Abstract
Non-lethal parasite infections are common in wildlife, but there is little information on their clinical consequences. Here, we pair infection data from a ubiquitous soil-transmitted helminth, the whipworm (genus Trichuris), with activity data from a habituated group of wild red colobus monkeys (Procolobus rufomitratus tephrosceles) in Kibale National Park, Uganda. We use mixed-effect models to examine the relationship between non-lethal parasitism and red colobus behaviour. Our results indicate that red colobus increased resting and decreased more energetically costly behaviours when shedding whipworm eggs in faeces. Temporal patterns of behaviour also changed, with individuals switching behaviour less frequently when whipworm-positive. Feeding frequency did not differ, but red colobus consumption of bark and two plant species from the genus Albizia, which are used locally in traditional medicines, significantly increased when animals were shedding whipworm eggs. These results suggest self-medicative plant use, although additional work is needed to verify this conclusion. Our results indicate sickness behaviours, which are considered an adaptive response by hosts during infection. Induction of sickness behaviour in turn suggests that these primates are clinically sensitive to non-lethal parasite infections.
Keywords: sickness behaviour, whipworm, primates, host–parasite interactions, Kibale National Park, Uganda
1. Introduction
Monitoring the health and abundance of wildlife is a primary focus of conservation, and parasitic diseases are increasingly being recognized as a major conservation concern [1–3]. However, while parasites that cause direct host mortality receive considerable research attention, the majority are non-lethal under ordinary circumstances. Often, non-lethal parasitic infections are caused by macroparasites (e.g. gastrointestinal helminths) that may not produce overt clinical symptoms but are instead responsible for chronic, low-intensity infections [4,5]. These infections may have long-term consequences, including reductions in host health and fitness [6,7].
Defining and measuring traits that predict fitness is difficult, especially in wildlife populations where intensive, long-term field monitoring is necessary [8]. However, considerable research has documented changes in host behaviour resulting from parasitism [9,10]. Parasite-induced behavioural changes can be complex (as in Toxoplasma, for example [11]), but most are subtle and involve reductions in host activity levels, which often manifest as lethargy, somnolence and reduced appetite [12]. Previously, these ‘sickness behaviours’ were considered maladaptive—a consequence of the parasite exerting pathological effects on the host [13]. Increasingly, however, sickness behaviours are being interpreted as adaptive changes by the host to conserve energy and fight infection [12–14].
Despite a basic understanding of the immunological underpinnings of sickness behaviour [15], little research has attempted to describe the presentation of sickness behaviours in wild animal populations [16,17]. Here, we examine behavioural variation in a wild primate associated with a gastrointestinal nematode, the whipworm (genus Trichuris). Whipworms are globally ubiquitous, soil-transmitted helminths of increasing concern to public health [18,19]. In humans, children aged 5–15 years often suffer the most pronounced symptoms, ranging from gastrointestinal upset to cognitive and developmental stunting. In severe infections, Trichuris dysentery syndrome may result in rectal prolapse and chronic dysentery [18–20]. Recently, ribosomal DNA sequencing revealed nearly identical sequences between a number of Ugandan primates (including monkeys and chimpanzees) and local people, suggesting that some parasite lineages are broadly transmissible among primates, including humans [21]. This is especially concerning given that whipworms are commonly found in African primates that share environments with humans [21–26].
We focus our study on wild red colobus monkeys (Procolobus rufomitratus ssp. tephrosceles), which are arboreal, predominantly leaf-eating primates. Currently, this taxon is considered endangered, with the largest and potentially only viable population existing in the location of our study, Kibale National Park (hereafter Kibale), Uganda [27]. Using behavioural data from a habituated group, we quantify changes in (i) activity, (ii) rate of behaviour change and (iii) feeding pattern associated with whipworm infection.
2. Material and methods
(a). Study site and data collection
This research was conducted in Kibale, a 795 km2 tropical rainforest in southwestern Uganda [28,29]. In the Kanyawara region, one habituated group of over 100 red colobus is the focus of long-term research and was used in this study [30]. Over 48 months from 2007 to 2011, we collected activity data from individuals that were recognizable based on physical characteristics and collars affixed as part of long-term research. Activity data were collected in the morning and early afternoon by selecting five easily visible adults every 30 min and recording their identity, sex and behaviour at the moment of observation. If the animal was feeding, the plant species and part being eaten were also recorded. After 30 min of observation, another five individuals were selected and the process was repeated for the duration of the field day.
Faecal samples were collected opportunistically when animals were observed defecating. In a field laboratory, these samples were subjected to a modified ethyl acetate sedimentation method using 1 g of faeces to concentrate nematode eggs, which are shed in the faeces of infected individuals [21,31–33]. Briefly, 1 g of fully formed faeces was mixed with water and strained through cheesecloth, pelleted by centrifugation and decanted, and then re-suspended in water and 3 ml ethyl acetate to remove lipids and debris. Samples were then pelleted again, and the decanted sediments were preserved in formalin before examination under 10× objective light magnification to determine whipworm infection status. Samples were classified as ‘positive’ when whipworm eggs were detected and ‘negative’ when the entire sediment was scanned without discovering eggs. While positive samples conclusively indicate whipworm infection, negative samples may indicate uninfected individuals or infected individuals that are not actively shedding eggs. The number of eggs per gram (EPG) of faeces was also recorded, as this is considered a proxy for adult nematode infection intensity in some species [34,35].
To determine whether the temporal pattern of red colobus behaviour varied with infection, three months of focal animal data were collected from 16 well-known adults (eight females and eight males) of the same red colobus group from June to September 2014 inclusively. Focal animals were observed over 30 min sampling periods, during which all activity and behaviour were recorded. A corresponding faecal sample was collected the same day and processed as described above.
(b). Analysis
All statistical analyses were conducted in R [36] unless otherwise specified. For each faecal sample, one week of behavioural data (3 days before the date of faecal sample collection and 4 days after) from the corresponding individual was examined. Only activity data collected during the week from which the sample was collected were used, to minimize temporal variability in faecal egg detection known from human infections [37]. Only samples from individually recognizable animals and individuals whose infection status changed through time (to control for intra-individual variation in behaviour) were included. Prior to performing further analysis, the relationship between whipworm infection (prevalence and EPG) and season (average rainfall and temperature in the Kanyawara region of Kibale) were examined using linear regression. There was no significant relationship (results not shown), and therefore the effects of seasonal patterns on our study were not considered further.
Red colobus behaviour was categorized into 11 activities (breastfeed, chase, copulate, feed, fight, groom, move, play, present, rest and vocalize). However, six behaviours were exceedingly rare (less than 1% of all observations: breastfeed, chase, fight, play, present and vocalize) and were therefore omitted from analyses. The five remaining behaviours (copulate, feed, groom, move and rest) were converted into proportions reflecting the number of events for that behaviour relative to the total number of observations. The relationship between whipworm infection and red colobus activity was evaluated using generalized linear mixed-effect models (GLMMs). Separate GLMMs were constructed for each behaviour, with behaviour a binomial response variable (e.g. feed/not feeding). Models containing infection as a binary variable (either positive or negative for whipworm eggs in faeces) outperformed those that used EPG. Including other, less frequent parasitic worm genera detected in the faecal samples (Oesophagostomum, n = 12; an unidentified strongyle, n = 5) also did not improve model performance. Information on infection by other parasite taxa was unavailable, although such data may have been informative in our models. Reported GLMMs therefore included individual identity as a random intercept, and contained two fixed effects: (i) sex and (ii) binary infection status. GLMMs were fitted with a binomial error distribution that was implemented in the lme4 library [38]. Statistical significance of fixed effects was assessed using likelihood ratio tests. To illustrate the relative frequency of behaviour, we used the equation
Here, pi indicates the number of observations during which a given behaviour was observed out of the total number of observations per individual. Relative change in behaviour was calculated in addition to absolute frequency to account for the infrequency of some behaviours relative to others. Relative change was only calculated for illustrative purposes; no statistical tests were performed on these scores.
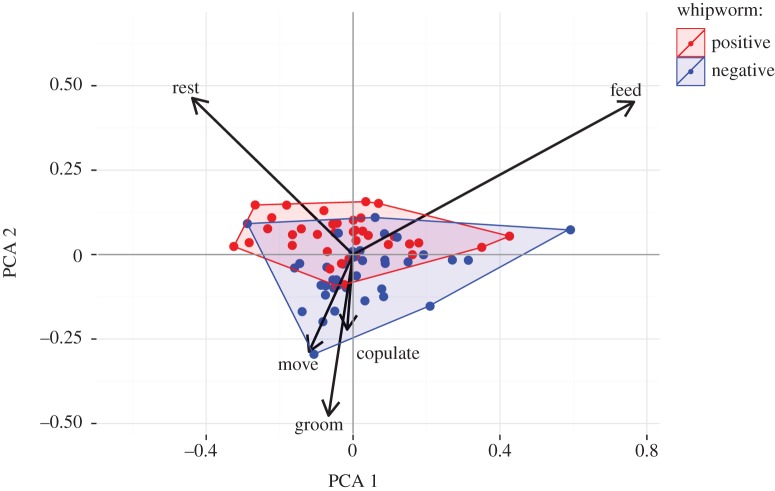
The relationship between all five behaviours was visualized by principal component analysis (PCA). Proportional mean activity per individual at whipworm-positive and -negative time points were transformed into individual scores for the first two components and plotted following standardization around zero on each axis. Infection status was colour coded and the minimum area of a given status was outlined using convex hulls. Each individual was represented twice in this analysis (once at whipworm-positive intervals and again at whipworm-negative intervals). PCA was used to visualize all activities together—the aforementioned analyses were performed with GLMMs as describe above.
Rate of behavioural switching was estimated from focal sample data, to determine whether the presence of whipworm was associated with changes in behaviour over time. To control for intra-individual variation in behaviour, only individuals for which infection status changed during the three-month focal period were included, resulting in a final sample size of 14 individuals. For each focal individual, we calculated the mean time (in seconds) per behaviour (breastfeed, chase, copulate, feed, fight, give groom, receive groom, move/travel, play and rest) over a 30 min focal period. The mean time an animal spent performing a behaviour was averaged within focal individuals at whipworm-positive and -negative intervals, and the probability of behaviour switching was evaluated using the Kaplan–Meier method [39]. Log-rank tests were used to assess whether there were significant difference between the curves of whipworm-positive and -negative individuals using the survival library [40,41].
Finally, to examine the relationship between diet composition and infection, the proportion of time red colobus spent feeding on a particular plant part (bark, flowers, fruit, leaves, seeds or other, with ‘other’ being leaf petioles, leaf buds, pith, dead wood and soil) was compared between whipworm-positive and -negative intervals using binomial tests. A Bonferroni correction was applied to critical p-values to account for multiple comparisons. To determine whether there was phylogenetic signal in shifts of diet composition, we also explored the taxonomic composition of plant families consumed at whipworm-positive and -negative intervals using the net relatedness index to quantify the phylogenetic structure of plants in the diet [42] (electronic supplementary material, Methods).
3. Results
In total, 371 faecal samples and 3032 individual activity recordings were included from 43 individuals (28 females, 15 males; electronic supplementary material, table S1). We recorded an average of 72 (±40.15 s.d.) observations per individual, 8.35 (±4.40 s.d.) observations per faecal sample and 8.63 (±4.05 s.d.) faecal samples per individual. A total of 155 (41.8%) faecal samples were positive for whipworm eggs. Males were more likely to have whipworm-positive samples (56%) than females (35%; χ2 = 13.46, p < 0.001). Mean whipworm EPG was 12.69 (±16.08 s.d.), and this also varied by sex, with males having higher EPG (17.5 EPG ±8.08 s.d.) than females (7.85 EPG ±8.82 s.d.; Wilcoxon rank-sum test p = 0.01).
GLMMs that included both sex and binary whipworm infection status had highest support for all behaviours except feeding (table 1). GLMMs revealed clear sex differences, with males copulating and resting more, and moving and grooming less (table 1; electronic supplementary material, figure S1). There was no difference in the proportion of time spent feeding between sexes. In both sexes, behaviour varied with whipworm status.
Table 1.
Relationship between infection status, sex and activity of focal group red colobus monkeys (Procolobus rufomitratus). Independent variables show the proportion of time spent performing each activity (copulating, grooming, moving, feeding and resting) by males and females while whipworm-positive or -negative (based on microscopic detection of whipworm eggs of the genus Trichuris). Likelihood ratio tests present the results of five independent generalized linear mixed models that included the individual's sex and infection status as fixed effects, and the individuals themselves as random effects. Statistical significance of fixed effects was tested using likelihood ratio tests comparing the fit between a full model and a reduced model missing a given fixed effect. Italics indicate statistically significant results.
| independent variables: infection, sex |
likelihood ratio test |
|||||||
|---|---|---|---|---|---|---|---|---|
| females |
males |
infection |
sex |
|||||
| dependent variable | negative | positive | negative | positive | χ2 | p-value | χ2 | p-value |
| copulating | 0.0198 | 0.0050 | 0.0443 | 0.0114 | 21.78 | <0.0001 | 4.69 | 0.0310 |
| grooming | 0.1032 | 0.0518 | 0.0563 | 0.0275 | 22.63 | <0.0001 | 11.48 | 0.0006 |
| feeding | 0.4774 | 0.4981 | 0.4732 | 0.4939 | 1.15 | 0.28119 | 0.02 | 0.8656 |
| moving | 0.0934 | 0.0533 | 0.0665 | 0.0375 | 15.02 | 0.0001 | 4.02 | 0.0345 |
| resting | 0.2806 | 0.3587 | 0.3350 | 0.4194 | 21.20 | <0.0001 | 7.72 | 0.0023 |
Whipworm-positive individuals copulated, groomed and moved less, and they rested more (table 1; electronic supplementary material, figure S1). Feeding did not vary with infection status. Relative time spent copulating differed most dramatically with infection status, being 71.72% (±4.2 s.e.) lower in females and 56.03% (±11.6 s.e.) lower in males when whipworm-positive (figure 1). Feeding (no significant difference) and moving (27.01% lower in females and 25.84% lower in males when whipworm-positive) changed the least with infection. Resting was the only behaviour that was significantly more frequent among whipworm-positive individuals, being 34.10% (±2.8 s.e) higher in females and 23.64% (±6.2 s.e.) higher in males (figure 1).
Figure 1.
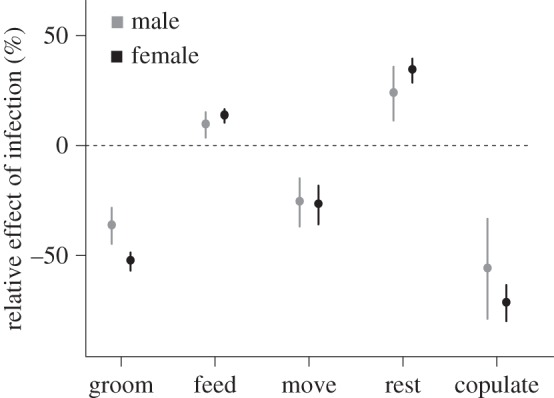
Mean relative change in the frequency of occurrence of each behaviour, separated by sex, as a function of infection (as determined by the presence of whipworm eggs in faeces). Negative values indicate a relative reduction in the frequency of a behaviour when individuals were whipworm-positive. Error bars, s.e.m.
Principal component (PC) 1 and PC 2 explained 65.59% and 21.41% of the total variance, respectively. Individuals occupied more negative values in PC 1 and more positive values in PC 2 when positive for whipworm (figure 2).
Figure 2.
Visualization by principal component analysis of all five behaviours (proportion of observations in which individuals were resting, feeding, moving, grooming and copulating), with first two principal components (PC 1 and PC 2) shown (cumulative proportion of explained variance = 86.9%). PC 1 loadings were: feed = 0.788; rest = −0.601; move = −0.127. PC 2 loadings were: feed = 0.361; rest = 0.630; move = −0.634; groom = −0.257. Data points represent the mean variation in behaviour of an individual at time points when negative for whipworm eggs (blue) or positive for whipworm eggs (red) in faeces, and convex hulls occupied by individuals when negative (blue) or positive (red) outlined and shaded. (Online version in colour.)
Focal animal data from 14 red colobus revealed that the same cohort of individuals switched behaviours less often when whipworm-positive than when whipworm-negative (χ2 = 11.96, p < 0.001). Within an individual, the average time to a switch in behaviour increased from 70.45 s (median = 63.5 s) when whipworm-negative to 222.08 s (median = 204.78 s) when whipworm-positive. This difference was highly significant in linear mixed models that used the log average switching time as the response variable, whipworm infection status as the predictor variable and individuals as random effects (F = 11.337, p < 0.001, d.f. = 1; figure 3; electronic supplementary material, figure S3).
Figure 3.
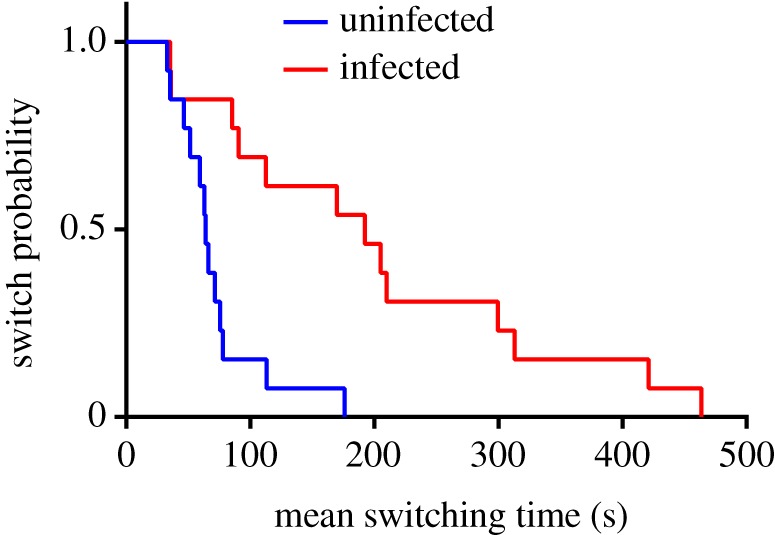
Kaplan–Meier curves of 14 individuals (eight females and six males) at intervals when negative (blue) and positive (red) for whipworm eggs in faeces. Each curve shows the mean time (seconds) required for an individual to switch behaviour in a 30 min focal period, with the percentage of individuals that have not switched behaviour shown on the y-axis. (Online version in colour.)
While the proportion of time spent feeding on most plant parts (flowers, fruit, leaves, seeds, other) was the same regardless of infection status, the proportion of time spent eating bark increased (p = 0.012 from binomial tests; table 2) when individuals were whipworm-positive (whipworm-negative = 3.84%, whipworm-positive = 7.39%). Specifically, red colobus consumed bark from nine plant species, of which consumption from seven increased during whipworm-positive periods (table 3). In addition, red colobus feeding frequency on the plant family Fabaceae differed significantly with infection status (whipworm-negative: 23.28%; whipworm-positive: 31.58%; Bonferroni corrected χ2 = 12.87, p < 0.05). This difference resulted from increased consumption of two species from the genus Albizia, for which consumption increased from 23.6% of all Fabaceae plant choices to 45.0% in whipworm-positive individuals (χ2 = 14.31, p < 0.001; electronic supplementary material, table S2).
Table 2.
Percentage of plant parts eaten by red colobus when individual faecal samples were positive or negative for whipworm eggs of the genus Trichuris. Non-parametric comparisons of two proportions were calculated using a χ2-test with Yates's continuity correction. p-values shown are adjusted for multiple comparisons using the Bonferroni correction method. Italics indicate statistically significant results.
| part | % negative (n = 886) | % positive (n = 731) | χ2 | p-value |
|---|---|---|---|---|
| bark | 3.84 | 7.39 | 9.130 | 0.012 |
| flowers | 1.58 | 0.82 | 1.320 | >0.500 |
| fruit | 1.35 | 1.64 | 0.072 | >0.500 |
| leaves | 91.99 | 88.37 | 5.595 | 0.108 |
| seeds | 0.11 | 1.09 | 5.311 | 0.126 |
| other | 1.13 | 0.68 | 0.446 | >0.500 |
Table 3.
Relative change in bark consumption, by species. Percentage relative change values (‘% change’) calculated by correcting for total number of observations and total number of bark feeding observations at intervals where individuals were positive and negative for whipworm. Positive relative change values indicate an increase in consumption of the species when positive for whipworm, while negative values indicate the opposite. ‘Chemical activity’ indicates species with reported pharmacological activity; ‘inconclusive’ signifies species that have been reported as potentially medicinal, but whose properties have not been characterized.
| traditional usage |
|||||
|---|---|---|---|---|---|
| species (family) | % change | chemical activity? | component | application | refs |
| Albizia grandibracteata (Fabaceae) | 33.11 | yes | bark | anti-helminthic | [43] |
| Trilepisium madagascariense (Moraceae) | 14.77 | yes | stem bark | anti-diarrhoeal | [44] |
| Celtis durandii (Cannabaceae) | −3.83 | inconclusive | leaves | fever, pain | [45] |
| Olea welwitschii (Oleaceae) | 3.56 | no | n.a. | n.a. | n.a. |
| Strombosia scheffleri (Olacaceae) | −3.83 | yes | bark | gastric pain | [46,47] |
| Prunus africana (Rosaceae) | 72.44 | yes | bark | stomach ailments | [46] |
| Eucalyptus sp. (Myrtaceae) | 71.57 | yes | extracted oil | antiseptic | [48] |
| Alangium chinense (Cornaceae) | 7.39 | yes | roots, stems | analgesic | [49] |
| Persea americana (Lauraceae) | 73.30 | yes | leaves, fruit rind | analgesic, vermifuge | [46,50] |
4. Discussion
We investigated the relationship between host behaviour and infection with a non-lethal gastrointestinal parasite, the whipworm, Trichuris spp. Individual behavioural profiles differed considerably with infectious status (electronic supplementary material, figure S1). Some of this variation may have resulted from differences in infection intensity, fitness or immunity between individuals, co-infections or social circumstance [51,52]. However, our results suggest that sex differences may also be important, with males more often whipworm-positive and with higher egg counts than females.
Regardless of sex, red colobus rested more, moved less, groomed less and copulated less when whipworm-positive. These patterns may indicate that red colobus modify their behaviour because of parasitism. However, it is also possible that red colobus may alter certain behaviours for reasons other than whipworm infection, and these factors may also affect whipworm egg shedding. For example, an increase in stress (perhaps from injury or antagonism) may cause increased resting behaviour but is also immunosuppressive, potentially facilitating parasite infection or the release of eggs. Another possibility is that red colobus with affinity for ‘risky’ behaviours are more likely to become infected with whipworm. For example, individuals that frequently groom conspecifics could be more susceptible to ingesting larvated parasite eggs adhered to fur [53]. Similarly, animals that move often might come into more frequent contact with infective stages that contaminate the soil and vegetation [53]. However, we suggest that our results are more consistent with sickness behaviours, and support an energetic trade-off, whereby ‘sick’ individuals favour low-energy states over strenuous activity. Specifically, we found that the most energetically expensive behaviours, copulating and grooming, showed the greatest reduction during infection (see [54] for estimates of energetic costs).
Anecdotes of lethargy during illness have been reported in chimpanzees. For example, a report from Kibale described increased resting and decreased feeding in animals experiencing a flu-like illnesses [17]. In a Tanzanian population, a sick female rested more and fed less than other group members, and constructed sleeping nests during the day [55]. Such behaviours could result directly from physiological impairment caused by infection. If so, this would imply a significant fitness cost of infection. However, an expanding body of evidence suggests that sickness behaviour has adaptive benefits to the host [12,14]. For example, early studies showed that rats repeatedly chose inactivity over exercise when injected with endotoxin (known to stimulate the immune response), suggesting that they were motivated to rest [56]. If sickness behaviour is an adaptive host response, then the behaviours observed in red colobus could help maintain homeostasis during infection.
We did not find evidence that feeding decreased with infection, which contrasts with previous studies [57]. However, we examined the frequency of feeding and not the rate of food intake. Another metric, such as bite rate, may have uncovered a different relationship. Nevertheless, we showed that whipworm-positive individuals consumed twice as much bark. Bark is highly fibrous, has low nutritional value and is relatively indigestible [58]. However, the bark from some plant species may have compounds with medicinal properties. For example, bark from Gongronema latifolium is eaten by Bossou chimpanzees in West Africa, and is also used by local people as a purge for symptoms associated with intestinal parasites [59]. Our results are consistent with predictions that bark consumption is a form of self-medication. Red colobus forage preferentially on barks with known medicinal properties, with seven of the nine species identified being used medicinally by people to treat a range of symptoms associated with helminth infection (diarrhoea and pain; table 3), and parts from two plant species consumed by red colobus (bark of Albizia grandibracteata and fruit rind of Persea americana) used directly for treatment of parasitic worms. Indeed, the bark of A. grandibracteata is also consumed by the chimpanzees of Kibale, has confirmed bioactive properties, and is used locally for anti-helminthic treatment and stomach pain [60]. If red colobus do consume barks for their medicinal benefits, investigations of the two species that lacked clear chemical activity (Celtis durandii and Olea welwitschii) may uncover new active compounds. In addition to the potential chemical effect of bark, its highly fibrous nature may increase gut motility, which could assist in purging intestinal nematodes [43].
Additional evidence supporting self-medication in whipworm-positive red colobus comes from shifts in dietary taxonomic composition. Although we did not find differences in the phylogenetic structure of plants consumed, we observed an 8.3% increase in consumption of plants in the legume family, Fabaceae (a.k.a. Leguminosae), in whipworm-positive individuals. In particular, consumption of two abundant species, A. grandibracteata and Albizia gummifera, nearly doubled when animals were whipworm-positive. Dried and crushed or boiled preparations of bark from both of these species are used locally as anti-parasitics and analgesics [46,61,62]. In addition, both the leaves and bark of A. grandibracteata have documented in vitro anti-helminthic activity because of the presence of cytotoxic saponins [46]. However, we note that our study was strictly observational, and further work should determine the efficacy of A. grandibracteata in reducing parasite burden or lessening symptoms within primate hosts. Evidence for self-medication in red colobus emphasizes the need for conservation efforts to consider the network of biological interactions in which they exist, including their parasites and the diversity of plant species that might be important for parasite regulation.
Finally, we found evidence that red colobus behavioural flexibility changed with whipworm status. Specifically, a given individual took longer to switch behaviours when it was whipworm-positive than when it was whipworm-negative. Reductions in the complexity of behavioural patterns have been associated with stressful conditions in a number of studies, and have also been used as a non-invasive indicator of health impairment [63,64]. In primates, fractal analysis has been used to show that health impairment by intestinal parasites results in increased periodicity of behaviour (i.e. reduced complexity) [65,66]. Complex or unpredictable behaviour is believed to be advantageous, because it allows organisms to adapt to changing environments [44,66]. Thus, while beneficial in the short term for mitigating the costs of infection, prolonged expression of sickness behaviours could have fitness costs.
6. Conclusion
We provide evidence that red colobus behaviour covaries with the presence of whipworm eggs in faeces, and that this relationship probably results from the induction of sickness behaviours. Specifically, we document that shedding of eggs in faeces was associated with more resting but less moving, grooming and copulating. In addition, red colobus ate plants with potential medicinal properties more frequently when shedding whipworm eggs. Self-medication is considered a truly adaptive response to parasitic infection because it is complex, has convergently evolved in a number of hosts and is beneficial to host fitness [13]. More generally, sickness behaviours may help animals cope with the burden of infection by concentrating limited energetic resources on critical immune functions or by impairing the parasite. However, although such behaviours may be adaptive in the short term, they may have longer-term fitness consequences. Our results support the idea that non-lethal parasite infections can alter host behaviour. How these changes in behaviour may translate into fitness consequences remains to be determined, but offers a potential avenue for future research in the field of wildlife parasitology.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We gratefully acknowledge the Uganda Wildlife Authority and Uganda National Council of Science and Technology for permission to conduct this research. We warmly thank Robert Basaija, Peter Tuhairwe, Clovice Kaganzi, Dr Patrick Omeja and Dr Dennis Twinomugisha for assistance in the field, Maxwell Farrell, Stephanie Shooner and Eric Pedersen for analytical advice, and Jeremy Hogan for thoughtful contributions to the manuscript.
Ethics
Permission to conduct this research was granted by the McGill Animal Care Committee (#5028), the Uganda National Council for Science and Technology (#RS242), and the Uganda Wildlife Authority.
Author's contributions
R.R.G. designed the study, participated in data collection, cleaned and analysed data, and drafted the manuscript. V.F. and T.L.G. contributed to data analysis and manuscript writing. C.A.C. collected long-term field data, and contributed to research design and manuscript writing. T.J.D. contributed to research design, data analysis and manuscript writing. All authors gave final approval for publication.
Competing interests
The authors do not have competing interests to declare.
Funding
This research was funded by the Canada Research Chairs Program (to C.A.C.) and the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to C.A.C., Alexander Graham Bell Award to R.R.G. and Michael Smith Foreign Study Supplement to R.R.G.), and the National Geographic Society (R.R.G.).
References
- 1.Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296, 2158–2162. ( 10.1126/science.1063699) [DOI] [PubMed] [Google Scholar]
- 2.Deem SL, Karesh WB, Weisman W. 2001. Putting theory into practice: wildlife health in conservation. Conserv. Biol. 15, 1224–1233. ( 10.1111/j.1523-1739.2001.00336.x) [DOI] [Google Scholar]
- 3.Thompson RCA, Lymbery AJ, Smith A. 2010. Parasites, emerging disease and wildlife conservation. Int. J. Parasitol. 40, 1163–1170. ( 10.1016/j.ijpara.2010.04.009) [DOI] [PubMed] [Google Scholar]
- 4.Hudson P, Dobson AP. 1995. Macroparasites: observed patterns. In Ecology of infectious diseases in natural populations (eds Grenfell BT, Dobson AP), pp. 144–176. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 5.Gulland FMD. 1995. The impact of infectious diseases on wild animal populations—a review. In Ecology of infectious disease in natural populations (eds Grenfell BT, Dobson AP), pp. 20–51. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 6.Anderson RM. 1978. The regulation of host population growth by parasitic species. Parasitology 76, 119–157. ( 10.1017/S0031182000047739) [DOI] [PubMed] [Google Scholar]
- 7.Anderson RM, May RM. 1979. Population biology of infectious disease: part 1. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 8.Barker JF. 2009. Defining fitness in natural and domesticated populations. In Adaptation and fitness in animal populations (eds van der Werf J, Graser H-U, Frankham R, Gondro C), pp. 3–14. New York, NY: Springer. [Google Scholar]
- 9.Minchella DJ, Scott ME. 1991. Parasitism—a cryptic determinant of animal community structure. Trends Ecol. Evol. 6, 250–254. ( 10.1016/0169-5347(91)90071-5) [DOI] [PubMed] [Google Scholar]
- 10.Poulin R. 1994. Meta-analysis of parasite-induced behavioural changes. Anim. Behav. 48, 137–146. ( 10.1006/anbe.1994.1220) [DOI] [Google Scholar]
- 11.Berdoy M, Webster JP, Macdonald DW. 2000. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. Lond. B 267, 1591–1594. ( 10.1098/rspb.2000.1182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hart BL. 1988. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 12, 123–137. ( 10.1016/S0149-7634(88)80004-6) [DOI] [PubMed] [Google Scholar]
- 13.Poulin R. 1995. ‘Adaptive’ changes in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383. ( 10.1016/0020-7519(95)00100-X) [DOI] [PubMed] [Google Scholar]
- 14.Johnson RW. 2002. The concept of sickness behavior: a brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 87, 443–450. ( 10.1016/S0165-2427(02)00069-7) [DOI] [PubMed] [Google Scholar]
- 15.Dantzer R. 2001. Cytokine-induced sickness behavior: where do we stand? Brain Behav. Immun. 15, 7–24. ( 10.1006/brbi.2000.0613) [DOI] [PubMed] [Google Scholar]
- 16.Weary DM, Huzzey JM, von Keyserlingk MAG. 2009. Using behavior to predict and identify ill health in animals. J. Anim. Sci. 87, 770–777. ( 10.2527/jas.2008-1297) [DOI] [PubMed] [Google Scholar]
- 17.Krief S, Huffman M, Sévenet T, Guillot J, Bories C, Hladik C, Wrangham R. 2005. Noninvasive monitoring of the health of Pan troglodytes schweinfurthii in the Kibale National Park, Uganda. Int. J. Primatol. 26, 467–490. ( 10.1007/s10764-005-2934-9) [DOI] [Google Scholar]
- 18.Stephenson LS, Holland CV, Cooper ES. 2000. The public health significance of Trichuris trichiura. Parasitology 121, S73–S95. ( 10.1017/S0031182000006867) [DOI] [PubMed] [Google Scholar]
- 19.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. 2006. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet 367, 1521–1532. ( 10.1016/S0140-6736(06)68653-4) [DOI] [PubMed] [Google Scholar]
- 20.Ojha SC, Jaide C, Jinawath N, Rotjanapan P, Baral P. 2014. Geohelminths: public health significance. J. Infect Dev. Ctries 8, 5–16. ( 10.3855/jidc.3183) [DOI] [PubMed] [Google Scholar]
- 21.Ghai R, Simons ND, Chapman CA, Omeja PA, Davies TJ, Ting N, Goldberg TL. 2014. Hidden population structure and cross-species transmission of whipworms (Trichuris sp.) in humans and non-human primates in Uganda. PLoS Negl. Trop. Dis. 8, e3256 ( 10.1371/journal.pntd.0003256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie TR, Greiner EC, Chapman CA. 2005. Gastrointestinal parasites of the colobus monkeys of Uganda. J. Parasitol. 91, 569–573. ( 10.1645/GE-434R) [DOI] [PubMed] [Google Scholar]
- 23.Mbora DNM, Munene E. 2006. Gastrointestinal parasites of critically endangered primates endemic to Tana River, Kenya: Tana River red colobus (Procolobus rufomitratus) and crested mangabey (Cercocebus galeritus). J. Parasitol. 92, 928–932. ( 10.2307/40058601) [DOI] [PubMed] [Google Scholar]
- 24.Weyher AH, Ross C, Semple S. 2006. Gastrointestinal parasites in crop raiding and wild foraging Papio anubis in Nigeria. Int. J. Primatol. 27, 1519–1534. ( 10.1007/s10764-006-9089-1) [DOI] [Google Scholar]
- 25.Teichroeb JA, Kutz SJ, Parkar U, Thompson RCA, Sicotte P. 2009. Ecology of the gastrointestinal parasites of Colobus vellerosus at Boabeng-Fiema, Ghana: possible anthropozoonotic transmission. Am. J. Phys. Anthropol. 140, 498–507. ( 10.1002/ajpa.21098) [DOI] [PubMed] [Google Scholar]
- 26.Murray S, Stem C, Boudreau B, Goodall J. 2000. Intestinal parasites of baboons (Papio cynocephalus anubis) and chimpanzees (Pan troglodytes) in Gombe National Park. J. Zoo Wildl. Med. 31, 176–178. ( 10.1638/1042-7260(2000)031%5B0176:IPOBPC%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 27.Struhsaker TT. 2005. Conservation of red colobus and their habitats. Int. J. Primatol. 26, 525–538. ( 10.1007/s10764-005-4364-0) [DOI] [Google Scholar]
- 28.Chapman CA, Struhsaker TT, Skorupa JP, Snaith TV, Rothman JM. 2010. Understanding long-term primate community dynamics: implications of forest change. Ecol. Appl. 20, 179–191. ( 10.1890/09-0128.1) [DOI] [PubMed] [Google Scholar]
- 29.Chapman CA, Lambert JE. 2000. Habitat alteration and the conservation of African primates: case study of Kibale National Park, Uganda. Am. J. Primatol. 50, 169–185. () [DOI] [PubMed] [Google Scholar]
- 30.Gogarten JE, Bonnell TR, Brown LM, Campenni M, Wasserman MD, Chapman CA. 2014. Increasing group size alters behaviour of a folivorous primate. Int. J. Primatol. 35, 590–608. ( 10.1007/s10764-014-9770-8) [DOI] [Google Scholar]
- 31.Young KH, Bullock SL, Melvin DM, Spruill CL. 1979. Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J. Clin. Microbiol. 10, 852–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia LS, Campbell J, Fritsche PTR, Hummert B, Johnston SP, Rachford FW, Rocha AJ, Shimizu R, Smith J. 2005. Procedures for the recovery and identification of parasites from the intestinal tract: approved guideline 109, 2nd edn Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- 33.Greiner EC, MacIntosh A. 2009. Collection methods and diagnostic procedures for primate parasitology. In Primate parasite ecology: the dynamics and study of host–parasite relationships (eds Huffman MA, Chapman CA), pp. 3–27. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 34.Seivwright LJ, Redpath SM, Mougeot F, Watt L, Hudson PJ. 2004. Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. J, Helminthol, 78, 69–76. ( 10.1079/JOH2003220) [DOI] [PubMed] [Google Scholar]
- 35.Cabaret JJ, Gasnier N, Jacquiet P. 1998. Faecal egg counts are representative of digestive-tract strongyle worm burdens in sheep and goats. Parasite 5, 137–142. ( 10.1051/parasite/1998052137) [DOI] [PubMed] [Google Scholar]
- 36.R Development Core Team. 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 37.Hall A. 1981. Quantitative variability of nematode egg counts in faeces: a study among rural Kenyans. Trans. R. Soc. Trop. Med. Hyg. 75, 682–687. ( 10.1016/0035-9203(81)90148-6) [DOI] [PubMed] [Google Scholar]
- 38.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. See http://arxiv.org/abs/1406.5823. [Google Scholar]
- 39.Bland JM, Altman DG. 1998. Survival probabilities (the Kaplan–Meier method). Br. Med. J. 317, 1572–1580. ( 10.1136/bmj.317.7172.1572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Therneau TM. 2000. Estimating the survival and hazard functions. In Modeling survival data: extending the cox model, pp. 7–31. New York, NY: Springer. [Google Scholar]
- 41.Therneau T. 2013. A package for survival analysis in S. Version 2.38. See http://CRAN.R-project.org/package=survival. [Google Scholar]
- 42.Webb CO. 2000. Exploring the phylogenetic structure of ecological communities: an example for rain forest trees. Am. Nat. 156, 145–155. ( 10.1086/303378) [DOI] [PubMed] [Google Scholar]
- 43.Huffman M, Page J, Sukhdeo MK, Gotoh S, Kalunde M, Chandrasiri T, Towers GHN. 1996. Leaf-swallowing by chimpanzees: a behavioral adaptation for the control of strongyle nematode infections. Int. J. Primatol. 17, 475–503. ( 10.1007/bf02735188) [DOI] [Google Scholar]
- 44.Goldberger AL. 1996. Fractal variability versus pathologic periodicity: complexity loss and stereotypy in disease. Perspect. Biol. Med. 40, 543–561. ( 10.1353/pbm.1997.0063) [DOI] [PubMed] [Google Scholar]
- 45.Teke GN, Kuiate JR, Kuete V, Teponno RB, Tapondjou LA, Vilaren G. 2010. Antidiarrheal activity of extracts and compound from Trilepisium madagascariense stem bark. Indian J. Parmacol. 42, 157–163. ( 10.4103/0253-7613.66839) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krief S, Hladik CM, Haxaire C. 2005. Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda. J. Ethnopharmacol. 101, 1–15. ( 10.1016/j.jep.2005.03.024) [DOI] [PubMed] [Google Scholar]
- 47.Pebsworth P, Krief S, Huffman MA. 2006. The role of diet in self-medication among chimpanzees in the Sonso and Kanyawara communities, Uganda. In Primates of western Uganda (eds Newton-Fisher NE, Notman H, Paterson JD, Reynolds V), pp. 105–133. New York, NY: Springer. [Google Scholar]
- 48.Moshi MJ, et al. 2009. Antimicrobial and brine shrimp toxicity of some plants used in traditional medicine in Bukoba District, north-western Tanzania. Tanzan J. Health Res. 11, 23–28. ( 10.4314/thrb.v11i1.43247) [DOI] [PubMed] [Google Scholar]
- 49.Cimanga K, Kambu K, Tona L, Apers S, de Bruyne T, Hermans N, Totte J, Pieters L, Vlietinck AJ. 2002. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 79, 213–220. ( 10.1016/S0378-8741(01)00384-1) [DOI] [PubMed] [Google Scholar]
- 50.Tang W, Eisenbrand G. 1992. Chinese drugs of plant origin: chemistry, pharmacology, and use in traditional and modern medicine, pp. 69–71. Berlin, Germany: Springer. [Google Scholar]
- 51.Schmid-Hempel P. 2003. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. Lond. B 270, 357–366. ( 10.1098/rspb.2002.2265) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lopes PC. 2014. When is it socially acceptable to feel sick? Proc. R. Soc. B 281, 20140218 ( 10.1098/rspb.2014.0218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunn CL, Altizer SM. 2006. Infectious diseases in primates: behavior, ecology and evolution, pp. 135–175. Oxford, UK: Oxford University Press. [Google Scholar]
- 54.Coelho A Jr, Bramblett C, Quick L, Bramblett S. 1976. Resource availability and population density in primates: a socio-bioenergetic analysis of the energy budgets of Guatemalan howler and spider monkeys. Primates 17, 63–80. ( 10.1007/bf02381567) [DOI] [Google Scholar]
- 55.Huffman M, Seifu M. 1989. Observations on the illness and consumption of a possibly medicinal plant Vernonia amygdalina by a wild chimpanzee in the Mahale Mountains National Park, Tanzania. Primates 30, 51–63. ( 10.1007/bf02381210) [DOI] [Google Scholar]
- 56.Miller NE. 1964. Some psychophysiological studies of motivation and of the behavioural effects of illness. Bull. Br. Psychol. Soc. 17, 1–20. [Google Scholar]
- 57.Crompton DW. 1984. Influence of parasitic infection on food intake. Fed. Proc. 43, 239–245. [PubMed] [Google Scholar]
- 58.Huffman MA. 1997. Current evidence for self-medication in primates: a multidisciplinary perspective. Yearb. Phys. Anthropol. 104, 171–200. () [DOI] [Google Scholar]
- 59.Huffman MA, Nakagawa N, Go Y, Imai H, Tomonaga M. 2013. Monkeys, apes, and humans, pp. 13–23. Tokyo, Japan: Springer. [Google Scholar]
- 60.Krief S, Thoison O, Sévenet T, Wrangham RW, Lavaud C. 2005. Triterpenoid saponin anthranilates from Albizia grandibracteata leaves ingested by primates in Uganda. J. Nat. Prod. 68, 897–903. ( 10.1021/np049576i) [DOI] [PubMed] [Google Scholar]
- 61.Burkill HM. 1985. Useful plants of West Tropical Africa, vol. 1–6 Kew, UK: Royal Botanic Gardens. [Google Scholar]
- 62.Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. 2009. Agroforestry database: a tree reference and selection guide version 4.0. See http://www.worldagroforestry.org/resources/databases/agroforestree. [Google Scholar]
- 63.Alados CL, Escos JM, Emlen JM. 1996. Fractal structure of sequential behaviour patterns: an indicator of stress. Anim. Behav. 51, 437–443. ( 10.1006/anbe.1996.0040) [DOI] [Google Scholar]
- 64.Maria GA, Escos J, Alados CL. 2004. Complexity of behavioural sequences and their relation to stress conditions in chickens (Gallus gallus domesticus): a non-invasive technique to evaluate animal welfare. Appl. Anim. Behav. Sci. 86, 93–104. ( 10.1016/j.applanim.2003.11.012) [DOI] [Google Scholar]
- 65.Alados CL, Huffman MA. 2000. Fractal long-range correlations in behavioural sequences of wild chimpanzees: a non-invasive analytical tool for the evaluation of health. Ethology 106, 105–116. ( 10.1046/j.1439-0310.2000.00497.x) [DOI] [Google Scholar]
- 66.MacIntosh AJJ, Alados CL, Huffman MA. 2011. Fractal analysis of behaviour in a wild primate: behavioural complexity in health and disease. J. R. Soc. Interface 8, 1497–1509. ( 10.1098/rsif.2011.0049) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.