [DOI] [PubMed] [Google Scholar]
- 3.Kleijn D, et al. 2009. On the relationship between farmland biodiversity and land-use intensity in Europe. Proc. R. Soc. B 276, 903–909. ( 10.1098/rspb.2008.1509) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiger F, et al. 2010. Persistent negative effects of pesticides on biodiversity and biological control potential on European farmland. Basic Appl. Ecol. 11, 97–105. ( 10.1016/j.baae.2009.12.001) [DOI] [Google Scholar]
- 5.Chaplin-Kramer R, O'Rourke ME, Blitzer EJ, Kremen C. 2011. A meta-analysis of crop pest and natural enemy response to landscape complexity. Ecol. Lett. 14, 922–932. () [DOI] [PubMed] [Google Scholar]
- 6.Caballero-Lopez B, Bommarco R, Blanco-Moreno JM, Sans FX, Pujade-Villar J, Rundlöf M, Smith HG. 2012. Aphids and their natural enemies are differently affected by habitat features at local and landscape scales. Biol. Control 63, 222–229. ( 10.1016/j.biocontrol.2012.03.012) [DOI] [Google Scholar]
- 7.Bommarco R, Kleijn D, Potts SG. 2013. Ecological intensification: harnessing ecosystem services for food security. Trends Ecol. Evol. 28, 230–238. ( 10.1016/j.tree.2012.10.012) [DOI] [PubMed] [Google Scholar]
- 8.Letourneau DK, Jedlicka JA, Bothwell SG, Moreno CR. 2009. Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 573–592. ( 10.1146/annurev.ecolsys.110308.120320) [DOI] [Google Scholar]
- 9.Haaland C, Naisbit RE, Bersier L-F. 2011. Sown wildflower strips for insect conservation: a review. Insect Conserv. Divers. 4, 60–80. ( 10.1111/j.1752-4598.2010.00098.x) [DOI] [Google Scholar]
- 10.Ekroos J, Olsson O, Rundlöf M, Wätzold F, Smith HG. 2014. Optimizing agri-environment schemes for biodiversity, ecosystem services or both? Biol. Conserv. 172, 65–71. ( 10.1016/j.biocon.2014.02.013) [DOI] [Google Scholar]
- 11.Kleijn D, et al. 2006. Mixed biodiversity benefits of agri-environment schemes in five European countries. Ecol. Lett. 9, 243–254. ( 10.1111/j.1461-0248.2005.00869.x) [DOI] [PubMed] [Google Scholar]
- 12.Batary P, Andras B, Kleijn D, Tscharntke T. 2011. Landscape-moderated biodiversity effects of agri-environmental management: a meta-analysis. Proc. R. Soc. B 278, 1894–1902. ( 10.1098/rspb.2010.1923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kampmann D, Lüscher A, Konold W, Herzog F. 2012. Agri-environment scheme protects diversity of mountain grassland species. Land Use Policy 29, 569–576. ( 10.1016/j.landusepol.2011.09.010) [DOI] [Google Scholar]
- 14.Whittingham MJ. 2011. The future of agri-environment schemes: biodiversity gains and ecosystem service delivery? J. Appl. Ecol. 48, 509–513. ( 10.1111/j.1365-2664.2011.01987.x) [DOI] [Google Scholar]
- 15.Korpela E-L, Hyvönen T, Lindgren S, Kuussaari M. 2013. Can pollination services, species diversity and conservation be simultaneously promoted by sown wildflower strips on farmland? Agric. Ecosyst. Environ. 179, 18–24. ( 10.1016/j.agee.2013.07.001) [DOI] [Google Scholar]
- 16.Haenke S, Scheid B, Schaefer M, Tscharntke T, Thies C. 2009. Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J. Appl. Ecol. 46, 1106–1114. ( 10.1111/j.1365-2664.2009.01685.x) [DOI] [Google Scholar]
- 17.Winkler K, Wäckers FL, Termorshuizen AJ, van Lenteren JC. 2010. Assessing risks and benefits of floral supplements in conservation biological control. Biocontrol 55, 719–727. ( 10.1007/s10526-010-9296-8) [DOI] [Google Scholar]
- 18.Wäckers FL, van Rijn PCJ. 2012. Pick and mix: selecting flowering plants to meet the requirements of target biological control insects. In Biodiversity and insect pests: key issues for sustainable management (eds Gurr GM, Wratten SD, Snyder WE, Read DMY), pp. 139–165. Chichester, UK: John Wiley & Sons, Ltd. [Google Scholar]
- 19.Tscharntke T, et al. 2012. Landscape moderation of biodiversity patterns and processes—eight hypotheses. Biol. Rev. 87, 661–685. ( 10.1111/j.1469-185X.2011.00216.x) [DOI] [PubMed] [Google Scholar]
- 20.Scheper J, Holzschuh A, Kuussaari M, Potts SG, Rundlöf M, Smith HG, Kleijn D. 2013. Environmental factors driving the effectiveness of European agri-environmental measures in mitigating pollinator loss—a meta-analysis. Ecol. Lett. 16, 912–920. ( 10.1111/ele.12128) [DOI] [PubMed] [Google Scholar]
- 21.Lundgren JG. 2009. Relationships of natural enemies and non-prey foods. Berlin, Germany: Springer Science+Business Media B.V. [Google Scholar]
- 22.Diehl E, Sereda E, Wolters V, Birkhofer K. 2013. Effects of predator specialization, host plant and climate on biological control of aphids by natural enemies: a meta-analysis. J. Appl. Ecol. 50, 262–270. ( 10.1111/1365-2664.12032) [DOI] [Google Scholar]
- 23.Bianchi FJJA, Booij CJH, Tscharntke T. 2006. Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc. R. Soc. B 273, 1715–1727. ( 10.1098/rspb.2006.3530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woltz JM, Isaacs R, Landis DA. 2012. Landscape structure and habitat management differentially influence insect natural enemies in an agricultural landscape. Agric. Ecosyst. Environ. 152, 40–49. ( 10.1016/j.agee.2012.02.008) [DOI] [Google Scholar]
- 25.Buntin GD, Flanders KL, Slaughter RW, DeLamar ZD. 2004. Damage loss assessment and control of the cereal leaf beetle (Coleoptera: Chrysomelidae) in winter wheat. J. Econ. Entomol. 97, 374–382. ( 10.1603/0022-0493-97.2.374) [DOI] [PubMed] [Google Scholar]
- 26.Ihrig RA, Herbert DA, Van Duyn JW, Bradley JR. 2001. Relationship between cereal leaf beetle (Coleoptera: Chrysomelidae) egg and fourth-instar populations and impact of fourth-instar defoliation of winter wheat yields in North Carolina and Virginia. J. Econ. Entomol. 94, 634–639. ( 10.1603/0022-0493-94.3.634) [DOI] [PubMed] [Google Scholar]
- 27.Bundesrat. 2013. Verordnung über die Direktzahlungen an die Landwirtschaft (Direktzahlungsverordnung, DZV) vom 23. Oktober 2013 (Stand am 1. Januar 2014).
- 28.Heimpel GE, Jervis MA. 2005. Does floral nectar improve biological control by parasitoids? In Plant-provided food and herbivore–carnivore interactions (eds Wäckers FL, van Rijn PCJ, Bruin J), pp. 267–304. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 29.Bianchi FJJA, Wäckers FL. 2008. Effects of flower attractiveness and nectar availability in field margins on biological control by parasitoids. Biol. Control 46, 400–408. ( 10.1016/j.biocontrol.2008.04.010) [DOI] [Google Scholar]
- 30.Fiedler AK, Landis DA, Wratten SD. 2008. Maximizing ecosystem services from conservation biological control: the role of habitat management. Biol. Control 45, 254–271. ( 10.1016/j.biocontrol.2007.12.009) [DOI] [Google Scholar]
- 31.Isaacs R, Tuell J, Fiedler A, Gardiner A, Landis D. 2009. Maximizing arthropod-mediated ecosystem services in agricultural landscapes: the role of native plants. Front. Ecol. Environ. 7, 196–203. ( 10.1890/080035) [DOI] [Google Scholar]
- 32.Géneau CE, Wäckers FL, Luka H, Daniel C, Balmer O. 2012. Selective flowers to enhance biological control of cabbage pests by parasitoids. Basic Appl. Ecol. 13, 85–93. ( 10.1016/j.baae.2011.10.005) [DOI] [Google Scholar]
- 33.Junge X, Jacot KA, Bosshard A, Lindemann-Matthies P. 2009. Swiss people's attitudes towards field margins for biodiversity conservation. J. Nat. Conserv. 17, 150–159. ( 10.1016/j.jnc.2008.12.004) [DOI] [Google Scholar]
- 34.Evans EW, Carlile NR, Innes MB, Pitigala N. 2013. Warm springs reduce parasitism of the cereal leaf beetle through phenological mismatch. J. Appl. Entomol. 137, 383–391. ( 10.1111/jen.12028) [DOI] [Google Scholar]
- 35.Schärer P. 1994. Analyse dichtebeeinflussender Faktoren beim Getreidehähnchen (Oulema sp., Chrysomelidae, Coleoptera) Bern, Switzerland: Haupt. [Google Scholar]
- 36.Meindl P, Kromp B, Bartl B, Ioannidou E. 2001. Arthropod natural enemies of the cereal leaf beetle (Oulema melanopus L.) in organic winter wheat fields in Vienna, Eastern Austria. IOBC/WPRS Bull. 24, 79–86. [Google Scholar]
- 37.Malschi D, Tritean N, Serbanescu R. 2010. Protective agroforestry belts and their environmental importance for sustainable agriculture development in Transylvania. Rom. Agric. Res. 27, 103–114. [Google Scholar]
- 38.ESRI. 2014. ArcGIS desktop: release 10.1. [Google Scholar]
- 39.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B. 2013. glmmADMB: generalized linear mixed models using AD model builder.
- 40.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer Science+Business Media LLC. [Google Scholar]
- 41.Venables WN, Ripley BD. 2002. Modern applied statistics with S, 4th edn New York, NY: Springer. [Google Scholar]
- 42.Bjornstad ON, Falck W. 2001. Nonparametric spatial covariance functions: estimation and testing. Environ. Ecol. Stat. 8, 53–70. ( 10.1023/a:1009601932481) [DOI] [Google Scholar]
- 43.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See http://www.r-project.org/.
- 44.Tylianakis JM, Didham RK, Wratten SD. 2004. Improved fitness of aphid parasitoids receiving resource subsidies. Ecology 85, 658–666. ( 10.1890/03-0222) [DOI] [Google Scholar]
- 45.Flückiger R, Schmidt MH. 2006. Contribution of sown wildflower areas to cereal aphid control: from local to landscape scale. IOBC/WPRS Bull. 29, 41–44. [Google Scholar]
- 46.Skirvin DJ, Kravar-Garde L, Reynolds K, Wright C, Mead A. 2011. The effect of within-crop habitat manipulations on the conservation biological control of aphids in field-grown lettuce. Bull. Entomol. Res. 101, 623–631. ( 10.1017/S0007485310000659) [DOI] [PubMed] [Google Scholar]
- 47.Balmer O, Pfiffner L, Schied J, Willareth M, Leimgruber A, Luka H, Traugott M. 2013. Noncrop flowering plants restore top-down herbivore control in agricultural fields. Ecol. Evol. 3, 2634–2646. ( 10.1002/ece3.658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wyss E. 1995. The effect of weed strips on aphids and aphidophagous predators in an apple orchard. Entomol. Exp. Appl. 75, 43–49. ( 10.1111/j.1570-7458.1995.tb01908.x) [DOI] [Google Scholar]
- 49.Baggen LR, Gurr GM. 1998. The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Biol. Control 11, 9–17. ( 10.1006/bcon.1997.0566) [DOI] [Google Scholar]
- 50.Wäckers FL, Romeis J, van Rijn P. 2007. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 52, 301–323. ( 10.1146/annurev.ento.52.110405.091352) [DOI] [PubMed] [Google Scholar]
- 51.Prasad RP, Snyder WE. 2006. Polyphagy complicates conservation biological control that targets generalist predators. J. Appl. Ecol. 43, 343–352. ( 10.1111/j.1365-2664.2006.01129.x) [DOI] [Google Scholar]
- 52.Griffiths GJK, Holland JM, Bailey A, Thomas MB. 2008. Efficacy and economics of shelter habitats for conservation biological control. Biol. Control 45, 200–209. ( 10.1016/j.biocontrol.2007.09.002) [DOI] [Google Scholar]
- 53.Pontin DR, Wade MR, Kehrli P, Wratten SD. 2006. Attractiveness of single and multiple species flower patches to beneficial insects in agroecosystems. Ann. Appl. Biol. 148, 39–47. ( 10.1111/j.1744-7348.2005.00037.x) [DOI] [Google Scholar]
- 54.Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992. ( 10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 55.Diehl E, Wolters V, Birkhofer K. 2012. Arable weeds in organically managed wheat fields foster carabid beetles by resource- and structure-mediated effects. Arthropod. Plant. Interact. 6, 75–82. ( 10.1007/s11829-011-9153-4) [DOI] [Google Scholar]
- 56.Concepcion ED, et al. 2012. Interactive effects of landscape context constrain the effectiveness of local agri-environmental management. J. Appl. Ecol. 49, 695–705. ( 10.1111/j.1365-2664.2012.02131.x) [DOI] [Google Scholar]
- 57.Aviron S, et al. 2009. Ecological cross compliance promotes farmland biodiversity in Switzerland. Front. Ecol. Environ. 7, 247–252. ( 10.1890/070197) [DOI] [Google Scholar]
- 58.Pe'er G, et al. 2014. EU agricultural reform fails on biodiversity. Science 344, 1090–1092. ( 10.1126/science.1253425) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.51cj4